Introduction
Osteoarthritis (OA), a common disease in many
middle-to-old-aged individuals, can cause pain and disability. The
imbalance of cartilage matrix synthesis and degradation are the
main pathological manifestations of OA, resulting in cartilage
degeneration (1). Chondrocytes
are the single cell type in cartilage which express
cartilage-specific proteins, such as type II collagen and
proteoglycans (2). Hence,
enhancing chondrocyte function by promoting chondrocyte
proliferation may prove to be beneficial in delaying the
progression of cartilage degradation.
The Wnt signaling pathway plays an important role in
the regulation of proliferation (3), differentiation and apoptosis
(4). The Wnt protein binds to
Frizzled family receptors and low density lipoprotein
receptor-related protein (LRP)5/6 can activate the Wnt signaling
pathway, which results in the activation of dishevelled (Dvl)
family proteins. The activation of Dvl leads to the inhibition of
glycogen synthase kinase (GSK)-3β. Without Wnt signaling, GSK-3β is
thought to phosphorylate and consequently induce the degradation of
β-catenin (5). When the kinase
activity of GSK-3β is suppressed, non-phosphorylated β-catenin can
accumulate in the cytoplasm and migrate to the nucleus, where it
interacts with the T-cell factor (TCF)/lymphoid enhancer factor
(LEF) family of transcription factors, thus altering the expression
of Wnt signaling target genes (6,7),
such as cyclin D1, which plays a key role in the G1/S transition in
the cell cycle and is a positive regulator of the G1/S transition
(8).
Bauhinia championi (Benth.) (BCB), a
dicotyledonous plant from the genus Bauhinia (Leguminosae),
has been used for dispelling wind, removing blood stasis,
activating blood circulation and relieving pain. The dry rattan of
BCB has been widely used for the treatment of OA in traditional
Chinese medicine (TCM). Previous studies have shown that
polysaccharides have vital biological activities in a number of
plants, such as inducing immunomodulatory, anti-tumorigenic and
wound-healing effects (9), as
well as promoting cell proliferation (10–13). In our previous study, BCB
polysaccharides (BCBPs) were shown to induce chondrocyte
proliferation by promoting the G1/S transition (19). However, it is yet not clear
whether BCBPs promote chondrocyte proliferation by activating the
Wnt/β-catenin signaling pathway. The aim of this study was to
determine whether BCBPs upregulate Wnt/β-catenin singaling, thus
promoting chondrocyte proliferation. Our results demonstrate that
BCBPs upregulate Wnt/β-catenin signaling in chondrocytes.
Materials and methods
Animals
Six four-week-old male Sprague-Dawley (SD) rats were
purchased from Slac Laboratory Animal Co. (Shanghai, China). The
care and use of the animals in this study complied with the
Guidance Suggestions for the Care and Use of Laboratory Animals
administered by the Ministry of Science and Technology, China
(14).
Extraction, isolation and purification of
BCBPs
Dried BCB powder (100 g) was extracted with
petroleum ether twice at 60–70°C. Subsequently, the extracted BCB
powder was dried again, extracted with 1,000 ml 80% (v/v) ethanol,
filtered, dried in air and extracted three times with boiling
distilled water (1:25, w/v) for 5 h. The extraction solution was
filtered and concentrated to 5% of the original volume with a
rotary evaporator under reduced pressure. Following deproteination
using the Sevag method (chloroform:1-butanol, 4:1) (15), the condensed solution was
precipitated by gradually adding 100% ethanol until the final
concentration reached 80%. Following overnight precipitation, the
precipitates were centrifuged and washed with 15–20 ml of ethanol,
acetone and ether. The BCBPs were obtained by freeze-drying the
precipitates, as previously described (16).
Analysis of monosaccharide
composition
BCBPs (20 mg) were hydrolyzed with 1 ml 4 M
trifluoroacetic acid (TFA) at 110°C for 2 h in a small sealed
ampoule filled with nitrogen. After being returned to room
temperature, the hydrolysate was neutralized with 0.6 M NaOH. A
total of 200 μl of stocked standard solution (2 mg/ml) or
hydrolyzed BCBP solution was mixed with 200 μl 0.6 M NaOH and 400
μl 0.4 M methanol solution of 1-phenyl-3-methyl-5-pyrazolone (PMP),
and kept in a water bath at 90°C for 70 min. After being cooled to
room temperature, 0.3 M HCl (400 μl) were added and allowed to
react with the mixture. Finally, each resulting solution was
extracted with 1 ml chloroform three times and filtered through a
0.45-μm membrane for high performance liquid chromatography
(HPLC).
BCBPs were analyzed on a HPLC system (Agilent 1200;
Agilent Technologies, Santa Clara, CA, USA), an Ultimate™ XB-C18
column (4.6×250 mm, 5 μm; Welch Materials Inc., Ellicott City, MD,
USA) and a UV detector. The analysis conditions were as follows:
mobile phase A:B, 82:18 (A, 0.1 M phosphate buffer; B,
acetonitrile); pH 6.7; flow rate, 1.0 ml/min; injection volume, 20
μl; UV detector, WL 245 nm; column temperature, 30°C. D-mannose
(Man), rhamnose (Rham), D-(+) glucuronic acid (GluUA), D-(+)
galacturonic acid (GalUA), D-glucose anhydrous (Glc), galactose
(Gal) and arabinose (Ara) were analyzed as the standard substances,
as previously described (17).
Isolation, culture and identification of
chondrocytes
The SD rats were sacrificed by cervical dislocation
and the bilateral knee joints were peeled off under sterile
conditions. The articular cartilage was cut out, and rinsed three
times in PBS (HyClone, Logan, UT, USA) containing penicillin and
streptomycin. The cartilage was cut into 1 mm3 sections,
transferred onto a dish with 0.2% collagenase II (Sigma, St. Louis,
MO, USA) and incubated in a 37°C 5% CO2 incubator.
Supernatant fluid was collected every 1.5 h, then centrifuged for 5
min (1,000 rpm), and the precipitate was resuspended in DMEM
containing 10% FBS, 50 mg/l vitamin C, 100 U/ml penicillin and 100
μg/ml streptomycin (HyClone). The cells were cultured in a 25
mm2 culture flask and placed in a 37°C 5% CO2
incubator after adjusting the cell density (3×105/ml;
termed P0). The cells were subcultured when they reached 80–90%
confluence, as determined under a microscope. Subcequently these
cells were named P1, P2 and P3, as previously described (18). The P2 chondrocytes were identified
by type II collagen immunohistochemistry.
Cell viability assay
A total of 100 μl 5×104/ml chondrocytes
were plated in a 96-well and cultured for 24 h. They were then
treated with 0, 50, 100 and 200 μg/ml BCBPs for 48 h. Subsequently,
20 μl 0.5% MTT (Sigma) were added to each well, followed by
incubation for 4 h at 37°C. The purple-blue MTT was dissolved in
150 μl DMSO with shaking for 10 min. The absorbance was measured at
490 nm on an ELISA plate reader (Model EXL800; BioTeK, Winooski,
VT, USA).
Western blot analysis
Following treatment with BCBPs, protein was
extracted from chondrocytes using lysis buffer on ice, and protein
was then measured using the BCA assay. Protein (20 μg) was
separated on a 10% SDS-PAGE gel and transferred onto PVDF
membranes. The membranes were blocked for 3 h at room temperature
in 5% non-fat dried milk, and incubated with primary antibodies
against Wnt-4, β-catenin, Frizzled-2 (Santa Cruz Biotechnology,
Inc., CA, USA), GSK-3β (Cell Signaling Technology, Inc., Beverly,
MA, USA ), cyclin D1, collagen II (Bio-Word Technology, Natong,
China) and β-actin (Santa Cruz Biotechnology, Inc.) overnight at
4°C with rocking. The membranes were incubated horseradish
peroxidase (HRP)-conjugated secondary antibody (Zhongshan
Goldenbridge Biotech, Beijing, China) at room temperature. The ECL
method was used to make the signal visible and β-actin was used as
the internal control.
RNA extraction and reverse transcription
PCR (RT-PCR)
Total RNA was isolated using TRIzol reagent
(Invitrogen, Grand Island, NY, USA). RNA (1 μg) was reverse
transcribed to cDNA according to the instructions provided by the
manufacturer. The obtained cDNA was used to determine the
expression of Wnt-4, β-catenin, Frizzled-2, GSK-3β, cyclin D1 and
collagen II; β-actin was used as an internal control. The primers
used for PCR are presented in Table
I. The conditions use for PCR were as follows: 94°C for 4 min,
94°C for 30 sec, 57°C/60°C/58°C/60°C/55°C/63°C/60°C for 45 sec,
72°C for 45 sec, for 35 cycles. The samples were analyzed by gel
electrophoresis (1.5% agarose) and were examined using a Gel
Documentation System (Model Gel Doc 2000; Bio-Rad, Hercules, CA,
USA).
 | Table ISequence of the primers used for
RT-PCR in this study. |
Table I
Sequence of the primers used for
RT-PCR in this study.
| Gene | Primer sequence
(5′→3′) |
|---|
| Wnt-4 |
| Forward | TCA GCC CAC AGG GTT
TCC A |
| Reverse | CGC TCG CCA GCA TGT
CTT T |
| β-catenin |
| Forward | AAG GAA GCT TCC AGA
CAT GC |
| Reverse | AGC TTG CTC TCT TGA
TTG CC |
| Frizzled-2 |
| Forward | TCG AGG CCA ATT CGC
AGT A |
| Reverse | CAG GAA GGA TGT GCC
GAT G |
| GSK-3β |
| Forward | AAA GTG CAT CGC TGG
CTT AT |
| Reverse | GTC GAC GGT TTG TTT
CCA AT |
| Cyclin D1 |
| Forward | AAT GCC AGA GGC GGA
TGA GA |
| Reverse | GCT TGT GCG GTA GCA
GGA GA |
| Collagen II |
| Forward | CCA GAG TGG AAG AGC
GGA GAC |
| Reverse | CAG TGG ACA GTA GAC
GGA GGA AAG |
| β-actin |
| Forward | CAC CCG CGA GTA CAA
CCT TC |
| Reverse | CCC ATA CCC ACC ATC
ACA CC |
Statistical analysis
Data are the means of three measurements and are
expressed as the means ± standard deviation. The data were
processed using the PASW package for Windows (version 18.0).
Statistical analysis of the data was performed using the Student’s
t-test and ANOVA. Values of P<0.05 were considered to indicate
statistically significant differences.
Results
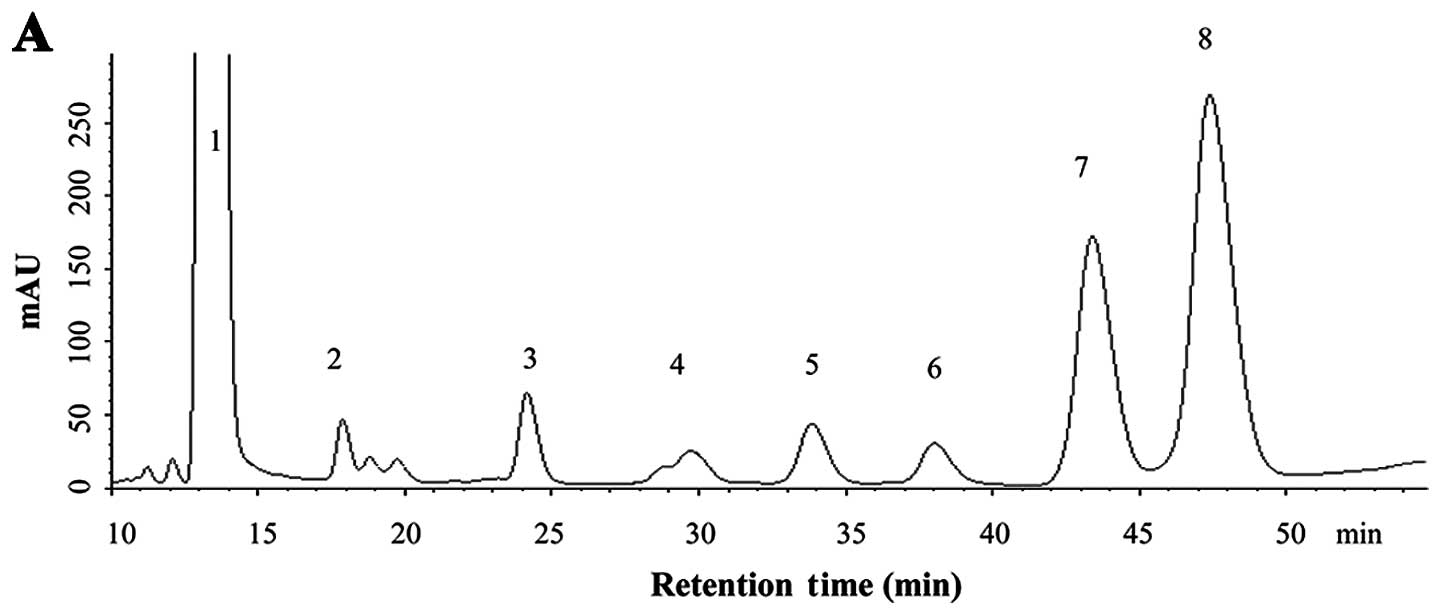
Monosaccharide composition of BCBPs
By comparing the retention time of unknown peaks to
peaks in the standard samples (Fig.
1B), the BCBPs monosaccharide composition was identified
(Fig. 1A), and contained at least
seven monosaccharides, including Man, Rham, GluUA, GalUA, Glc, Gal
and Ara. Among these, the contents of Ara and Gal were higher than
those of the other monosaccharides; the content of Ara was the
highest, while the separation of Rham, GluUA, GalUA, Glc, Gal and
Ara was easier than that of Man.
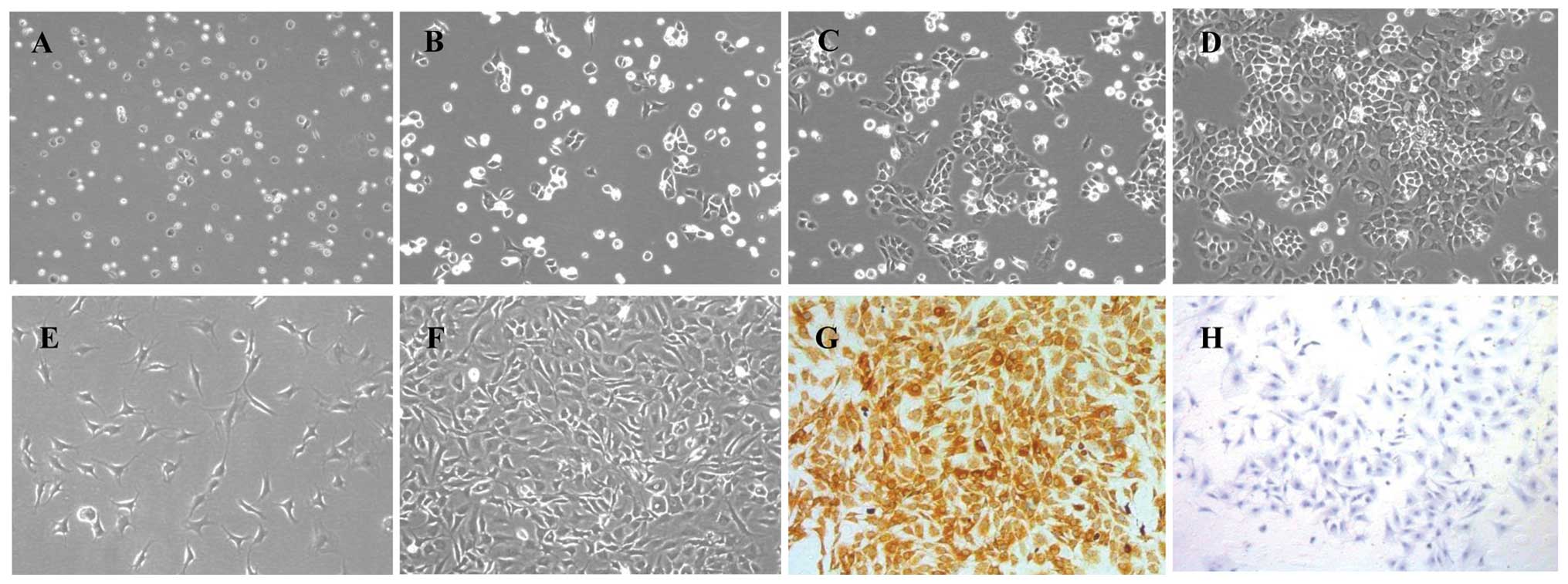
Morphological observation and
identification of chondrocytes
The primary chondrocytes resembled small balls when
first suspended in DMEM. After 24 h, the majority of the cells were
nestled against the culture flask, and their volumes became larger
(Fig. 2A and B). Three days
later, the cells grew to be tufted (Fig. 2C). The cells had typical
characteristics of chondrocytes, having a ‘flagstone’ pattern
(Fig. 2D) when confluent in a
single cell layer. The P2 and P3 chondrocytes grew much more
rapidly and became confluent cells within four to five days
(Fig. 2E and F). Collagen type
II, one of the main secreted proteins in the chondrocyte
extracellular matrix, has been commonly used for the identification
of chondrocytes by immunohistochemistry. The cytoplasm stained
brown represented the positive expression in chondrocytes.
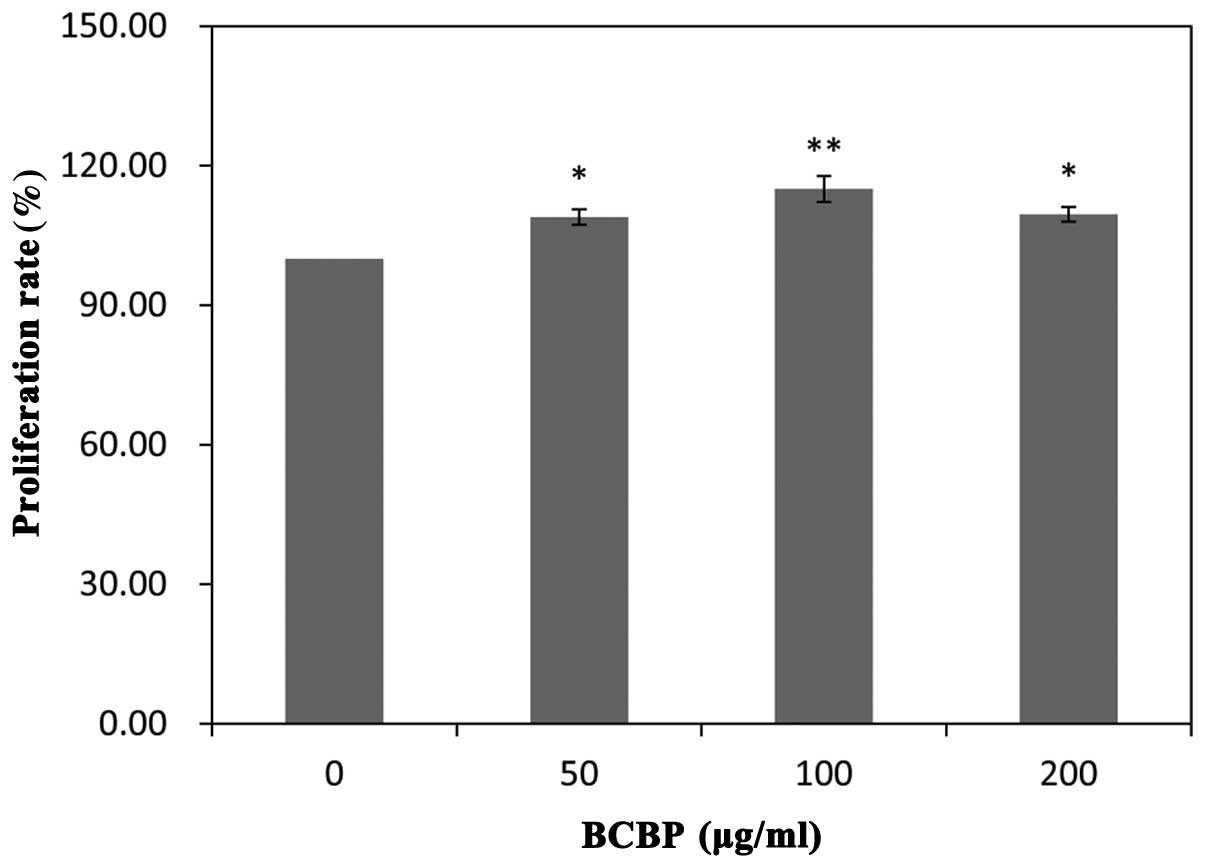
BCBPs promote chondrocyte viability
Chondrocyte viability was detected by MTT assay. As
shown in Fig. 3, following
treatment with 50, 100 and 200 μg/ml of BCBPs, the cell viability
increased by 8.96±1.66, 15.75±2.75 and 9.58±1.59, respectively when
compared with the untreated group (treated with 0 μg/ml BCBPs)
(P<0.01, P<0.05). These results confirmed that treatment with
BCBPs promoted chondrocyte proliferation in a time-dependent
manner, as shown in our previous study (19).
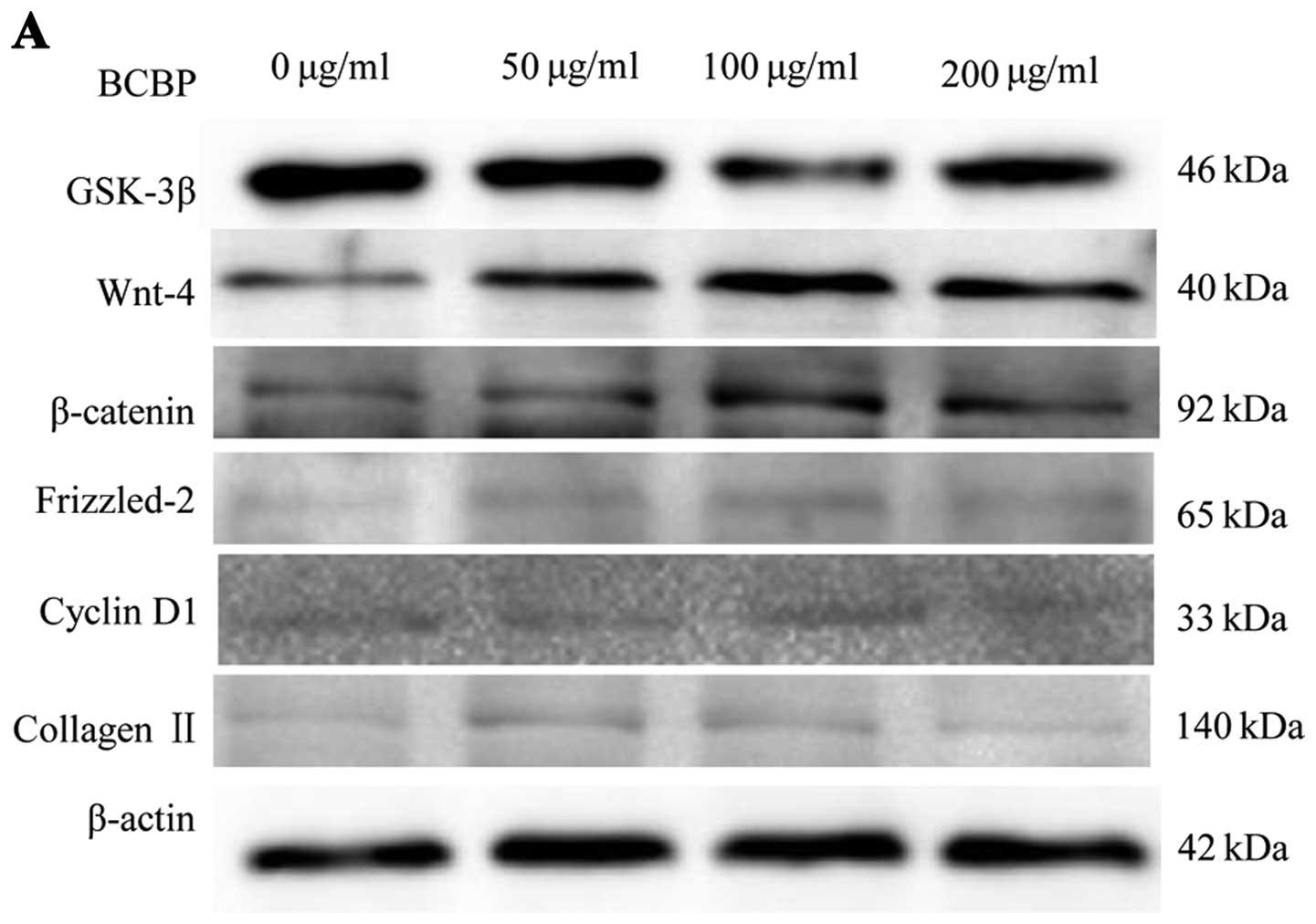
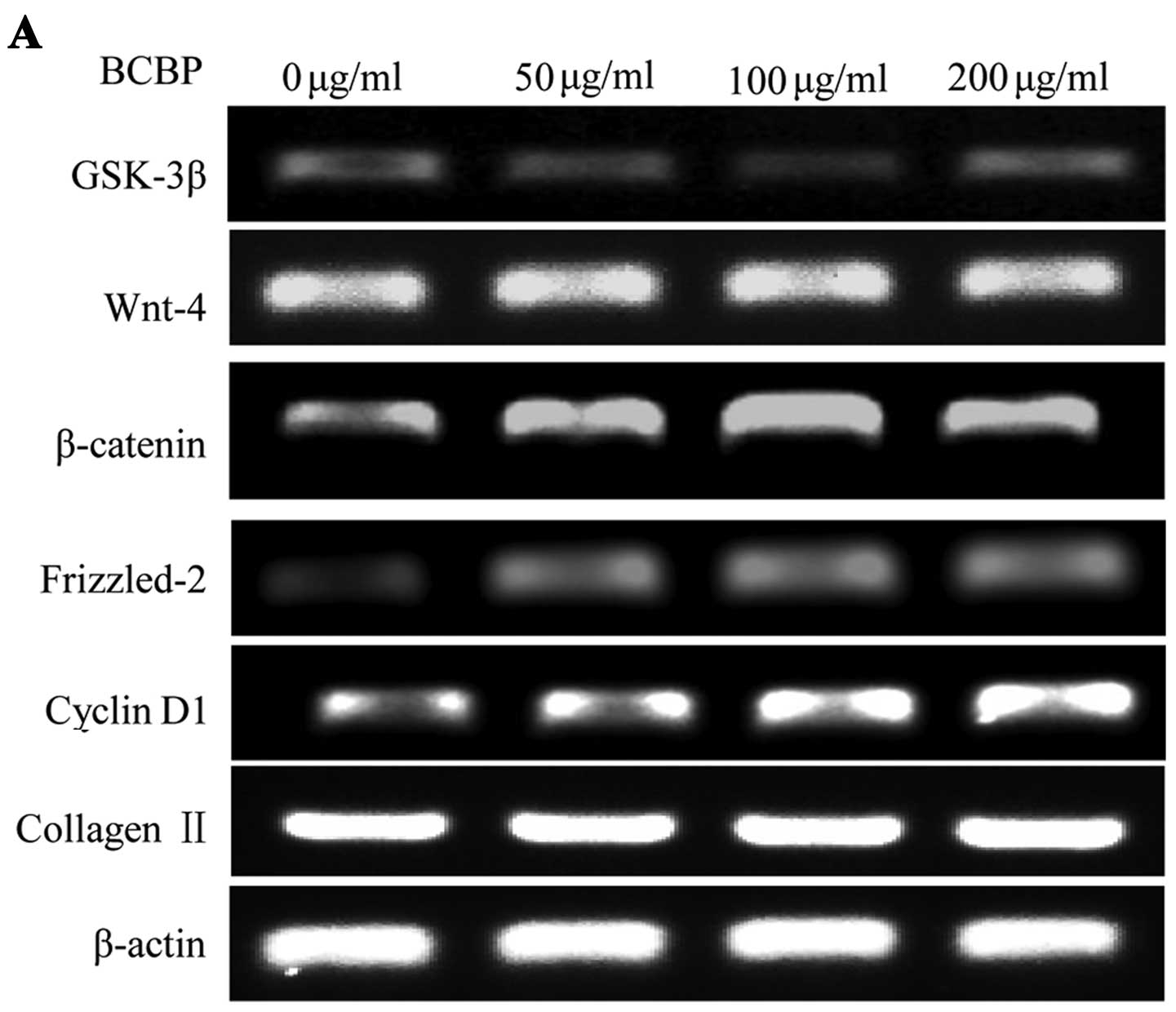
Protein and mRNA expression of GSK-3β,
Wnt-4, Frizzled-2, β-catenin, cyclin D1 and collagen II in
chondrocytes following treatment with BCBPs
To further determine the effects of BCBPs on the
Wnt/β-catenin signaling pathway in chondrocytes, we examined the
protein and mRNA expression of GSK-3β, Wnt-4, β-catenin,
Frizzled-2, cyclin D1 and collagen II by western blot analysis and
RT-PCR, respectively. Following treatment with BCBPs, the protein
levels of Wnt-4, β-catenin, Frizzled-2, cyclin D1 and collagen II
significantly increased compared with the control group (P<0.01,
P<0.05). Compared with the control group (P<0.01, P<0.05),
the protein expression of GSK-3β in the BCBP-treated chondrocytes
was significantly downregulated (Fig.
4). The mRNA expression levels of GSK-3β, Wnt-4, β-catenin,
Frizzled-2, cyclin D1 and collagen II were similar to their
respective protein levels (Fig.
5). Taken together, our results indicate that BCBPs upregulate
the protein and mRNA levels of Wnt-4, β-catenin, Frizzled-2, cyclin
D1 and collagen II, and downregulate the levels of GSK-3β.
Discussion
The Wnt/β-catenin signaling pathway plays an
important role in a number of cellular events, such as cell
proliferation, migration and differentiation. The activation of
this pathway in different cells and the consequences of such an
activation have been extensively discussed. A number of studies
have reported that the Wnt/β-catenin signaling pathway is necessary
for normal and abnormal cell proliferation, such as neural
progenitor cells (20), glial
cells (21) and a variety of of
tumor cells (22). A number of
studies have demonstrated that the Wnt/β-catenin signaling pathway
is closely related to cartilage function, including cartilage
development, chondrocyte differentiation and plays an important
role in the progression of OA (23,24). In this study, our results revealed
that BCBPs upregulated the protein and mRNA expression of Wnt-4,
β-catenin, Frizzled-2, cyclin D1 and collagen II, whereas BCBPs
downregulated GSK-3β expression levels, suggesting that BCBPs
activate the Wnt/β-catenin signaling pathway, thus promoting
chondrocyte proliferation.
Currently, the therapeutic strategies for OA are
exercise combined with the use of analgesics and non-steroidal
anti-inflammatory drugs (25);
however, these treatments only focus on alleviating the symptoms,
which are mainly pain and inflammation, and fail to obtain good
results. Non-steroidal anti-inflammatory drugs have several
side-effects, such as gastrointestinal-related toxicities and
cardiovascular risks. Corticosteroids are also applied for the
treatment of OA, but their substantial toxicities limit long term
usage. Chinese herbs have been used in the treatment of OA for
thousands of years (26). BCBPs,
extracted from BCB are one of the main effective elements used for
OA therapy.
Chondrocytes, the only cell type that is present in
mature cartilage, are important to maintain the balance of
cartilage matrix synthesis, as well as the function of the
articular cartilage. In recent years, certain studies have
demonstrated that osteoarthritic chondrocytes have a low
proliferative activity (27,28). Thus, promoting chondrocyte
proliferation may be an efficient method for the treatment of OA.
In our previous study, we demonstrated that BCBPs promoted
chondrocyte proliferation (19);
however, the precise molecular mechanisms responsible for the
effects of BCBPs on chondrocyte proliferation have not yet been
fully elucidated. In this study, we investigated the mechanisms
responsible for the effects of BCBPs on the Wnt/β-catenin signaling
pathway in chondrocytes to build a scientific foundation for the
use of BCBPs in the treatment of OA.
The Wnt/β-catenin signaling pathway plays a crucial
role in the processes of cell proliferation and differentiation
(29), the early stage of
cartilage formation, chondrocyte differentiation and endochondral
bone formation. The key factor of the Wnt/β-catenin signaling
pathway is the stable accumulation of β-catenin in the cytosol and
its transfer to the nucleus, binding to the transcription factors,
TCF and LEF. These factors regulate the expression of cyclin D1,
which is an important factor in cell proliferation and
differentiation (30). The
activation of the Wnt/β-catenin signaling pathway activates cyclin
D1, accelerating cell cycle progression. Our results demonstrated
that BCBPs increased chondrocyte viability, and significantly
upregulated the protein and mRNA expression of Wnt-4, Frizzled-2
and β-catenin in the chondrocytes, whereas the expression of GSK-3β
in the chondrocytes was significantly downregulated, suggesting
that the BCBPs activated the Wnt/β-catenin signaling pathway by
inhibiting the activity of GSK-3β, causing non-phosphorylated
β-catenin accumulation and transfer to the nucleus, where it
interacts with TCF/LEF to enhance the expression of cyclin D1.
In conclusion, our results demonstrated that the
activation of the Wnt/β-catenin signaling pathway induced by
treatment with BCBPs contributed to chondrocyte proliferation.
Apart from the Wnt/β-catenin signaling pathway, there are multiple
signaling pathways controlling the proliferation of chondrocytes.
Hence, additional studies are required to explore the cross-talk
function of the signaling pathways during the process of
chondrocyte proliferation.
Acknowledgements
This project was financially supported by NSFC
(81202912), the Natural Science Foundation of Fujian Province
(2011J05074&2011J01035), the Developmental Fund of Chen Keji
Integrative Medicine (CKJ20110003).
References
|
1
|
Pitsillides AA and Beier F: Cartilage
biology in osteoarthritis-lessons from developmental biology. Nat
Rev Rheumatol. 7:654–663. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu Q, Zhu M, Rosier RN, Zuscik MJ, O’Keefe
RJ and Chen D: Beta-catenin, cartilage, and osteoarthritis. Ann N Y
Acad Sci. 1192:344–350. 2010.PubMed/NCBI
|
|
3
|
Tao HY, He B, Liu SQ, et al: Effect of
carboxymethylated chitosan on the biosynthesis of NGF and
activation of the Wnt/β-catenin signaling pathway in the
proliferation of Schwann cells. Eur J Pharmacol. 702:85–92.
2013.PubMed/NCBI
|
|
4
|
Lu W, Tinsley HN, Keeton A, Qu Z, Piazza
GA and Li Y: Suppression of Wnt/β-catenin signaling inhibits
prostate cancer cell proliferation. Eur J Pharmacol. 602:8–14.
2009.
|
|
5
|
Akiyama T: Wnt/β-catenin signaling.
Cytokine Growth Factor Rev. 11:273–282. 2000.
|
|
6
|
Li X, Peng J, Wu M, et al: BMP2 promotes
chondrocyte proliferation via the Wnt/β-catenin signaling pathway.
Mol Med Rep. 4:621–626. 2011.PubMed/NCBI
|
|
7
|
Blom AB, van Lent PL, van der Kraan PM and
van den Berg WB: To seek shelter from the Wnt in osteoarthritis?
Wnt-signaling as a target for osteoarthritis therapy. Curr Drug
Targets. 11:620–629. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vlad-Fiegen A, Langerak A, Eberth S and
Müller O: The Wnt pathway destabilizes adherens junctions and
promotes cell migration via β-catenin and its target gene cyclin
D1. FEBS Open Bio. 2:26–31. 2012.PubMed/NCBI
|
|
9
|
Schepetkin IA and Quinn MT: Botanical
polysaccharides: Macrophage immunomodulation and therapeutic
potential. Int Immunopharmacol. 6:317–333. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu F, Li X, Cai L, et al: Achyranthes
bidentata polysaccharides induce chondrocyte proliferation via
the promotion of the G1/S cell cycle transition. Mol Med Rep.
7:935–940. 2013.
|
|
11
|
Gescher K and Deters AM: Typha
latifolia L. fruit polysaccharides induce the differentiation
and stimulate the proliferation of human keratinocytes in vitro. J
Ethnopharmacol. 137:352–358. 2011. View Article : Google Scholar
|
|
12
|
Liu H, Fan Y, Wang W, Liu N, Zhang H, Zhu
Z and Liu A: Polysaccharides from Lycium barbarum leaves:
isolation, characterization and splenocyte proliferation activity.
Int J Bio Macromol. 51:417–422. 2012.
|
|
13
|
Yao H, Chen Y, Li S, Huang L, Chen W and
Lin X: Promotion proliferation effect of a polysaccharide from
Aloe barbadensis Miller on human fibroblasts in vitro. Int J
BioMacromol. 45:152–156. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
The Ministry of Science and Technology of
the People’s Republic of China. Guidance Suggestion for the Care
and Use of Laboratory Animals. 2006.
|
|
15
|
Sevag MG: A new physical de-proteination
method for representation of biologically effective
substances-isolation of carbohygrates in chicken-protein and
pneumococci. Biochemis Zeitschrift. 273:419–429. 1934.
|
|
16
|
Li X, Zhang H and Xu H: Analysis of
chemical components of shiitake polysaccharides and its
anti-fatigue effect under vibrational. Int J Bio Macromol.
45:377–380. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang Q, Wang S, Xie Y, Sun J and Wang J:
HPLC analysis of Ganoderma lucidum polysaccharides and its effect
on antioxidant enzymes activity and Bax, Bcl-2 expression. Int J
Biol Macromol. 46:167–172. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li X, Du M, Liu X, Chen W, Wu M, Lin J and
Wu G: Milimeter wave treatment promote chondrocyte proliferation by
upregulating the expression of cyclin-dependent kinase 2 and cyclin
A. Int J Mol Med. 26:77–84. 2010.PubMed/NCBI
|
|
19
|
Cai L, Ye H, Yu F, Li H, Chen J and Liu X:
Effects of Bauhinia championii (Benth.) Benth
polysaccharides on the proliferation and cell cycle of
chondrocytes. Mol Med Rep. 7:1624–1630. 2013.
|
|
20
|
Hirsch C, Campano LM, Wöhrle S and Hecht
A: Canonical Wnt signaling transiently stimulates proliferation and
enhances neurogenesis in neonatal neural progenitor cultures. Exp
Cell Res. 313:572–587. 2007. View Article : Google Scholar
|
|
21
|
Chen Y, Guan Y, Liu H, et al: Activation
of the Wnt/β-catenin signaling pathway is associated with glial
proliferation in the adult spinal cord of ALS transgenic mice.
Biochem Biophys Res Commun. 420:397–403. 2012.
|
|
22
|
Kang K, Lee KM, Yoo JH, Lee HJ, Kim CY and
Nho CW: Dibenzocyclooctadie-ne lignans, gomisins J and N inhibit
the Wnt/β-catenin signaling pathway in HCT116 cells. Biochem
Biophys Res Commun. 428:285–291. 2012.PubMed/NCBI
|
|
23
|
Kitagaki J, Iwamoto M, Liu JG, Tamamura Y,
Pacifci M and Enomoto-lwamoto M: Activation of β-catenin -LEF/TCF
signal pathway in chondrocytes stimulates ectopic endochondral
ossification. Osteoarthritis Cartilage. 11:36–43. 2003.
|
|
24
|
Andrade AC, Nilsson O, Barnes KM and Baron
J: Wnt gene expression in the post-natal growth plate: regulation
with chondrocyte differentiation. Bone. 40:1361–1369. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Van Vijven JP, Luijsterburq PA, Verhagen
AP, van Osch GJ, Kloppenburg M and Bierma-Zeinstra SM: Symptomatic
and chondroprotective treatment with collagen derivatives in
osteoarthritis: a systematic review. Osteoarthritis Cartilage.
20:809–821. 2012.PubMed/NCBI
|
|
26
|
Kang M, Junq I, Hur J, et al: The
analgesic and anti-inflammatory effect of WIN-34B, a new herbal
formula for osteoarthritis composed of Lonicera japonica
Thunb and Anemarrhena asphodeloides BUNGE in vivo. J
Ethnopharmacol. 131:485–496. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chan BY, Fuller ES, Russell AK, et al:
Increased chondrocyte sclerostin may protect against cartilage
degradation in osteoarthritis. Osteoarthritis Cartilage.
19:874–885. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang JG, Xia C, Zheng XP, Yi TT, Wang XY,
Song G and Zhang B: 17β-Estradiol promotes cell proliferation in
rat osteoarthritis model chondrocytes via PI3K/Akt pathway. Cell
Mol Biol Lett. 16:564–575. 2011.
|
|
29
|
Wang C, Yuan X and Yang S: IFT80 is
essential for chondrocyte differentiation by regulating Hedgehog
and Wnt signaling pathways. Exp Cell Res. 319:623–632. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bueno MJ and Malumbres M: MicroRNAs and
the cell cycle. Biochim Biophys Acta. 1812:592–601. 2011.
View Article : Google Scholar : PubMed/NCBI
|