Introduction
Estrogen has been shown to play critical role in
bone homeostasis that is maintained by the coupled actions of two
main bone cell types, bone-forming osteoblasts and bone-resorbing
osteoclasts (1). The biological
functions of estrogen are mediated by its binding to estrogen
receptor (ER)-α and ER-β, which are widely expressed in a variety
of tissues and cell types, e.g., liver tissue (2), heart tissue (3), breast epithelial cells (4), ovarian cells (5) and endothelial cells (6). As osteoblasts also express ER-α and
ER-β, estrogen can directly bind to its receptors and initiate
signals to regulate a number of osteoblast cellular responses, such
as proliferation (7),
differentiation (8), calcified
matrix formation (9) and the
secretion of osteoclastic mediators (1). Importantly, previous studies have
demonstrated that estrogen regulates the production of two
osteoclast differentiation-related mediators, receptor activator of
nuclear factor-κB ligand (RANKL) and osteoprotegerin (OPG), in
osteoblasts (10). However, as
the levels of ER-α and ER-β in osteoclasts are very low (11,12), the direct effects of estrogen on
osteoclast functions through their binding to ERs remain
unclear.
It is well known that estrogen replacement therapy
(ERT) is a common treatment for women who have declining estrogen
levels due to natural- or surgical-induced menopause that lead to a
reduction in bone mass (13).
Although ERT has proven to be effective for the prevention of
menopausal-mediated bone loss, prolonged estrogen treatment
increases undesirable systemic hormonal side-effects, such as the
development of breast cancer, heart attack, strokes and dementia
(13,14). In this regard, dietary
phytoestrogens, which are naturally occurring plant-derived
non-steroidal polyphenolic compounds, e.g., isoflavonoids,
coumestans, lignans and stilbenes, are of particular interest as
they are considered safe for human consumption and have estrogenic
potency (15,16). Of note, as soy isoflavones,
particularly genistein (4′,5,7-trihydroxyisoflavone) and daidzein
(4′,7-dihydroxyisoflavone) have a similar chemical structure to
mammalian estrogen, they can bind to ER-α and ER-β, and may thus
mediate an increase in osteoblastic proliferation and
differentiation via the canonical ER-dependent signaling pathway
(16). Moreover, in our previous
study, we demonstrated that both soybean and black soybean
(Rhynchosia volubilis), which contain significant
concentrations of isoflavones, stimulate osteoblast functions, such
as cell proliferation and differentiation through their binding to
ERs (17).
Osteoblasts interact closely with osteoclasts, and
paracrine factors that are released from osteoblasts mediate
osteoclast functions, including osteoclast differentiation
(18). The osteoclast
differentiation factor, RANKL, binds to its receptor, RANK, that is
expressed in pre-osteoclasts, initiating osteoclast differentiation
through previously identified mechanism(s), i.e., membrane adaptor
protein tumor necrosis factor (TNF) receptor-associated factors
(TRAFs) signal to increase the expression/activity of MAP kinases
that transactivate multiple osteoclastic transcription factors,
including nuclear factor of activated T cells c1 (NFATc1) (19,20). However, considering that
osteoclasts express low levels of ERs, the anti-differentiation
effects of estrogen and phytoestrogen on these cells may mainly be
regulated by the modulation of various osteoclastic mediators
produced by osteoblasts. Therefore, in this study, we investigated
the indirect effects of soybean, which contains high concentrations
of isoflavones, on the inhibition of osteoclast differentiation.
Our data demonstrate that the increased secretion of OPG as opposed
to that of RANKL in the conditioned medium (CM) of MC3T3-E1
osteoblasts incubated with soybean extracts, inhibits RANKL-induced
osteoclast differentiation through the suppression of NFATc1
activation. The data presented in this study elucidate the indirect
role of soybean extracts in inhibiting osteoclast
differentiation.
Materials and methods
Preparation of soybean extracts
Soybean extracts were prepared from the seeds of
soybean (product of Yangjoo-si, Gyunggi-do, Korea), as previously
described (17). Briefly, soybean
(100 g) was extracted under reflux with 70% methanol (100 ml, 5
times). The solvents were evaporated and freeze-dried for 72 h to
yield 13.6 g of soybean extracts. The extracts were dissolved in
dimethyl sulfoxide (DMSO), which was subjected to membrane (0.45
μm) filtration (EMD Millipore, Billerica, MA, USA).
Cell culture
Murine MC3T3-E1 subclone 14 pre-osteoblastic cells
[American Type Culture Collection (ATCC), Manassas, VA, USA] were
maintained in ascorbic acid-free α-minimum essential medium (α-MEM)
containing 10% fetal bovine serum (FBS) (both from Invitrogen,
Carlsbad, CA, USA) at 37°C in 5% CO2. To induce the
differentiation of pre-osteoblasts into mature osteoblasts,
MC3T3-E1 pre-osteoblasts were incubated with 50 μg/ml ascorbic acid
and 10 mM β-glycerophosphate (both from Sigma-Aldrich, St. Louis,
MO, USA) for 3 days, followed by treatment with soybean extracts or
17β-estradiol (E2) or a combination of daidzein and genistein (D/G)
(positive controls) for 6 days.
In addition to osteoblasts, murine RAW264.7
pre-osteoclasts (ATCC) were maintained in α-MEM (Invitrogen)
supplemented with 10% FBS. To induce differentiation into mature
osteoclasts, the cells were treated with 30 ng/ml of soluble RANKL
(PeproTech, Rocky Hill, NJ, USA), followed by treatment with the
mixed medium of CM collected from MC3T3-E1 osteoblasts treated with
0.001–0.1 mg/ml soybean extracts and osteoclast culture medium
(1:1, v/v) for 3 days, as previously described (18).
Quantitative reverse transcription
polymerase chain reaction (qRT-PCR)
qRT-PCR was performed using cDNA prepared from total
RNA fractions of cell lysates, as previously described (21). The following primer sets were
used: matrix metalloproteinase-9 (MMP-9) forward,
5′-CTGGACAGCCAGACAC TAAAG-3′ and reverse,
5′-CTCGCGGCAAGTCTTCAGAG-3′; and mouse glyceraldehyde 3′-phosphate
dehydrogenase (GAPDH) forward, 5′-TTGTCAAGCTCATTTCCTGGT ATG-3′ and
reverse, 5′-GCCATGTAGGCCATGAGGTC-3′. mRNA expression was normalized
to the levels of GAPDH.
Western blot analysis
Western blot analysis was performed as previously
described (21). Cell lysates,
prepared in radio-immunoprecipitation assay (RIPA) buffer, were
resolved by electrophoresis on a 10–12% SDS-PAGE gel. The resultant
bands were blotted onto nitrocellulose membranes, probed with
anti-murine β-actin, MMP-9 and NFATc1 (Abcam, Cambridge, MA, USA),
and detected by enhanced chemiluminescence reagent (Thermo
Scientific, Waltham, MA, USA).
Quantification of OPG and RANKL
levels
OPG and RANKL levels were quantified using the OPG
and RANKL murine ELISA kits (BD Biosciences, San Jose, CA, USA) in
accordance with the manufacturer’s instructions.
Tartate-resistant acid phosphatase (TRAP)
staining
TRAP staining was performed using the leukocyte acid
phosphatase staining kit 387-A (Sigma-Aldrich) according to the
manufacturer’s instructions. Briefly, the fixed cells were treated
with fast garnet GBC base/sodium nitrite (1:1, v/v) at room
temperature for 3 min; subsequently, substrate solution (2.5 mM
naphthol-ASBI phosphate, 100 mM acetate solution, pH 5.0 and 50 mM
tartrate solution) was added followed by incubation for 30 min at
37°C in the dark. TRAP-stained giant multinucleated cells were
observed under a light microscope.
siRNA and transfection
RAW264.7 cells were transfected with 20 nM siRNA for
NFATc1, or non-targeting, control siRNA, using Lipofectamine
RNAiMAX (all from Invitrogen), as previously described (21).
Statistical analyses
Statistical comparisons were performed using one-way
ANOVA coupled with the Duncan’s multiple range test. A value of
p<0.05 was considered to indicate a statistically significant
difference.
Results
Treatment with soybean extracts increases
the secretion of OPG as opposed to that of RANKL from MC3T3-E1
osteoblasts
We wished to investigate whether treatment with
soybean extracts can modulate the secretion of RANKL and OPG by
osteoblasts, which are critical paracrine factors for osteoclast
differentiation (10,22). MC3T3-E1 pre-osteoblasts were
differentiated into mature osteoblasts by treatment with ascorbic
acid (50 μg/ml) and β-glycerophosphate (10 mM) for 3 days, followed
by incubation in soybean extracts [0.001 mg/ml, low concentration
of soybean extracts (LS); 0.1 mg/ml, high concentration of soybean
extracts (HS)], E2 (10−9 M), or a combination of
daidzein and genistein (D/G, 0.1×10−8 M/each) for 6
days. The concentration of E2 was based on the normal plasma
estrogen concentration (50–500 pg/ml: 0.18-1.8×10−9 M)
in healthy adult women (23). In
addition, as in our previous study, we demonstrated that the potent
estrogenic effects of soybean on osteoblastic function are mediated
by the synergism of the combination of daidzein and genistein at
0.1×10−8 M/each (17),
we employed these isoflavones at a concentration of
0.1×10−8 M/each as the controls in all subsequent
experiments. ELISA revealed that the secretion of RANKL and OPG
increased when the cells differentiated into osteoblasts following
treatment with ascorbic acid and β-glycerophosphate, while RANKL
and OPG were detected at minimal levels (or not detected) in the CM
of undifferentiated cells (Table
I). Moreover, we observed a significant increase in the
secretion of OPG from osteoblasts that were incubated with E2 [182%
vs. differentiated control (+C)], D/G (142% vs. +C), LS (188% vs.
+C) or HS (194% vs. +C). Conversely, the secretion of RANKL was
attenuated in the CM of cells in response to all the compounds
(~51% vs. +C), apart from HS (114% vs. +C), which induced a modest
increase.
 | Table ILevels of OPG and RANKL and the
OPG/RANKL ratio in the conditioned medium of MC3T3-E1 osteoblasts
in response to treatment with soybean extracts, estrogen or a
combination of isoflavones. |
Table I
Levels of OPG and RANKL and the
OPG/RANKL ratio in the conditioned medium of MC3T3-E1 osteoblasts
in response to treatment with soybean extracts, estrogen or a
combination of isoflavones.
| Treatment | OPG (ng/ml) | RANKL (ng/ml) | OPG/RANKL ratio (% of
+C) |
|---|
| −C |
1.7±1.3d | ND1 | NA2 |
| +C |
32.4±2.6c |
4.3±0.3a |
100±8.7d |
| E2 |
59.0±12.8a |
3.3±0.4b |
237.3±32.0b |
| D/G |
46.1±5.7b |
2.2±0.4c |
278.1±14.3a |
| LS |
60.9±2.0a |
2.8±0.3b,c |
288.7±6.7a |
| HS |
62.8±4.3a |
4.9±0.7a |
170.1±6.1c |
As the OPG/RANKL ratio is considered an important
parameter in the regulation of osteoclast differentiation (24), we then analyzed the relative
abundance of OPG and RANKL (Table
I). The secreted OPG/RANKL ratio significantly increased
following treatment with E2, D/G, LS or HS. Of note, LS (288.7% vs.
+C) increased the ratio to a greater extent than HS (170.1% vs.
+C). These results suggest that a lower concentration of soybean
extracts (0.001 vs. 0.1 mg/ml) is more effective at stimulating the
secretion of OPG as opposed to RANKL, thus exerting inhibitory
effects on osteoclast differentiation.
CM of soybean-treated osteoblasts
attenuates RANKL-induced osteoclast differentiation
To investigate the indirect inhibitory effects of
soybean on osteoclast differentiation, RAW264.7 pre-osteoclasts
treated with or without soluble RANKL (30 ng/ml), an inducer of
osteoclast differentiation, were exposed to CM collected from
MC3T3-E1 osteoblasts treated with either E2, D/G, LS or HS. As
expected, the number of TRAP-positive giant multinucleated
osteoclasts significantly increased in the cells following
treatment with RANKL (Fig. 1A).
However, the RANKL-induced osteoclast formation was significantly
attenuated by treatment with CM of MC3T3-E1 osteoblasts incubated
with E2 (E-CM), or D/G (D/G-CM) (Fig.
1A). Similarly, CM of MC3T3-E1 osteoblasts following treatment
with soybean extracts (S-CM) also attenuated the effects of RANKL,
while S-CM-mediated decreases in osteoclast formation were evident
in the cells treated with CM of a lower concentration of soybean
extracts (LS-CM) compared with that of a high concentration of
soybean extracts (HS-CM).
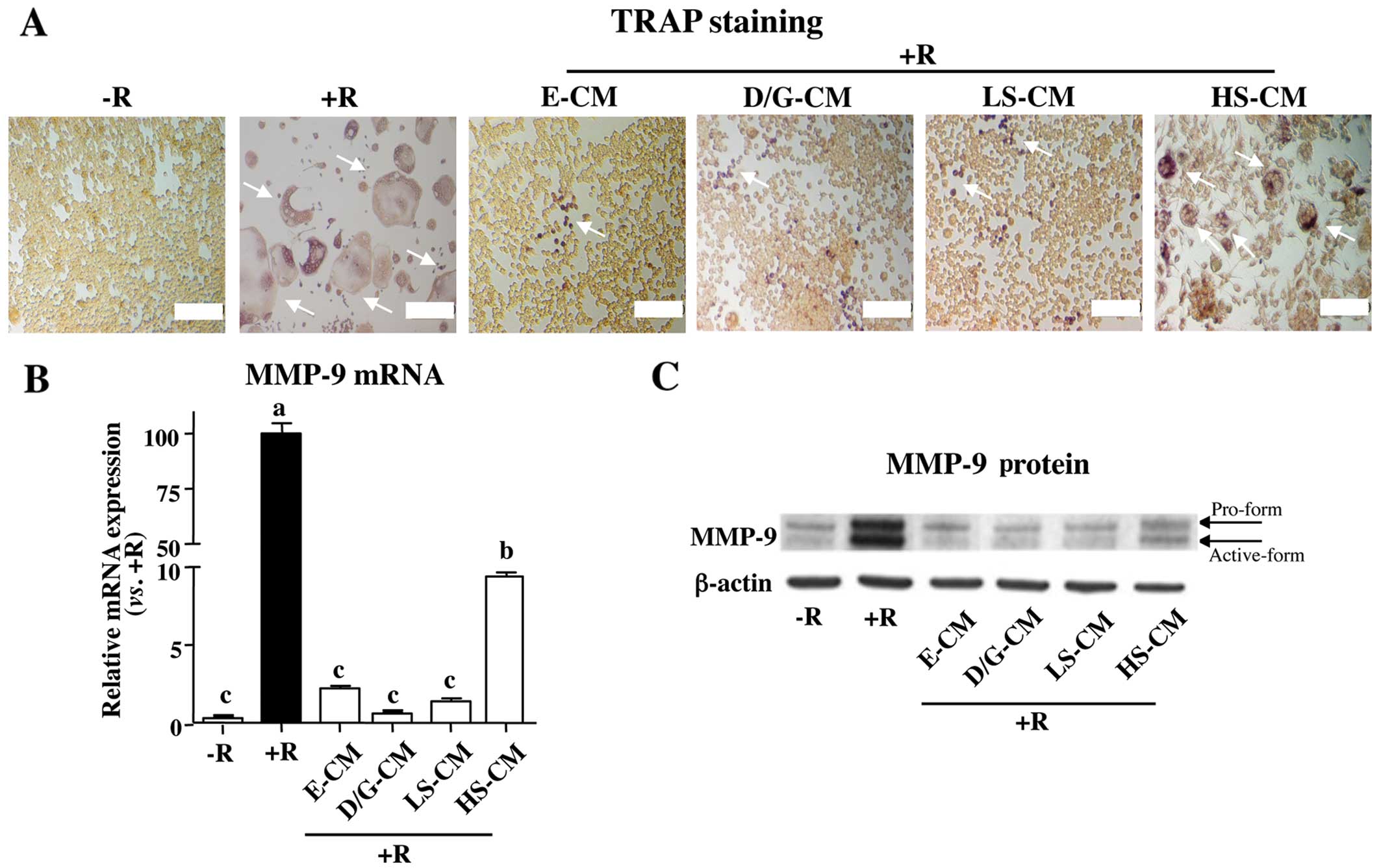 | Figure 1Conditioned medium (CM) of
soybean-treated MC3T3-E1 osteoblasts suppresses receptor activator
of nuclear factor-κB ligand (RANKL)-induced osteoclast
differentiation. RAW264.7 pre-osteoclasts were incubated with or
without RANKL (30 ng/ml), followed by treatment with CM collected
from MC3T3-E1 osteoblasts incubated with soybean extracts (0.001
mg/ml, LS; 0.1 mg/ml, HS), estrogen (E, 10−9 M), or a
combination of isoflavones (0.1×10−8 M/each, D/G). (A)
Osteoclast formation was assessed by tartate-resistant acid
phosphatase (TRAP) staining. The number of TRAP-positive giant
multinucleated osteoclasts (indicated by arrows) significantly
increased in the cells following treatment with RANKL. The number
of these cells decreased following treatment with E, D/G, LS and
HS. However, LS was more effective than HS. Matrix
metalloproteinase-9 (MMP-9) mRNA and protein levels were quantified
by (B) qRT-PCR and (C) western blot analysis, respectively.
Different letters indicate significant difference at p<0.05.
Similar results were obtained when the experiment was repeated (in
triplicate) using different cell preparations. Scale bar, 100 μm.
R, RANKL; D/G, daidzein and genistein. |
To further delineate the effects of S-CM-mediated
decreases in osteoclast differentiation, we determined the
expression levels of MMP-9, a potential biomarker of osteoclast
differentiation (25). Similar to
the changes observed with TRAP staining, treatment with RANKL
significantly increased MMP-9 mRNA and protein levels (Fig. 1B and C). However, the
RANKL-induced increase in MMP-9 expression was attenuated in the
cells exposed to all compounds tested. Again, LS-CM was more
effective at suppressing MMP-9 expression than HS-CM. Taken
together, these results suggest that osteoclast differentiation is
indirectly regulated by the key paracrine factors, OPG and RANKL,
that are produced and secreted by osteoblasts.
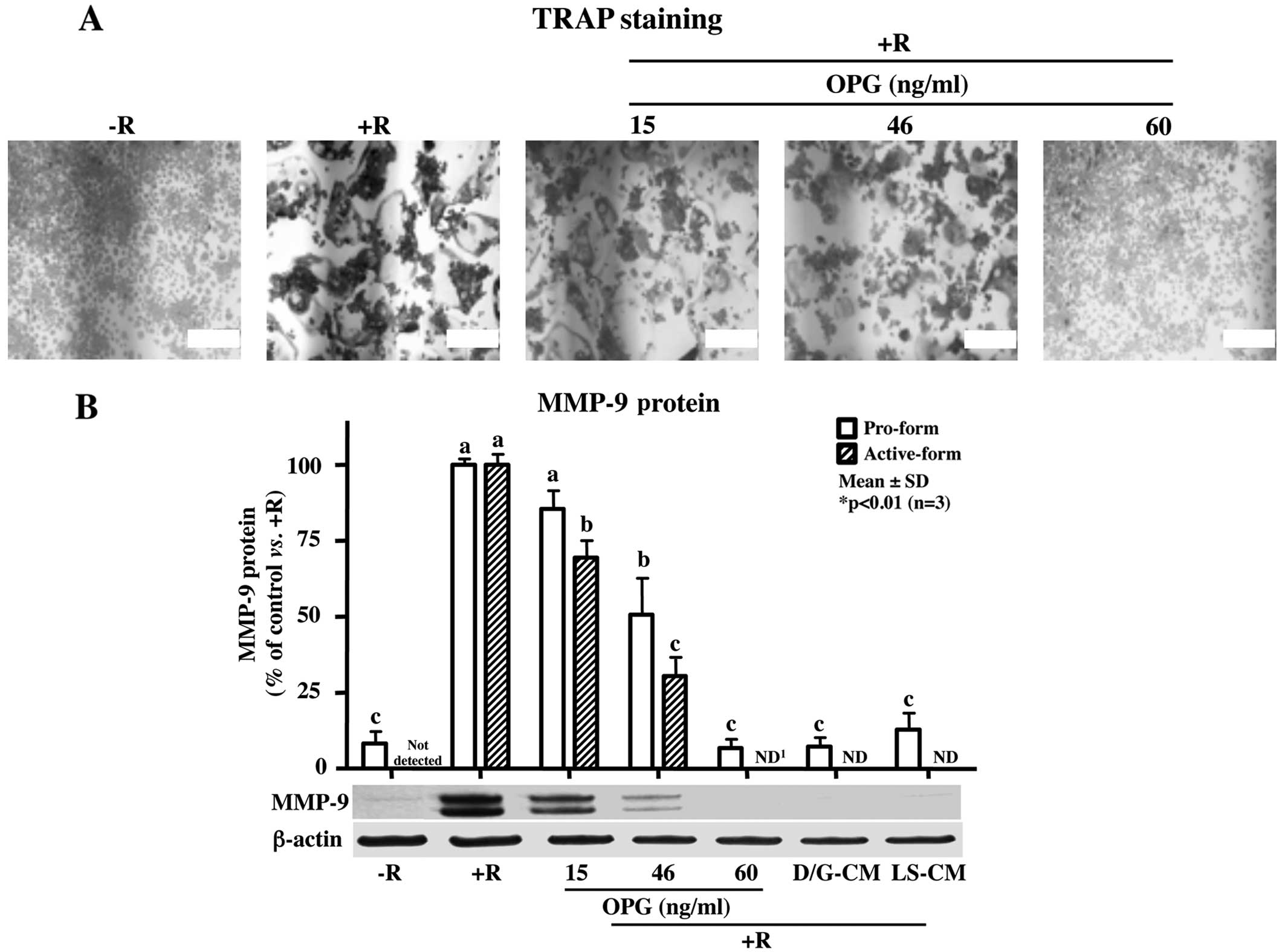
Recombinant OPG peptides attenuate
RANKL-induced osteoclast differentiation
As previous studies have demonstrated that OPG, a
decoy receptor of RANKL that binds to RANK, decreases the binding
ability of RANKL to RANK and subsequently inhibits osteoclast
differentiation (22,24), we then investigated whether
exogenous OPG, and at what concentrations, inhibits osteoclast
formation. TRAP staining revealed that treatment with OPG peptides
significantly decreased osteoclast formation induced by RANKL in a
dose-dependent manner (Fig. 2A).
Consistent with the changes observed with TRAP staining, the
RANKL-induced increase in MMP-9 protein expression was markedly
attenuated by treatment with OPG peptides at 60 ng/ml (Fig. 2B), while the addition of LS-CM
with OPG concentrations of >60 ng/ml, completely inhibited
RANKL-induced TRAP-positive giant multinucleated osteoclasts and
MMP-9 expression. Of note, although the OPG concentration of 46
ng/ml was not sufficient to suppress RANKL-induced osteoclast
formation and MMP-9 expression, treatment with D/G-CM, which
contains 46.1 ng/ml OPG, completely blocked both osteoclast
formation and MMP-9 expression, suggesting that other regulators in
addition to OPG, are involved in the regulation of osteoclast
differentiation and formation.
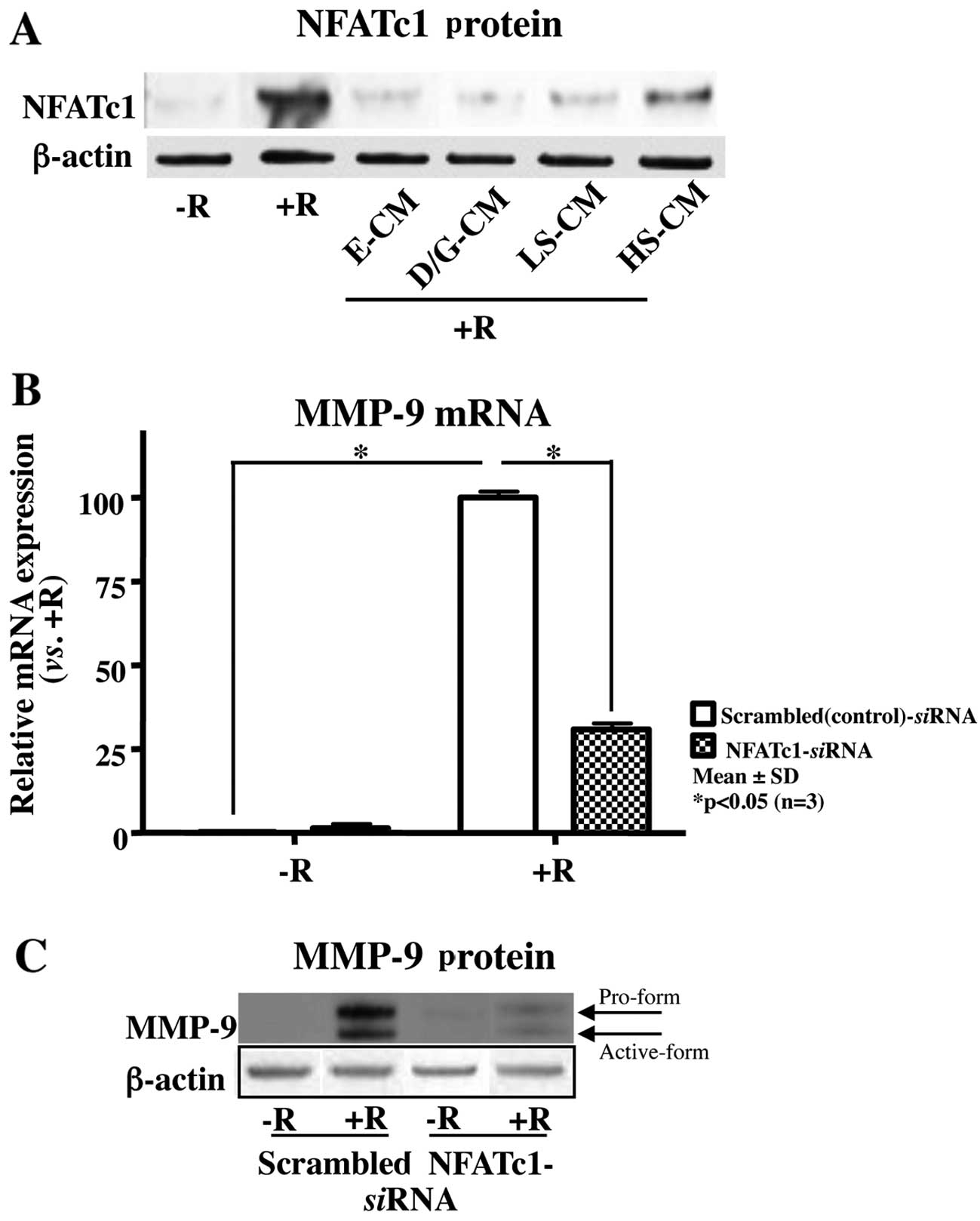
CM of soybean-treated osteoblasts
attenuates RANKL-induced osteoclast differentiation by suppressing
the activation of the transcription factor, NFATc1
Since NFATc1 is a critical transcription factor that
is responsible for osteoclast differentiation (20), we examined whether S-CM can alter
RANKL-induced NFATc1 expression. Western blot analysis revealed
that all the treatments, including S-CM, markedly attenuated the
RANKL-induced increase in NFATc1 expression (Fig. 3). In a similar manner, LS-CM was
more effective at decreasing the expression levels of NFATc1 than
HS-CM (Fig. 3A). To further
identify NFATc1 as the mediator responsible for RANKL-induced
osteoclast differentiation in our culture system, we transfected
the cells with siRNA targeting NFATc1 or scrambled (control) siRNA,
followed by treatment with RANKL. The silencing of NFATc1 markedly
attenuated the RANKL-induced MMP-9 mRNA and protein expression
(Fig. 3B). Taken together, these
results suggest that CM collected from MC3T3-E1 osteoblasts
incubated with a lower concentration of soybean extracts was more
effective at inhibiting osteoclast differentiation by suppressing
the activation of NFATc1 compared to incubation with a high
concentration of soybean extracts.
Discussion
Soybean is a major dietary source of isoflavones,
particularly daidzein and genistein that can bind to ER-α and ER-β,
due to their structural similarity to estrogen (16). Accordingly, these isoflavones have
been shown to inhibit osteoclast differentiation and bone
resorption in vitro and in vivo (16,26–28). However, coupled with the low
expression of ER-α and ER-β in osteoclasts (12), the inhibitory effects of
isoflavones on osteoclast differentiation are likely regulated by
various paracrine factors, including OPG and RANKL, released from
osteoblasts. In this study, we demonstrate that the
soybean-mediated increase in the levels of secreted OPG as opposed
to RANKL in osteoblast-CM attenuates RANKL-induced osteoclast
differentiation.
A number of studies have shown soy isoflavones to
exert direct inhibitory effects on osteoclast differentiation via
previously suggested cellular mechanisms (29,30): i) an increased extracellular
calcium concentration that directly stimulates osteoclast
apoptosis; ii) an inhibition of protein tyrosine kinase Src, that
is a critical factor in inducing osteoclast differentiation; and
iii) an activation of protein tyrosine phosphatase that is a
negative regulator of osteoclast differentiation. However, our
preliminary experiments revealed that direct treatment with
estrogen and a combination of isoflavones failed to suppress
RANKL-induced osteoclast formation (data not shown). In addition,
the partial inhibition of osteoclast formation by treatment with
soybean extracts may be due to other components of soybeans,
individually or in conjunction with isoflavones, as the
RANKL-induced osteoclast formation was not attenuated by the
combination of daidzein and genistein, which is the concentration
of these two isoflavones that is similar to soybean (data not
shown). Instead, we demonstrated that osteoclast differentiation
was significantly attenuated in cells following treatment with CM
collected from osteoblasts incubated with soybean extracts, as well
as estrogen or a combination of isoflavones, which were employed as
the controls. These results demonstrate that although the direct
inhibitory effects of soybean extracts on osteoclast formation
remain feasible, the inhibitory effects of soybean extracts on
osteoclast differentiation in our culture system are likely
mediated indirectly through paracrine factors produced by
osteoblasts. Of note, such an effect was evident following
treatment with low concentrations of soybean extracts or the
combination of isoflavones (daidzein and genistein) at
0.1×10−8 M/each, which is a concentration of these two
isoflavones that is similar to soybean at 0.001 mg/ml, suggesting
that the synergistic effects of daidzein and genistein and a low
concentration of soybean likely led to an upregulation of paracrine
factors in osteoblasts, which in effect modulated an increase in
the OPG/RANKL ratio.
As noted above, OPG is able to bind to RANKL as a
decoy receptor, resulting in the inhibition of osteoclast
differentiation (10,22,24). In the present study, we
demonstrated that recombinant OPG at 60 ng/ml is sufficient to
inhibit the RANKL-induced osteoclast differentiation, whereas
osteoclast differentiation was not completely suppressed by OPG
peptides at 46 ng/ml. These results are in agreement with those of
previous studies, demonstrating that an exogenous OPG concentration
up to 100 ng/ml inhibits RANKL-induced RAW264.7 osteoclast
differentiation (31). Of note,
however, the combination of isoflavones, which induced the
secretion of OPG at ~46 ng/ml, was sufficient to inhibit the
RANKL-induced osteoclast differentiation. Conversely, although a
high concentration of soybean extracts increased the OPG secretion
by >60 ng/ml, osteoclast differentiation was not completely
suppressed as treatment also highly stimulated RANKL levels that
induced a decrease in the OPG/RANKL ratio (278% D/G vs. 170% HS).
Hence, our study further demonstrates that although a certain
concentration of OPG has the ability to inhibit osteoclast
differentiation, the OPG/RANKL ratio is a critical parameter in the
inhibition of osteoclast differentiation.
Among diverse cellular mediators that are associated
with RANKL-mediated osteoclast differentiation, the importance of
the transcription factor, NFATc1, has been suggested by previous
studies, demonstrating that NFATc1-deficient cells fail to
differentiate from pre-osteoclasts into osteoclasts (32). The results from the present study
further support the key role of NFATc1 in RANKL-induced MMP-9
expression and osteoclast differentiation.
In conclusion, the present study demonstrates the
indirect inhibitory effects of soybean extracts on osteoclast
differentiation. Although further studies are required to elucidate
the direct and/or indirect inhibitory effects of other functional
components of soybeans, e.g., lecithins, lectins, linoleic acid and
saponins, on osteoclast differentiation, the indirect effects that
we observed are likely caused by the synergistic action of daidzein
and genistein. Furthermore, a low concentration of soybean extracts
markedly mediated an increase in the OPG/RANKL ratio, which in
effect inhibited osteoclast differentiation by suppressing NFATc1
activation.
Acknowledgements
The authors thank Dr Taj Mann for providing superb
editorial assistance. This study was supported by a 2006 grant from
Kyung Hee University (KHU-20060554) and by a grant from the Agenda
Program (PJ008559), Rural Development Administration, Republic of
Korea.
References
|
1
|
Spelsberg TC, Subramaniam M, Riggs BL and
Khosla S: The actions and interactions of sex steroids and growth
factor/cytokines on the skeleton. Mol Endocrinol. 13:819–828. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Barton M and Shapiro D: Transient
administration of estradiol-17 beta establishes an autoregulatory
loop permanently inducing estrogen receptor mRNA. Proc Natl Acad
Sci USA. 85:7119–7123. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Babiker FA, De Windt LJ, van Eickels M,
Grohe C, Meyer R and Doevendans PA: Estrogenic hormone action in
the heart: regulatory network and function. Cardiovasc Res.
53:709–719. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kang KS, Morita I, Cruz A, Jeon YJ, Trosko
JE and Chang CC: Expression of estrogen receptors in a normal human
breast epithelial cell type with luminal and stem cell
characteristics and its neoplastically transformed cell lines.
Carcinogenesis. 18:251–257. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lindgren PR, Cajander S, Bäckström T,
Gustafsson JA, Mäkelä S and Olofsson JI: Estrogen and progesterone
receptors in ovarian epithelial tumors. Mol Cell Endocrinol.
221:97–104. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Couse JF, Lindzey J, Grandien K,
Gustafsson JA and Korach KS: Tissue distribution and quantitative
analysis of estrogen receptor-alpha (ERalpha) and estrogen
receptor-beta (ERbeta) messenger ribonucleic acid in the wild-type
and ERalpha-knockout mouse. Endocrinology. 138:4613–4621. 1997.
|
|
7
|
Ernst M, Schmid C and Froesch ER: Enhanced
osteoblast proliferation and collagen gene expression by estradiol.
Proc Natl Acad Sci USA. 85:2307–2310. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Okazaki R, Inoue D, Shibata M, Saika M,
Kido S, Ooka H, Tomiyama H, Sakamoto Y and Matsumoto T: Estrogen
promotes early osteoblast differentiation and inhibits adipocyte
differentiation in mouse bone marrow stromal cell lines that
express estrogen receptor (ER) alpha or beta. Endocrinology.
143:2349–2356. 2002.
|
|
9
|
Balica M, Boström K, Shin V, Tillisch K
and Demer LL: Calcifying subpopulation of bovine aortic smooth
muscle cells is responsive to 17 beta-estradiol. Circulation.
95:1954–1960. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bord S, Ireland DC, Beavan SR and Compston
JE: The effects of estrogen on osteoprotegerin, RANKL, and estrogen
receptor expression in human osteoblasts. Bone. 32:136–141. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kameda T, Mano H, Yuasa T, Mori Y,
Miyazawa K, Shiokawa M, Nakamaru Y, Hiroi E, Hiura K, Kameda A,
Yang NN, Hakeda Y and Kumegawa M: Estrogen inhibits bone resorption
by directly inducing apoptosis of the bone-resorbing osteoclasts. J
Exp Med. 186:489–495. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Collier FM, Huang WH, Holloway WR, Hodge
JM, Gillespie MT, Daniels LL, Zheng MH and Nicholson GC:
Osteoclasts from human giant cell tumors of bone lack estrogen
receptors. Endocrinology. 139:1258–1267. 1998.PubMed/NCBI
|
|
13
|
Grodstein F, Stampfer MJ, Colditz GA,
Willett WC, Manson JE, Joffe M, Rosner B, Fuchs C, Hankinson SE,
Hunter DJ, Hennekens CH and Speizer FE: Postmenopausal hormone
therapy and mortality. N Engl J Med. 336:1769–1775. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Compston JE: Sex steroids and bone.
Physiol Rev. 81:419–447. 2001.PubMed/NCBI
|
|
15
|
Keinan-Boker L, van Der Schouw YT, Grobbee
DE and Peeters PH: Dietary phytoestrogens and breast cancer risk.
Am J Clin Nutr. 79:282–288. 2004.PubMed/NCBI
|
|
16
|
Setchell KD and Lydeking-Olsen E: Dietary
phytoestrogens and their effect on bone: evidence from in vitro and
in vivo, human observational, and dietary intervention studies. Am
J Clin Nutr. 78(Suppl 3): 593S–609S. 2003.PubMed/NCBI
|
|
17
|
Kim J, Um SJ, Woo J, Kim JY, Kim HA, Jang
KH, Kang SA, Lim BO, Kang I, Choue RW and Cho Y: Comparative effect
of seeds of Rhynchosia volubilis and soybean on MG-63 human
osteoblastic cell proliferation and estrogenicity. Life Sci.
78:30–40. 2005.
|
|
18
|
Qu Q, Härkönen PL, Mönkkönen J and
Väänänen HK: Conditioned medium of estrogen-treated osteoblasts
inhibits osteoclast maturation and function in vitro. Bone.
25:211–215. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wong BR, Josien R, Lee SY, Vologodskaia M,
Steinman RM and Choi Y: The TRAF family of signal transducers
mediates NF-kappaB activation by the TRANCE receptor. J Biol Chem.
273:28355–28359. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ishida N, Hayashi K, Hoshijima M, Ogawa T,
Koga S, Miyatake Y, Kumegawa M, Kimura T and Takeya T: Large scale
gene expression analysis of osteoclastogenesis in vitro and
elucidation of NFAT2 as a key regulator. J Biol Chem.
277:41147–41156. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park K, Elias PM, Oda Y, Mackenzie D,
Mauro T, Holleran WM and Uchida Y: Regulation of cathelicidin
antimicrobial peptide expression by an endoplasmic reticulum (ER)
stress signaling, vitamin D receptor-independent pathway. J Biol
Chem. 286:34121–34130. 2011. View Article : Google Scholar
|
|
22
|
Simonet WS, Lacey DL, Dunstan CR, Kelley
M, Chang MS, Lüthy R, Nguyen HQ, Wooden S, Bennett L, Boone T,
Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G,
Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D,
Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R
and Boyle WJ: Osteoprotegerin: a novel secreted protein involved in
the regulation of bone density. Cell. 89:309–319. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Santanam N, Shern-Brewer R, McClatchey R,
Castellano PZ, Murphy AA, Voelkel S and Parthasarathy S: Estradiol
as an antioxidant: incompatible with its physiological
concentrations and function. J Lipid Res. 39:2111–2118.
1998.PubMed/NCBI
|
|
24
|
Khosla S: Minireview: the OPG/RANKL/RANK
system. Endocrinology. 142:5050–5055. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sundaram K, Nishimura R, Senn J, Youssef
RF, London SD and Reddy SV: RANK ligand signaling modulates the
matrix metalloproteinase-9 gene expression during osteoclast
differentiation. Exp Cell Res. 313:168–178. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Karieb S and Fox SW: Phytoestrogens
directly inhibit TNF-α-induced bone resorption in RAW264.7 cells by
suppressing c-fos-induced NFATc1 expression. J Cell Biochem.
112:476–487. 2011.
|
|
27
|
Tyagi AM, Srivastava K, Sharan K, Yadav D,
Maurya R and Singh D: Daidzein prevents the increase in
CD4+CD28null T cells and B lymphopoesis in
ovariectomized mice: a key mechanism for anti-osteoclastogenic
effect. PLoS One. 6:e212162011.PubMed/NCBI
|
|
28
|
García Palacios V, Robinson LJ, Borysenko
CW, Lehmann T, Kalla SE and Blair HC: Negative regulation of
RANKL-induced osteoclastic differentiation in RAW264.7 cells by
estrogen and phytoestrogens. J Biol Chem. 280:13720–13727.
2005.
|
|
29
|
Lorget F, Kamel S, Mentaverri R, Wattel A,
Naassila M, Maamer M and Brazier M: High extracellular calcium
concentrations directly stimulate osteoclast apoptosis. Biochem
Biophys Res Commun. 268:899–903. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gao YH and Yamaguchi M: Suppressive effect
of genistein on rat bone osteoclasts: Involvement of protein kinase
inhibition and protein tyrosine phosphatase activation. Int J Mol
Med. 5:261–267. 2000.PubMed/NCBI
|
|
31
|
Fu YX, Gu JH, Zhang YR, Tong XS, Zhao HY,
Yuan Y, Liu XZ, Bian JC and Liu ZP: Osteoprotegerin influences the
bone resorption activity of osteoclasts. Int J Mol Med.
31:1411–1417. 2013.PubMed/NCBI
|
|
32
|
Takayanagi H, Kim S, Koga T, Nishina H,
Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J, Wagner
EF, Mak TW, Kodama T and Taniguchi T: Induction and activation of
the transcription factor NFATc1 (NFAT2) integrate RANKL signaling
in terminal differentiation of osteoclasts. Dev Cell. 3:889–901.
2002. View Article : Google Scholar : PubMed/NCBI
|