Introduction
The processes and mechanisms underlying brain
injuries due to ischemia and anoxia remain to be elucidated.
Additionally, few clinical treatments are currently available for
these types of injury. Activins, which are widely distributed in
the body, possess biological activity and are capable of regulating
the restoration, differentiation and survival of injured cells.
These molecules also promote tissue regeneration, participate in
the processes of various diseases and exhibit marked tissue and
organ specificity (1).
Investigations into neuronal disorders have led to an increased
interest in activin A (ActA), which acts as a recognized strong
protectin in the nervous system (2). ActA is a member of the transforming
growth factor (TGF) β1 super-family (4–6),
and transmits signals by binding to serine-threonine receptors on
cell membranes (7). The
activation of different ligands of ActA receptors may lead to
different physiological and pathological effects in vivo.
ActA has been shown to exert its effects through stimulation of the
Smad signal transduction pathway (3), including Smad2 and Smad3.
Receptor-regulated Smad proteins then form a multimer with Smad4,
which translocates into the nucleus and co-regulates target gene
transcription and other biological functions with intranuclear
cofactors (8–10).
It is clear that exogenous activin plays a
protective role in the nervous system. However, few studies have
focused on the protective effect of ActA on neurons. Therefore, the
aim of the present study was to elucidate the protective mechanism
of action of ActA in an in vitro oxygen/glucose deprivation
(OGD) model system. To this end, PC12 cells were differentiated
into neuron-like cells and were then exposed to different
concentrations of exogenous ActA. The effect of this treatment was
assessed under anoxic and ischemic conditions on PC12 survival and
caspase-3 expression, a key apoptotic regulatory protein.
Materials and methods
PC12 cell culture
PC12 cells (Beijing Chinese Redbud Biological Co.
Ltd., Beijing, China) were plated into polylysine-coated flasks
containing Dulbecco’s modified Eagle’s medium (DMEM; Gibco,
Carlsbad, CA, USA). The cells were then incubated in a 5%
CO2 incubator at 37°C, and their media were changed
after two days. When the cells reached 85% confluence, they were
used for the experiments.
PC12 cell differentiation
Glass coverslips were scratched and placed into
24-well plates. PC12 cells were inoculated into these wells at a
concentration of 5×104 cells/ml and cultivated for 24 h.
Nerve growth factor (NGF) diluted in DMEM (0.05 mg/ml) was then
added to the culture well. The morphological changes following NGF
treatment were observed after 0, 24, 36 and 72 h using an inverted
microscope (Olympus, Tokyo, Japan). Neurites that were >2-fold
the length of the cell bodies were used to classify the cells as
neuron-like, as were multiple neuritis. The rate of differentiation
into neuron-like cells was calculated by observation. Images were
obtained from five wells to give the mean value.
Immunocytochemistry
Following treatment, cells adhering to the glass
coverslips were removed from the 24-well plates and washed in 0.01
M phosphate-buffered saline (PBS). The cells were then fixed in 4%
paraformaldehyde, and exposed (10 min) to 0.1% Triton X-100/PBS
(PBST) to permeabilize the cells. Goat serum (10% in PBS) was
subsequently added for 30 min following the removal of the PBST to
block non-specific binding sites in the cells. One drop of rabbit
anti-rat microtubule-associated protein 2 antibody (1 mg/ml,
Beijing Bo’ao Biological Co., Ltd., Beijing, China) was then added
to the coverslips, which were incubated overnight in a humidified
container at 4°C. The following morning, the coverslips were washed
and a drop of FITC-labeled goat anti-rabbit IgG (2 mg/ml; Beijing
Bo’ao Biotechnology Co., Ltd., Beijing, China) was added to the
cells and incubated at 37°C for 20 min. The coverslips were sealed
with anti-quenching mounting medium (Beijing Bo’ao Biotechnology
Co., Ltd.). A negative control was created by using PBS instead of
the primary antibody. The cells were observed under a fluorescence
microscope (Olympus).
Establishment of the oxygen/glucose
deprivation (OGD) model
The model was established using an improved method,
as described by Guo et al (24). Briefly, cell culture flasks were
placed in sealable glassware. Sterile water (100 ml) was injected
into the glassware and sodium sulfoxylate (40 g) was also placed in
the glassware for oxygen removal. The glassware was then sealed
with a rubber tube to keep the atmosphere in the bottle at
CO2 5%, N2 95%. Glucose-free fetal bovine
serum (10%) DMEM was added to the cell culture bottles, as well as
sodium sulfoxylate (final concentration 1 mM), in order to continue
culturing of PC12 cells. The hypoxia chamber was then placed in an
incubator and maintained at 37°C.
Exogenous ActA and detection of the cell
survival rate using the MTT assay
Cells were cultured for 3, 6, 9, 12 and 16 h in
hypoxic conditions, and suspended (100 μl) in wells of culture
plates at a density of 5×104 cells/ml. Cells were then
incubated in 5% CO2 at 37°C, and recombinant human ActA
(rhActA) was added at concentrations of 10, 20, 30, 50 or 100
μmol/ml. When the cells reached confluence, the MTT solution (5
mg/ml; 20 μl) was added to each well and cultured for a further 4
h. The MTT solution was then removed and dimethyl sulfoxide (150
μl) was added to each well. After the plates were agitated on a
table concentrator for 10 min, the optical density at 490 nm was
measured to determine the cell survival rate (%), calculated as:
(light absorbance at 490 nm × 100% in the experimental
group)/(light absorbance at 490 nm × 100% in the control group).
Experiments were repeated three times.
Hoechst 33342 staining assay
Following cell culture for 3, 6, 9, 12, 16 and 24 h,
scratched coverslips were placed into 12-well plates. Cells
(5×104/ml) were then added to each well and were
subjected to OGD, as described above. The cells were fixedy by
removing the culture medium and replacing it with 0.5 ml 95%
ethanol. After being washed twice with PBS, 0.5 ml of Hoechst 33342
staining solution was added to the cells. The coverslips were
mounted in a fluorescence anti-quenching mounting medium, and the
cells were observed under a fluorescence microscope. Hoechst 33342
staining revealed that the nuclei of apoptotic cells appeared
bright blue and condensed. The cells were quantified as percentage
cells undergoing apoptosis, calculated as: apoptotic cell
number/total cell number under the ×100 field of vision × 100.
Counts were repeated three times to establish a mean value.
Western blot analysis of ActAIIR, Smad3,
Smad4 and caspase-3 in PC12 cells
Culture medium was replaced with 1X sodium dodecyl
sulfate (SDS) sample buffer after PC12 cells under OGD were exposed
to rhActA. The cells were scraped from the plates and transferred
to centrifuge tubes, where they were sonicated and boiled for 5
min. Samples were centrifuged at 12,000 rpm for 5 min and the
supernatant was removed. The pellets were resuspended, separated
using electrophoresis, and transferred to a membrane. The membrane
was then placed in a blocking solution (5% skim milk powder) and
probed with rabbit anti-rat primary antibodies (1:200) against
ActAIIR, Smad3, Smad4, pro-caspase-3, active caspase-3 and mouse
anti-rat β-actin monoclonal antibody (200 μg/ml, Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA). The membrane was
incubated overnight at 4°C and washed three times the following
morning (5 min each). Following this washing step, the membranes
were incubated with horseradish peroxidase (HRP)-conjugated goat
anti-rabbit secondary antibody (1:1,000, Santa Cruz Biotechnology)
and incubated subsequent to agitation for 2 h at 37°C. The
membranes were then washed three times in 0.1%
Tween-20/Tris-buffered saline (TTBS) cleaning solution, (5 min),
and then in TBS alone for 5 min. Band absorbance was measured with
a gelatin image analysis system (Olympus) and the absorbance ratio
was calculated against the absorbance for the β-actin band.
Experiments were repeated three times.
Statistical analysis
Results are shown as the mean ± SD. Statistical
analysis was conducted using SPSS 13.0 software (SPSS; Chicago, IL,
USA). The difference between groups was analyzed using a one-way
analysis of variance, and multiple comparisons were conducted using
the SNK-q method. P<0.05 indicated a statistically significant
difference.
Results
Morphological analysis of differentiated
PC12 cells
PC12 cell morphology
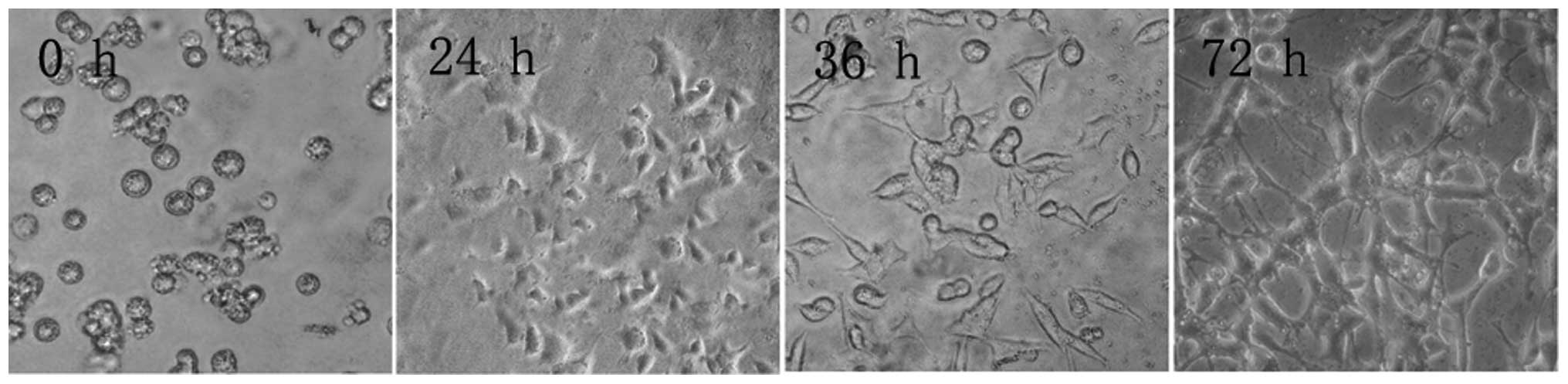
Microscopy revealed that after 12 h in culture, PC12
cells had still not adhered to the culture flask. After 36 h, the
cells had adhered and began to rapidly divide, until confluence was
reached after 72 h (Fig. 1).
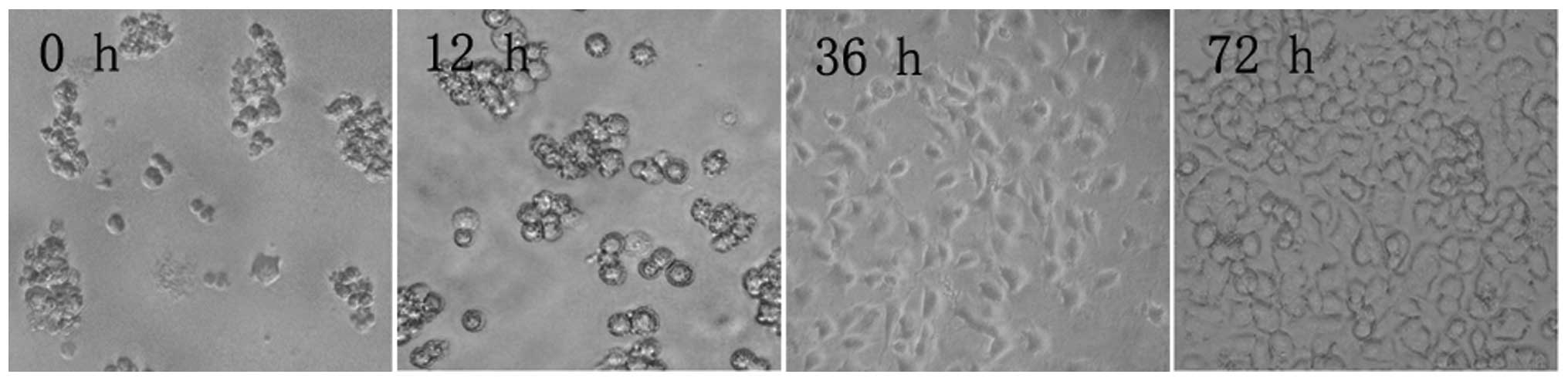 | Figure 1PC12 cell morphology at different time
points during culture (inverted microscope, ×100). After 12 h, PC12
cells did not adhere to the flask walls, but congregated, assuming
a semi-suspension state. After 36 h, PC12 cells assumed an
elliptical or polygonal shape, tending to congregate in clusters.
After 72 h, PC12 cells entered an exponential growth phase, in
which cells grew rapidly, no longer congregated, and had a strong
optical refraction. |
Nerve growth factor-induced
differentiation of PC12 cells into neuron-like cells
NGF induced PC12 cells to differentiate into
neuron-like cells after 24 h. Synapses formed between PC12 cells,
and after 36 h axons appeared to grow longer and thicker. After 72
h, the length of the axons was >5-fold the length of the cell
bodies. These axons were interlaced into a network and demonstrated
neuronal characteristics. These neuronal-like cells accounted for
>95% of the culture (Fig.
2).
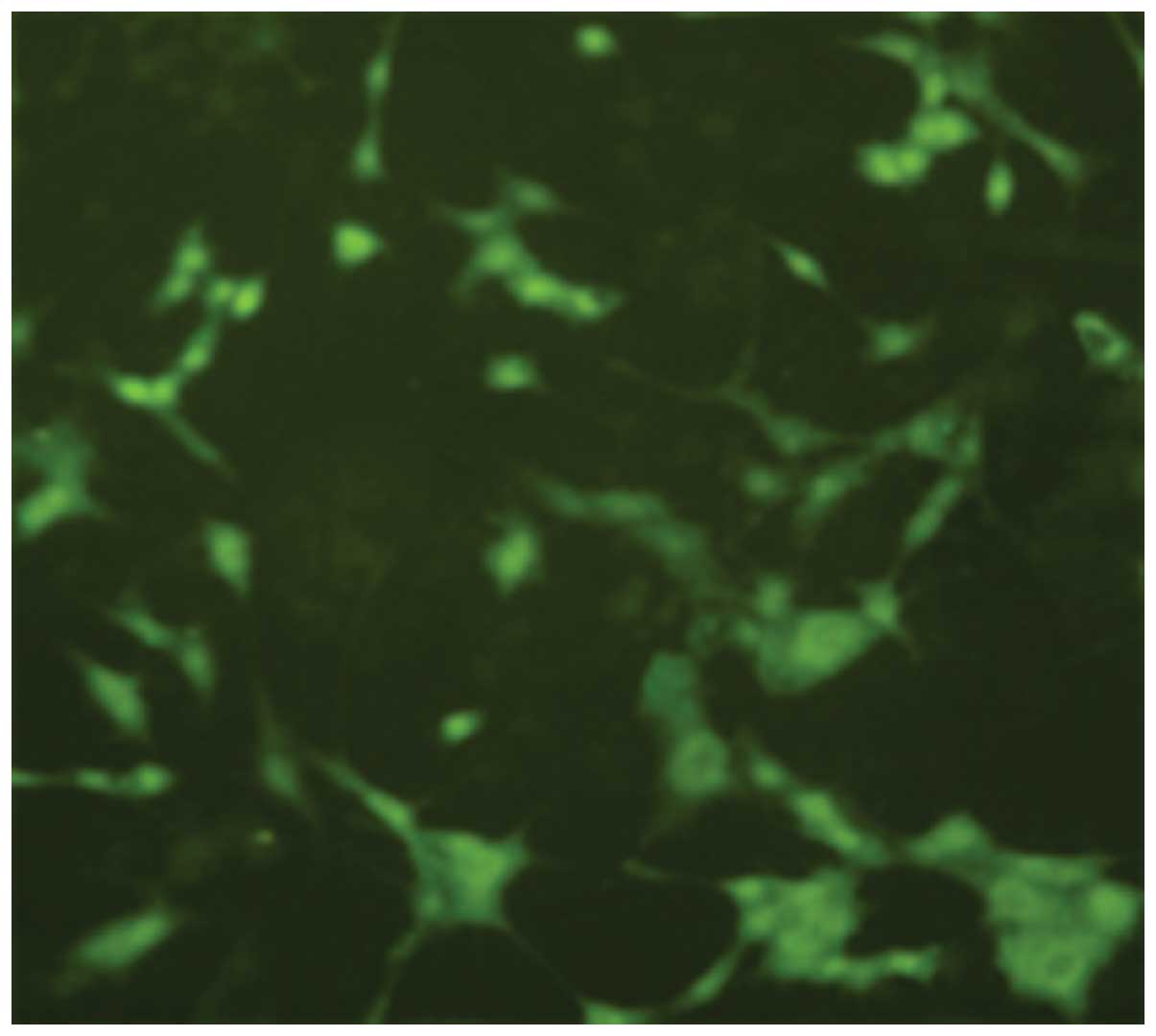
After 72 h post-NGF induction of PC12 cells,
immunofluorescence staining showed that cells expressed
microtubule-associated protein 2 (MAP2) (Fig. 3).
Rate of cell survival after PC12 cells
were subjected to OGD
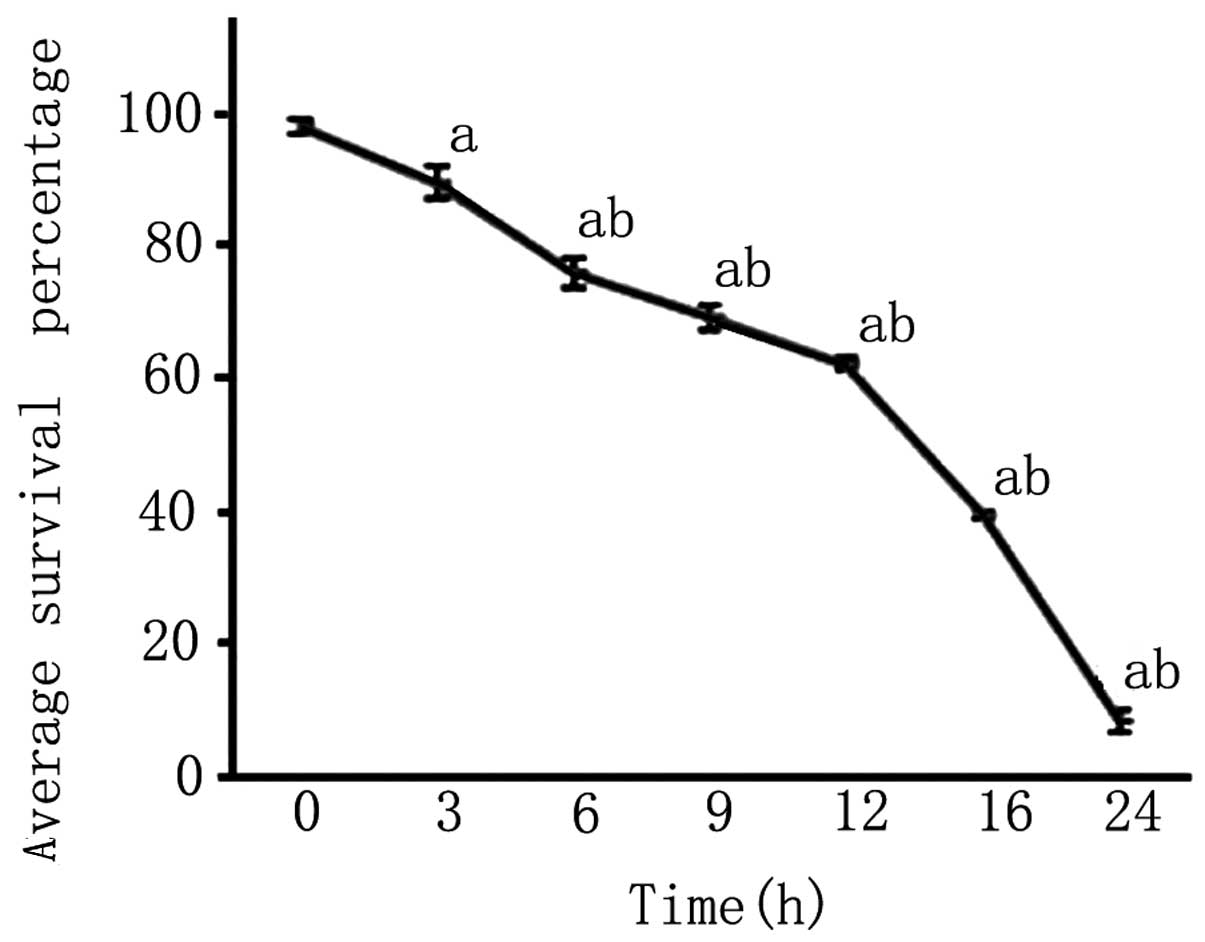
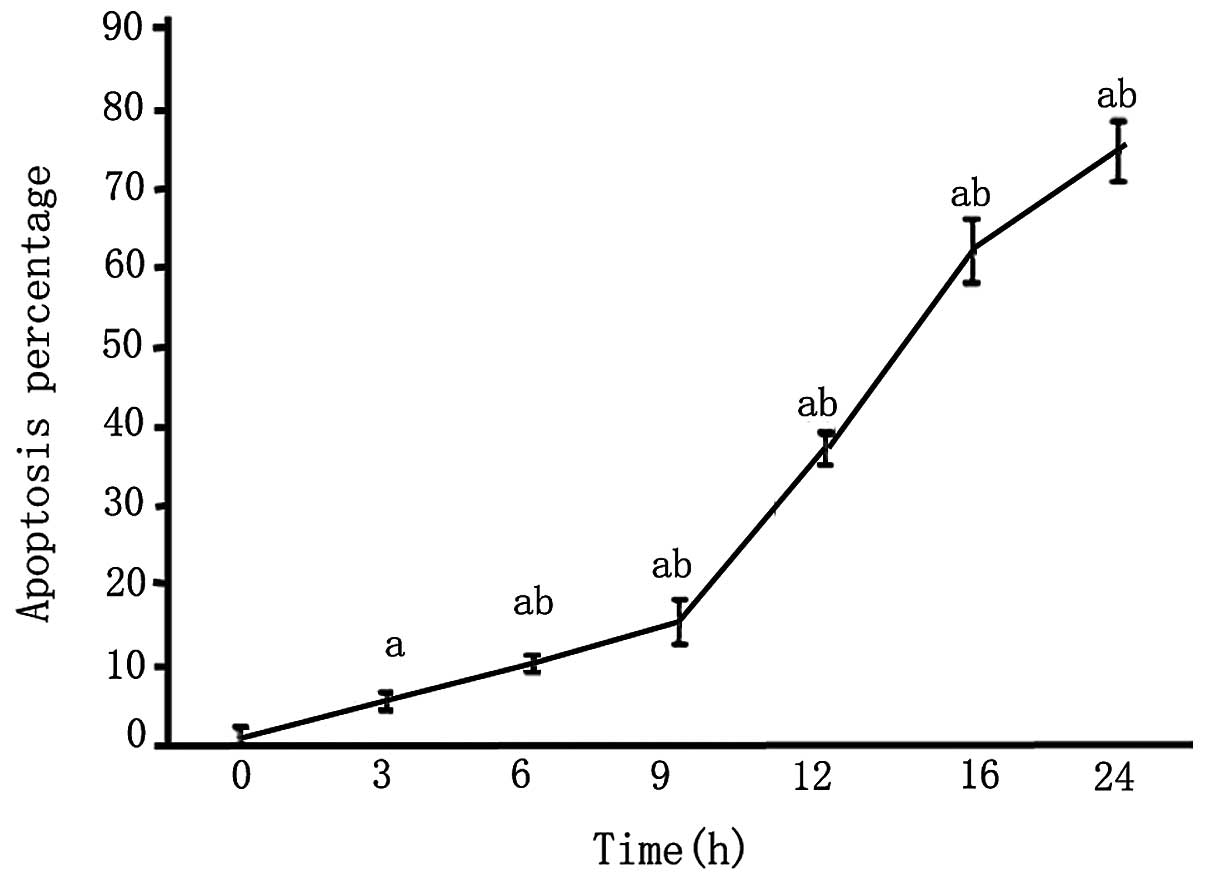
An MTT assay revealed that after 3 h of OGD, the
survival rate of PC12 cells was reduced and continued to decrease
with the length of OGD (P<0.05; Fig. 4).
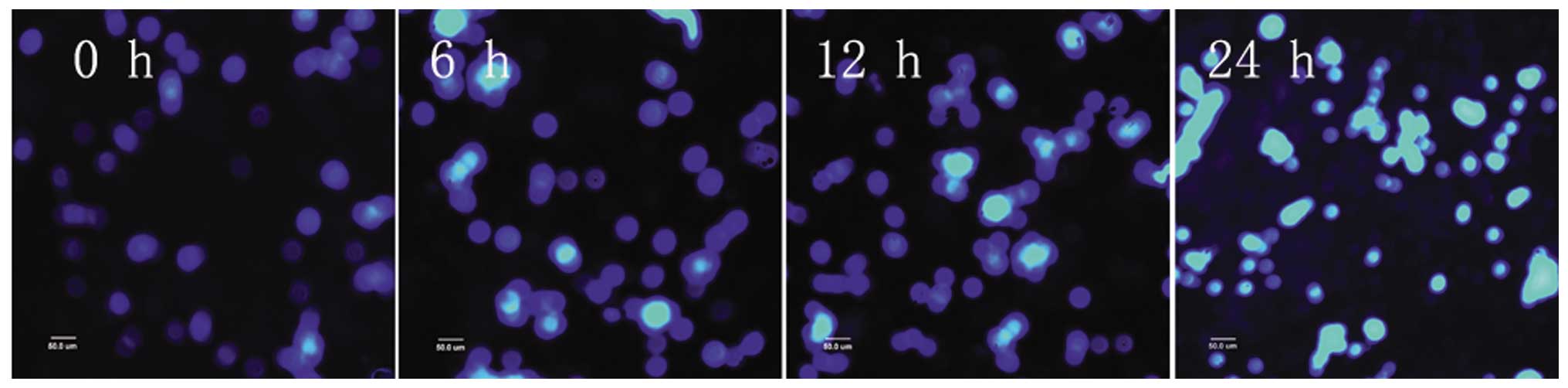
After PC12 cells were subjected to 3 and 6 h of OGD,
apoptotic cells were observed, as detected by condensed nuclei
visualized by Hoechst 33342 staining. After 9 h, apoptotic cells
increased in number. After 12 h of OGD, the majority of cells
appeared bright, pyknotic, heavily stained and exhibited apoptotic
blebs. The majority of the cells died after 24 h of OGD and very
few normal cells were evident (Figs.
5 and 6).
The effect of exogenous ActA on injured
PC12 cells after OGD
rhActA increased the survival rate of
PC12 cells after OGD
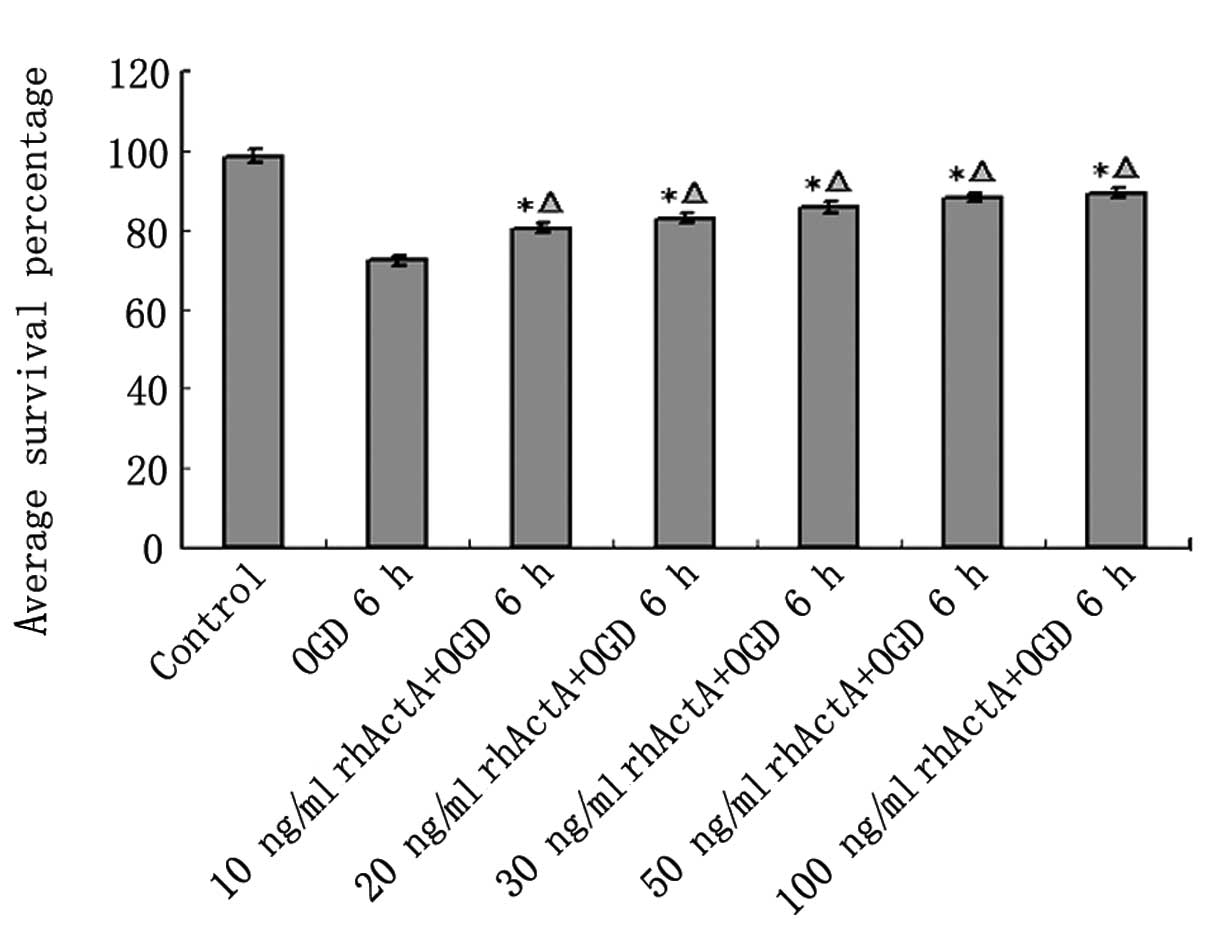
As shown above, the cell survival rate of PC12 cells
under OGD was markedly lower than that of the control group.
However, this cell death was decreased after 24 h of rhActA
stimulation. The cell survival rate after 6 h was increased in the
rhActA + OGD group compared with the OGD group. Furthermore, the
survival rate was positively correlated with the final
concentration of rhActA. The cell survival rate in the rhActA (100
ng/ml) group was slightly higher than that of the 50 ng/ml group,
however, this difference was not significant. A comparison of the
cell survival rate in each group is shown in Fig. 7.
rhActA prevented apoptosis after OGD
in PC12 cells
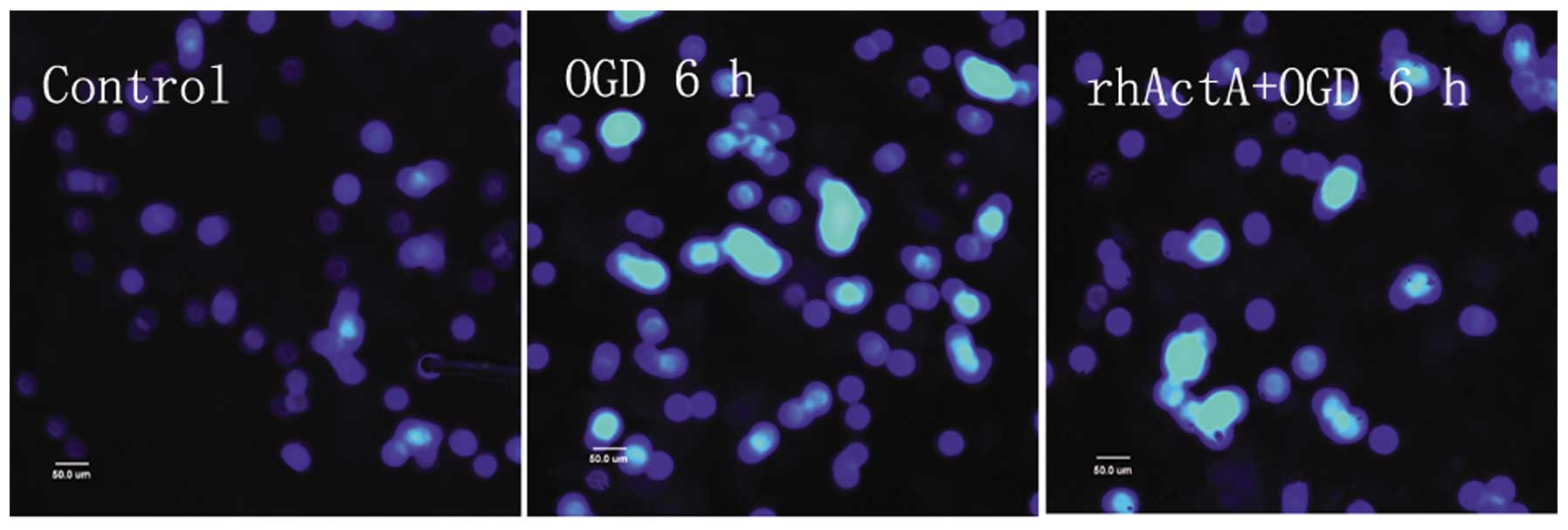
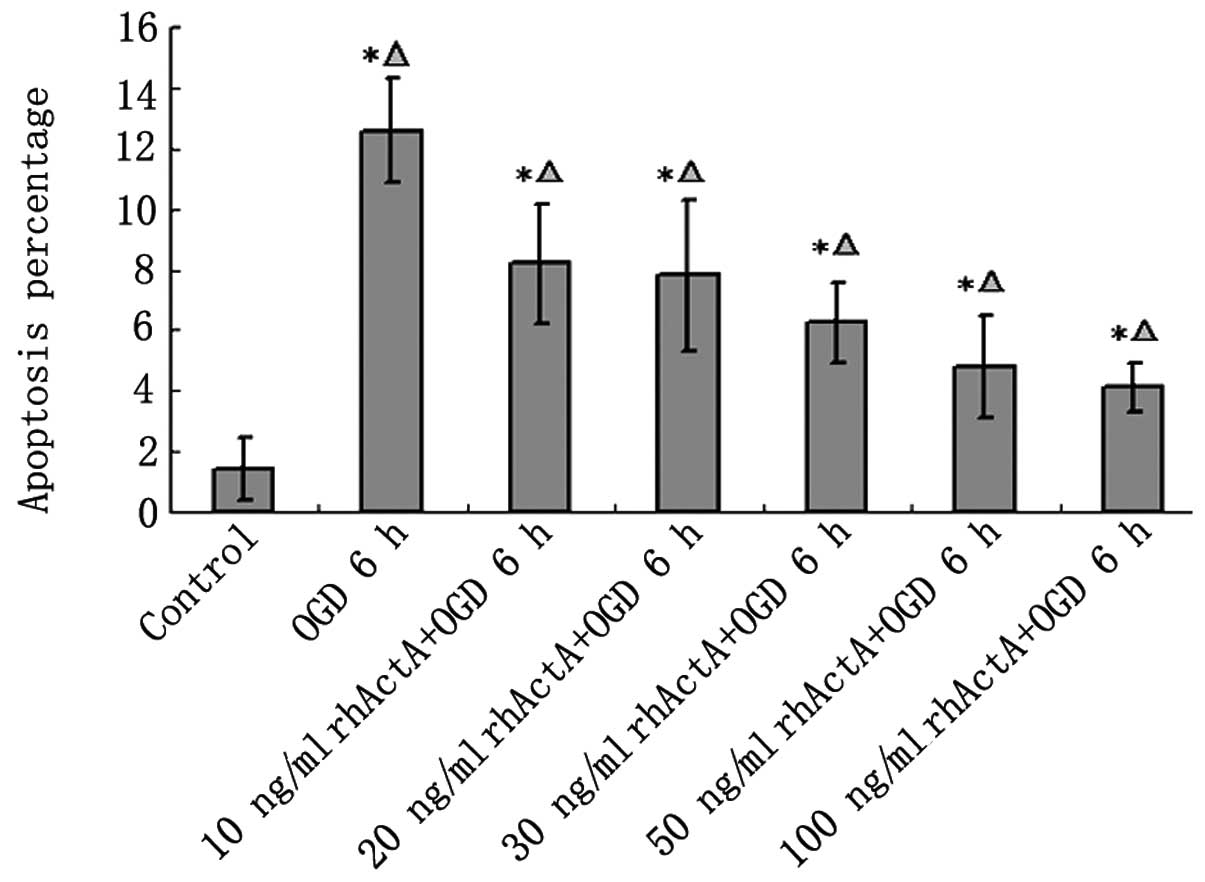
Although most of the nuclei in the control group
were not condensed or showed signs of apoptosis, a small number of
apoptotic cells were observed (Fig.
8). Apoptosis was evident in the OGD 6 h group in which the
endonuclear chromatin was unevenly distributed showing typical
bright, condensed and densely stained forms. Fewer apoptotic cells
were visible in the rhActA + OGD 6 h group compared with those in
the OGD 6 h group. The apoptotic rate in the OGD 6 h group was
significantly higher than that in the control group (Fig. 9). After 24 h of rhActA
stimulation, PC12 cells exhibited less cell damage compared with
that observed in the untreated cells. Furthermore, consistent with
the MTT results above, the apoptotic rate appeared to depend on the
rhActA concentration. The apoptotic rate in the rhActA (100 ng/ml)
group was slightly lower than that observed in the 50 ng/ml group,
although this difference was not significant.
Effect of exogenous ActA on regulating
caspase-3 protein expression in PC12 cells following OGD
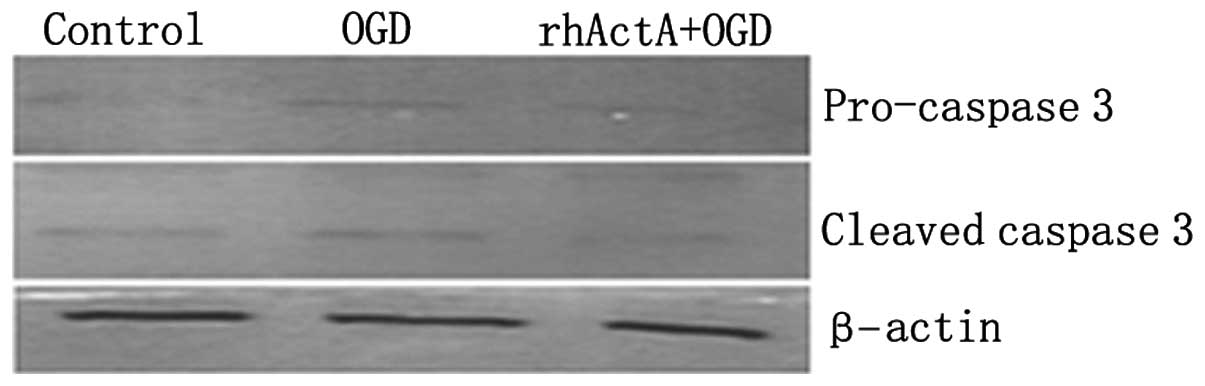
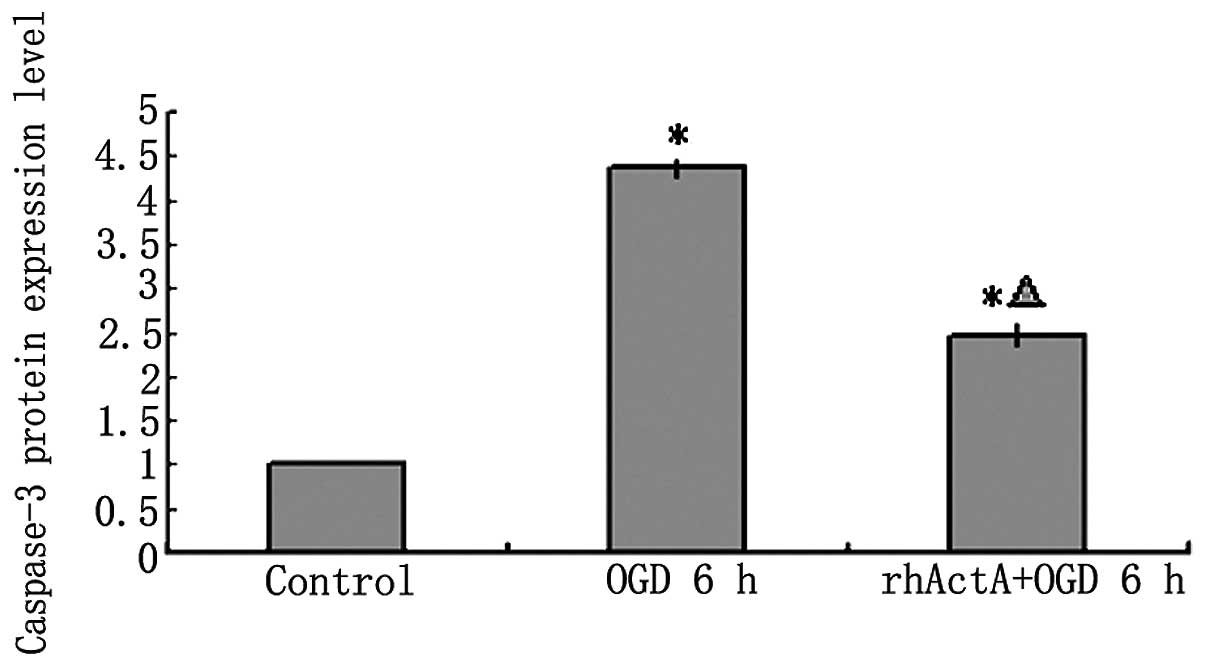
On the basis of the above results, the rhActA (100
ng/ml) + OGD 6 h group was used for subsequent experiments. Results
showed that the expression of pro-caspase-3 and active caspase-3
after 6 h of OGD alone was higher than that of the control group
not experiencing deprivation (P<0.05) (Figs. 10 and 11). Furthermore, caspase-3 expression
after 6 h of OGD alone showed a marked increase compared with that
in cells co-stimulated with rhActA (P<0.05).
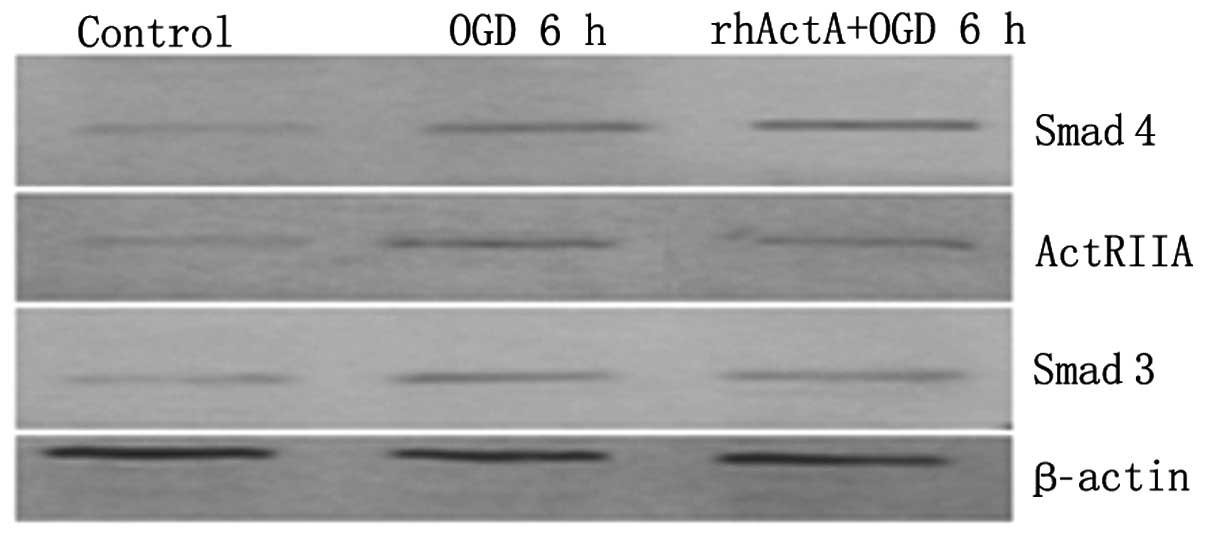
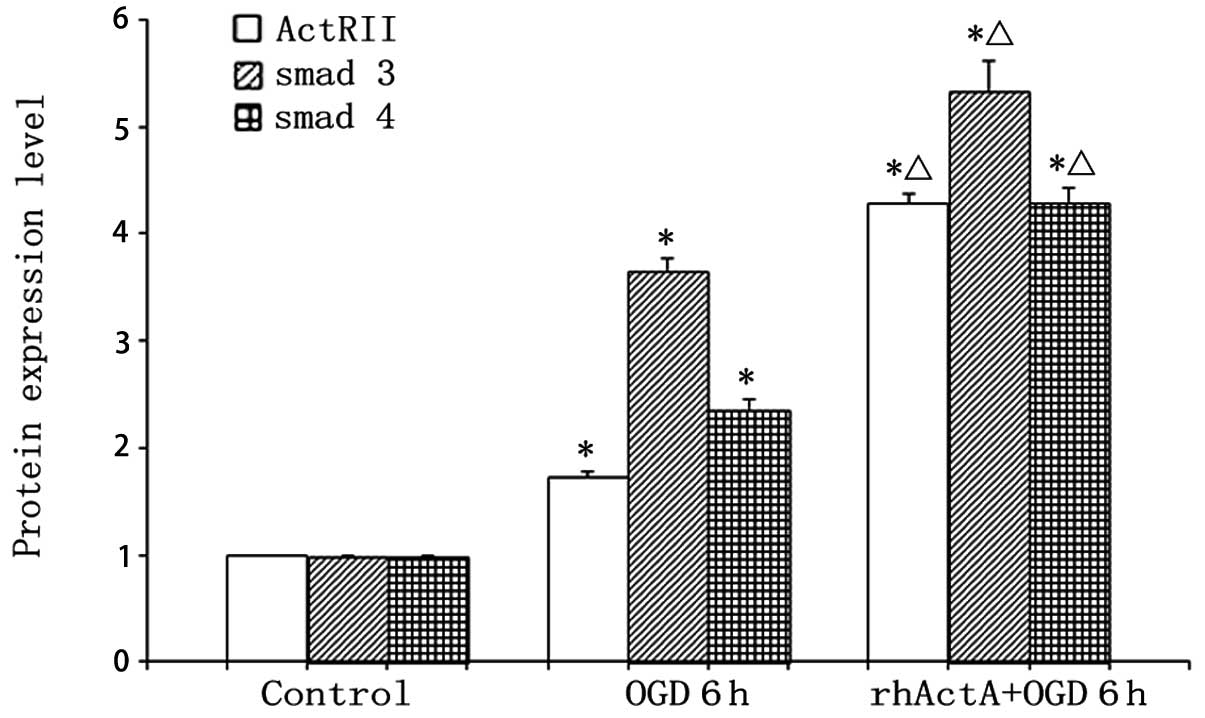
Effect of exogenous ActA on ActRIIA,
Smad3 and Smad4 protein expression after PC12 cells were subjected
to OGD
Protein expression of ActRIIA, Smad3 and Smad4 in
the OGD 6 h group was higher than that in the control group
(P<0.05) (Figs. 12 and
13). By contrast, pretreatment
with rhActA significantly decreased the expression of ActRIIA after
6 h of OGD (P<0.05) compared with the OGD 6 h group.
Discussion
Results of the present study have shown that ActA is
a fundamental regulator of histiocytes, participating in histiocyte
growth, as well as aiding in the maintenance of their normal
function. The biological activity of ActA is restricted to target
cell types, suggesting its tissue specificity. As a neuronal growth
factor, ActA has a protective role against many factors, inducing
nerve injuries. It has been found that ActA plays an active role in
neuronal repair after brain injury (2). Shoji and colleagues (11) have found that activins can
increase the synapse number and dendritic crest neck length of
seahorse neurons cultured in vitro. Other brain injuries,
such as those caused by acute anoxia/ischemia, also cause ActA to
increase in expression. Therefore, ActA has been described as an
early transient ischemic and anoxic regulatory gene (12). It is confirmed that calcitonin
gene-related peptide (CGRP) has a nerve-protective function and is
capable of regulating axonal regeneration (13). ActA has been shown to stimulate
the expression of DRG in vitro (14), and DRG and CGRP in vivo
(15). An increasing number of
studies suggest that recombinant activins likely alleviate
post-ischemic nerve injury. This mechanism may occur because an
activin induces basic fibroblast growth factor (bFGF) expression,
and dopaminergic neuron generation. Thus, there may be a
synergistic protective effect between recombinant activin and bFGF
(2,16). Of note, previous studies (17) have also revealed that activins
regulate apoptosis. For example, ActA has been found to prevent
apoptosis in hepatocytes. As a regulatory factor of hepatocyte
proliferation, it is able to induce DNA synthesis by affecting
mitogens and regulate hepatocyte growth. This effect has been shown
to be mediated by activin type I and type II receptors on the cell
surface (17,18). The dose-dependent inhibition of
ActA on apoptosis confirms the role of ActA in promoting cell
survival.
Caspases are cysteine-aspartic proteases that share
amino acid similarity and secondary structure cysteine proteases.
These proteins are closely associated with ukaryotic apoptosis.
Caspase-3 activation is a pivotal point of a number of apoptotic
pathways. Furthermore, caspase-3 may be an important effector in
ischemic neuronal apoptosis (19)
and directly leads to cell death (20). Administration of a caspase-3
inhibitor after 9 h of mouse cerebral ischemia has been shown to
effectively reduce cerebral infarction size (21). Furthermore, after 2 h of
unilateral middle cerebral artery occlusion in mice, the occurrence
of cerebral post-ischemic neuronal apoptosis has been shown to be
closely associated with caspase-3 activity, as well as increasing
split products. Following ischemic reperfusion, caspase-3 protein
expression has also been shown to increase to different extents
(22,23).
In the present study, we established a novel
neuronal-like OGD model that can be used to study the effects of
anoxia and ischemia in vitro. This was accomplished by
inducing the differentiation of PC12 cells with NGF prior to oxygen
and glucose deprivation. Using this novel OGD model, we have
demonstrated that there was an increased amount of apoptosis over
time, indicating that the OGD model was successful.
We also demonstrated that ActA pretreatment
prevented apoptosis and promoted cell viability after OGD.
Caspase-3 protein expression in cells was increased after OGD,
which was partially abrogated by ActA pretreatment. Additionally,
we found that ActA pretreatment increased the expression of
ActRIIA, Smad3 and Smad4, even after OGD. This finding suggests
that activation of the ActA/Smad signal transduction pathway might
prevent neuronal injury due to ischemia and anoxia. The ActA/Smads
pathway activation induced by transient ischemic injury in the
course of cerebral ischemia may therefore also play a protective
role. It is likely through the ActA/Smad signal transduction that
ActA protects against cell apoptosis and protects neurons.
In conclusion, our study results suggest that ActA
potentially plays a protective role in ischemic and anoxic neuronal
injury through the ActA/Smad signal transduction pathway. This
protective effect may be mediated in part by downregulating
caspase-3 expression. However, how the activation of the ActA/Smad
signal transduction pathway inhibits apoptotic processes and
caspase-3 expression remains to be determined. Additional studies
are required to elucidate the underlying mechanism of action of the
protective effects of ActA.
Acknowledgements
This study was financially supported by the National
Natural Science Foundation of China (no. 30971037), Postdoctoral
Foundation of China (no. 2012MS10489), the Natural Science
Foundation of Jilin Provincial Science and Technology Department
(no. 201015240) and the Department of Education of Jilin Province
Project (no. 2013361).
References
|
1
|
Massagué J: TGFbeta in cancer. Cell.
134:215–230. 2008.
|
|
2
|
Tretter YP, Hertel M, Munz B, ten
Bruggencate G, Werner S and Alzheimer C: Induction of activin A is
essential for the neuroprotective action of basic fibroblast growth
factor in vivo. Nat Med. 6:812–815. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lin X, Duan X, Liang YY, et al: PPM1A
functions as a Smad phosphatase to terminate TGFbeta signaling.
Cell. 125:915–928. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ling N, Ueno N, Ying SY, et al: Inhibins
and activins. Vitam Horm. 44:1–46. 1988. View Article : Google Scholar
|
|
5
|
Munz B, Tretter YP, Hertel M, Engelhardt
F, Alzheimer C and Werner S: The roles of activins in repair
processes of the skin and the brain. Mol Cell Endocrinol.
180:169–177. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ageta H, Murayama A, Migishima R, et al:
Activin in the brain modulates anxiety-related behavior and adult
neurogenesis. PLoS One. 3:e18692008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tsuchida K, Nakatani M, Uezumi A, Murakami
T and Cui X: Signal transduction pathway through activin receptors
as a therapeutic target of musculoskeletal diseases and cancer.
Endocr J. 55:11–21. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schneyer AL, Sidis Y, Gulati A, Sun JL,
Keutmann H and Krasney PA: Differential antagonism of activin,
myostatin and growth and differentiation factor 11 by wild-type and
mutant follistatin. Endocrinology. 149:4589–4595. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou WJ and Hu ZP: Expression of Smad 2
and Smad 4 proteins in brain tissue following cerebral
ischemia/reperfusion in gerbils. Guoji Shenjing Bing Xue Shenjing
Waike Zazhi. 35:112–114. 2008.(In Chinese).
|
|
10
|
Tsuchida K, Nakatani M, Hitachi K, et al:
Activin signaling as an emerging target for therapeutic
interventions. Cell Commun Signal. 7:152009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shoji-Kasai Y, Ageta H, Hasegawa Y,
Tsuchida K, Sugino H and Inokuchi K: Activin increases the number
of synaptic contacts and the length of dendritic spine necks by
modulating spinal actin dynamics. J Cell Sci. 120:3830–3837. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mukerji SS, Katsman EA, Wilber C, Haner
NA, Selman WR and Hall AK: Activin is a neuronal survival factor
that is rapidly increased after transient cerebral ischemia and
hypoxia in mice. J Cereb Blood Flow Metab. 27:1161–1172. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li XQ, Verge VM, Johnston JM and Zochodne
DW: CGRP peptide and regenerating sensory axons. J Neuropathol Exp
Neurol. 63:1092–1103. 2004.PubMed/NCBI
|
|
14
|
Cruise BA, Xu P and Hall AK: Wounds
increase activin in skin and a vasoactive neuropeptide in sensory
ganglia. Dev Biol. 271:1–10. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu P, Van Slambrouck C, Berti-Mattera L
and Hall AK: Activin induces tactile allodynia and increases
calcitonin gene-related peptide after peripheral inflammation. J
Neurosci. 25:9227–9235. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Müller MR, Zheng F, Werner S and Alzheimer
C: Transgenic mice expressing dominant-negative activin receptor IB
in forebrain neurons reveal novel functions of activin at
glutamatergic synapses. J Biol Chem. 281:29076–29084.
2006.PubMed/NCBI
|
|
17
|
Yasuda H, Mine T, Shibata H, et al:
Activin A: an autocrine inhibitor of initiation of DNA synthesis in
rat hepatocytes. J Clin Invest. 92:1491–1496. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen W, Woodruff TK and Mayo KE: Activin A
induced Hep G2 liver cell apoptosis: involvement of activin
receptors and smad proteins. Endocrinology. 141:1263–1272.
2000.PubMed/NCBI
|
|
19
|
Yuan J and Yankner BA: Apoptosis in the
nervous system. Nature. 407:802–809. 2000. View Article : Google Scholar
|
|
20
|
Gurney ME, Tomasselli AG and Heinrikson
RL: Neurobiology. Stay the executioner’s hand. Science.
288:283–284. 2000.
|
|
21
|
Lee JM, Grabb MC, Zipfel GJ and Choi DW:
Brain tissue responses to ischemia. J Clin Invest. 106:723–731.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Benchoua A, Guégan C, Couriaud C, et al:
Specific caspase pathways are activated in the two stages of
cerebral infarction. J Neurosci. 21:7127–7134. 2001.PubMed/NCBI
|
|
23
|
Harrison DC, Davis RP, Bond BC, et al:
Caspase mRNA expression in a rat model of focal cerebral ischemia.
Brain Res Mol Brain Res. 89:133–146. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guo HL, Xu ZX and Li XH: Use of RNAi
silencing to target preconditioned glial cell line-derived
neurotrophic factor in neuronal apoptosis. Neural Regen Res.
6:510–516. 2011.
|