Introduction
Benign prostatic hyperplasia is a leading cause of
partial bladder outlet obstruction (BOO) in elderly men. This may
eventually lead to the occurrence of detrusor dysfunction (1). Animal experimental studies have
demonstrated that the bladder undergoes three sequential stages
(hypertrophy, compensation and decompensation) in partial BOO
(2). Detrusor overactivity (DO)
then occurs during the filling phase. This is followed by the
transition from compensation to decompensation, characterized by
decreased detrusor contractility during the voiding phase (3). To date, a number of studies have
shown that changes occur in detrusor contractility in partial BOO
(2,4–7).
However, little is known about the changes in detrusor
contractility that occur due to the compensation and decompensation
of bladder function during the filling and voiding phase.
Animal experiments have indicated that the DO is an
indicator of a rapid but transient contraction of the bladder
smooth muscles during the filling phase, thus termed as a phasic
contraction (8). The contractile
response of the bladder shows a biphasic pattern during the voiding
phase: initial phasic contraction and prolonged sustained tension,
thus termed as a tonic contraction. Presumably, this may play a
role in efficient voiding and the resulting residual volume (RV)
(9,10). Thus, the bladder smooth muscles
undergo two phasic contractions followed by one tonic one,
involving different types of kinetics (8). However, little is known about the
underlying mechanisms through which the transition from the
compensation to decompensation of bladder function occurs. Certain
studies have suggested that the decreased microvascular perfusion
and the impaired energy metabolism are involved in the alterations
in detrusor contractility (11,12).
As shown in the contraction of all the other smooth
muscles, the bladder smooth muscles are contracted in an adenosine
triphosphate (ATP)-dependent manner as a main source of metabolic
energy (8). Each smooth muscle
cell contains only a small amount of ATP and is unable to import
ATP. This strongly suggests that signaling pathways are involved in
the control of the uptake and subsequent use of ATP (13). In further detail, it has been
suggested that the AMP-activated kinase (AMPK) plays a key role in
regulating the energy balance and thereby mediating the unlimited
supply of ATP (14). To date,
however, no studies have proposed a theory to explain the
mechanisms through which energy is supposedly supplied to the
bladder. Furthermore, AMPK is definitely involved in the
pathophysiology of various cardiovascular diseases, such as
pathological cardiac hypertrophy or heart failure (13). It has also been reported to be
present in the bladder (15).
However, little is known about its role in the ATP-dependent
process in the bladder.
Previous studies have indicated that there are
alterations in the degree of the expression of signaling proteins,
such as extracellular signal-regulated kinase (ERK) and protein
kinase C (PKC) (16,17). These signaling proteins are
involved in the transduction of intracellular signals and the
control of cell growth and differentiation in response to
mechanical stimuli in BOO. This phenomenon is termed as
mechanotransduction (18).
Furthermore, it has been suggested that ERK and PKC are also
involved in the AMPK phosphorylation pathway in the heart (19,20). However, little is known about
their involvement in the AMPK phosphorylation pathway in the
bladder.
To date, it has been well established that there is
a temporal difference in the onset of compensation of bladder
function, followed by its decompensation; animal experimental
studies have revealed that the compensation of bladder function
occurs within two weeks following the onset of BOO and
decompensation subsequently occurs. In a preliminary animal study,
however, the decompensation also occurred one week following the
onset of BOO. This suggests that the compensation or decompensation
prevalently occurs due to a difference in the degree of AMPK
activity rather than a temporal difference in the time point of the
onset between the two events (7).
Given the above background, we conducted this study
to examine whether the degree of detrusor contractility is
associated with the compensation or decompensation of bladder
function depending on the RV during the first two weeks after the
onset of BOO in an animal experimental model of partial BOO based
on three cystometric parameters: the degree of DO, maximal pressure
(MP) and RV%. We speculated that the degree of DO and MP are
indicators of the two phasic contractions of the bladder during the
filling and voiding phases, respectively, and the RV% is an
indicator of tonic contraction during the voiding phase. Moreover,
we also examined whether the degree of the phosphorylation and
expression of signaling proteins (AMPK, ERK1/2 and PKC) is
associated with the prevalence of bladder compensation or
decompensation.
Materials and methods
Experimental animals
For the current experimental study, 27 female
Sprague-Dawley (SD) rats weighing 230–260 g were obtained from a
commercial breeder (Orient Bio Inc., Seongnam, Korea) and then kept
in a vivarium with free access to water and food with diurnal light
cycling. Urethral constriction was induced as previously described
(7). All the experimental
procedures were performed in accordance with the Guide for the Care
and Use of Laboratory Animals of the Korean National Institutes of
Health, for which the study protocol was approved by the Ethics
Committee of the Inha University College of Medicine (INHA
11108-116).
We randomly assigned the SD rats into two groups:
the sham-operated group (n=7) and the group with partial BOO
(n=20). The sham-operated group comprised SD rats that underwent
sham operation (no BOO was induced) and served as the control
group. Moreover, the group with BOO comprised SD rats who underwent
surgery to induce partial BOO.
For the induction of partial BOO, the SD rats in the
group with BOO were anesthetized with ketamine (Ketamine
50®; Yuhan Corp., Korea; 75 mg kg−1
intraperitoneally) and xylazine (Rompun®; Bayer Korea
Ltd., Korea; 15 mg kg−1 intraperitoneally). Through a
lower midline incision, the bladder was approached and the proximal
urethra exposed. A 3/0 Novafil (monofilament polybutester; Davis
& Geck, Wayne, NJ, USA) ligature was placed around the urethra
and then tied after a steel rod of 0.9 mm in diameter was
intraluminally placed. After the knot was tied, the steel rod was
removed. This was followed by the repositioning of the bladder and
the closure of the abdominal wall.
To perform a cystometric analysis in the SD rats in
both groups, a catheter was placed in the bladder for the
measurement of intravesical pressure (IVP), followed by the
recording of the intraabdominal pressure (IAP), three days prior to
the cystometric analysis, as previously described (21,22). In further detail, a polyethylene
catheter (PE-50; Becton Dickinson, Parsippany, NJ, USA) with a cuff
was placed in the bladder dome through a lower abdominal incision.
Moreover, an abdominal balloon (Latex; Dawoo Medical, Incheon,
Korea) was placed around the cuff of the catheter and then
displaced proximal to the bladder. This was followed by the
connection to another catheter using a silk tie. These catheters
were then tunneled subcutaneously and then anchored to the skin of
the back with a silk ligature and their free end was sealed.
Cystometric analysis
The conscious rats were placed in a metabolic cage
(Nalgene metabolic cage; Nalge Co., Rochester, NY, USA) without
restraint. For cystometric analysis, the indwelling IVP catheter
was attached to a two-way valve that was connected to a pressure
transducer (Research Grade Blood Pressure Transducer; Harvard
Apparatus, Holliston, MA, USA), as well as an infusion pump (PHD
22/2000 programmable syringe pump; Harvard Apparatus). Moreover, it
was also directly connected to another pressure transducer. Thus,
the IVP was recorded synchronously with the micturition volume (MV)
using a fluid collector connected to a force displacement
transducer (Research Isometric Transducer; Harvard Apparatus). This
was followed by the infusion of saline into the bladder at a rate
of 20 ml/h at room temperature in both groups. Subsequently, data
recording and analysis were performed using Acq Knowledge 3.8.1
software (Biopac Systems Inc., Goleta, CA, USA) at a sampling rate
of 100 Hz and an MP150 data acquisition system (Biopac Systems
Inc.).
In the current experimental study, we measured
cystometric parameters, such as the degree of DO and MP from three
reproducible micturition cycles and then averaged the results for a
comparison between the two groups.
Subgroup analysis
Based on a cut-off value of a mean RV% of 25%, we
subdivided our experimental animals into two subgroups: the
subgroup with bladder compensation (mean RV%, <25%) and the
subgroup with bladder decompensation (mean RV%, >25%). Thus, we
attempted to examine whether detrusor contractility is associated
with the compensation or decompensation of bladder function
depending on the RV during the first two weeks after the onset of
BOO.
Protein assay by western blot
analysis
In the current study, we assayed the levels of
signaling proteins, such as AMPK, ERK1/2 and PKC using the BCA
protein assay kit (Pierce, Rockford, IL, USA). The signaling
proteins were resolved by 8–12% SDS-PAGE and then transferred onto
PVDF membranes (Millipore, Milford, MA, USA) and their activity was
blocked in TBS-T buffer (40 mM Tris-HCl pH 7.4, 25 mM NaCl, 0.1%
Tween-20) containing 5% skim milk. This was followed by the
incubation of the membranes with primary antibodies (Cell Signaling
Technology, Danvers, MA, USA) against the signaling proteins and
their respective phosphorylated forms. Following incubation with
the primary antibodies, the PVDF membranes were incubated with goat
anti-rabbit-HRP conjugated secondary antibodies (Santa Cruz, CA,
USA). This was followed by the visualization of the specific
protein bands using ECL reagent (Pierce). The degree of the
alteration in the phospholylation of each signaling protein was
expressed as the ratio of the level of the phosphorylated form to
that of the non-phosphorylated one.
Statistical analysis
All data are expressed as the means ± standard
errors of the mean (SEM). We performed a simple regression analysis
using Pearson’s correlation coefficient to identify the
correlations between the frequency and degree of DO and MP.
Moreover, we also performed the Shapiro-Wilk W test to examine
whether the urodynamic parameters followed the normal distribution.
Statistical significance was analyzed using an unpaired Student’s
t-test or one-way analysis of variance (ANOVA), accompanied by
Tukey’s post-hoc test for multiple comparisons. Statistical
analysis was performed using GraphPad Prism software version 5.03,
2009 (Graph Pad Software, San Diego, CA, USA). A P-value <0.05
was considered to indicate a statistically significant
difference.
Results
General characteristics
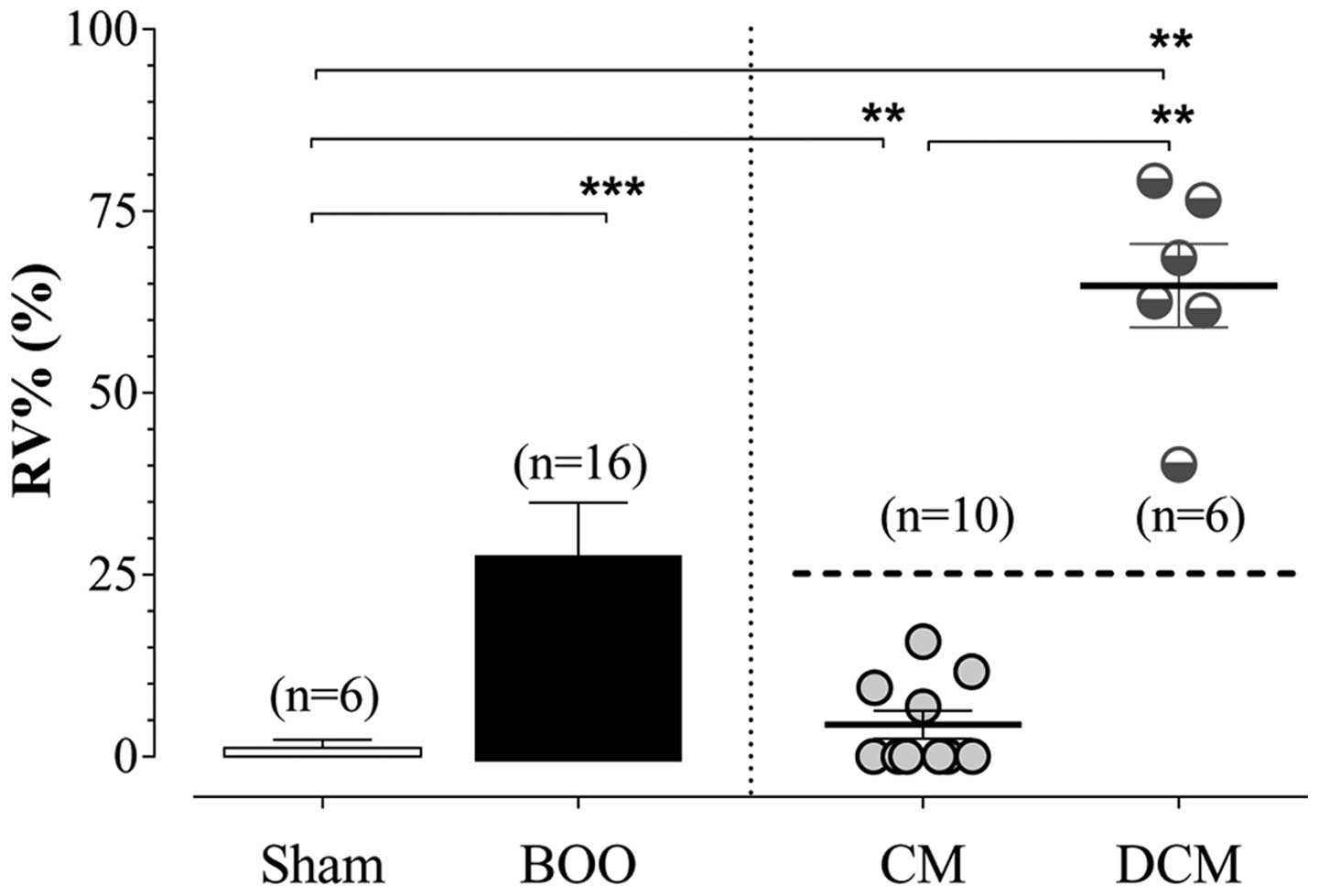
In the current study, there were 10 SD rats (n=10)
(62.5%) in the subgroup with bladder compensation and 6 SD rats
(n=6) (37.5%) in the subgroup with bladder decompensation (Fig. 1).
Moreover, the degree of compliance was significantly
lower in the group with BOO compared with the sham-operated group
(0.38±0.01 ml/cmH2O vs. 9.16±0.83 ml/cmH2O)
(P<0.0001). However, there was no significant difference in the
degree of compliance between the subgroup with bladder
decompensation and the subgroup with bladder compensation
(0.39±0.02 ml/cmH2O vs. 0.38±0.02 ml/cmH2O)
(P>0.05).
Changes in cystometric parameters
As compared with the sham-operated group, in the
group with BOO, we observed a significant increase in threshold
pressure (TP) (P<0.01), bladder capacity (BC) (P<0.05) and
micturition interval (MI) (P<0.05). As compared with the
subgroup with bladder compensation, the subgroup with bladder
decompensation showed a significant decrease in MP (P<0.05) and
MV (P<0.001) and a significant increase in RV (P<0.01).
However, there were no significant differences in other cystometric
parameters, including BP, TP, BC and MI between the subgroup with
bladder compensation and the subgroup with bladder decompensation
(Table I).
 | Table ISummary of cystometric parameters. |
Table I
Summary of cystometric parameters.
| Group/subgroup | BP | TP | MP | BC | MV | RV | MI |
|---|
| Sham (n=6) | 10.1±0.72 | 24.5±3.6 | 65.5±7.7 | 1.27±0.12 | 1.24±0.11 | 0.03±0.03 | 3.90±0.35 |
| BOO (n=16) | 15.4±1.9 |
44.0±3.2b | 88.1±9.5 |
2.26±0.24a | 1.46±0.17 | 0.80±0.28 |
6.88±0.76a |
| CM (n=10) |
17.0±2.5b |
40.3±4.5a |
103.4±12.9a | 1.91±0.22 | 1.81±0.20 | 0.10±0.04 | 5.81±0.67 |
| DCM (n=6) | 12.7±2.7 |
50.1±3.6b |
62.7±3.3c |
2.84±0.46a |
0.88±0.03b,e |
1.96±0.44b,d |
8.67±1.51a |
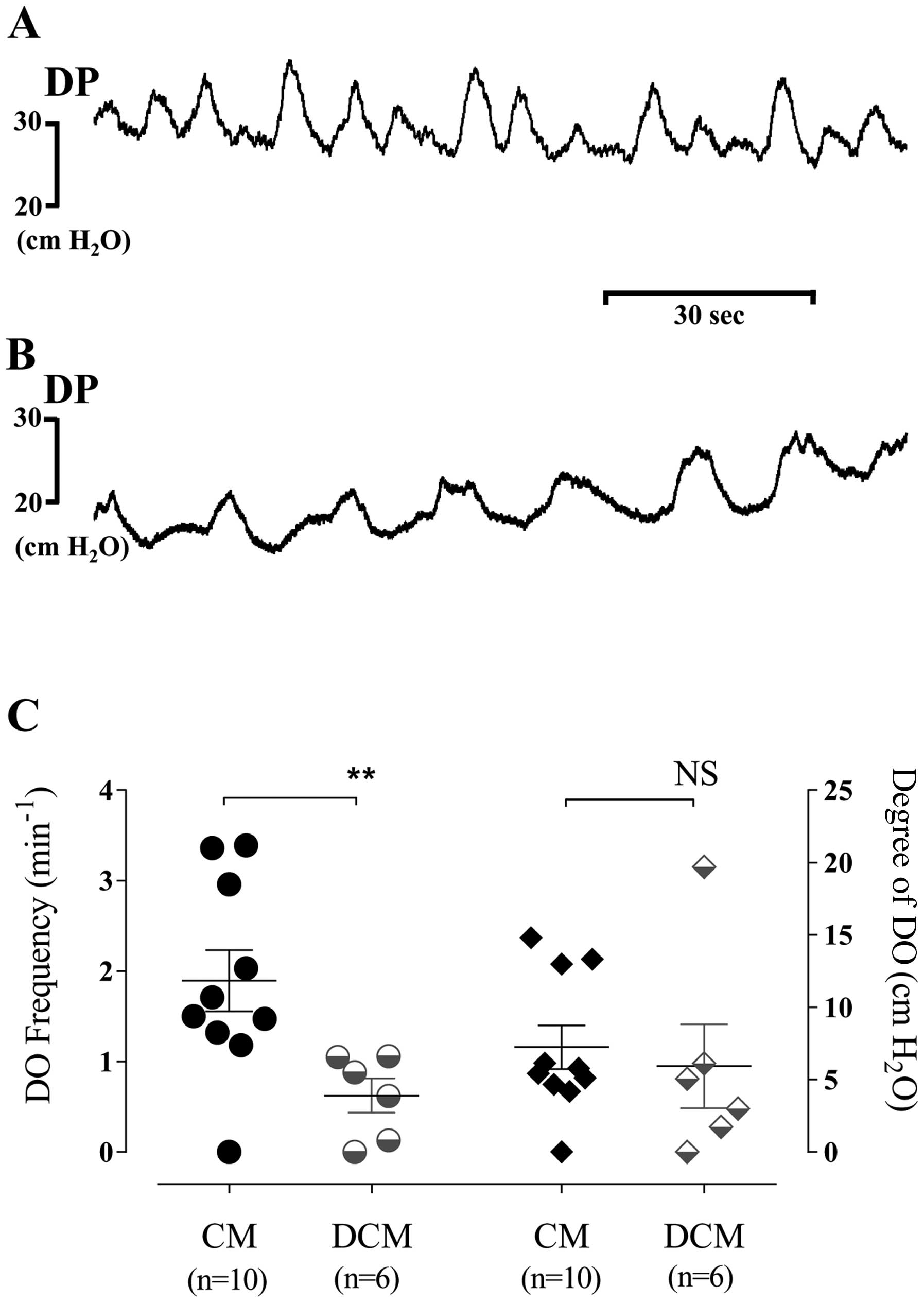
No DO occurred during the filling phase in the
sham-operated group. However, DO occurred in 87.5% (14/16) of the
SD rats in the group with BOO. In the group with BOO, the mean
frequency and degree of DO were 1.42±0.27 min−1 and
6.75±1.39 cm H2O, respectively. In addition, the
frequency of DO was significantly lower in the subgroup with
bladder decompensation as compared with the subgroup with bladder
compensation. There was no significant difference observed in the
degree of DO between the subgroup with bladder decompensation and
the subgroup with bladder compensation (Fig. 2).
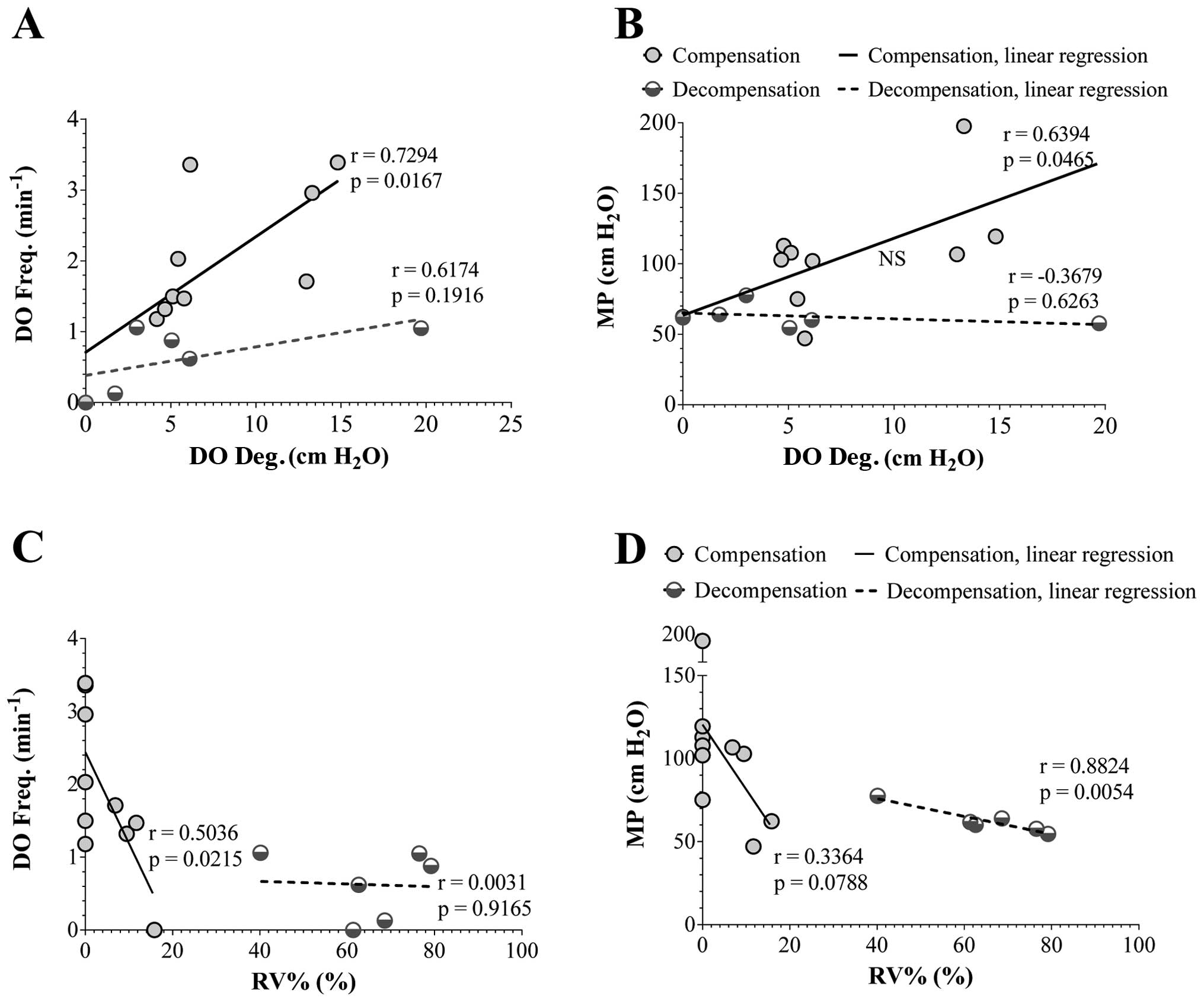
Univariate analysis revealed a significant positive
correlation between the frequency and degree of DO (r=0.73,
P<0.05) and MP (r=0.64, P<0.05) and a significant negative
correlation between the frequency of DO and RV% (r=0.50,
P<0.05). However, a significant negative correlation was
observed between MP and RV% in the subgroup with bladder
decompensation (r=0.88, P<0.01), which was not observed in the
subgroup with bladder compensation (Fig. 3).
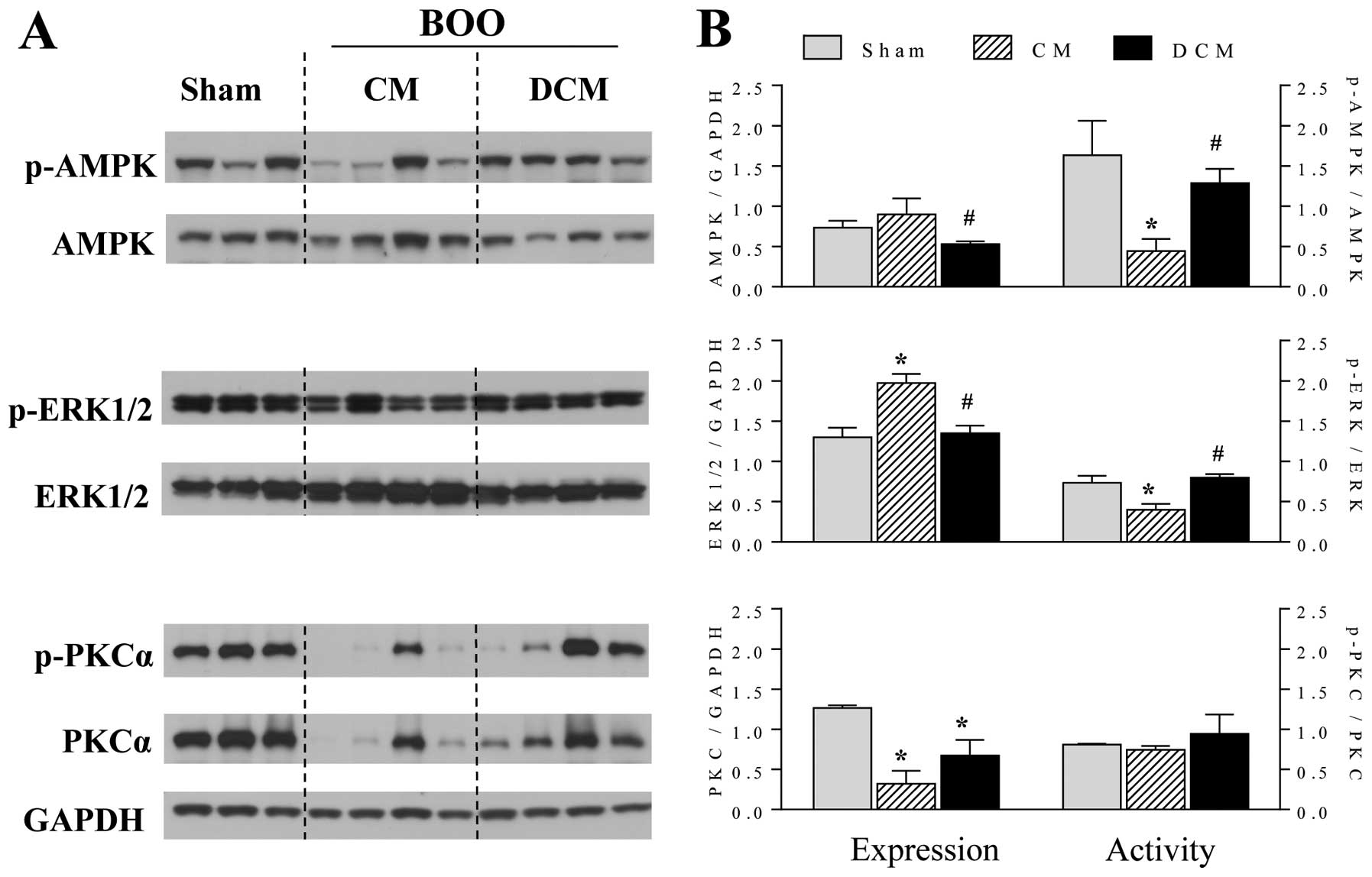
Degree of expression and phosphorylation
of signaling proteins
As shown by western blot analysis, there were
significant differences in the degree of expression and
phosphorylation of signaling proteins between the subgroup with
decompensation and the subgroup with compensation (Fig. 4). That is, the degree of
phosphorylation of AMPK and ERK1/2 was significantly lower in the
subgroup with bladder compensation as compared with the
sham-operated group and the subgroup with bladder decompensation.
However, there was no significant difference between the subgroup
with bladder decompensation and the sham-operated group.
Furthermore, the degree of ERK1/2 expression was significantly
higher in the subgroup with bladder compensation.
The expression of PKC was decreased in both the
subgroup with bladder compensation and the subgroup with bladder
decompensation, significantly lower as compared with the
sham-operated group. Moreover, there was no significant difference
observed in the degree of phosphorylation of PKC between the
subgroup with bladder compensation and the subgroup with bladder
decompensation (Fig. 4). These
findings indicate that PKC is not involved in two-phase bladder
contraction.
Discussion
The decompensation of the bladder in partial BOO is
postulated to be a process of progressive deterioration in
contractility and voiding function of the bladder smooth muscles
(8,23). However, its definition has not
been fully established in a clinical and experimental setting.
Levin et al defined the decompensation of the bladder as the
condition where the mean bladder contractility was smaller by 80%
as compared with normal controls when there were contractile
responses of the isolated bladder strips to stimulation in an in
vitro setting (24). However,
this does not apply to our animal model. Patients are clinically
diagnosed with voiding problems based on a RV of >100 ml when
the normal bladder capacity is 400 ml (25). We therefore divided our
experimental animals based on a cut-off value of a mean RV of 25%.
Thus, our study differs from previous reports.
Presumably, the initial contractile response of the
urinary bladder determining the MP of the bladder may be associated
with the intracellular concentration of ATP. It is also
hypothesized that the subsequent tonic phase may be associated with
mitochondrial respiration. Partial BOO induces a marked decrease in
the tissue ATP content, oxidative glucose metabolism and in the
activity of mitochondrial enzymes (26). The sustained tonic component is
highly dependent on the deprivation of glucose and oxygen as
compared with the peak pressure response. This explains the
mechanisms through which the voiding difficulty occurs, despite a
lack of decreased DO during the early phase of decompensation
(24).
AMPK is a phylogenetically conserved
serine-threonine kinase that is involved in the regulation of
diverse cellular pathways through which the cellular energy is
consumed and chronic muscular pathology due to energy deprivation
occurs (13). Previous studies
have suggested that not only is AMPK activated when the cellular
AMP-to-ATP ratio is increased (14), indicating the state of
intracellular energy deprivation, but also that it is produced in
the presence of metabolic stress involved in reducing ATP synthesis
or increasing ATP consumption. There is no direct evidence
indicating that AMPK is involved in the decompensation of bladder
function in partial BOO. According to certain studies however, the
ATP consumption is reduced in BOO and this is accompanied by an
increase in AMP synthesis and a decrease in ATP synthesis (9,26).
In the current study, the degree of AMPK phosphorylation was
significantly lower in the compensated bladder, accompanied by the
appropriate level of the contractility of bladder smooth muscles,
which indicates that there was a decreased need for the production
of extra ATP. Furthermore, the degree of AMPK expression was
restored to the normal level in the decompensated bladder,
accompanied by decreased bladder contractility. This suggests that
there would be a greater need for the production of ATP for
efficient voiding in the decompensated bladder as compared with the
compensated one. However, this was notably observed at two weeks
after the onset of BOO. Moreover, further studies are warranted to
examine the changes in the degree of AMPK expression that occur
during the acute period or after longer periods of time. It has
been reported that ERK1/2 MAP kinase is involved in the in
vitro generation of the force of smooth muscles in such
conditions that cytosolic Ca2+ levels are decreased to a
near resting level. However, little is known about the in
vivo involvement of ERK1/2 MAP kinase in the function of smooth
muscles (16).
PKC isoforms play a key role in the regulation of
cell growth through several intracellular signaling pathways
(9,20). Chang et al demonstrated
that the degree of PKCα activity is lower in the decompensated
bladder as compared with the compensated one (27). This is not in agreement with our
results. Presumably, this may be due to the difference in criteria
for differentiating between compensation and decompensation. Chang
et al defined the decompensation of the bladder solely based
on the daily voiding frequency and the volume of voiding without
considering the residual urine at each cycle (27). In the current experimental study
however, we analyzed the cystometric parameters after the removal
of residual urine at the end of each cycle of awake cystometry.
This may be noteworthy in that rigorous criteria for
differentiating between the compensation and decompensation of the
bladder should be applied. The degree of PKC expression was
decreased in both subgroups (with bladder compensation and
decompensation). This indicates that PKC may be involved in the
dysfunctional signal transduction pathways through which the
downstream targets of contraction (e.g., Ca2+
sensitization) are regulated. However, this warrants further
investigation.
In conclusion, our results indicate that the degree
of DO is associated with the compensation or decompensation of
bladder function, depending on the RV during the first two weeks
after the onset of BOO in an animal experimental model of partial
BOO. Moreover, it can also be concluded that AMPK and ERK1/2 are
involved in the compensation or decompensation of bladder function.
Our results also suggest however, that PKC is not involved in
two-phase bladder contraction. Finally, alterations in the activity
of signaling proteins, such as AMPK and ERK1/2 may prove to be
helpful in the treatment of patients with voiding difficulty.
Acknowledgements
We are grateful for the copy editing and
proofreading of this manuscript by the Medical Consulting Group
Inc. This study was supported by an Inha University research grant
and by Astellas Pharmaceuticals. The funders had no role in study
design, data collection and analysis, decision to publish, or
preparation of the manuscript.
References
|
1
|
Roehrborn CG: BPH progression: concept and
key learning from MTOPS, ALTESS, COMBAT, and ALF-ONE. BJU Int.
101(Suppl 3): S17–S21. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Levin RM, Haugaard N, O’Connor L, Buttyan
R, Das A, Dixon JS and Gosling JA: Obstructive response of human
bladder to BPH vs. rabbit bladder response to partial outlet
obstruction: a direct comparison. Neurourol Urodyn. 19:609–629.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Levin RM, Levin SS, Zhao Y and Buttyan R:
Cellular and molecular aspects of bladder hypertrophy. Eur Urol.
32(Suppl 1): S15–S21. 1997.
|
|
4
|
Levin RM, Longhurst PA, Monson FC, Kato K
and Wein AJ: Effect of bladder outlet obstruction on the
morphology, physiology, and pharmacology of the bladder. Prostate
Suppl. 3:9–26. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Levin RM, Monson FC, Haugaard N, Buttyan
R, Hudson A, Roelofs M, Sartore S and Wein AJ: Genetic and cellular
characteristics of bladder outlet obstruction. Urol Clin North Am.
22:263–283. 1995.PubMed/NCBI
|
|
6
|
O’Connor LT Jr, Vaughan ED Jr and Felsen
D: In vivo cystometric evaluation of progressive bladder outlet
obstruction in rats. J Urol. 158:631–635. 1997.PubMed/NCBI
|
|
7
|
Kang YJ, Jin LH, Park CS, Shin HY, Yoon SM
and Lee T: Early sequential changes in bladder function after
partial bladder outlet obstruction in awake sprague-dawley rats:
focus on the decompensated bladder. Korean J Urol. 52:835–841.
2011. View Article : Google Scholar
|
|
8
|
Andersson KE and Arner A: Urinary bladder
contraction and relaxation: physiology and pathophysiology. Physiol
Rev. 84:935–986. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yoon JY, Zderic SA, Duckett JW and Levin
RM: Effect of partial outlet obstruction on the biphasic response
to field stimulation at different concentrations of calcium.
Pharmacology. 49:167–172. 1994. View Article : Google Scholar
|
|
10
|
Chien CT, Yu HJ, Lin TB and Chen CF:
Neural mechanisms of impaired micturition reflex in rats with acute
partial bladder outlet obstruction. Neuroscience. 96:221–230. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schröder A, Chichester P, Kogan BA,
Longhurst PA, Lieb J, Das AK and Levin RM: Effect of chronic
bladder outlet obstruction on blood flow of the rabbit bladder. J
Urol. 165:640–646. 2001.PubMed/NCBI
|
|
12
|
Lin AT, Chen MT, Yang CH and Chang LS:
Blood flow of the urinary bladder: effects of outlet obstruction
and correlation with bioenergetic metabolism. Neurourol Urodyn.
14:285–292. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dyck JR and Lopaschuk GD: AMPK alterations
in cardiac physiology and pathology: enemy or ally? J Physiol.
574:95–112. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dolinsky VW and Dyck JR: Role of
AMP-activated protein kinase in healthy and diseased hearts. Am J
Physiol Heart Circ Physiol. 291:H2557–H2569. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zheng QY, Jin FS, Yao C, Zhang T, Zhang GH
and Ai X: Ursolic acid-induced AMP-activated protein kinase (AMPK)
activation contributes to growth inhibition and apoptosis in human
bladder cancer T24 cells. Biochem Biophys Res Commun. 419:741–747.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aitken KJ, Block G, Lorenzo A, Herz D,
Sabha N, Dessouki O, Fung F, Szybowska M, Craig L and Bägli DJ:
Mechanotransduction of extracellular signal-regulated kinases 1 and
2 mitogen-activated protein kinase activity in smooth muscle is
dependent on the extracellular matrix and regulated by matrix
metalloproteinases. Am J Pathol. 169:459–470. 2006. View Article : Google Scholar
|
|
17
|
Persson K, Sando JJ, Tuttle JB and Steers
WD: Protein kinase C in cyclic stretch-induced nerve growth factor
production by urinary tract smooth muscle cells. Am J Physiol.
269:C1018–C1024. 1995.PubMed/NCBI
|
|
18
|
Mirone V, Imbimbo C, Longo N and Fusco F:
The detrusor muscle: an innocent victim of bladder outlet
obstruction. Eur Urol. 51:57–66. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shibata R, Ouchi N, Ito M, Kihara S,
Shiojima I, Pimentel DR, Kumada M, Sato K, Schiekofer S, Ohashi K,
Funahashi T, Colucci WS and Walsh K: Adiponectin-mediated
modulation of hypertrophic signals in the heart. Nat Med.
10:1384–1389. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nishino Y, Miura T, Miki T, Sakamoto J,
Nakamura Y, Ikeda Y, Kobayashi H and Shimamoto K: Ischemic
preconditioning activates AMPK in a PKC-dependent manner and
induces GLUT4 up-regulation in the late phase of cardioprotection.
Cardiovasc Res. 61:610–619. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee T, Andersson KE, Streng T and Hedlund
P: Simultaneous registration of intraabdominal and intravesical
pressures during cystometry in conscious rats - effects of bladder
outlet obstruction and intravesical PGE2. Neurourol Urodyn.
27:88–95. 2008. View Article : Google Scholar
|
|
22
|
Jin LH, Lee HJ, Shin HY, Choi BH, Yoon SM,
Park CS and Lee T: Development and changes with age of detrusor
overactivity in spontaneous hypertensive rats as observed by
simultaneous registrations of intravesical and intraabdominal
pressures. Int Neurourol J. 15:192–198. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kato K, Monson FC, Longhurst PA, Wein AJ,
Haugaard N and Levin RM: The functional effects of long-term outlet
obstruction on the rabbit urinary bladder. J Urol. 143:600–606.
1990.PubMed/NCBI
|
|
24
|
Levin RM, Haugaard N, Hypolite JA, Wein AJ
and Buttyan R: Metabolic factors influencing lower urinary tract
function. Exp Physiol. 84:171–194. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
McNeill SA, Hargreave TB,
Geffriaud-Ricouard C, Santoni J and Roehrborn CG: Postvoid residual
urine in patients with lower urinary tract symptoms suggestive of
benign prostatic hyperplasia: pooled analysis of eleven controlled
studies with alfuzosin. Urology. 57:459–465. 2001. View Article : Google Scholar
|
|
26
|
Hypolite JA, Longhurst PA, Haugaard N and
Levin RM: Effect of partial outlet obstruction on 14C-adenine
incorporation in the rabbit urinary bladder. Neurourol Urodyn.
16:201–208. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chang S, Hypolite JA, Mohanan S, Zderic
SA, Wein AJ and Chacko S: Alteration of the PKC-mediated signaling
pathway for smooth muscle contraction in obstruction-induced
hypertrophy of the urinary bladder. Lab Invest. 89:823–832. 2009.
View Article : Google Scholar : PubMed/NCBI
|