Introduction
Branch retinal vein occlusion (BRVO) is the second
most common cause of retinal vascular abnormality after diabetic
retinopathy and a frequent cause of visual loss (1). In a pooled analysis using existing
data from 11 individual population-based studies, the prevalence of
BRVO was found to be 4.42 per 1,000 individuals (95% CI 3.65,
5.19). The prevalence of BRVO is greater in Asians (2,3).
Visual loss in BRVO, either short or long term, may be the result
of the presence of macular edema, macular non-perfusion, retinal
neovascularization, vitreous or intraretinal hemorrhage, tractional
retinal detachment or a combination of these disorders (4). Macular edema is the most frequent
cause of visual impairment in patients with BRVO (5). Thus, understanding the cellular and
molecular factors that underlie the pathogenesis of macular edema
with BRVO is of critical importance.
The aqueous humor (AH) is an important intraocular
fluid responsible for the supply of nutrients to and the removal of
metabolic wastes from the avascular tissues of the eye. It is known
that protein levels in AH are altered in various eye diseases,
including anterior and posterior segment disorders. In addition, a
number of studies have demonstrated that some proteins whose
expression is altered in AH correlate with the mechanisms or
prognosis of several eye disorders (6). A number of cytokines and other
factors in the AH have been suggested to be involved in the
pathogenesis of macular edema due to BRVO, such as vascular
endothelial growth factor (VEGF) and interleukin-6 (IL-6) (5). However, the pathogenesis of macular
edema with BRVO is complex; thus, the measurement of these
cytokines may not provide enough information as to the disease
process. A comprehensive list of the proteins whose expression is
altered in the AH of patients with macular edema due to BRVO is
still lacking.
Proteomic analysis is a valuable method for
elucidating the molecular nature of AH (7). High resolution 2-dimensional (2D)
polyacrylamide gel electrophoresis (PAGE) is a technique used for
the analysis of several hundred proteins in tissues, fluids or
cells using only a few microliters of sample and is therefore ideal
for analyzing limited volumes of AH. Some researchers have used
this technology to explore the pathogenesis of various eye diseases
(6,8–12).
In this study, we used proteomics as a means to identify
disease-specific proteins in AH. Through comparative analyses of
the proteomes in patients with cataract (controls) and those with
macular edema due to BRVO, it may be possible to obtain a better
understanding of the molecular events involved in the development
of macular edema due to BRVO and to generate essential data
required for the identification of novel biomarkers and/or
treatments. The proteomic techniques used include protein
separation by 2-DE and characterization by mass spectrometry (MS)
of peptides, amino acid sequencing and bioinformatics analysis.
Enzyme-linked immunosorbent assay (ELISA) was used to validate the
results of proteomics.
Materials and methods
Patients and controls
Twelve AH samples were included in this study, 6
from patients with BRVO-induced macular edema (mean age, 53±4.98
years; 3 males and 3 females) and 6 from age-matched patients with
cataract without BRVO (mean age, 53.5±2.35 years; 3 males and 3
females). The disease course was between 6 and 16 months (mean,
10±3.406 months). Clinical data from the patients are summarized in
Table I.
 | Table IData from patients with BRVO and the
controls. |
Table I
Data from patients with BRVO and the
controls.
| No. | Age (years) | Gendera | Course of disease
(months) | Baseline central
macular thickness (μm) |
|---|
| Patients | 1 | 56 | F | 16 | 496 |
| 2 | 47 | F | 6 | 523 |
| 3 | 60 | M | 10 | 457 |
| 4 | 48 | F | 8 | 539 |
| 5 | 52 | M | 11 | 431 |
| 6 | 55 | M | 9 | 511 |
| Controls | 1 | 56 | M | | |
| 2 | 52 | F | | |
| 3 | 50 | M | | |
| 4 | 54 | M | | |
| 5 | 56 | F | | |
| 6 | 53 | F | | |
The study followed the tenets of the Declaration of
Helsinki, and informed written consent was obtained from all
patients and controls after we explained the nature and possible
consequences of the study. The protocol for this research project
was approved by the Ethics Committee of the First Affiliated
Hospital of Nanjing Medical University, Nanjing, China.
All participants went through a standard examination
including best-corrected visual acuity, slit lamp biomicroscopy,
optical coherence tomography (OCT), fundus photo, and fluorescein
angiography (FFA). The presence of macular edema was confirmed with
FFA and OCT in all patients. No patient had been treated previously
for BRVO. None of the controls had any eye diseases other than
cataract.
In both groups of examined patients (controls and
BRVO), a certain degree of cataract was present. Patients with
severe cataract determining blindness or unacceptable vision were
not included in the control group.
Sample collection
AH samples were obtained from the eyes of patients
with BRVO just before an intravitreal injection of bevacizumab
(Avastin, treatment for macular edema due to BRVO) was
administered. All sample collections were performed using a
standard sterilization procedure as previously described (12). A mean volume of 100 μl of AH was
collected by anterior chamber limbal paracentesis with a 27-gauge
needle attached to an insulin syringe. The intravitreal injection
of bevacizumab was then administered through the pars plana.
Antibiotic ointment was administered after surgery for 4 days.
Immediately after collection, the AH samples were transferred to
sterile plastic tubes and stored at −80°C until analysis.
AH samples from patients before cataract surgery
were obtained for this study. AH samples from 6 controls (cataract
patients without other eye diseases) were also collected as
previously described (8). A total
of 100–200 μl of sample from each patient sample was pooled. All
samples were stored at −80°C until analysis.
Sample preparation
AH samples from patients or controls were pooled to
ensure there was sufficient protein in the extracts for
matrix-assisted laser desorption ionization
time-of-flight/time-of-flight mass spectrometry (MALDI-TOF/TOF
MS).
Excess salts followed by precipitation of proteins
using the ProteoExtract™ Protein Precipitation kit (Calbiochem, San
Diego, CA, USA) were removed in each of the pooled samples. The
samples were processed according to the manufacturer’s
instructions. The protein concentrations of the AH samples were
determined using the Bradford method (Bio-Rad Protein Assay;
Bio-Rad, Hercules, CA, USA).
2-DE
2-DE was performed as previously described (13–15). Twenty-four centimeter, pH 4–7, NL
IPG strips (Amersham Bioscience, Uppsala, Sweden) were rehydrated
with 80 μg solubilized protein (for silver staining) in a
rehydration buffer. After isoelectric focusing, the IPG strips were
equilibrated. They were then loaded onto pre-cast 12.5% homogeneous
polyacrylamide gels for electrophoresis, ran in an Ettan-Dalt II
system (Amersham Biosciences, San Francisco, CA, USA) and
visualized.
Image analysis
The stained gels were scanned and the resulting
images were analyzed using ImageMaster™ 2D Platinum software
(version 5.0, Amersham Bioscience, Swiss Institute of
Bioinformatics, Geneva, Switzerland) for spot detection,
quantification, comparison and analyses, as previously described
(12,13). The relative intensities of the
spots were used for a comparison between the BRVO and control
groups. The statistical comparisons between the intensity of the
control and the BRVO protein spots were conducted using the
Student’s t-test (ImageMaster™ 2D platinum software, with p<0.05
considered to be significant). The commonly differentially
expressed spots (2-fold increase or decrease) were further
identified by MALDI-TOF/TOF MS.
Protein identification
Protein identification was performed as previously
described (12,14,16,17). In brief, the common differentially
expressed protein spots were excised and the proteins within were
reduced, alkylated and digested with trypsin. Digests were
immediately spotted onto 600 μm anchorchips (Bruker Daltonics,
Bremen, Germany). The Bruker Peptide Calibration Mixture was
spotted for external calibration. MALDI-TOF MS and tandem TOF/TOF
MS were carried out on a time-of-flight Ultraflex II mass
spectrometer (Bruker Daltonics). Using the MASCOT search engine
[http://www.matrixscience.com; Database:
NCBInr 20100409 (10,820,686 sequences; 3,689,795,467 residues);
Taxonomy: Homo sapiens (human) (231,301 sequences)] based on
the Swiss-Prot protein database, peptide mass fingerprinting was
performed for the identification of proteins from tryptic fragment
sizes using the assumption that peptides are monoisotopic. One
missed trypsin cleavage was allowed. A mass tolerance of 100 parts
per million (ppm) was the window of error allowed for matching the
peptide mass values.
Gene Ontology analysis
All the proteins identified in this experiment were
subjected to Gene Ontology (www.geneontology.org/) for molecular function,
biological process and cellular component analysis.
ELISA
Concentrations of alpha crystallin A chain (CRYAA)
in the AH samples were verified and quantified using commercially
available human cytokine ELISA kits from Uscn Life Science Inc.
(Catalog no. E9662h; Wuhan, China). The recommended protocol of the
manufacturer was followed in all cases. Briefly, standards and AH
samples were added to antibody-coated 96-well plates and incubated
for 2 h at room temperature, followed by the addition of
biotin-conjugated polyclonal antibody specific for CRYAA and
incubation for an additional 1 h. The plates were then washed and
incubated with avidin conjugated to horseradish peroxidase for 1 h
at 37°C. Subsequently, a tetramethylbenzidine substrate solution
was added to each well. The enzyme-substrate reaction was
terminated by the addition of sulfuric acid solution. The color
change was measured by spectrophotometry at a wavelength of 450 nm.
A standard curve was plotted from measurements made with the
standard solution (from 0.78 to 50 ng/ml for CRYAA) and was used to
determine the concentration of CRYAA in each sample. The
concentration of CRYAA in the samples was determined by comparing
the OD of the samples to the standard curve. All measurements were
performed in duplicate. CRYAA concentrations were calculated as per
nanogram of protein.
Statistical analysis
The protein spots were visualized using ImageMaster™
2D Platinum software as described in the image analysis section.
The variation in protein spot intensity within a sample map and
between 2 sample maps was analyzed using the Student’s t test. The
ELISA results were also analyzed using the Student’s t test.
Results
Protein content in AH from patients with
BRVO and controls
A total of 12 AH samples were included in this
study, 6 from patients with BRVO and 6 from age-matched cataract
patients without BRVO. There was no statistically significant
difference between the 2 groups as regards age (p=0.074). Clinical
data from the patients are summarized in Table I.
The mean total protein level in AH from patients
with BRVO was 1.124 mg/ml, while that from the controls was 0.545
mg/ml. Total protein levels in the patients with BRVO were
significantly greater than those of the controls.
2-DE patterns
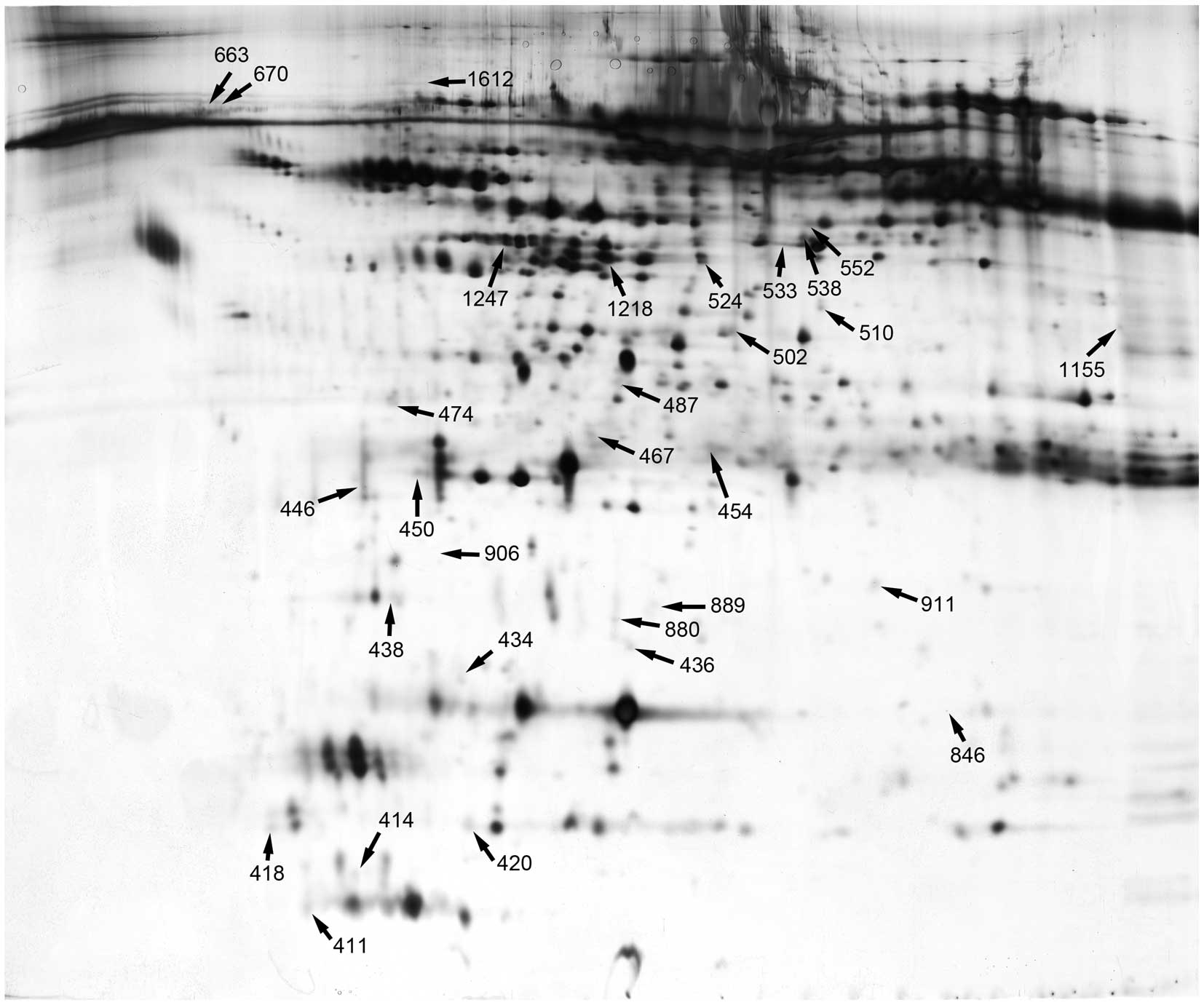
Figs. 1 and
2 depict the 2D gel images from
patients and the controls. Gel images from patients with BRVO
displayed more spots and more intensely silver stained spots than
the gel images from the controls. There were significant
differences in relative spot volumes (% volume) in the gel
patterns; patients with BRVO showed greater volumes than the
controls. The stained gels were scanned and the resulting images
were analyzed using ImageMaster™ 2D Platinum software for spot
detection, quantification, comparison and analyses. A total of 56
protein spots were altered by >2-fold in the 2D gels from
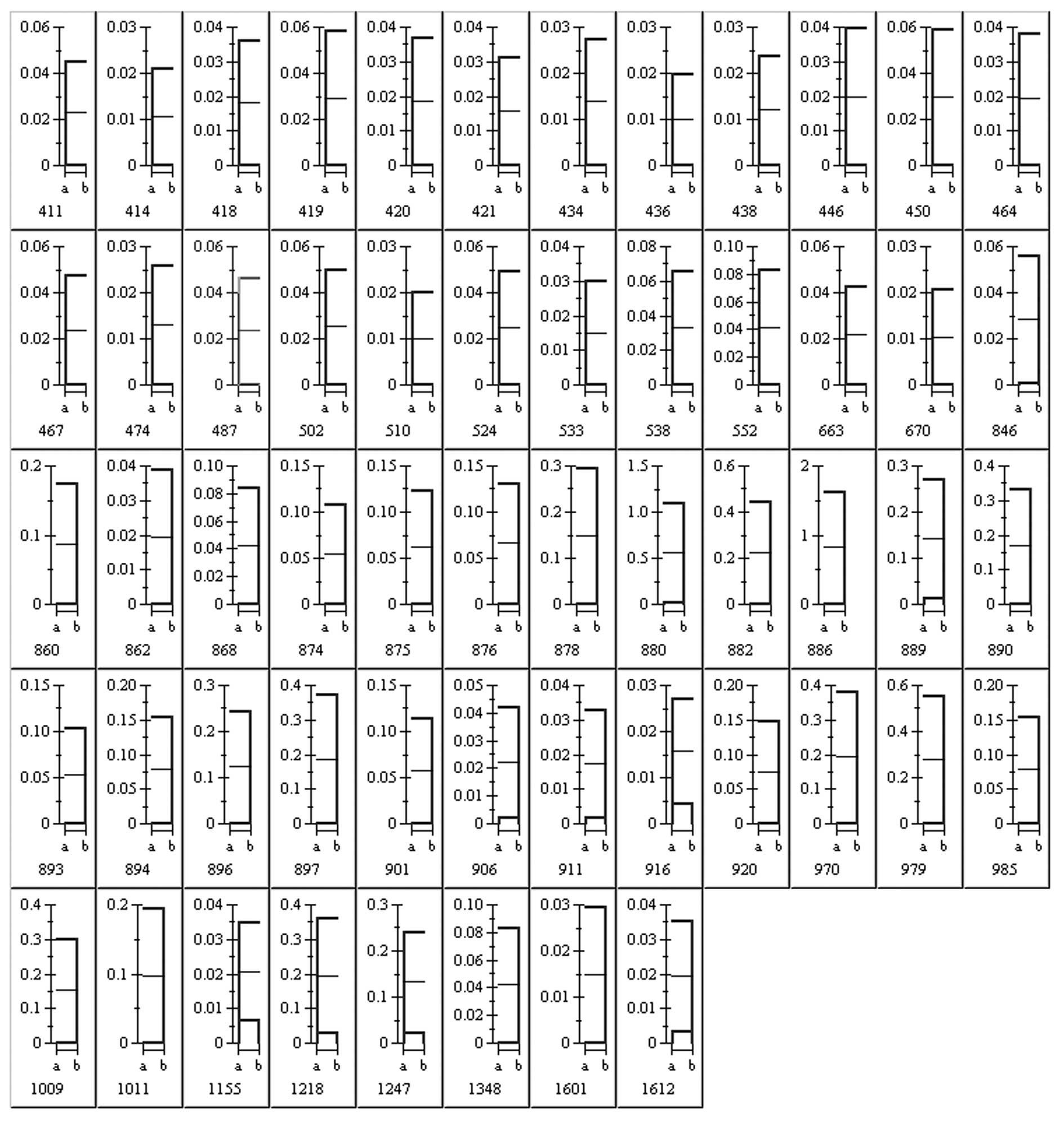
patients with BRVO-induced macular edema (Fig. 3).
Identification of proteins
Based on the results presented above, 56 protein
spots were isolated for further analysis. Each spot was acquired
from the gel and digested extensively with trypsin. The resulting
peptides were applied to a MALDI TOF/TOF MS for measurements. A
total of 49 protein spots were identified by MS, including
fibroblast growth factor-4 (FGF-4), hepatoma-derived growth factor
(HDGF) and crystallins. Many of these proteins have been implicated
in angiogenesis, oxidative stress and collagen synthesis. The
identified proteins [name, function, molecular weight (MW),
isoelectric point (PI) and sequence coverage] are listed in
Table II.
 | Table IIIdentified proteins associated with
BRVO-induced macular edema. |
Table II
Identified proteins associated with
BRVO-induced macular edema.
| ID | Accession
no.a | Definition | Name | Molecular
functionb | Biological
process | Cellular
component | Mascot score | Nominal mass
(Mr) | Calculated pI
value | Sequence
coveragec | No. of matched
peptides | Ratiod |
|---|
| 411 | NP_085915 | Lens intrinsic
membrane protein 2, 19 kDa isoform 1 | LIM2 | Structural
constituent of eye lens | Cell-cell junction
assembly | Cell
junction/integral to membrane | 161 | 24,514 | 10.04 | 30% | 11 | Bs only |
| 414 | AAH57766 | Ninjurin-2 | NINJ2 | Protein
binding | Nervous system
development/neuron cell-cell adhesion/tissue regeneration | Integral to plasma
membrane | 172 | 17,461 | 9.52 | 53% | 15 | Bs only |
| 418 | NP_034601 | Neuron-specific
calcium-binding protein hippocalcin | Hpca | Actin
binding/calcium ion binding | | ND | 200 | 22,527 | 4.87 | 72% | 14 | Bs only |
| 420 | NP_037370 | DnaJ homolog
subfamily C member 15 | DNAJC15 | Heat shock protein
binding | ND | Cytoplasm/focal
adhesion/integral to membrane/nucleus | 443 | 16,373 | 10.08 | 100% | 27 | Bs only |
| 434 | BAC81643 | PMS2-C
terminal-like | PMS2CL | ATP
binding/mismatched DNA binding | Mismatch
repair | ND | 182 | 21,851 | 5.85 | 58% | 14 | Bs only |
| 436 | AAF37290 | p37 TRAP/SMCC/PC2
subunit | Mediator of RNA
polymerase II transcription subunit 27 (MED27) | Protein
binding/transcription co-activator activity/transcription regulator
activity | Regulation of
transcription from RNA polymerase II promoter/transcription
initiation from RNA polymerase II promoter |
Cytoplasm/nucleolus/transcription factor
complex | 202 | 31,540 | 9.45 | 52% | 17 | Bs only |
| 438 | AAH45686 | ANAPC5 protein | ANAPC5 | Binding | ND | ND | 123 | 36,007 | 7.18 | 40% | 10 | Bs only |
| 446 | BAG62828 | cDNA FLJ58860,
highly similar to Homo sapiens trichoplein, mRNA | | ND | ND | ND | 300 | 27,353 | 9.69 | 60% | 33 | Bs only |
| 450 | NP_001138549 | LRRC10-like protein
ENSP00000367315 | | Protein
binding | ND | ND | 201 | 32,864 | 6.88 | 63% | 17 | Bs only |
| 454 | AAH20843 | HAVCR2 protein | Hepatitis A virus
cellular receptor 2 | ND | ND | Integral to
membrane | 119 | 16,594 | 5.15 | 27% | 9 | Bs only |
| 467 | AAK07550 | PNAS-139 | | ND | Regulation of actin
filament polymerization | Cytoskeleton | 170 | 22,952 | 5.83 | 50% | 13 | Bs only |
| 474 | EAW87342 | hCG15971, isoform
CRA_b | | ND | ND | ND | 228 | 13,907 | 5.24 | 78% | 16 | Bs only |
| 487 | Q6NV95 | Putative protein
PABPC1-like | | ND | ND | ND | 146 | 30,256 | 8.88 | 43% | 16 | Bs only |
| 502 | AAP49001 | Programmed death
ligand 2 type III isoform | PDCD1LG2 | Receptor
activity | Immune
response | Endomembrane
system/extracellular region/integral to membrane/plasma
membrane | 96 | 2,1017 | 6.94 | 25% | 8 | Bs only |
| 510 | AAF64257 | BM-001
(cyclin-L1) | CCNL1 | ND | Regulation of
transcription/transcription | Nuclear speck | 121 | 37,420 | 11.18 | 29% | 13 | Bs only |
| 524 | NP_001092 | Actin, cytoplasmic
1 (β actin) | ACTB | ATP binding/kinesin
binding/nitric-oxide synthase binding/nucleotide binding/protein
binding/protein kinase binding/structural constituent of
cytoskeleton |
Axonogenesis/cellular component
movement | MLL5-L complex/NuA4
histone acetyltransferase complex/axon/cortical
cytoskeleton/cytoplasm/cytoskeleton/cytosol/protein
complex/ribonucleoprotein complex/soluble fraction | 109 | 42,052 | 5.29 | 31% | 11 | Bs only |
| 533 | BAG58981 | cDNA FLJ55029,
highly similar to creatine kinase, ubiquitous mitochondrial (EC
2.7.3.2) | | Kinase
activity | ND | ND | 61 | 16,823 | 7.82 | 21% | 5 | Bs only |
| 538 | NP_000468 | Isoform 1 of serum
albumin precursor | ALB | DNA
binding/antioxidant activity/chaperone binding/copper ion
binding/drug binding/fatty acid binding/metal ion binding/oxygen
binding/protein binding/pyridoxal phosphate binding/toxin
binding | Cellular response
to starvation/hemolysis by symbiont of host
erythrocytes/maintenance of mitochondrion location/negative
regulation of apoptosis/negative regulation of programmed cell
death/transport | Extracellular
region/extracellular space/platelet α granule lumen/protein
complex | 62 | 71,317 | 5.92 | 11% | 9 | Bs only |
| 552 | NP_878907 | Prolyl
4-hydroxylase, alpha III subunit precursor | P4HA3 | L-ascorbic acid
binding/iron ion binding/metal ion binding/oxidoreductase activity,
acting on paired donors, with incorporation or reduction of
molecular oxygen/oxidoreductase activity, acting on single donors
with incorporation of molecular oxygen, incorporation of two atoms
of oxygen/procollagen-proline 4-dioxygenase activity | Oxidation
reduction | Endoplasmic
reticulum/endoplasmic reticulum lumen | 64 | 61,430 | 6.05 | 16% | 8 | Bs only |
| 663 | EAW48192 | Minichromosome
maintenance deficient isoform CRA_b | | | ND | ND | 183 | 63,435 | 5.89 | 34% | 22 | Bs only |
| 670 | NP_786923 | Oligodendrocyte
transcription factor 3 | OLIG3 | DNA
binding/transcription regulator activity | Regulation of
transcription/transcription |
Cytoplasm/nucleus/plasma membrane | 340 | 29,567 | 9.54 | 56% | 36 | Bs only |
| 846 | AAU34193 | AKNA transcript
F2 | AKNA | ND | ND | ND | 130 | 45,166 | 10.66 | 29% | 10 | −92.434 |
| 860 | NP_000385 | Alpha crystallin A
chain | CRYAA | Protein
binding/structural constituent of eye lens/unfolded protein
binding | M phase specific
microtubule process/actin filament
organization/anti-apoptosis/camera-type eye development/lens fiber
cell morphogenesis/mitochondrion organization/negative regulation
of intracellular transport/protein homooligomerization/protein
refolding/response to heat/response to stimulus/visual
perception | Cytoplasm | 72 | 20,011 | 5.77 | 45% | 7 | Cs only |
| 862 | Q14917 | SPTAN1 protein
(fragment) | SPTAN1 | Actin
binding/protein binding/calcium ion binding/calmodulin
binding/structural constituent of cytoskeleton | Actin filament
capping | Cell
cortex/cytoplasm/cytoskeleton/cytosol/membrane
fraction/spectrin | 63 | 54,468 | 5.72 | 24% | 8 | Cs only |
| 868 | A6NMY6 | Putative Annexin
A2-like protein | ANXA2P2 | Calcium ion
binding/calcium-dependent phospholipid binding/phospholipase
inhibitor activity | ND | Basement
membrane/melanosome | 65 | 20,146 | 6.76 | 39% | 13 | Cs only |
| 874 | NP_001876 | E3
ubiquitin-protein ligase TTC3/DCRR1 | TTC3 | Protein
binding/ubiquitin-protein ligase activity/zinc ion binding | Protein K48-linked
ubiquitination/ubiquitin-dependent protein catabolic process | Nucleus | 83 | 222,852 | 8.38 | 8% | 18 | Cs only |
| 875 | Q5HYJ3 | Protein FAM76B | FAM76B | ND | ND | ND | 157 | 39,753 | 9.35 | 42% | 19 | Cs only |
| 880 | NP_000385 | Alpha crystallin A
chain | CRYAA | Protein
binding/structural constituent of eye lens/unfolded protein
binding | M phase specific
microtubule process/actin filament
organization/anti-apoptosis/camera-type eye development/lens fiber
cell morphogenesis/mitochondrion organization/negative regulation
of intracellular transport/protein homooligomerization/protein
refolding/response to heat/response to stimulus/visual
perception | Cytoplasm | | 20,011 | 5.77 | 45% | 9 | −69.84 |
| 882 | NP_001876 | Alpha crystallin B
chain | CRYAB | Cytoskeletal
protein binding/microtubule binding/protein binding/protein
homodimerization activity/structural constituent of eye
lens/unfolded protein binding |
Aging/anti-apoptosis/camera-type eye
development/glucose metabolic process/microtubule polymerization or
depolymerization/muscle contraction/muscle organ
development/negative regulation of cell growth/negative regulation
of intracellular transport/oxygen and reactive oxygen species
metabolic process/protein folding/protein
homooligomerization/response to estradiol stimulus/response to
heat/response to hydrogen peroxide/stress-activated MAPK
cascade | Golgi apparatus/Z
disc/actin filament bundle/cell surface/contractile
fiber/cytoplasm/insoluble fraction/microtubule cytoskeleton/plasma
membrane/soluble fraction | 83 | 20,146 | 6.76 | 52% | 10 | Cs only |
| 886 | NP_000385 | Alpha crystallin A
chain | CRYAA | Protein
binding/structural constituent of eye lens/unfolded protein
binding | M phase-specific
microtubule process/actin filament
organization/anti-apoptosis/camera-type eye development/lens fiber
cell morphogenesis/mitochondrion organization/negative regulation
of intracellular transport/protein homooligomerization/protein
refolding/response to heat/response to stimulus/visual
perception | Cytoplasm | 93 | 20,011 | 5.77 | 45% | 9 | Cs only |
| 889 | BAH11709 | cDNA FLJ55428,
highly similar to regulator of G-protein signaling 7 | | Signal transducer
activity | G-protein coupled
receptor protein signaling pathway | Heterotrimeric
G-protein complex | 155 | 46,564 | 8.56 | 24% | 11 | −21.561 |
| 890 | CAM25565 | Kinesin family
member C1 | KIFC1 | ND | ND | ND | 147 | 22,613 | 10.33 | 46% | 10 | Cs only |
| 893 | EAX10319 | hCG2040067 | | ND | ND | ND | 26 | 6,487 | 11.35 | 51% | 2 | Cs only |
| 894 | NP_001876 | Alpha-crystallin B
chain | CRYAB | Cytoskeletal
protein binding/microtubule binding/protein binding/protein
homodimerization activity/structural constituent of eye
lens/unfolded protein binding |
Aging/anti-apoptosis/camera-type eye
development/glucose metabolic process/microtubule polymerization or
depolymerization/muscle contraction/muscle organ
development/negative regulation of cell growth/negative regulation
of intracellular transport/oxygen and reactive oxygen species
metabolic process/protein folding/protein
homooligomerization/response to estradiol stimulus/response to
heat/response to hydrogen peroxide/stress-activated MAPK
cascade | Golgi apparatus/Z
disc/actin filament bundle/cell surface/contractile
fiber/cytoplasm/insoluble fraction/microtubule cytoskeleton/plasma
membrane/soluble fraction | 180 | 20,146 | 6.76 | 55% | 13 | Cs only |
| 896 | BAB15368 | Growth
hormone-regulated TBC protein 1 | GRTP1 | Rab GTPase
activator activity | Regulation of Rab
GTPase activity | Intracellular Golgi
apparatus/ | 95 | 29,398 | 5.74 | 25% | 7 | Cs only |
| 897 | NP_006794 | AP-3 complex
subunit mu-2 | AP3M2 | Protein
binding | Intracellular
protein transport/vesicle-mediated transport | Clathrin adaptor
complex | 207 | 47,175 | 7.15 | 25% | 18 | Cs only |
| 906 | NP_001998 | Fibroblast growth
factor 4 precursor | FGF4 | Growth factor
activity/heparin binding | Cell-cell
signaling/chondroblast differentiation/fibroblast growth factor
receptor signaling pathway/mesenchymal cell proliferation/positive
regulation of ERK1 and ERK2 cascade/positive regulation of cell
division/positive regulation of cell proliferation/signal
transduction | Extracellular
region | 191 | 22,148 | 9.73 | 61% | 12 | −19.061 |
| 911 | NP_004485 | Hepatoma-derived
growth factor isoform a | HDGF | DNA binding/growth
factor activity/heparin binding/nucleotide binding | Cell
proliferation/regulation of transcription/signal
transduction/transcription |
Cytoplasm/extracellular space/nucleus | 216 | 26,886 | 4.7 | 68% | 16 | −20.769 |
| 920 | NP_060011 | Beta crystallin
S | CRYGS | Structural
constituent of eye lens | Lens development in
camera-type eye/morphogenesis of an epithelium | | 68 | 21,392 | 6.44 | 45% | 9 | Cs only |
| 970 | NP_000487 | Beta-crystallin
B2 | CRYBB2 | Structural
constituent of eye lens | Response to
stimulus/visual perception | | 413 | 23,479 | 6.5 | 75% | 30 | Cs only |
| 979 | NP_000487 | Beta crystallin
B2 | CRYBB2 | Protein
homodimerization activity/structural constituent of eye
lens/structural molecule activity | Camera-type eye
development/response to stimulus/visual perception | | 110 | 23,479 | 6.5 | 65% | 13 | Cs only |
| 985 | AAH20718 | CFI protein | CFI | Peptidase
activity/scavenger receptor activity/serine-type endopeptidase
activity | Complement
activation, classical pathway/innate immune
response/proteolysis | Extracellular
region/extracellular space/membrane | 62 | 44,315 | 8.49 | 33% | 9 | Cs only |
| 1009 | BAA20881 | HLA-A26 | HLA-A26 | ND | Antigen processing
and presentation/immune response | MHC class I protein
complex | 162 | 24,393 | 9.54 | 67% | 17 | Cs only |
| 1011 | AAG49447 | LYST-interacting
protein LIP8 (centrobin) | CNTROB | Protein domain
specific binding | Centriole
replication/centrosome separation/cytokinesis | Centriole | 73 | 13,652 | 10.43 | 23% | 6 | Cs only |
| 1155 | Q8NB50 | Isoform 2 of Zinc
finger protein 62 homolog | ZFP62 | Metal ion
binding/nucleic acid binding/zinc ion binding | Regulation of
transcription |
Intracellular/nucleus | 62 | 58,765 | 9.34 | 23% | 11 | 5.58146 |
| 1218 | NP_000468 | Isoform 1 of serum
albumin precursor | ALB | DNA
binding/antioxidant activity/chaperone binding/copper ion
binding/drug binding/fatty acid binding/metal ion binding/oxygen
binding/protein binding/pyridoxal phosphate binding/toxin
binding | Cellular response
to starvation/hemolysis by symbiont of host
erythrocytes/maintenance of mitochondrion location/negative
regulation of apoptosis/negative regulation of programmed cell
death/transport | extracellular
region/extracellular space/platelet α granule lumen/protein
complex | 245 | 71,317 | 5.92 | 47% | 33 | 12.2397 |
| 1247 | CAI56771 | Protein-cysteine
N-palmitoyltransferase HHAT (hypothetical protein) | HHAT | GTP
binding/acyltransferase activity | Multicellular
organismal development | Endoplasmic
reticulum membrane/integral to membrane | 106 | 9,436 | 7.82 | 40% | 7 | 10.786 |
| 1348 | NP_000468 | Isoform 1 of serum
albumin precursor | ALB | DNA
binding/antioxidant activity/chaperone binding/copper ion
binding/drug binding/fatty acid binding/metal ion binding/oxygen
binding/protein binding/pyridoxal phosphate binding/toxin
binding | Cellular response
to starvation/hemolysis by symbiont of host
erythrocytes/maintenance of mitochondrion location/negative
regulation of apoptosis/negative regulation of programmed cell
death/transport | Extracellular
region/extracellular space/platelet α granule lumen/protein
complex | 92 | 71,317 | 5.92 | 25% | 16 | Cs only |
| 1612 | EAW90919 | Dynamin 3, isoform
CRA_e | | ND | ND | ND | 377 | 46,798 | 8.21 | 67% | 32 | −10.998 |
Gene Ontology analysis
All the proteins identified in this study were
subjected to Gene Ontology (www.geneontology.org/) for molecular function,
biological process and cellular component analysis. The results are
listed in Table II.
Aqueous levels of CRYAA in BRVO patients
and controls
Aqueous levels of CRYAA in the AH of BRVO patients
or controls were below the minimum detectable concentration (data
not shown).
Discussion
BRVO is a common cause of retinal vascular
abnormality and a frequent cause of visual loss. Macular edema is
the most frequent cause of visual impairment in patients with BRVO.
Therefore, understanding the cellular and molecular factors that
underlie the pathogenesis of macular edema with BRVO is of
particular importance. However, the majority of studies on BRVO
have focused on the treatment and only a few studies have
emphasized the pathogenesis of macular edema due to BRVO (5,18–22,26).
To assess the severity of macular edema with BRVO by
obtaining a sample of the AH or vitreous fluid at surgery is of
critical importance. Several cytokines and other factors in the
ocular fluid have been suggested to be involved in the pathogenesis
of macular edema due to BRVO, such as VEGF and IL-6 (5,18,19). However, the surgical harvesting of
vitreous fluid is associated with the risk of vitreous haemorrhage,
retinal tears and retinal detachment, whereas it is difficult to
obtain vitreous samples for diagnostic or investigative purposes
without performing surgery. On the other hand, obtaining AH samples
is a far easier and less risky procedure. AH can be collected
directly from patients and AH proteins may manifest discrete
changes in patients with BRVO.
To date, only a few studies have examined the
changes in AH protein epxression in patients with BRVO. The exact
changes in protein expression that occur in AH in patients with
BRVO are unclear. Therefore, it was considered of importance to
study the changes in AH protein expression in patients with BRVO.
Such information may provide new insight into the mechanisms of
BRVO and identify potential biomarkers of this condition. AH is
valuable for understanding eye disorders and certain studies have
tried to identify the majority of the proteins in AH of patients
with cataract (7,23–25). Previous studies have suggested
that AH proteins activate signaling cascades, which subsequently
regulate cellular functions, including mitosis, differentiation,
motility, apoptosis and angiogenesis. Such proteins may play a
vital role in the pathology of macular edema secondary to BRVO.
Noma et al found that the aqueous level of VEGF reflected
its vitreous level (26); thus,
in this study, we investigated the pathogenesis of macular edema
induced by BRVO by measuring alterations in protein expression in
AH samples from patients with BRVO.
Proteomic analysis is a valuable method for
elucidating the molecular nature of AH. Some studies have used this
technology to explore the pathogenesis of various eye diseases
(6,8–12).
In this study, we conducted proteomic analysis of AH from patients
with macular edema induced by BRVO and age-matched patients with
cataract (controls). The abnormal expression and distribution of
proteins in AH were identified. The patterns of 2-DE gels in the
patients with BRVO differed from the controls, which indicated that
the protein content in the AH changes with the development of BRVO.
Proteomics revealed that 56 protein spots were altered by
>2-fold, which suggests that there are complex mechanisms
involved in the pathogenesis of BRVO.
Several of the proteins identified by proteomics
have been implicated in angiogenesis, oxidative stress and collagen
synthesis. Such proteins may play a vital role in the pathogenesis
of macular edema induced by BRVO.
FGF-4 was found to be downregulated in this study.
FGF-4 is a member of the fibroblast growth factor family and it
induces the proliferation, migration and survival of several cell
types, including endothelial cells. FGF-4 induces vascular
permeability, therapeutic angiogenesis and arteriogenesis
comparable to that of VEGF (27).
Other results point to an indirect angiogenic activity of FGF-4
through the autocrine induction of VEGF secretion (28,29). In a previous study, the induction
of the angiogenic morphotype and the parallel modulations of the
biosynthetic phenotype in human umbilical vein endothelial cells
were completely suppressed by a neutralizing antibody directed
against VEGF (28).
HDGF was found to be downregulated in this study,
which is mitogenic for vascular smooth muscle and aortic
endothelial cells. HDGF is a highly expressed vascular endothelial
cell protein in vivo and is a potent endothelial mitogen and
regulator of endothelial cell migration by mechanisms distinct from
VEGF. As previously demonstrated, with the chick chorioallantoic
membrane (CAM), a bioassay for angiogenesis, exogenous recombinant
HDGF significantly stimulated blood vessel formation and a
dose-dependent reorganization of cells within the CAM into a more
compact, linear alignment reminiscent of tube formation (30).
Some crystallins were identified to be downregulated
in this study. Alpha crystallins are chaperones belonging to the
small heat shock protein family. Certain studies have suggested
that the expression of alphaA and alphaB crystallin is related to
oxidative stress (31,32). AlphaB-crystallin (CRYAB) plays an
important role as a chaperone for VEGF-A in angiogenesis. The
attenuation of intraocular angiogenesis has been observed in CRYAB
knockout [CRYAB (−/−)] mice in 2 models of intraocular disease:
oxygen-induced retinopathy and laser-induced choroidal
neovascularization. VEGF-A protein expression was low in CRYAB
(−/−) mouse retinas compared with wild-type mouse retinas. CRYAB
(−/−) retinal pigment epithelial (RPE) cells showed low VEGF-A
secretion under serum-starved conditions compared with wild-type
cells. CRYAB can bind to VEGF-A but not transforming growth
factor-β in cultured RPE cells. CRYAB and VEGF-A are co-localized
in the endoplasmic reticulum in RPE cells under chemical hypoxia.
Endothelial cell apoptosis in newly formed vessels was greater in
CRYAB (−/−) than wild-type mice (33). Ghosh et al found that human
CRYAB peptides have strong interactions with FGF-2 and VEGF, which
are both related with angiogenesis. Chaperone assays confirmed the
ability of CRYAB to protect against the aggregation of FGF-2 and
VEGF (34). α- and β-crystallin
isoforms are overexpressed with diabetes, as shown by proteomics
and confirmed by immunoblotting (35). These data suggest that crystallins
may function together with VEGF during angiogenesis.
Complement factor I (CFI) was also downregulated in
our study. In a previous study, CFI was found to be increased in
proliferative diabetic retinopathy vitreous compared with
non-diabetic vitreous by a comprehensive proteomic analysis
[one-dimensional SDS-PAGE and nano-liquid chromatography
(LC)/MS/MS] (36). Thus, CFI may
also play a role in angiogenesis.
FGF-4, platelet-derived growth factor (PDGF),
crystallins and CFI, which are tightly associated with
angiogenesis, were all downregulated in this study. This may be due
to the fact that the patients in this study had a long course of
disease and the angiogenic tissues had become quiescent. Further
investigations are required.
Albumin, anaphase promoting complex subunit 5
(ANAPC5) and β-actin were found to be all upregulated in this
study. Human serum albumin (HSA) is the most abundant protein in
the circulatory system, and one of its principal functions is to
transport fatty acids (37).
Albumin is also a very abundant and important circulating
antioxidant (38). HSA inhibits
endothelial apoptosis in a highly specific manner (39). Increased vascular disease occurs
with low albumin, possibly reflecting the specific inhibition of
endothelial apoptosis reported for tissue culture (40). Serum albumin has been
significantly associated with the severity of retinopathy and
neuropathy in patients with type 2 diabetes (41). It has been reported that the
expression of ANAPC5 may represent an important event in the
pathogenesis of vascular proliferative diseases (42).
Actins are highly conserved proteins that are
involved in cell motility, structure and integrity. β-actin is a
major constituent of the contractile apparatus and one of the two
non-muscle cytoskeletal actins. β-actin as a transcription factor
also stimulates endothelial nitric oxide synthase (eNOS) expression
(43).
Of note, some proteins identified in this study may
participate in collagen synthesis, the cytoskeleton and
organization of the actin cytoskeleton, such as prolyl
4-hydroxylase, alpha polypeptide III (P4HA3); spectrin, alpha,
non-erythrocytic 1, alpha-fodrin (SPTAN1); actin related protein
2/3 complex, subunit 2, 34 kDa (ARPC2, also known as PNAS-139).
These events are critical for driving a wide range of cellular
processes, including motility, endocytosis and intracellular
trafficking. Previous studies have demonstrated that the level of
IL-6 is increased in the AH of diabetic patients with macular edema
and in patients with macular edema inducedy by BRVO (5,44).
IL-6 can induce an increase in endothelial permeability in
vitro by rearranging actin filaments and by changing the shape
of endothelial cells (45). Thus,
actin filaments may participate in the pathogenesis of macular
edema due to BRVO.
Other proteins identified play crucial roles in
photoreceptor or retina functions. Some proteins identified in this
study may control cell cycle progression and/or apoptosis, immune
responses and oxidative stress. Further investigations are rquired
to fully understand the exact association between changes in AH
protein expression and BRVO.
Although a comparison of AH samples from patients
with BRVO with and without macular edema may be more useful to
analyze the proteins in patients with BRVO that may be involved in
the development of macular edema, it is not easy to obtain AH
samples from patients with BRVO without macular edema. Moreover, AH
from normal healthy adults cannot be obtained ethically. Therefore,
we used patients with cataract as the controls in this study, as
previously done by others (6,8–11).
Therefore, it is possible that some of the proteins identified in
our study are present due to the underlying cataract condition.
However, the patients with BRVO were all senile and age-matched
with the controls and all had cataract which did not require
surgery. Thus, the 2 groups were comparable.
Due to the use of pooled samples, the results in
this study do not provide any information as to the variation
between patients within a group. Thus, it is possible that
patient-to-patient variability exists within the current study.
However, the use of pooled samples should reduce the component of
patient-to-patient variation and reveal overall differences between
patients and controls, as previously described (45). The use of lesser numbers of pooled
AH is a disadvantage in this aspect. The influence of
patient-to-patient variation will be addressed in a subsequent
study. Our results may prove valuable for future research in the
pathogenesis in BRVO.
In conclusion, the results of the present study
revealed that the proteomic composition of AH was differed
significantly between the patients with macular edema with BRVO and
the controls. The proteins identified may serve as potential
biomarkers for macular edema induced by BRVO.
Acknowledgements
This study was supported by grants from the National
Basic Research Program of China (973 Program, no. 2011CB510200) and
the National Natural Science Foundation of China (no.
81170855).
References
|
1
|
Lim JW: Intravitreal bevacizumab and
cytokine levels in major and macular branch retinal vein occlusion.
Ophthalmologica. 225:150–154. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rogers S, McIntosh RL, Cheung N, et al:
The prevalence of retinal vein occlusion: pooled data from
population studies from the United States, Europe, Asia, and
Australia. Ophthalmology. 117:313–319. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou JQ, Xu L, Wang S, et al: The 10-year
incidence and risk factors of retinal vein occlusion: the Beijing
eye study. Ophthalmology. 120:803–808. 2013.PubMed/NCBI
|
|
4
|
McIntosh RL, Mohamed Q, Saw SM and Wong
TY: Interventions for branch retinal vein occlusion: an
evidence-based systematic review. Ophthalmology. 114:835–854. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Noma H, Funatsu H, Yamasaki M, et al:
Pathogenesis of macular edema with branch retinal vein occlusion
and intraocular levels of vascular endothelial growth factor and
interleukin-6. Am J Ophthalmol. 140:256–261. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Duan X, Lu Q, Xue P, et al: Proteomic
analysis of aqueous humor from patients with myopia. Mol Vis.
14:370–377. 2008.PubMed/NCBI
|
|
7
|
Chowdhury UR, Madden BJ, Charlesworth MC
and Fautsch MP: Proteome analysis of human aqueous humor. Invest
Ophthalmol Vis Sci. 51:4921–4931. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Richardson MR, Segu ZM, Price MO, et al:
Alterations in the aqueous humor proteome in patients with Fuchs
endothelial corneal dystrophy. Mol Vis. 16:2376–2383.
2010.PubMed/NCBI
|
|
9
|
Funding M, Vorum H, Honore B, Nexo E and
Ehlers N: Proteomic analysis of aqueous humour from patients with
acute corneal rejection. Acta Ophthalmol Scand. 83:31–39. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Izzotti A, Longobardi M, Cartiglia C and
Sacca SC: Proteome alterations in primary open angle glaucoma
aqueous humor. J Proteome Res. 9:4831–4838. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Duan X, Xue P, Wang N, Dong Z, Lu Q and
Yang F: Proteomic analysis of aqueous humor from patients with
primary open angle glaucoma. Mol Vis. 16:2839–2846. 2010.PubMed/NCBI
|
|
12
|
Yao J, Liu X, Yang Q, et al: Proteomic
analysis of the aqueous humor in patients with wet age-related
macular degeneration. Proteomics Clin Appl. 7:550–560. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yao JQ, Liu QH, Chen X, et al: Hsp90
inhibitor 17-allylamino-17-demethoxygeldanamycin inhibits the
proliferation of ARPE-19 cells. J Biomed Sci. 17:302010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gong Y, Chen N, Wang FQ, Wang ZH and Xu
HX: Serum proteome alteration of severe sepsis in the treatment of
continuous renal replacement therapy. Nephrol Dial Transplant.
24:3108–3114. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun ZL, Zhu Y, Wang FQ, et al: Serum
proteomic-based analysis of pancreatic carcinoma for the
identification of potential cancer biomarkers. Biochim Biophys
Acta. 1774:764–771. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu J, Wang F, Gong Y, et al: Proteomic
analysis of changes induced by nonylphenol in Sprague-Dawley rat
Sertoli cells. Chem Res Toxicol. 22:668–675. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Su Y, Liu R, Sheng J, et al: Malignant
progression in O6-methylguanine-DNA
methyltransferase-deficient esophageal cancer cells is associated
with Ezrin protein. DNA Cell Biol. 31:856–866. 2012.PubMed/NCBI
|
|
18
|
Noma H, Minamoto A, Funatsu H, et al:
Intravitreal levels of vascular endothelial growth factor and
interleukin-6 are correlated with macular edema in branch retinal
vein occlusion. Graefes Arch Clin Exp Ophthalmol. 244:309–315.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Noma H, Funatsu H, Mimura T and Shimada K:
Increase of aqueous inflammatory factors in macular edema with
branch retinal vein occlusion: a case control study. J Inflamm
(Lond). 7:442010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee WJ, Kang MH, Seong M and Cho HY:
Comparison of aqueous concentrations of angiogenic and inflammatory
cytokines in diabetic macular oedema and macular oedema due to
branch retinal vein occlusion. Br J Ophthalmol. 96:1426–1430. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Noma H, Funatsu H, Mimura T and Eguchi S:
Vascular endothelial growth factor receptor-2 in macular oedema
with retinal vein occlusion. Ophthalmic Res. 48:56–58. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fonollosa A, Garcia-Arumi J, Santos E, et
al: Vitreous levels of interleukine-8 and monocyte chemoattractant
protein-1 in macular oedema with branch retinal vein occlusion. Eye
(Lond). 24:1284–1290. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bennett KL, Funk M, Tschernutter M, et al:
Proteomic analysis of human cataract aqueous humour: Comparison of
one-dimensional gel LCMS with two-dimensional LCMS of unlabelled
and iTRAQ®-labelled specimens. J Proteomics. 74:151–166.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Escoffier P, Paris L, Bodaghi B, Danis M,
Mazier D and Marinach-Patrice C: Pooling aqueous humor samples:
bias in 2D-LC-MS/MS strategy? J Proteome Res. 9:789–797. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Richardson MR, Price MO, Price FW, et al:
Proteomic analysis of human aqueous humor using multidimensional
protein identification technology. Mol Vis. 15:2740–2750.
2009.PubMed/NCBI
|
|
26
|
Noma H, Funatsu H, Yamasaki M, et al:
Aqueous humour levels of cytokines are correlated to vitreous
levels and severity of macular oedema in branch retinal vein
occlusion. Eye (Lond). 22:42–48. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rissanen TT, Markkanen JE, Arve K, et al:
Fibroblast growth factor 4 induces vascular permeability,
angiogenesis and arteriogenesis in a rabbit hindlimb ischemia
model. Faseb J. 17:100–102. 2003.
|
|
28
|
Deroanne CF, Hajitou A, Calberg-Bacq CM,
Nusgens BV and Lapiere CM: Angiogenesis by fibroblast growth factor
4 is mediated through an autocrine up-regulation of vascular
endothelial growth factor expression. Cancer Res. 57:5590–5597.
1997.PubMed/NCBI
|
|
29
|
Hajitou A, Deroanne C, Noel A, et al:
Progression in MCF-7 breast cancer cell tumorigenicity: compared
effect of FGF-3 and FGF-4. Breast Cancer Res Treat. 60:15–28. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Everett AD, Narron JV, Stoops T, Nakamura
H and Tucker A: Hepatoma-derived growth factor is a pulmonary
endothelial cell-expressed angiogenic factor. Am J Physiol Lung
Cell Mol Physiol. 286:L1194–L1201. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kumar PA, Haseeb A, Suryanarayana P,
Ehtesham NZ and Reddy GB: Elevated expression of alphaA- and
alphaB-crystallins in streptozotocin-induced diabetic rat. Arch
Biochem Biophys. 444:77–83. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rao NA, Saraswathy S, Wu GS, Katselis GS,
Wawrousek EF and Bhat S: Elevated retina-specific expression of the
small heat shock protein, alphaA-crystallin, is associated with
photoreceptor protection in experimental uveitis. Invest Ophthalmol
Vis Sci. 49:1161–1171. 2008. View Article : Google Scholar
|
|
33
|
Kase S, He S, Sonoda S, et al:
alphaB-crystallin regulation of angiogenesis by modulation of VEGF.
Blood. 115:3398–3406. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ghosh JG, Shenoy AK Jr and Clark JI:
Interactions between important regulatory proteins and human alphaB
crystallin. Biochemistry. 46:6308–6317. 2007. View Article : Google Scholar
|
|
35
|
Fort PE, Freeman WM, Losiewicz MK, Singh
RS and Gardner TW: The retinal proteome in experimental diabetic
retinopathy: up-regulation of crystallins and reversal by systemic
and periocular insulin. Mol Cell Proteomics. 8:767–779. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gao BB, Chen X, Timothy N, Aiello LP and
Feener EP: Characterization of the vitreous proteome in diabetes
without diabetic retinopathy and diabetes with proliferative
diabetic retinopathy. J Proteome Res. 7:2516–2525. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kragh-Hansen U, Watanabe H, Nakajou K,
Iwao Y and Otagiri M: Chain length-dependent binding of fatty acid
anions to human serum albumin studied by site-directed mutagenesis.
J Mol Biol. 363:702–712. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Roche M, Rondeau P, Singh NR, Tarnus E and
Bourdon E: The antioxidant properties of serum albumin. FEBS Lett.
582:1783–1787. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zoellner H, Siddiqui S, Kelly E and
Medbury H: The anti-apoptotic activity of albumin for endothelium
is inhibited by advanced glycation end products restricting
intramolecular movement. Cell Mol Biol Lett. 14:575–586. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bolitho C, Bayl P, Hou JY, et al: The
anti-apoptotic activity of albumin for endothelium is mediated by a
partially cryptic protein domain and reduced by inhibitors of
G-coupled protein and PI-3 kinase, but is independent of radical
scavenging or bound lipid. J Vasc Res. 44:313–324. 2007. View Article : Google Scholar
|
|
41
|
Iwasaki T, Togashi Y and Terauchi Y:
Significant association of serum albumin with severity of
retinopathy and neuropathy, in addition to that of nephropathy, in
Japanese type 2 diabetic patients. Endocr J. 55:311–316. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Autieri MV: Expression of
anaphase-promoting complex 5 in balloon angioplasty-injured rat
carotid arteries and mitogen-stimulated human vascular smooth
muscle cells. Biochem Biophys Res Commun. 282:723–728. 2001.
View Article : Google Scholar
|
|
43
|
Ou H, Shen YH, Utama B, et al: Effect of
nuclear actin on endothelial nitric oxide synthase expression.
Arterioscler Thromb Vasc Biol. 25:2509–2514. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Funatsu H, Yamashita H, Noma H, Mimura T,
Yamashita T and Hori S: Increased levels of vascular endothelial
growth factor and interleukin-6 in the aqueous humor of diabetics
with macular edema. Am J Ophthalmol. 133:70–77. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Diz AP, Truebano M and Skibinski DO: The
consequences of sample pooling in proteomics: an empirical study.
Electrophoresis. 30:2967–2975. 2009. View Article : Google Scholar : PubMed/NCBI
|