Introduction
Allergic rhinitis (AR), a very common chronic
illness that affects patients of all ages, initiates the allergic
reaction induced by the release of preformed mediators and the
generation of inflammatory mediators from mast cells and
eosinophils (1). The levels of
interleukin (IL)-l, IL-5, IL-6, macrophage-inflammatory protein-2
(MIP-2), and granulocyte-macrophage colony-stimulation factor
(GM-CSF) are increased during the early and late phase of AR
(2,3). The cytokine messenger RNA (mRNA)
expression of IL-3, IL-4, IL-5 and GM-CSF has been shown to be
increased in the nasal mucosa of patients with AR following
allergen provocation and is associated with tissue eosinophilia
(4). Activated endothelial cells
express intercellular adhesion molecule-1 (ICAM-1) and vascular
cell adhesion molecule-1 (VCAM-1) on their cell surfaces (5). GM-CSF is an important activating
factor for eosinophils and neutrophils, and is known as a
pleiotropic and pro-inflammatory cytokine (6). GM-CSF induced the expression of
IL-32 in eosinophils (7).
IL-32 is associated with AR, cancer, infection and
chronic inflammation (7,8–10).
IL-32 is a cytokine produced mainly by immune cells, including T
lymphocytes, natural killer cells, epithelial cells, mast cells,
keratinocytes, eosinophils and blood monocytes (11,12). IL-32 has previously been shown to
contribute to pro-inflammatory cytokine synthesis (7). Several pathways have been shown to
be involved in inflammatory processes, including the
phosphatidylinositide 3-kinase (PI3K)/Akt pathway, nuclear factor
(NF)-κB/AP-1 pathway, p38 mitogen-activated protein kinase (MAPK)
and caspase-1 pathways (7,12,13).
Viral-induced IL-32 expression seems to be mediated by
cyclooxygenase-2 (COX-2) pathways (14).
Thymic stromal lymphopoietin (TSLP) is a central
factor in allergic inflammation and allergy-related diseases,
including atopic dermatitis (AD), asthma and AR (15–17). TSLP is expressed and produced by
caspase-1 and NF-κB in mast cells (18,19). Recently, we reported that IL-32
induced TSLP production through the activation of NF-κB and
caspase-1 in monocytes (20).
Traditional Korean medicine (TKM) has been used for
thousands of years. Naju Jjok (Polygonum tinctorium Lour.,
NJJ) is a species of flowering plant in the buckwheat family
(commonly known as Chinese indigo) and has been used for its
detoxifying, antibacterial, anticancer, antioxidant,
anti-inflammatory and anti-allergic properties (21–24) and traditionally, as a textile dye.
Tryptanthrin, kaempferol and indirubin are the main components of
NJJ. Tryptanthrin and kaempferol have antibacterial properties
(23). Kaempferol has been shown
to inhibit the production of IL-8 and tumor necrosis factor (TNF)-α
and the infiltration of eosinophils in allergic reactions (41,42). Indirubin has been shown to inhibit
inflammatory reactions and allergic contact dermatitis (43,44) However, the effects of NJJ on AR
have not yet been fully elucidated. In this study, we evaluated the
anti-allergic and anti-inflammatory effects of NJJ on mice with
ovalbumin (OVA)-induced AR, as well as on the GM-CSF-stimulated
human eosinophilic cell line, Eol-1.
Materials and methods
Materials
OVA, o-phthaldialdehyde (OPA), avidin
peroxidase (AP), 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic
acid) substrate tablets (ABTS),
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
and bicinchoninic acid (BCA) were purchased from Sigma (St. Louis,
MO, USA). Fetal bovine serum (FBS), Roswell Park Memorial Institute
(RPMI)-1640, and streptomycin were purchased from Gibco-BRL (Grand
Island, NY, USA). Anti-mouse immunoglobulin E
(IgE)/TSLP/IL-4/interferon (IFN)-γ/MIP-2/ICAM-1 antibody (Ab),
biotinylated anti-mouse IgE/TSLP/IL-4/IFN-γ/MIP-2/ICAM-1 Ab,
recombinant mouse IgE/TSLP/IL-4/IFN-γ/MIP-2/ICAM-1, anti-human IL-8
Ab, biotinylated anti-human IL-8 Ab and recombinant human
IL-8/GM-CSF were purchased from Pharmingen (San Diego, CA, USA).
The IL-32 Abs was obtained from BioLegend (San Diego, CA, USA) and
Acris (Herford, Germany). Abs against COX-2, caspase-1 and actin
were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). The caspase-1 assay kit was supplied by R&D Systems Inc.
(Minneapolis, MN, USA).
Preparation of NJJ
NJJ was obtained from Naju (Korea). The whole plant
material of NJJ was extracted with distilled water (DW) at 80°C for
3 h. The filtered crude extracts were lyophilized and reduced to
powder. The yield of dried extract from the starting materials was
approximately 18% (w/w). The NJJ was dissolved in DW and filtered
with a 0.22-μm syringe filter.
OVA-induced animal model of AR
We maintained 6-week-old female BALB/c mice (Charles
River Laboratories, Inc., Wilmington, MA, USA) under pathogen-free
conditions. Mouse care and experimental procedures were performed
under the approval of the Animal Care and Use Committee of Kyung
Hee University [KHUASP(SE)-11-037]. We sensitized the mice on days
1, 5 and 14 by an intraperitoneal (i.p.) injection of 100 μg OVA
emulsified in 20 mg aluminum hydroxide (Sigma) and then challenged
the mice with 1.5 mg OVA. NJJ was orally (1, 10, or 100 mg/kg) or
intranasally (i.n., 2 μl of 40 μg/nostril) administered prior to
i.n. OVA challenge for 10 days. Nasal symptoms were evaluated by
counting the number of nasal rubs that occurred in the 10 min
following OVA i.n. provocation. The mice were divided into 3
groups: i) the normal group, in which the mice were not
sensitized/challenged with OVA; ii) the control group, in which the
mice were sensitized/challenged with OVA; and the iii) the NJJ
group, in which the mice were sensitized/challenged with OVA and
administered NJJ. There were 5 mice in each group.
Enzyme-linked immunosorbent assay
(ELISA)
The Eol-1 cells (3×105) were treated with
NJJ (1, 10, or 100 μg/ml) for 1 h prior to stimulation with GM-CSF
and incubated for 24 h. Cytokines in serum, nasal mucosal tissue
and spleen tissue, as well as in the cell supernatants were
measured by ELISA. ELISA was performed by coating 96-well plates
with 1 mg/well of capture Ab. Before the subsequent steps in the
assay, the coated plates were washed twice with 1X PBS containing
0.05% Tween-20 (PBST). All reagents and coated wells used in this
assay were incubated for 2 h at room temperature. The standard
curve was generated from known concentrations of cytokines, as
provided by the manufacturer. Following exposure to the medium, the
assay plates were exposed sequentially to each of the
biotin-conjugated secondary antibodies, as well as AP and ABTS
substrate solution containing 30% H2O2. The
plates were read at an absorbance of 405 nm. IL-32 was analyzed
according to the manufacturer’s specifications. Appropriate
specificity controls were included, and all samples were run in
duplicate. Cytokine levels in the spleen and nasal mucosa were
divided according to the total protein levels. Protein levels were
determined using a BCA kit.
Histamine assay
Histamine serum levels were measured by the OPA
spectrofluorometric procedure. The fluorescent intensity was
measured at 440 nm (excitation at 360 nm) using a
spectrofluorometer.
Histological examination
Mice were euthanized by carbon dioxide inhalation.
Nasal mucosa tissues from the euthanized mice were removed. Tissue
samples were immediately fixed with 10% formaldehyde and embedded
in paraffin. The section of the nasal mucosa samples were
4-μm-thick. Each section was stained with hematoxylin and eosin
(H&E, for eosinophils) prior to dewaxing and dehydration. The
number of eosinophils on both sides of the septal mucosa was
counted. The sections were coded and randomly analyzed by 2 blinded
observers.
Culture of Eol-1 cells
Human Eol-1 cells were a kind gift from Dr H. Bae
(Kyung Hee University, Seoul, Korea). The Eol-1 cells were grown in
RPMI-1640 supplemented with 100 U/ml penicillin, 100 mg/ml
streptomycin and 10% heat-inactivated FBS at 37°C 5% CO2
and 95% humidity. The Eol-1 cells were treated with NJJ (1, 10, or
100 μg/ml) for 1 h prior to stimulation with GM-CSF.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Eol-1 cells (3×106) were treated with NJJ
(1, 10, or 100 mg/ml) for 1 h prior to stimulation with GM-CSF and
incubated for 4 h. Total RNA was isolated from the cells and nasal
mucosal tissue according to the manufacturer’s specifications using
an easy-BLUE™ RNA extraction kit (iNtRON Biotech, Sungnam, Korea).
The concentration of total eluted RNA was determined by
spectrophotometry. Total RNA (2.5 mg) was heated at 65°C for 10 min
and then chilled on ice. Each sample was reverse-transcribed to
cDNA for 90 min at 37°C using the cDNA synthesis kit (Amersham
Pharmacia Biotech, Piscataway, NJ, USA). PCR was performed using
the following primers: mouse TSLP (5′-TAT GAG TGG GAC CAA AAG TAC
CG-3′ and 5′-GGG ATT GAA GGT TAG GCT CTG G-3′); mouse GAPDH (5′-TTC
ACC ACC ATG GAG AAG GC-3′ and 5′-GGC ATG GAC TGT GGT CAT GA-3′);
human IL-8 (5′-CGA TGT CAG TGC ATA AAG ACA-3′ and 5′-TGA ATT CTC
AGC CCT CTT CAA AAA-3′); human IL-32 (5′-TGA CAT GAA GAA GCT GAA
GGC-3′ and 5′-CAT GAC CTT GTC ACA AAA GCT C-3′); and human GAPDH
(5′-TCG ACA GTC AGC CGC ATC TTC TTT-3′ and 5′-ACC AAA TCC GTT GAC
TCC GAC CTT-3′) was used to verify whether equal amounts of RNA
were used for reverse transcription and PCR amplification under the
different experimental conditions. The annealing temperature was
60°C for TSLP, IL-8, IL-32 and GAPDH. Products were electrophoresed
on a 1.5% agarose gel and visualized by staining with ethidium
bromide.
Confocal laser scanning microscopy
The tissue samples were fixed with 4% formaldehyde
and embedded in paraffin. After dewaxing and dehydration, the
sections were blocked with bovine serum albumin followed by 60 min
of incubation with an anti-mouse c-Kit (Santa Cruz Biotechnology,
Inc.) at a concentration of 1 μg/ml. The secondary antibody,
fluorescein isothiocyanate (FITC)-conjugated anti-mouse IgG
(Invitrogen, Carlsbad, CA, USA), was added to the incubation medium
for 30 min. The mounting medium containing
4′,6-diamidino-2-phenylinodole (DAPI; Vector Laboratories,
Burlingame, CA, USA) was used to counterstain the DNA. All
specimens were examined under a confocal laser-scanning microscope.
The numbers of mast cells on both sides of the septal mucosa were
counted. The sections were coded and randomly analyzed by 2 blinded
observers.
Western blot analysis
Western blot analysis was used to analyze the nasal
mucosal tissue extracts which were prepared by the detergent lysis
procedure. Samples were heated at 95°C for 5 min, and briefly
cooled on ice. Following centrifugation at 15,000 × g for 5 min, 50
mg aliquots were resolved by 12% SDS-PAGE. Resolved proteins were
electrotransferred overnight onto nitrocellulose membranes in 25 mM
Tris, pH 8.5, 200 mM glycerin, 20% methanol at 25 V. The blots were
blocked for at least 2 h with PBST containing 5% non-fat dry milk
and then incubated with primary antibodies for 1 h at room
temperature. Blots were developed by peroxidase-conjugated
secondary antibodies, and proteins were visualized by enhanced
chemiluminescence procedures (Amersham Biosciences, Piscataway, NJ,
USA) according to the manufacturer’s instructions.
Caspase-1 assay
The Eol-1 cells (3×106) were treated with
NJJ (1, 10 or 100 μg/ml) for 1 h prior to stimulation with GM-CSF
and incubated for 2 h. Caspase-1 assay was used to analyze the
nasal mucosal tissue and cell extracts. Caspase-1 activity was
measured according to the manufacturer’s specifications using the
caspase assay kit (R&D Systems Inc.). Equal amounts of total
protein were quantified by the BCA protein quantification kit
(Sigma) in each lysate. The catalytic activity of caspase-1 from
the cell lysates was measured by the proteolytic cleavage of
WEHD-pNA for 4 h at 37°C. The plates were read at 405 nm.
MTT assay
The Eol-1 cells (2×105 cells/ml) were
cultured in microplate wells for 24 h following treatment with NJJ
(1, 10, or 100 μg/ml) and incubated with 20 μl of MTT solution (5
mg/ml) for an additional 4 h at 37°C in an atmosphere of 5%
CO2 and 95% air. Consecutively, 250 μl of DMSO were
added to extract the MTT formazan and the absorbance of each well
at 540 nm was read using an automatic microplate reader.
Statistical analysis
The experiments shown are a summary of the data from
at least 3 experiments and statistical analyses were performed
using SPSS statistical software (SPSS 11.5; SPSS Inc., Chicago,
USA). The effects of treatment were analyzed by one-way ANOVA and
Tukey’s multiple range tests, and a value of P<0.05 was used to
indicate a statistically significant difference.
Results
Effects of NJJ on IL-32 levels
Recently, we reported that IL-32 is an important
factor in patients and animals with AR and is expressed in
eosinophils (7). Thus, in this
study, we examined the effects of NJJ on IL-32 levels in animal
models of AR and GM-CSF-stimulated Eol-1 cells. IL-32 levels in
serum and nasal mucosal tissues of mice with AR were significantly
increased compared with those of normal mice (Fig. 1A and B). However, the increased
IL-32 levels significantly decreased following the administration
of NJJ (Fig. 1A and B).
Eosinophil infiltration increased following OVA challenge and
significantly decreased following the administration of NJJ
(Fig. 1C). To assess the
regulatory effects of NJJ on IL-32 expression in the in
vitro model, we measured IL-32 production and mRNA expression
in GM-CSF-stimulated Eol-1 cells. The protein and mRNA levels of
IL-32 were significantly inhibited by treatment with NJJ (Fig. 1D and F). IL-8 production was
analyzed to examine the activity of GM-CSF. IL-8 production and
mRNA expression were decreased following treatment with NJJ
(Fig. 1E and F). NJJ had no
effect on cell viability (data not shown).
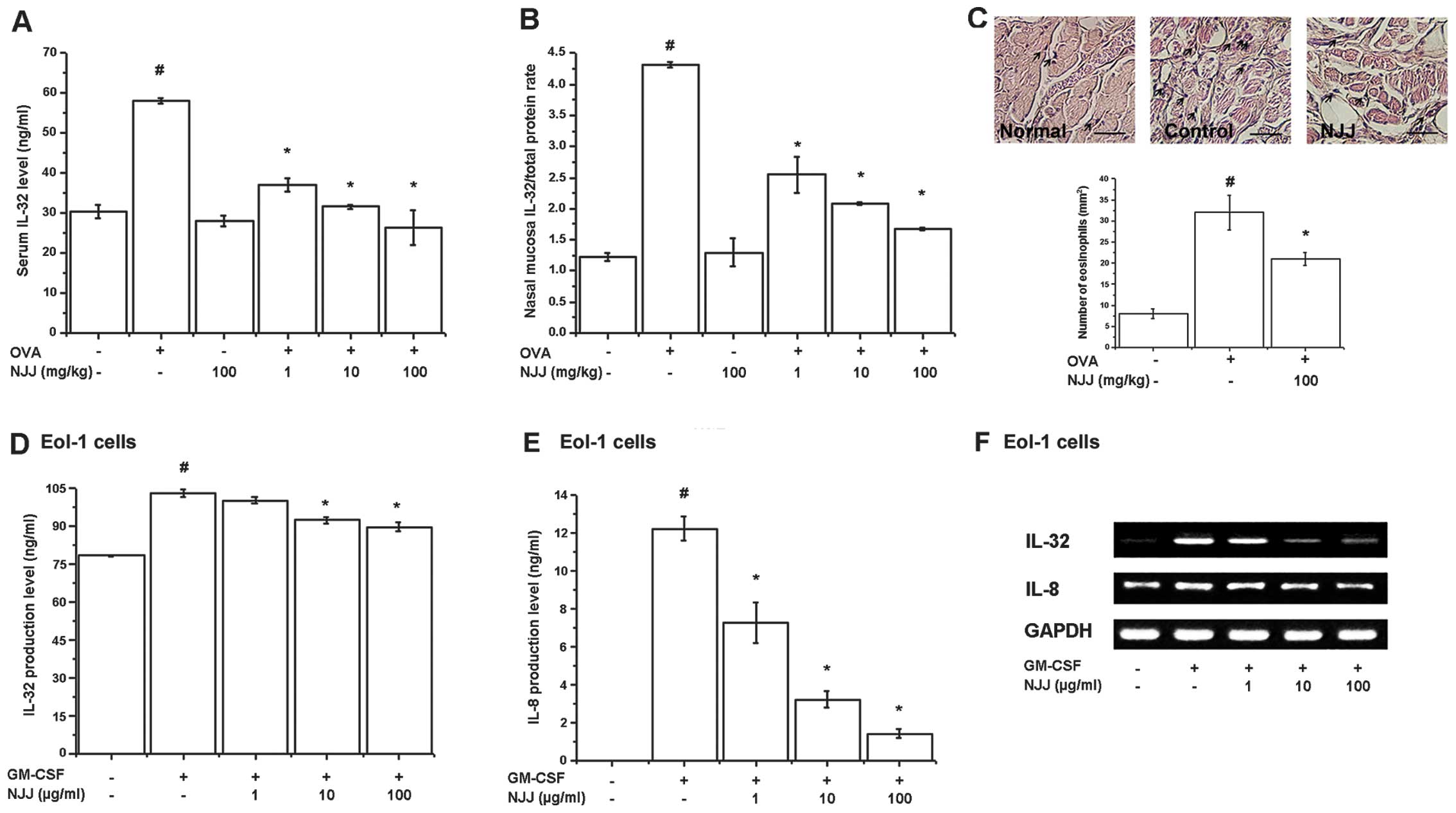 | Figure 1Effects of Naju Jjok (NJJ) on
interleukin (IL)-32 levels. NJJ was administered orally for 10 days
prior to the i.n. ovalbumin (OVA) challenge. (A and B) IL-32 levels
were measured by ELISA in the serum and nasal mucosal tissue. (C)
Nasal mucosal tissue was stained with hematoxylin and eosin
(H&E) for eosinophils (black arrow, upper panel). Five randomly
selected tissue sections per mouse were counted (lower panel). The
absolute number of cells was counted as the mean ± SEM.
#P<0.05, significantly different from the
OVA-unsensitized mice. *P<0.05, significantly
different from the OVA-sensitized mice. Normal, OVA
unsensitization; control, OVA sensitization; NJJ, OVA sensitization
and NJJ (100 mg/kg) administration. (Original magnification, ×400;
scale bar, 50 μm). N=5. (D and E) Eol-1 cells were treated with NJJ
(1, 10, or 100 μg/ml) and then stimulated with
granulocyte-macrophage colony-stimulation factor (GM-CSF). IL-32
and IL-8 levels were measured by ELISA. (F) mRNA levels were
measured by RT-PCR. #P<0.05, significantly different
from the unstimulated cells. *P<0.05, significantly
different from the GM-CSF-stimulated cells. |
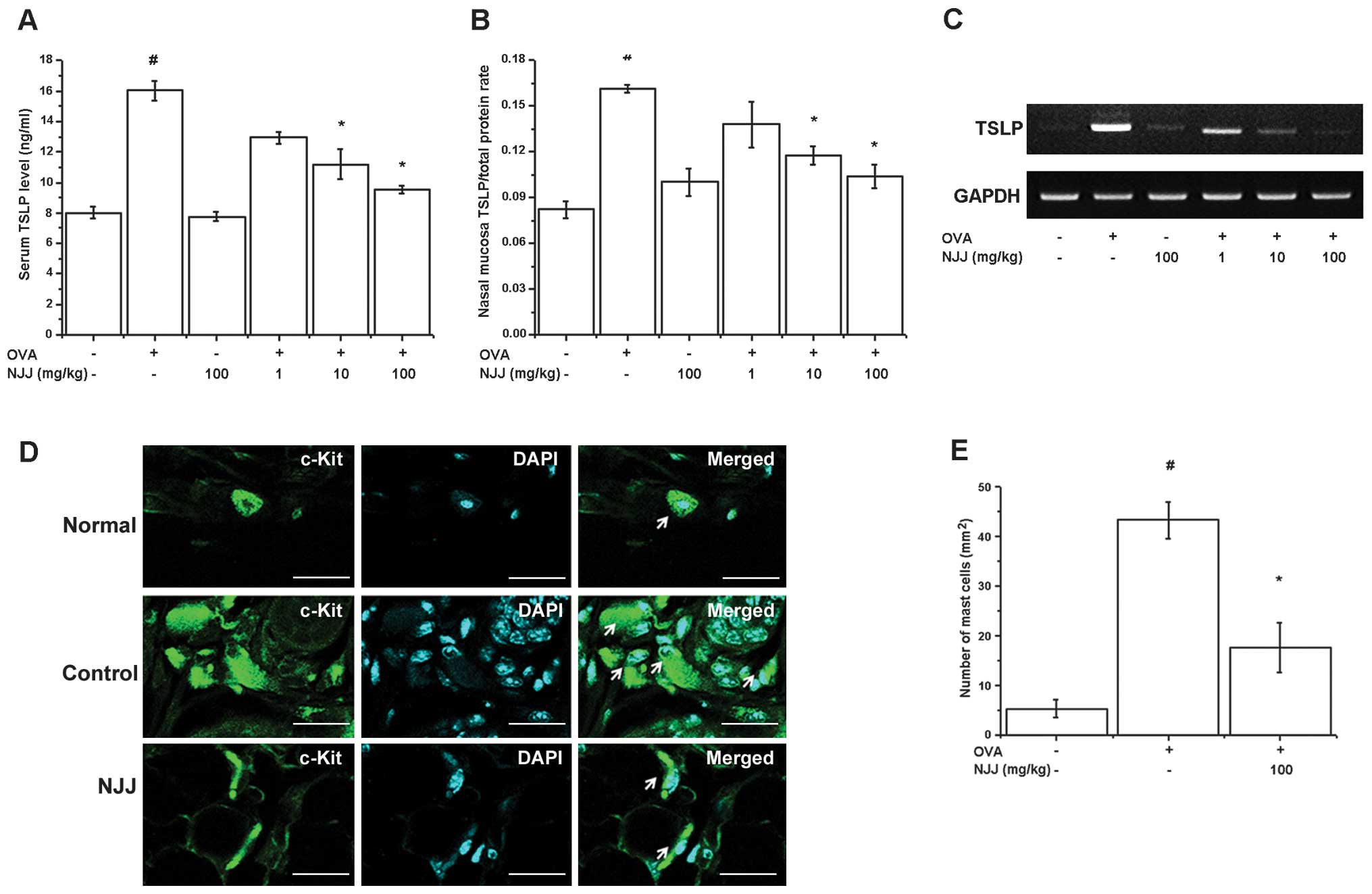
Effect of NJJ on TSLP levels
TSLP is expressed in the nasal mucosa of mice with
AR (25) and is produced in
activated mast cells (26). In
our study, mice administered NJJ showed significantly decreased
levels of TSLP in serum and nasal mucosal tissue (Fig. 2A and B). The oral administration
of NJJ reduced TSLP mRNA expression (Fig. 2C). TSLP is expressed in mast cells
(18,19). Mast cells play a central role in
AR (1). Thus, we determines
whether the oral administration of NJJ suppresses mast cell
infiltration in mice with AR. The respective numbers of mast cells
in the nasal mucosa of the mice with AR were significantly higher
than those in the OVA-unsensitized mice; however, the oral
administration of NJJ significantly reduced the infiltration of
mast cells (Fig. 2D and E).
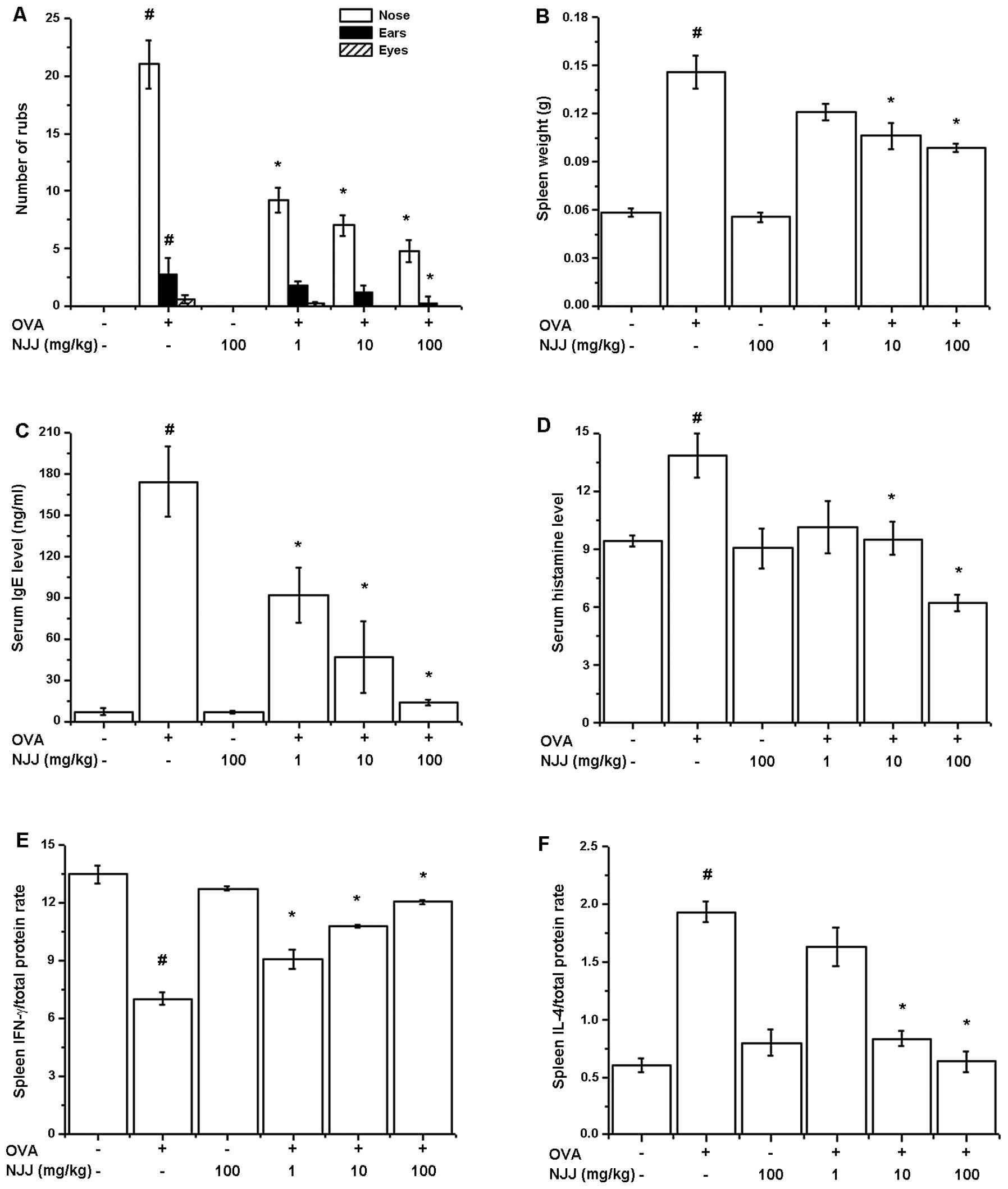
Effect of oral administration of NJJ on
clinical symptoms in mice with AR
To investigate the regulatory effects of NJJ in our
animal model of AR, NJJ was administrated orally prior to OVA i.n.
challenge for 10 days. The oral administration of NJJ significantly
reduced the clinical symptoms (nasal rubs, spleen weight and levels
of histamine, IFN-γ, IL-4 in spleen) in mice with AR (Fig. 3A). Spleen weight increased
following challenge with OVA; this was reduced following the oral
administration of NJJ (Fig. 3B).
The IgE and histamine levels which were increased by OVA were also
reduced by the oral administration of NJJ (Fig. 3C and D). To investigate the
Th1/Th2 immune reactions in mice administered NJJ, we measured the
IFN-γ and IL-4 levels in the spleen extracts. As shown in Fig. 3E and F, NJJ significantly
increased IFN-γ levels, while decreasing IL-4 levels compared with
the control group.
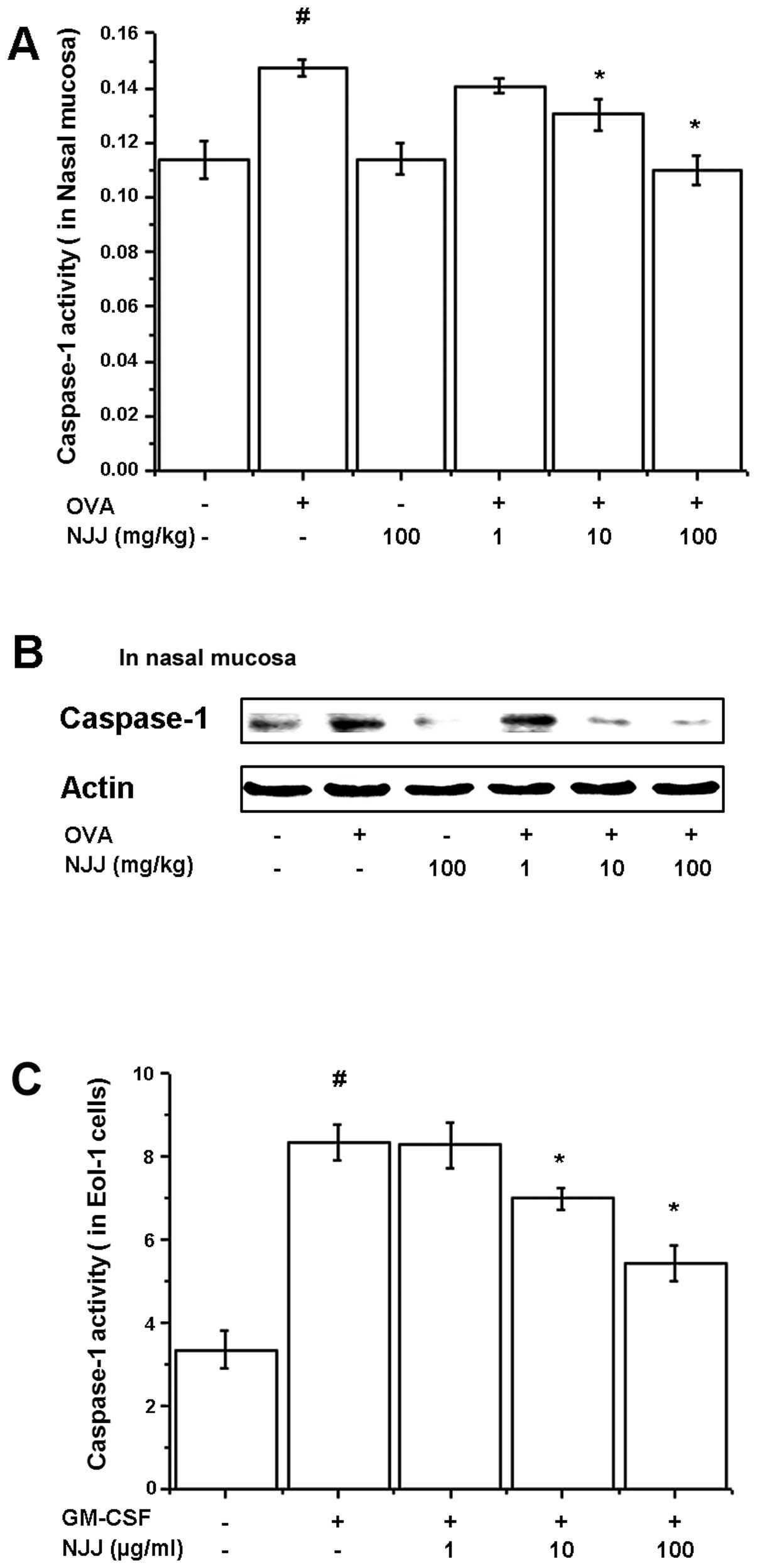
Effects of NJJ on activation of
caspase-1
To investigate the regulatory mechanisms of NJJ on
IL-32 and TSLP production, caspase-1 assay and western blot
analysis for caspase-1 were performed on the nasal mucosal tissues
and GM-CSF-stimulated Eol-1 cell extracts. OVA-induced and
GM-CSF-induced caspase-1 activities were decreased by NJJ (Fig. 4A and C). The active form of
caspase-1 in the nasal mucosal tissue was reduced following the
oral administration of NJJ (Fig.
4B).
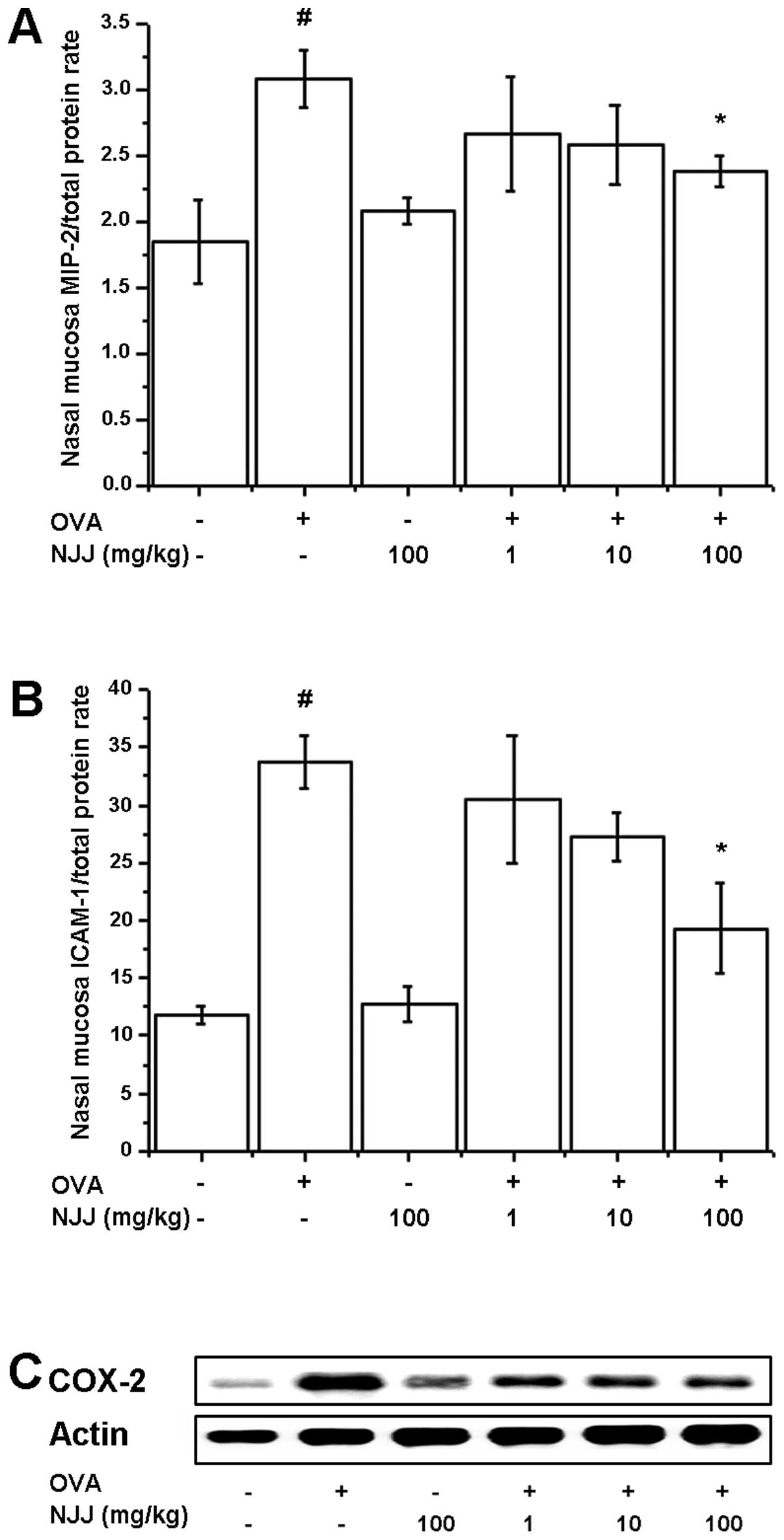
Effects of NJJ on inflammatory markers in
mice with AR
To investigate the effects of NJJ on
inflammation-related protein levels, we analyzed the MIP-2, ICAM-1
and COX-2 levels in the nasal mucosal tissue of mice with AR. The
increased levels of MIP-2, ICAM-1 and COX-2 in the mice with AR
were significantly reduced following the oral administration of NJJ
(Fig. 5).
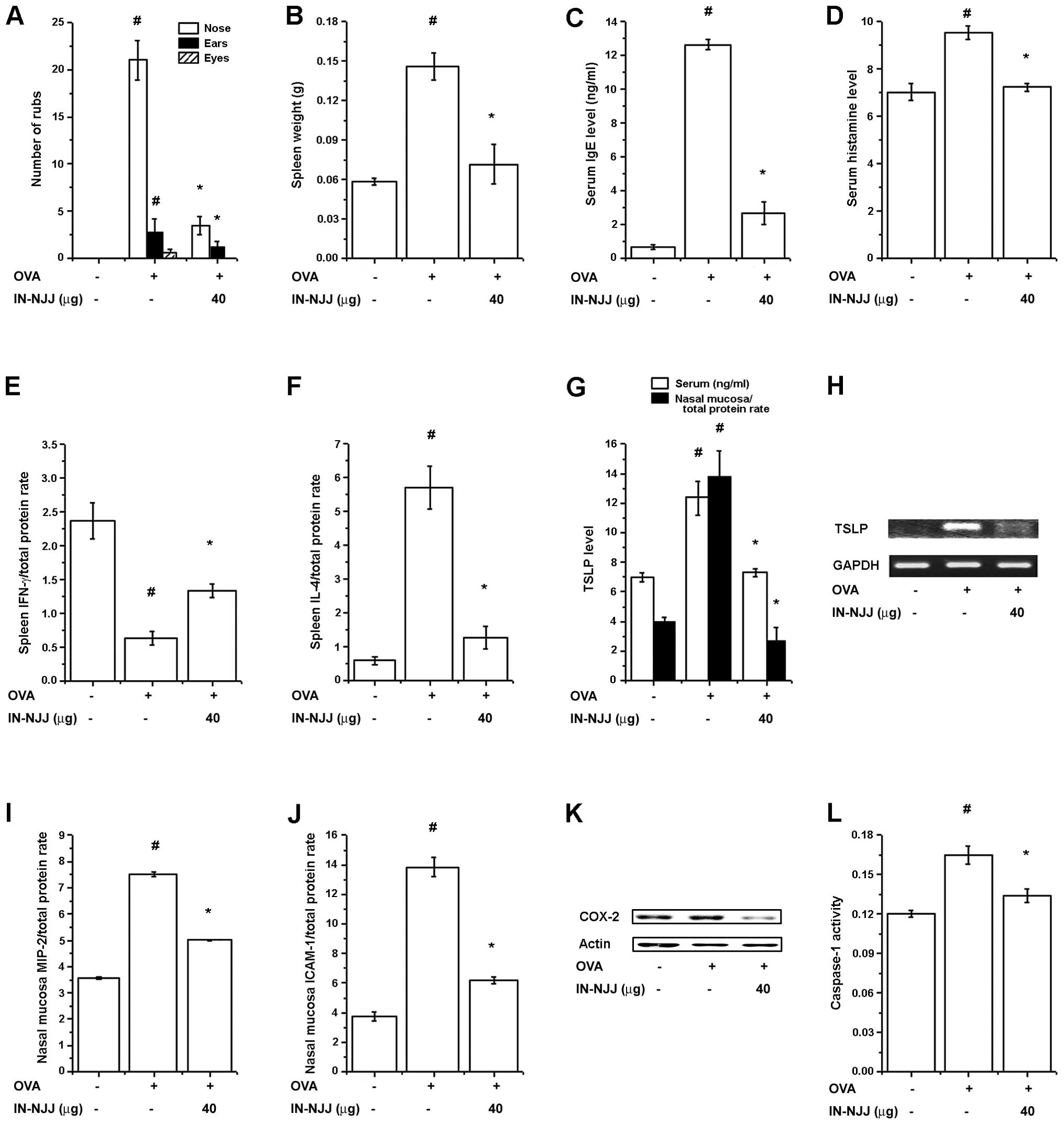
Effects of i.n. administration of NJJ on
clinical symptoms in mice with AR
To investigate the effects of the i.n.
administration of NJJ (IN-NJJ) on clinical symptoms in mice with
AR, IN-NJJ was directly administered into the nasal cavity prior to
OVA i.n. challenge for 10 days. The clinical symptoms (spleen
weight, serum IgE and histamine levels, IFN-γ levels, ICAM-1
levels) were decreased following IN-NJJ administration (Fig. 6A). The spleen weight was
significantly decreased following IN-NJJ administration (Fig. 6B). Serum IgE and histamine levels
which were increased by OVA were reduced in the IN-NJJ-administered
mice (Fig. 6C and D). As shown in
Fig. 6E and F, IN-NJJ
administration significantly increased the IFN-γ levels, while
decreasing the IL-4 levels compared with the control group. We
measured the TSLP levels in the mice with AR. As shown in Fig. 6G, the IN-NJJ-administered mice
showed significantly decreased levels of TSLP compared with the
untreated mice with AR. TSLP mRNA levels were also significantly
decreased following IN-NJJ administration (Fig. 6H). The levels of MIP-2, ICAM-1 and
COX-2 in the nasal mucosal tissue were significantly reduced by
IN-NJJ administration (Fig.
6I–K). In addition, caspapae-1 activity, which was increased by
OVA was decreased following IN-NJJ administration (Fig. 6L).
Discussion
AR is an inflammatory disease of the nasal mucosa in
which a number of cells and mediators play a part. These mediators
may then contribute to the influx of mast cells, eosinophils,
basophils, neutrophils and monocytes in the late response (27,28). Eosinophils are innate effector
cells and important in immune responses against helminth parasitic
infections. They contribute to the pathology associated with
allergic inflammatory conditions (29,30). Eosinophil accumulation in health
and disease is a hallmark characteristic of mucosal immunity and
type 2 helper T cell inflammation (31). The eosinophil granule proteins
play a central role in the development of rhinitis (32). IL-32 released from eosinophils has
been shown to significantly increase the levels of IgE and
inflammatory cytokines, including IL-1β, IL-18 and GM-CSF in AR
(7). Despite the absence of a
Th1-cell predominant inflammation, other Th2-type inflammatory
disorders, such as AR, asthma and AD have been linked with
increased levels of IL-32 (33).
An increase in the levels of IL-4, IgE, eosinophil cationic protein
(ECP) and eosinophil infiltration by IL-32 stimulation has been
demonstrated in an animal model of AR (7). These data suggest that IL-32 is an
attractive target in the treatment of AR. Accordingly, we
hypothesized that NJJ mediates anti-allergic effects, at least
partly by suppressing the production of IL-32. In this study, our
results demonstrated that NJJ relieved the clinical symptoms of AR
and decreased the levels of IgE. NJJ reduced the levels of IL-32
and the number of infiltrating eosinophils in the mice with AR. NJJ
induced a dose-related inhibition of the production and mRNA
expression of IL-32 in the GM-CSF-stimulated Eol-1 cells.
Therefore, we suggest that NJJ exerts anti-allergic effects. Its
mechanisms of action may be associated with the decrease in IL-32
expression.
Recently, we reported that IL-32 significantly
increased TSLP production in monocytes (20). TSLP is a critical regulator of
innate and adaptive immune responses associated with Th2
cytokine-mediated inflammation, including AR. TSLP is expressed not
only in epithelial cells but also in fibroblasts, endothelial cells
and smooth muscle cells at nasal mucosal sites (34). Moon and Kim reported that TSLP is
expressed in activated mast cells (18). Pyeongwee-San extract and
hesperidin have been shownt o alleviate the symptoms of AR and to
inhibit TSLP production and mRNA expression in mast cells (19,35). AR is an IgE-mediated disease.
Antigen-specific IgE binds to high affinity receptors (FcɛRI) on
tissue mast cells, basophils and dendritic cells. The early phase
response mediators induce the characteristic symptoms of AR, such
as watery rhinorrhea, sneezing and itching, within minutes of
allergen exposure. This is followed by the late phase response,
involving the infiltration of inflammatory cells due to the release
of cytokines and chemokines, resulting in congestion and
inflammation (36). In this
study, we demonstrated that the number of infiltrating mast cells
in nasal mucosal tissue was reduced following the administration of
NJJ. Therefore, it can be deduced that NJJ inhibits mast cell
functions.
Caspase-1 plays a central role in innate immunity
and in several important inflammatory diseases (37). Caspase-1 activation is involved in
inflammation and the regulation of immune responses and
differentiation (20,35). In an animal model of AR, caspase-1
activity was shown to increase compared with normal mice (35). Caspase-1 levels in allergic
asthmatic patients are also higher than those in normal individuals
(38). IL-1β, IL-18, histamine
and IgE have been shown to be upregulated through the activation of
caspase-1 in kanamycin-administered NC/Nga mice (39). IL-32 and TSLP production are also
increased by the activation of caspase-1 (7,20).
The activation of caspase-1 promotes COX-2-dependent inflammatory
reactions (40). As previously
demonstrated, COX-2 expression is reduced in caspase-1 knockout
mice (40). Thus, the suppression
of caspase-1 activity is associated with a reduction in
inflammation. In this study, we observed that NJJ inhibited the
activation of caspase-1 in the mice with AR and in the Eol-1 cells.
Therefore, we hypothesized that the anti-allergic effects of NJJ
may be derived from the downregulation of caspase-1 activity.
TKM has been used to treat symptoms of AR for
decades and is still generally used for treatment of AR in Korea.
TKM therapy is usually considered to be safe and effective in a
large population. NJJ has been known for thousands of years to have
antibacterial and anti-inflammatory effects. Tryptanthrin,
kaempferol and indirubin are the main components of NJJ.
Tryptanthrin and kaempferol have antibacterial properties (23). Kaempferol has been shown to
inhibit the production of IL-8 and tumor necrosis factor (TNF)-α
and the infiltration of eosinophils in allergic reactions (41,42). Indirubin has been shown to inhibit
inflammatory reactions and allergic contact dermatitis (43,44). In this study, we found that the
oral and nasal administration of NJJ significantly reduced the
levels of IgE, IL-4, ICAM-1, MIP-2 and COX-2 in mice with AR.
Therefore, the above data suggest that the combination of various
active compounds in NJJ have a synergistic effect in AR. However,
the active components of NJJ should be isolated in further studies
to clarify whether the components themselves may also be effective
in the treatment of AR.
In conclusion, to our knowledge, we report for the
first time that NJJ regulates the production of IL-32 and TSLP, as
well as that of histamine, IgE, IL-4, MIP-2, ICAM-1 and COX-2 in an
animal model of AR. The anti-allergic effects of NJJ are also
associated with the inhibition of infiltrating inflammatory cells
and the activation of caspase-1. Therefore, our data suggest the
possible therapeutic application of NJJ in the treatment of
allergic inflammatory diseases.
Acknowledgements
This study was supported by Naju City (2012) and the
Basic Science Research Program through the National Research
Foundation of Korea (NRF) funded by the Ministry of Education,
Science and Technology (2012R1A1A3005103).
References
|
1
|
Varney VA, Jacobson MR, Suddarick RM, et
al: Immunohistology of nasal mucosa following allergen induced
rhinitis. Am Rev Respir Dis. 146:170–175. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sim TC, Grant JA, Hilsmeier KA, Fukuda Y
and Alam R: Pro-inflammatory cytokines in nasal secretions of
allergic subjects after antigen challenge. Am J Respir Crit Care
Med. 149:339–344. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gupta S, Feng L, Yoshimura T, Redick J, Fu
SM and Rose CE Jr: Intra-alveolar macrophage-inflammation peptide 2
induces rapid neutrophil localization in the lung. Am J Respir Cell
Mol Biol. 15:656–663. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Durham SR, Ying S, Varney VA, et al:
Cytokine messenger RNA expression for IL-3, IL-4, IL-5 and
granulocyte/macrophase colony stimulating factors in the nasal
mucosa after local allergen provocation. J ImmunoI. 148:2390–2394.
1992.
|
|
5
|
Bacon AS, McGill JI, Anderson DF, Baddeley
S, Lightman SL and Holgate ST: Adhesion molecules and relationship
to leukocyte levels in allergic eye disease. Invest Ophthalmol Vis
Sci. 39:322–330. 1998.PubMed/NCBI
|
|
6
|
Tai PC and Spry CJ: The effects of
recombinant granulocyte-macrophage colony-stimulating factor
(GM-CSF) and interleukin-3 on the secretory capacity of human blood
eosinophils. Clin Exp Immunol. 80:426–434. 1990.
|
|
7
|
Jeong HJ, Shin SY, Oh HA, Kim MH, Cho JS
and Kim HM: IL-32 up-regulation is associated with inflammatory
cytokine production in allergic rhinitis. J Path. 224:553–563.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Marcondes AM, Mhyre AJ, Stirewalt DL, Kim
SH, Dinarello CA and Deeg HJ: Dysregulation of IL-32 in
myelodysplastic syndrome and chronic myelomonocytic leukemia
modulates apoptosis and impairs NK function. Proc Natl Acad Sci
USA. 105:2865–2870. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bae S, Kang D, Hong J, et al:
Characterizing antiviral mechanism of interleukin-32 and a
circulating soluble isoform in viral infection. Cytokine. 58:79–86.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Heinhuis B, Koenders MI, van Riel PL, et
al: Tumour necrosis factor alpha-driven IL-32 expression in
rheumatoid arthritis synovial tissue amplifies an inflammatory
cascade. Ann Rheum Dis. 70:660–667. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim SH, Han SY, Azam T, Yoon DY and
Dinarello CA: Interleukin-32: a cytokine and inducer of TNF alpha.
Immunity. 22:131–142. 2005.PubMed/NCBI
|
|
12
|
Netea MG, Azam T, Ferwerda G, et al: IL-32
synergizes with nucleotide oligomerization domain (NOD) 1 and NOD2
ligands for IL-1beta and IL-6production through a caspase
1-dependent mechanism. Proc Natl Acad Sci USA. 102:16309–16314.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nishida A, Andoh A, Shioya M,
Kim-Mitsuyama S, Takayanagi A and Fujiyama Y: Phosphatidylinositol
3-kinase/Akt signaling mediates interleukin-32alpha induction in
human pancreatic periacinar myofibroblasts. Am J Physiol
Gastrointest Liver Physiol. 294:G831–G838. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Heinhuis B, Koenders MI, van den Berg WB,
Netea MG, Dinarello CA and Joosten LA: IL-32 contains a typical
alpha-helix bundle structure that resembles the focal adhesion
targeting region of focal adhesion kinase-1. J Biol Chem.
287:5733–5743. 2012. View Article : Google Scholar
|
|
15
|
Ying S, O’Connor B, Ratoff J, et al:
Expression and cellular provenance of thymic stromal lymphopoietin
and chemokines in patients with severe asthma and chronic
obstructive pulmonary disease. J Immunol. 181:2790–2798. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Soumelis V, Reche PA, Kanzler H, et al:
Human epithelial cells trigger dendritic cell mediated allergic
inflammation by producing TSLP. Nat Immunol. 3:673–680.
2002.PubMed/NCBI
|
|
17
|
Bunyavanich S, Melen E, Wilk JB, et al:
Thymic stromal lymphopoietin (TSLP) is associated with allergic
rhinitis in children with asthma. Clin Mol Allergy. 9:12011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Moon PD and Kim HM: Thymic stromal
lymphopoietin is expressed and produced by caspase-1/NF-κB pathway
in mast cells. Cytokine. 54:239–243. 2011.PubMed/NCBI
|
|
19
|
Song YH, Nam SY, Choi Y, Kim JH, Kim YS
and Jeong HJ: Socioeconomic impact of traditional Korean medicine,
Pyeongwee-San (KMP6) as an anti-allergic inflammatory drug. TANG.
2:52–60. 2012.
|
|
20
|
Jeong HJ, Nam SY, Oh HA, et al:
Interleukin-32-induced thymic stromal lymphopoietin plays a
critical role in macrophage differentiation through the activation
of caspase-1 in vitro. Arthritis Res Ther. 14:R2592012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Iwaki K, Satomi KM, Kohno K, Ushio S and
Fukuda S: Antimicrobial activity of Polygonum tinctorium
Lour: extract against oral pathogenic bacteria. J Nat Med.
60:121–125. 2006.
|
|
22
|
Satomi KM, Kimoto T, Micallef MJ, et al:
Prevention of azoxymethane-induced intestinal tumors by a crude
ethyl acetate-extract and tryptanthrin extracted from Polygonum
tinctorium Lour. Anticancer Res. 21:3295–3300. 2001.PubMed/NCBI
|
|
23
|
Kataoka M, Hirata K, Kunikata T, et al:
Antibacterial action of tryptanthrin and kaempferol, isolated from
the indigo plant (Polygonum tinctorium Lour.), against
Helicobacter pylori-infected Mongolian gerbils. J
Gastroenterol. 36:5–9. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim HM, Hong DR and Lee EH: Inhibition of
mast cell-dependent anaphylactic reactions by the pigment of
Polygonum tinctorium (Chung-Dae) in rats. Gen Pharmacol.
31:361–365. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Miyata M, Hatsushika K, Ando T, et al:
Mast cell regulation of epithelial TSLP expression plays an
important role in the development of allergic rhinitis. Eur J
Immunol. 38:1487–1492. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Han NR, Kim HM and Jeong HJ: Thymic
stromal lymphopoietin is regulated by the intracellular calcium.
Cytokine. 59:215–217. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Spada CS, Nieves AL, Krauss AH and
Woodward DF: Comparison of leukotriene B4 and D4 effects on human
eosinophil and neutrophil motility in vitro. J Leukoc Biol.
55:183–191. 1994.PubMed/NCBI
|
|
28
|
Sugimoto H, Shichijo M, Iino T, et al: An
orally bioavailable small molecule antagonist of CRTH2, ramatroban
(BAY u3405), inhibits prostaglandin D2-induced eosinophil migration
in vitro. J Pharmacol Exp Ther. 305:347–352. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Melvin TA and Ramanathan M Jr: Role of
innate immunity in the pathogenesis of allergic rhinitis. Curr Opin
Otolaryngol Head Neck Surg. 20:194–198. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Isobe Y, Kato T and Arita M: Emerging
roles of eosinophils and eosinophil-derived lipid mediators in the
resolution of inflammation. Front Immunol. 3:2702012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Moshkovits I, Shik D, Itan M, et al:
CMRF35-like molecule 1 (CLM-1) regulates eosinophil homeostasis by
suppressing cellular chemotaxis. Mucosal Immunol. Jul 3–2013.(Epub
ahead of print). View Article : Google Scholar
|
|
32
|
Bystrom J, Patel SY, Amin K and
Bishop-Bailey D: Dissecting the role of eodinophil cationic protein
in upper airway disease. Curr Opin Allergy Clin Immunol. 12:18–23.
2012. View Article : Google Scholar
|
|
33
|
Soyka MB, Treis A, Eiwegger T, et al:
Regulation and expression of IL-32 in chronic rhinosinusitis.
Allergy. 67:790–798. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nomura K, Kojima T, Fuchimoto J, Obata K,
Keira T, Himi T and Sawada N: Regulation of interleukin-33 and
thymic stromal lymphopoietin in human nasal fibroblasts by
proinflammatory cytokines. Laryngoscope. 122:1185–1192. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Oh HA, Kim HM and Jeong HJ: Alleviation of
allergic rhinitis symptoms with Pyeongwee-San extract (KMP6).
Immunopharmacol Immunotoxicol. 34:135–142. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vashisht P and Casale T: Omalizumab for
treatment of allergic rhinitis. Expert Opin Biol Ther. 13:933–945.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sollberger G, Strittmatter GE,
Garstkiewicz M, Sand J and Beer HD: Caspase-1: the inflammasome and
beyond. Innate Immun. May 15–2013.(Epub ahead of print).
|
|
38
|
Grzegorczyk J, Kowalski ML, Pilat A and
Iwaszkiewicz J: Increased apoptosis of peripheral blood mononuclear
cells in patients with perennial allergic asthma/rhinitis: relation
to serum markers of apoptosis. Mediators Inflamm. 11:225–233. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Han NR, Kim HM and Jeong HJ: Kanamycin
activates caspase-1 in NC/Nga mice. Exp Dermatol. 20:659–663. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cunha TM, Talbot J, Pinto LG, et al:
Caspase-1 is involved in the genesis of inflammatory
hypernociception by contributing to peripheral IL-1β maturation.
Mol Pain. 6:632010.PubMed/NCBI
|
|
41
|
Lee EJ, Ji GE and Sung MK: Quercetin and
kaempferol suppress immunoglobulin E-mediated allergic inflammation
in RBL-2H3 and Caco-2 cells. Inflamm Res. 59:847–854. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gong JH, Shin D, Han SY, Kim JL and Kang
YH: Kaempferol suppresses eosionphil infiltration and airway
inflammation in airway epithelial cells and in mice with allergic
asthma. J Nutr. 142:47–56. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kunikata T, Tatefuji T, Aga H, Iwaki K,
Ikeda M and Kurimoto M: Indirubin inhibits inflammatory reactions
in delayed-type hypersensitivity. Eur J Pharmacol. 410:93–100.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kim MH, Choi YY, Yang G, Cho IH, Nam D and
Yang WM: Indirubin, a purple 3,2-bisindole, inhibited allergic
contact dermatitis via regulating Thelper(Th)-mediated immune
system in DNCB-induced model. J Ethnopharmacol. 145:214–219. 2013.
View Article : Google Scholar : PubMed/NCBI
|