Introduction
Myocardial infarction is one of the most common
ischemic heart diseases and a major cause of morbidity and
mortality worldwide. It is a clinical syndrome arising from the
sudden and persistent interruption of the myocardial blood supply,
eventually leading to the loss of cardiomyocytes. Although a number
of drugs, including angiotensin-converting enzyme inhibitors,
calcium channel blockers and angiotensin II receptor antagonists,
has been widely used to treat myocardial ischemia in western
medicine, the usefulness of these drugs has been limited due to
their serious adverse effects, such as cardiac depression or even,
proarrhythmic effects (1). There
is great interest in the development of new types of
cardioprotective drugs, which may attenuate the effects of
myocardial infarction in clinical practice.
Cumulative evidence has demonstrated that cell death
in myocardial infarction is mainly mediated by reactive oxygen
species (ROS). A previous study demonstrated that the accumulation
of oxygen free radicals following ischemia not only destroyed
cellular structures, but further caused mitochondrial dysfunction,
activating apoptotic signaling cascades (2). The fatty acids are attacked by an
excess of free radicals in myocardial membranes. This causes a
chain reaction of lipid peroxidation, which has a harmful effect on
the myocardium during acute myocardial infarction (3). In addition, oxidative stress is
intimately involved in the occurrence of myocardial ischemia.
Therefore, the reduction of ROS may attenuate the effects of acute
myocardial infarction.
Apoptosis has been shown to participate in the
pathogenesis of myocardial injuries during myocardial infarction.
Caspase-3 serves as an ‘apoptotic effector’ in response to
apoptotic stimuli (4). It has
previously been reported that a dramatic elevation of caspase-3 can
cause damage in isoproterenol-induced Wistar rats with acute
myocardial infarction (5). In
addition, proteins of the Bcl-2 family have been shown to play a
central role in the regulation of cellular apoptosis. Bcl-2 is a
cytosolic protein and serves as an anti-apoptotic molecule, whereas
another member of the family, Bax, functions as a pro-apoptotic
protein (6). These findings
suggest that the regulation of caspase-3, Bcl-2 and Bax proteins
may attenuate the cardiac damage occuring in myocardial
infarction.
Nuclear factor-κB (NF-κB), which is found among most
eukaryotes, is the hub of the related signal transduction pathway
and is present in myocytes. Its levels can reflect the pathological
changes occuring during acute myocardial infarction (7,8).
The activation of NF-κB can lead to the release of pro-inflammatory
cytokines, such as tumor necrosis factor-α (TNF-α) and
interleukin-1β (IL-1β). During myocardial infarction, high levels
of NF-κB p65, TNF-α and IL-1β in cells can promote myocardial
necrosis and cellular apoptosis, eventually leading to serious
heart failure. Thus, drugs that suppress the NF-κB signaling
pathway and the release of pro-inflammatory factors may be used for
the treatment of diverse cardiac disorders.
Huperzine A (HupA) is a compound found in the plant
Qian Ceng Ta (Huperzia serrata), used in traditional Chinese
medicine (9). It has been
previously demonstrated to upregulate the cholinergic
anti-inflammatory pathway and to inhibit immunosenescence and
age-related disorders, such as Alzheimer’s disease, by suppressing
acetylcholinesterase (AChE) activity (10–13). Additionally, HupA has been
reported to reduce oxidative damage in D-galactose-treated rats
(14). However, it has not been
well established whether HupA can protect against acute myocardial
infarction in rats. Based on the above data, we hypothesized that
HupA exerts cardioprotective effects in rats with acute myocardial
infarction, and further explored the potential cardioprotective
mechanisms involved.
Materials and methods
Ethics statement
The experimental protocols for animal handling were
approved by the Animal Ethics Committee of the Medical School of
Chinese PLA, Beijing, China.
Animals and induction of acute myocardial
infarction
We purchased adult male Wistar rats (250–300 g) from
the Beijing Animal Center (Beijing, China). The rats were housed in
polypropylene cages under a controlled environment (12:12 h
light/dark cycle, 50–70% humidity, 24°C) with free access to water
and food. The rat model of acute myocardial infarction was
established as previously described with minor modifications
(15). Briefly, the animals were
anesthetized intraperitoneally (i.p.) with sodium pentobarbital (40
mg/kg). They were then intubated and artificially ventilated with a
respirator. The standard electrocardiogram (II) was procured by a
transducer attached to a multi-channel recorder (BL-420F; Chengdu
Tai Meng Science and Technology Co. Ltd., Chengdu, China), with
electrodes subcutaneously inserted into the limbs of the animals. A
5–0 silk suture of 1–2 mm was selected to encircle the left
anterior descending coronary artery under the left atrial
appendage. The sham-operated animals underwent identical surgical
procedures with the exception of the coronary artery ligation.
Efforts were made to minimize the number of animals used and their
suffering. Successful ligation was confirmed by an ST-segment
elevation and regional cyanosis of the myocardial surface.
Drug administration
HupA (98% pure; Sigma-Aldrich, St. Louis, MO, USA)
was dissolved in physiological saline solution. The rats were
randomly divided into 4 groups, 2 referred to as contorl and 2 as
treated: i) the control sham-operated group, in which the rats were
injected with physiological saline (0.1 ml/100 g, i.p.) and
underwent identical surgery with the exception of the coronary
artery ligation; ii) the control vehicle-treated group, in which
the rats were injected with physiological saline (0.1 ml/100 g,
i.p.) and underwent the ligation of the left coronary artery; and
iii) 2 HupA treatment groups, which were subjected to the ligation
of the left coronary artery and treated with 167 and 500 μg/kg
HupA, respectively. The rats were injected with physiological
saline solution or HupA 7 consecutive days. Thirty minutes after
the final injection, the rats underwent left coronary artery
ligation.
Measurement of infarct size
The hearts were rapidly excised and the left
ventricles were sliced into 2-mm thick sections from the apex to
the atrioventricular groove 6 h after the coronary artery was
ligated, followed by incubation with 1% triphenyltetrazolium
chloride (TTC) (Sigma-Aldrich) solution at 3°C for 30 min. The
normal myocardium was stained brick red, while the area without
color corresponded to the ischemic heart muscle. The volume and
weight of the rat hearts as a percentage of the left ventricle were
used to calculate the size of the infarcted area.
Quantification of activities of cardiac
marker enzymes
Six hours after the ligation of the coronary artery,
the blood samples were collected in order to determine the
activities of the myocardial-specific enzymes, creatine kinase
(CK), the MB isoenzyme of creatine kinase (CK-MB), lactate
dehydrogenase (LDH) and cardiac troponin T (cTnT). The colorimetric
method was employed to determine the activities of CK, CK-MB and
LDH following the manufacturer’s instructions (Nanjing Jiancheng
Bioengineering Institute, Nanjing, China). Serum cTnT levels were
measured using the Elecsys 2010 immunoassay (Roche Diagnostics
GmbH, Mannheim, Germany).
Measurement of malondialdehyde (MDA),
superoxide dismutase (SOD), glutathione (GSH) and glutathione
peroxidase (GSH-PX) activities
The enzymatic activities of MDA, SOD, GSH and GSH-PX
in the heart homogenates were measured according to the
instructions provided with the relevant commercially-available
assay kits (Nanjing Jiancheng Bioengineering Institute). The
concentration of MDA, a presumptive marker of oxidant-mediated
lipid peroxidation, was quantified using the thiobarbituric acid
(TBA)-reacting substances assay, followed by measuring the
absorbance at a wavelength of 532 nm. SOD activity was measured in
the heart homogenates by calculating the rate of inhibition of
nucleotide oxidation. The activities of GSH-PX and GSH were
determined by quantifying the rate of oxidation of the reduced
glutathione to the oxidized glutathione by
H2O2. One unit of GSH-PX was represented as
the quantity that reduced the level of GSH by 1 μM in 1 min/mg
protein. Quantification of protein concentrations in the heart
homogenates was performed using the Coomassie Brilliant Blue method
and bovine serum albumin as the standard.
Caspase-3 activity assay
The cleavage of the chromogenic caspase substrate,
acetyl-Asp-Glu-Val-Asp-p-nitroanilide (Ac-DEVD-pNA), by
caspase-3 was used to measure the activity of the enzyme. The
amount of caspase-3 was measured by the colorimetric approach using
a commercial kit (Beyotime Institute of Biotechnology, Beijing,
China). The protein samples from the heart muscles were acquired as
described below in ‘Western blot analysis’. Approximately 50 μg of
protein was added to the reaction buffer containing
Ac-DEVD-pNA (2 mM), incubated at 37°C for 4 h, and the
absorbance of yellow pNA was calculated by a spectrometer at
a wavelength of 405 nm. The activity of caspase-3, normalized as to
the total protein activity in heart muscle, was then expressed as a
fold change, compared with the baseline caspase-3 activity of the
control (sham-operated) group.
Western blot analysis
Western blot analysis was performed on the heart
samples from each group. Briefly, heart muscle from different
samples was homogenized in ice-cold RIPA buffer. Following
centrifugation at 13,200 × g for 20 min at 4°C, the supernatant was
collected and the total protein level was measured using a standard
BCA method (Beyotime Institute of Biotechnology).
Proteins (50 μg) were separated in 8 or 10%
SDS-polyacryl-amide gels and transferred onto nitrocellulose
membranes (Millipore, Billerica, MA, USA). The membranes were
blocked with 5% fat-free milk for 2 h at room temperature and then
incubated with a primary antibody overnight at 4°C. The primary
antibodies used were as follows: rabbit anti-caspase-3 (1:300),
rabbit anti-Bcl-2 (1:200), rabbit anti-Bax (1:200) (all from Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and mouse anti-GAPDH
(1:2,000; Kang Chen, Shanghai, China). The membranes were then
rinsed and incubated with horseradish peroxidase-conjugated goat
anti-rabbit antibody (1:5,000) or goat anti-mouse antibody
(1:5,000) (both from Santa Cruz Biotechnology, Inc.) for 2 h. The
detection of immunolabeled protein bands was performed using an
enhanced chemiluminescence (ECL) kit (Pierce, Rockford, IL, USA),
followed by exposure to an X-ray film. GAPDH was used as the
loading control. The quantification of protein band intensities was
carried out using Quantity One software (Bio-Rad, Hercules, CA,
USA).
Measurement of NF-κB, TNF-α and IL-1β
levels
The nuclear levels of p65 may positively correlate
with the activation of the NF-κB pathway. The NF-κB/p65 ActivELISA
kit (Imgenex, San Diego, CA, USA) was used to measure the levels of
NF-κB-free p65 in the nuclear lysates following the manufacturer’s
instructions. The quantification of TNF-α and IL-β was carried out
using the TNF-α and IL-1β Quantikine Rat immunoassay kits following
the manufacturer’s instructions (R&D Systems, Minneapolis, MN,
USA).
Statistical analysis
Data are expressed as the means ± SD from 6 rats in
each group. Experimental results were analyzed using a one-way
ANOVA followed by Dunnett’s test for individual comparisons between
the means of each group. All statistical analyses were performed
using SPSS 13.0 software. A value of P<0.05 was considered to
indicate a statistically significant difference.
Results
Effects of HupA on myocardial infarct
size in a rat model of acute myocardial infarction
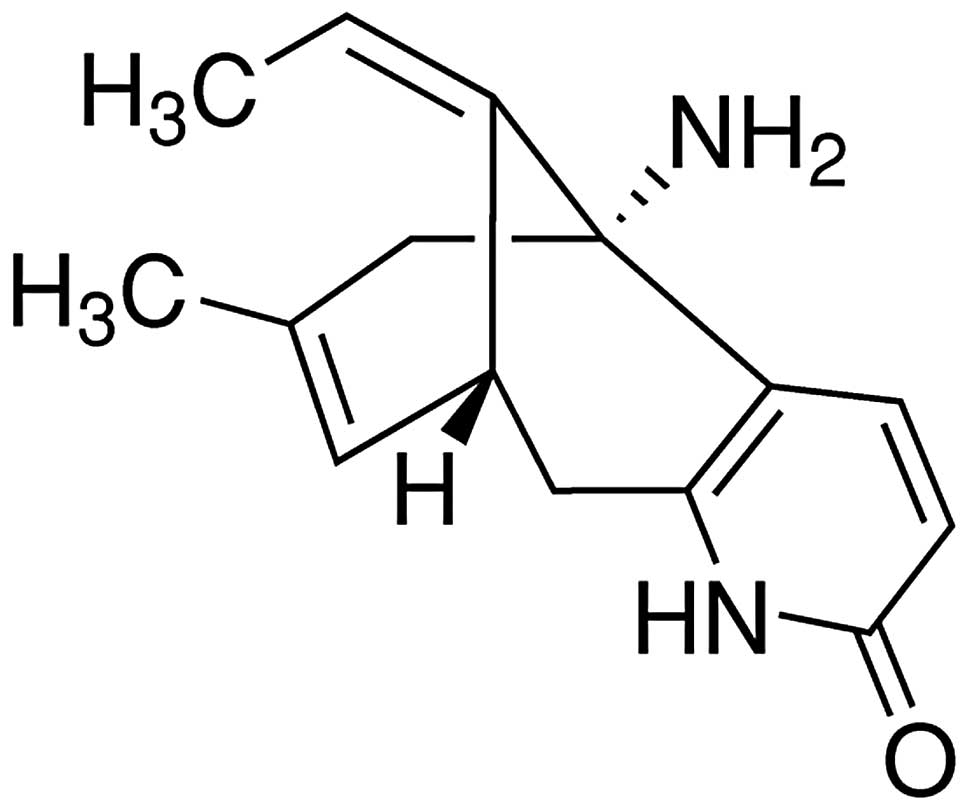
The chemical structure of HupA is illustrated in
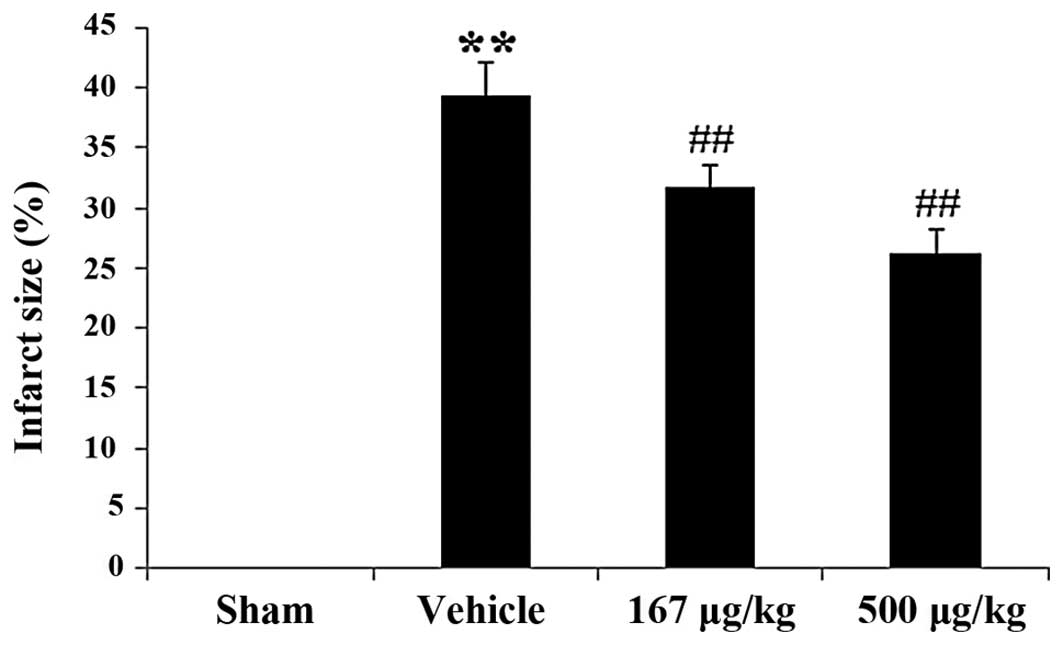
Fig. 1. The infarct size in the
vehicle-treated group was 39.31±2.78%. Following treatment with
HupA at concentrations of 167 and 500 μg/kg, the size of the
infarcted area significantly decreased to 31.77±1.74% (P<0.01)
and 26.21±1.91% (P<0.01), respectively, in comparison with the
vehicle-treated group (Fig.
2).
Effects of HupA on serum CK, CK-MB, LDH
and cTnT levels in a rat model of acute myocardial infarction
The measurements of serum CK, CK-MB, LDH and cTnT
levels in the control and treated groups are summarized in Table I. The rats with acute myocardial
infarction showed a significant increase in serum levels of CK,
CK-MB, LDH and cTnT (P<0.01) as compared with the sham-operated
control group. Pre-treatment with 167 and 500 μg/kg HupA
significantly decreased (P<0.01) the serum levels of CK, CK-MB,
LDH and cTnT in the rats subjected to myocardial infarction vs. the
vehicle-treated group.
 | Table IEffects of HupA on serum levels of CK,
CK-MB, LDH and cTnT in rats subjected to acute myocardial
infarction. |
Table I
Effects of HupA on serum levels of CK,
CK-MB, LDH and cTnT in rats subjected to acute myocardial
infarction.
| Group | CK | CK-MB | LDH | cTnT |
|---|
| Sham-operated
group | 0.24±0.05 | 78.08±2.61 | 1838±405.69 | 0.058±0.03 |
| Vehicle-treated
group | 0.58±0.07a | 186.16±6.11a | 3748.5±398.27a | 0.3±0.05a |
| HupA (167 μg/kg) | 0.41±0.06b | 115.87±7.29b |
3165.67±405.44b | 0.19±0.05b |
| HupA (500 μg/kg) | 0.29±0.08b | 93.45±7.42b | 2455.5±319.19b | 0.17±0.04b |
Effects of HupA on the activities of MDA,
SOD, GSH-PX and GSH in a rat model of acute myocardial
infarction
Table II
demonstrates the activities of MDA, SOD, GSH-PX and GSH in the rat
hearts from the control and treated groups. Rats subjected to acute
myocardial infarction exhibited a marked increase in MDA levels, an
indicator of lipid peroxidation, as compared with the sham-operated
control rats (P<0.01). The administration of 167 and 500 μg/kg
HupA to the rats with myocardial infarction significantly reduced
ischemia-mediated lipid peroxidation in comparison with the
vehicle-treated group (P<0.01 for both HupA concentrations).
Furthermore, the activities of antioxidants and antioxidative
enzymes were measured in the heart homogenates from the control and
treated groups. Rats with acute myocardial infarction showed a
significant reduction in SOD, GSH-PX and GSH activities (P<0.01)
vs. the control rats. However, following treatment with HupA (167
or 500 μg/kg), a significant elevation in the activities of SOD,
GSH-PX and GSH was observed in the infarcted rat heart as compared
with the vehicle-treated group (P<0.01 for both HupA
concentrations).
 | Table IIEffects of HupA on MDA, SOD, GSH-PX
and GSH activities in the hearts of rats subjected to acute
myocardial infarction. |
Table II
Effects of HupA on MDA, SOD, GSH-PX
and GSH activities in the hearts of rats subjected to acute
myocardial infarction.
| Group | MDA | SOD | GSH-PX | GSH |
|---|
| Sham-operated
group | 25.19±1.07 | 6.29±0.73 | 6.29±0.73 | 34.81±2.69 |
| Vehicle-treated
group | 16.29±0.80a | 3.34±0.18a | 3.34±0.18a | 23.61±2.15a |
| HupA (167 μg/kg) | 20.90±1.00b | 4.87±0.48b | 4.87±0.48b | 28.85±1.79b |
| HupA (500 μg/kg) | 22.22±1.24b | 5.25±0.25b | 5.25±0.25b | 30.54±2.21b |
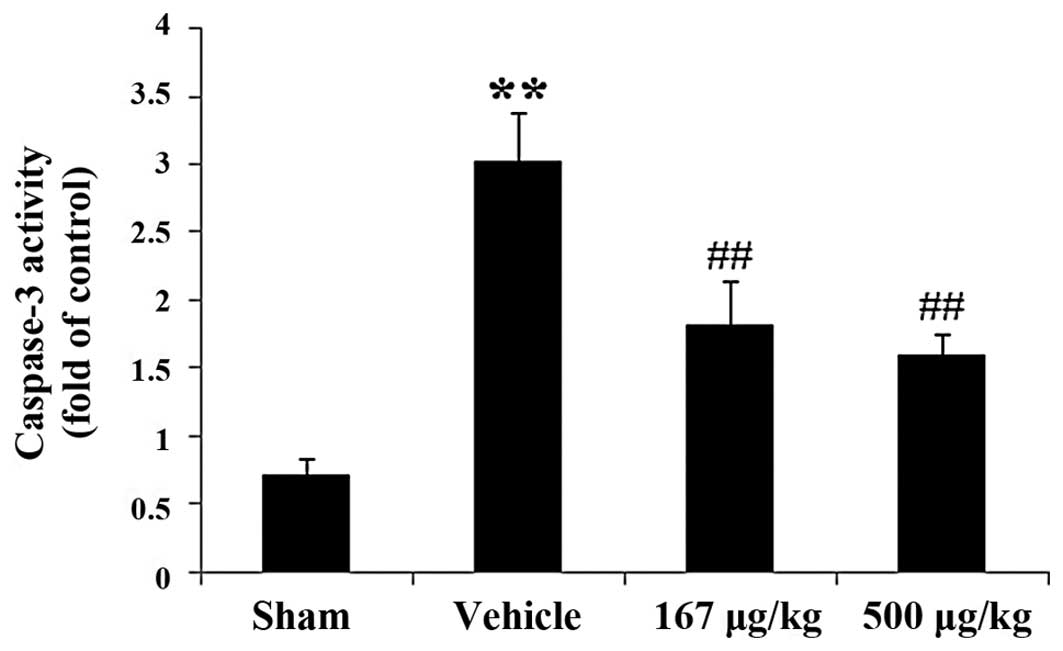
Effects of HupA on caspase-3 activity in
a rat model of acute myocardial infarction
In order to explore whether HupA attenuates the
apoptotic damage induced by myocardial infarction, the activity of
caspase-3, an effector of the apoptotic signaling pathway, was
determined by colorimetric analysis. Caspase-3 activity was
markedly elevated by 319.92% (P<0.01) in the vehicle-treated
group as compared with the sham-operated group (Fig. 3). In the HupA-treated (167 and 500
μg/kg) groups, there was a significant reduction in caspase-3
activity by 39.80% (P<0.01) and 47.03% (P<0.01),
respectively, as compared with the vehicle-treated group.
Effects of HupA on the protein expression
of caspase-3, Bcl-2 and Bax in a rat model of acute myocardial
infarction
To corroborate the fact that HupA prevents cardiac
damage induced by acute myocardial infarction in rats, western blot
analysis was carried out to determine the expression levels of the
apoptosis-related proteins, caspase-3, Bcl-2 and Bax, in the rat
hearts. Western blot analysis with caspase-3 antibody exhibited a
specific band of 20 kDa, characteristic of caspase-3 (Fig. 4A). The protein expression of
caspase-3 in the vehicle-treated group with myocardial infarction
was markedly increased in the rat hearts as compared with the
sham-operated control group (P<0.01). Nevertheless, upon
treatment with HupA (167 or 500 μg/kg) a marked reduction in
caspase-3 protein expression was observed in the infarcted rats
(P<0.01 for both HupA concentrations) in comparison to the
vehicle-treated group (Fig. 4B).
Thus, these results further confirmed that HupA treatment reduced
the caspase-3 protein level in a rat model of acute myocardial
infarction, in line with the caspase-3 activity measurements. In
addition, proteins of the Bcl-2 family, such as Bcl-2 and Bax, are
considered to play a pivotal role in the regulation of apoptotic
cascades. Fig. 4A shows that
these proteins were detected in bands of 26 kDa for Bcl-2 and 23
kDa for Bax. The statistical comparison revealed a statistically
significant reduction in Bcl-2 and a marked elevation in Bax
protein levels in the vehicle-treated rats (P<0.01) as compared
with the sham-operated group. However, upon treatment with HupA
(167 or 500 μg/kg), we observed strikingly elevated and markedly
decreased levels of Bcl-2 and Bax, respectively (P<0.01 for both
HupA concentrations) in the infarcted rat hearts as compared with
the vehicle-treated group (Fig. 4C
and D).
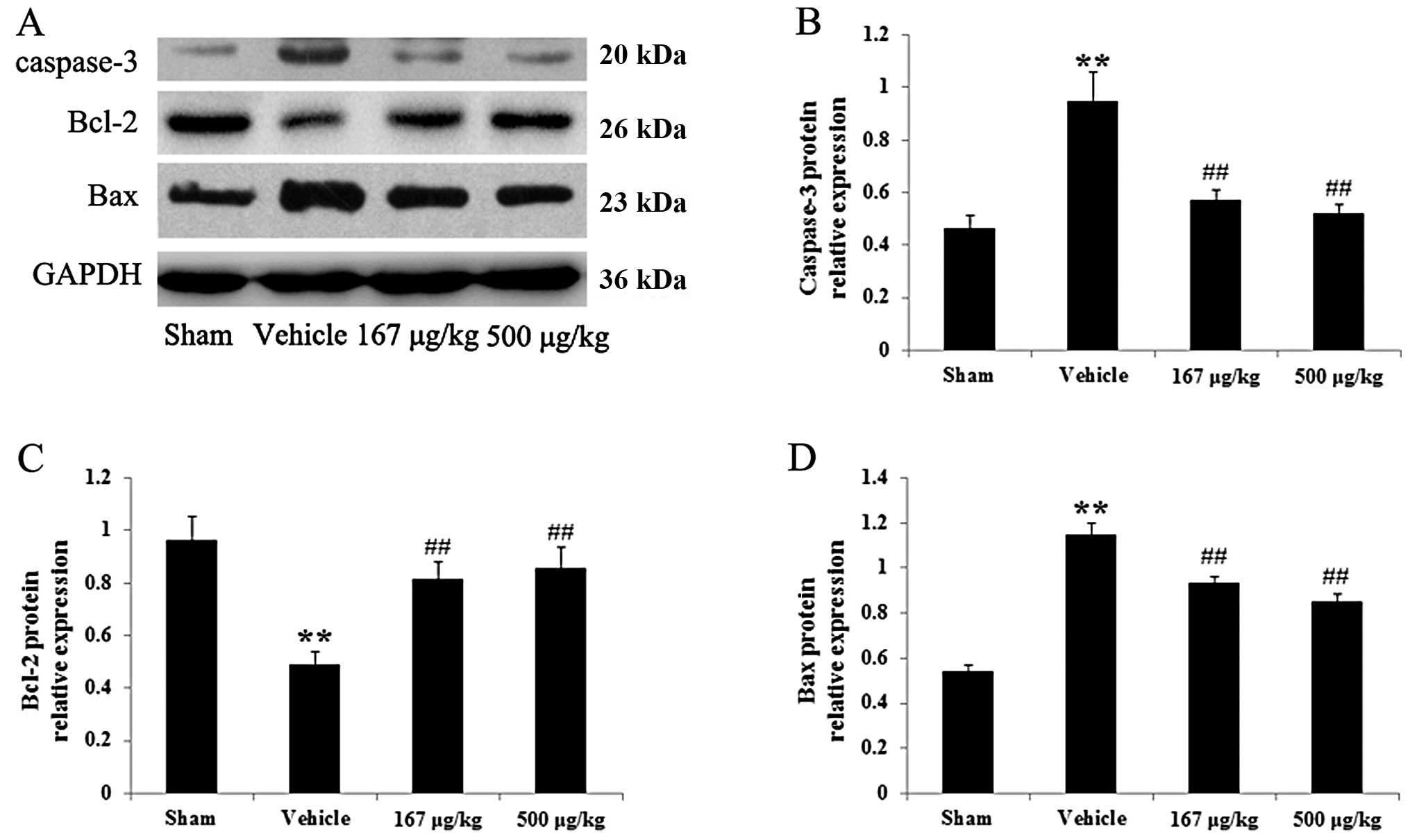 | Figure 4Effects of huperzine A (HupA) on the
protein expression of caspase-3, Bcl-2 and Bax in the hearts of
rats in the control group and group subjected to acute myocardial
infarction (means ± SD, n=6). (A) Representative images of
immunoblots with antibodies against caspase-3, Bcl-2 and Bax in the
hearts of rats from the different groups. Caspase-3, 20 kDa; Bcl-2,
26 kDa; Bax, 23 kDa; GAPDH, 36 kDa. (B–D) Quantitative analysis of
the protein levels of caspase-3, Bcl-2 and Bax in the hearts of
rats from the different groups. The data were normalized to the
loading control GAPDH. **P<0.01 vs. sham-operated
group; ##P<0.01 vs. vehicle-treated group. Sham,
sham-operated; vehicle, vehicle-treated; 167 μg/kg, HupA (167
μg/kg)-treated; 500 μg/kg, HupA (500 μg/kg)-treated. |
Effects of HupA on protein expression of
NF-κB p65, TNF-α and IL-1β in a rat model of acute myocardial
infarction
Table III shows
the effects of HupA on the protein levels of the inflammatory
response molecules, NF-κB p65, TNF-α and IL-1β, in a rat model of
acute myocardial infarction. It was found that the levels of NF-κB
p65 (P<0.01), TNF-α (P<0.01) and IL-1β (P<0.01) were
markedly increased in the vehicle-treated group with myocardial
infarction. However, treatment with HupA (167 or 500 μg/kg) induced
a reduction in NF-κB p65 (P<0.01), TNF-α (P<0.01) and IL-1β
(P<0.01) levels in comparison to the group subjected to
myocardial infarction.
 | Table IIIEffects of HupA on NF-κB p65, TNF-α
and IL-1β levels in the hearts of rats subjected to acute
myocardial infarction. |
Table III
Effects of HupA on NF-κB p65, TNF-α
and IL-1β levels in the hearts of rats subjected to acute
myocardial infarction.
| Group | NF-κB p65 | TNF-α | IL-1β |
|---|
| Sham-operated
group | 13.17±1.12 | 84.47±2.33 | 2.16±0.13 |
| Vehicle-treated
group | 45.32±1.02a | 410.92±8.56a | 6.25±0.27a |
| HupA (167 μg/kg) | 35.17±0.71b | 377.76±15.74b | 4.20±0.23b |
| HupA (500 μg/kg) | 25.98±0.88b | 254.73±7.29b | 3.54±0.26b |
Discussion
To our knowledge, the results of the present study
revealed for the first time that: i) HupA has the potential to
decrease the myocardial infarct size and the activities of CK,
CK-MB, LDH in serum, as well as those of cTnT in a rat model of
acute myocardial infarction; ii) HupA markedly inhibited lipid
peroxidation (MDA production), but increased the activities of
endogenous antioxidant enzymes (SOD and GSH), as well as those of
the non-enzymatic scavenger, GSH-PX, in a rat model of acute
myocardial infarction; iii) HupA treatment of rats with myocardial
infarction resulted in a marked decrease in caspase-3 and Bax
levels, and in an elevated Bcl-2 expression at the protein level;
iv) HupA inhibited the protein expression of the major inflammatory
factors, NF-κB p65, TNF-α and IL-1β, in the infarcted rat hearts.
These findings support the hypothesis that the cardioprotective
effects induced by HupA are associated with its antioxidant,
anti-apoptotic and anti-inflammatory properties.
HupA, which is isolated from numerous plants of the
Lamiaceae family, is a major phenolic monoterpene and has various
pharmacological actions. It has been widely used in the food
industry and clinical dentistry. The present study extended the
spectrum of the therapeutic effects of HupA and demonstrated its
potential as a potent cardioprotective agent in a rat model of
acute myocardial infarction.
In ischemic heart disease, the infarct size and the
activities of myocardial-specific enzymes, such as CK, CK-MB, LDH
and cTnT, are known as important parameters for evaluating cardiac
injury. Previous studies have demonstrated a prominent elevation of
infarct size and of the activities of myocardial-specific enzymes,
including CK, CK-MB and LDH, in rats with acute myocardial
infarction (5,16). The activity of serum cTnT is a
very specific and sensitive indicator of myocardial infarction.
cTnT is a contractile protein that is rarely found in serum but is
abundantly released when myocardial necrosis occurs (17). Consistent with previous reports,
the present study illustrated that the infarct size and the
activities of CK, CK-MB, LDH and cTnT significantly increased in
rats subjected to myocardial infarction. Moreover, the activities
of these enzymes were significantly reduced following treatment
with HupA, demonstrating the cardioprotective effects of HupA.
It is well established that the excessive generation
of oxygen free radicals is a pivotal factor that exacerbates
cellular damage during ischemic attack. Under normal conditions,
the generation of oxygen free radicals is kept under homeostatic
control by endogenous antioxidant enzymes, such as SOD and GSH-PX,
and by low-molecular weight antioxidants. The first line of
cellular defense against oxidative injury may be the anti-oxidative
system. A number of drugs based on antioxidants and radical
scavengers has been shown to contribute to a favorable outcome in
the therapy of ischemic diseases (18,19). Furthermore, the liberation of
lipid aldehydes and peroxides is facilitated by cellular membrane
damage. MDA is synthesized during the oxidation of fatty acids
within myocardial membranes and causes a ripple effect of lipid
peroxidation. Therefore, MDA may serve as an indicator of
structural oxidative injury of cell membranes. The present study
demonstrated that HupA induced a significant reduction in MDA, as
well as in SOD, GSH and GSH-PX activities in the infarcted rat
hearts. This result indicated that the cardioprotective effects of
HupA against acute myocardial infarction are associated with its
antioxidant properties.
The cardiac impairment of ischemic patients occurs
via oxidative stress and could lead to mitochondrial dysfunction
and hence, activate an apoptotic cascade. To further explore the
protective effects of HupA against cardiac injury induced by acute
myocardial infarction in rats, we measured the levels of
apoptosis-related proteins in the rat hearts. Caspases are
evolutionarily conserved cysteinyl proteases with a central role in
the apoptotic signaling pathway; among them, caspase-3 is a
critical molecule in the caspase-dependent apoptotic cascade.
Caspase-3 has previously been reported to activate a variety of
substrates that presumably cause DNA fragmentation and cell death
(20). An increasing number of
studies has revealed the upregulation of caspase-3 following acute
myocardial infarction (5,15). The present study demonstrated that
treatment with HupA markedly reduced caspase-3 activity in the
infarcted rat hearts. In agreement with our findings, Wang and Tang
(9) also reported that HupA
significantly decreased the expression level of caspase-3 in
Alzheimer’s disease. In addition to caspases, proteins of the Bcl-2
family have been shown to be involved in the modulation of
ischemia-induced apoptosis in rat myocytes. Bcl-2 itself acts as a
repressor of apoptosis, while another member of the family, Bax,
functions as a promoter of cell death (6). It has previously been reported that
myocardial infarction induces a marked reduction in the expression
of the anti-apoptotic protein, Bcl-2, and an increase in the
expression of the apoptotic-promoting molecule, Bax (21,22). In agreement with previous studies,
our findings demonstrated a significant downregulation of Bcl-2 and
an upregulation of Bax in rats subjected to acute myocardial
infarction. However, the alterations in the levels of Bcl-2 and Bax
caused by ischemic damage were prevented by the administration of
HupA. In addition, our results demonstrated a reduction in
caspase-3 activity in the rats subjected to myocardial infarction
treated with HupA, suggesting that the cadioprotective effects of
HupA are associated with its anti-apoptotic properties in acute
myocardial infarction in rats.
Previous studies have suggested that the
inflammatory response participates in the pathogenesis of ischemic
heart diseases, and NF-κB is regarded as an important factor in
this respect (23,24). The activation of NF-κB leads to
the release of pro-inflammatory cytokines, such as TNF-α and IL-1β.
The inhibition of the NF-κB pathway has previously been reported to
improve adverse left ventricular remodeling and cardiac dysfunction
following myocardial infarction (25). Our study revealed that HupA
inhibited the excessive activation of NF-κB p65 and reduced the
release of TNF-α and IL-1β in rats subjected to myocardial
infarction. Consistently, Ruan et al (14) found that HupA exerted
anti-inflammatory effects against D-galactose-treated rats. In
addition, a previous study illustrated that HupA inhibited the
nuclear translocation of NF-κB and decreased the expression of
pro-inflammatory factors following transient focal cerebral
ischemia (26). Taken together,
these findings suggest that HupA exerts cardioprotective effects
against acute myocardial infarction through anti-inflammatory
mechanisms.
In conclusion, the present study demonstrates that
HupA attenuates the damage induced by acute myocardial infarction.
The cardioprotective effects of HupA may be associated with its
antioxidan, anti-apoptotic and anti-inflammatory properties. To our
knowledge, our study presents the first evidence of the potential
cardioprotective profile and related mechanisms of action of HupA.
Our results also support the hypothesis that HupA may be a
promising cardioprotective agent for the treatment of acute
myocardial infarction. However, more detailed studies are required
to fully elucidate its effects and mechanisms of action.
References
|
1
|
Yang B, Lin H, Xiao J, et al: The
muscle-specific microRNA miR-1 regulates cardiac arrhythmogenic
potential by targeting GJA1 and KCNJ2. Nat Med. 13:486–491. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chan PH: Mitochondria and neuronal
death/survival signaling pathways in cerebral ischemia. Neurochem
Res. 29:1943–1949. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Priscilla DH and Prince PS:
Cardioprotective effect of gallic acid on cardiac troponin-T,
cardiac marker enzymes, lipid peroxidation products and
antioxidants in experimentally induced myocardial infarction in
Wistar rats. Chem Biol Interact. 179:118–124. 2009. View Article : Google Scholar
|
|
4
|
Tanaka M, Mokhtari GK, Terry RD, et al:
Overexpression of human copper/zinc superoxide dismutase (SOD1)
suppresses ischemia-reperfusion injury and subsequent development
of graft coronary artery disease in murine cardiac grafts.
Circulation. 110:II200–II206. 2004. View Article : Google Scholar
|
|
5
|
Guo J, Li HZ, Wang LC, et al: Increased
expression of calcium-sensing receptors in atherosclerosis confers
hypersensitivity to acute myocardial infarction in rats. Mol Cell
Biochem. 366:345–354. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mao X, Ji C, Sun C, et al: Topiramate
attenuates cerebral ischemia/reperfusion injury in gerbils via
activating GABAergic signaling and inhibiting astrogliosis.
Neurochem Int. 60:39–46. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Muller DN, Mervaala EM, Dechend R, et al:
Angiotensin II (AT(1)) receptor blockade reduces vascular tissue
factor in angiotensin II-induced cardiac vasculopathy. Am J Pathol.
157:111–122. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Speir E: Cytomegalovirus gene regulation
by reactive oxygen species. Agents in atherosclerosis. Ann NY Acad
Sci. 899:363–374. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang R and Tang XC: Neuroprotective
effects of huperzine A. A natural cholinesterase inhibitor for the
treatment of Alzheimer’s disease. Neurosignals. 14:71–82. 2005.
|
|
10
|
Pollak Y, Gilboa A, Ben-Menachem O,
Ben-Hur T, Soreq H and Yirmiya R: Acetylcholinesterase inhibitors
reduce brain and blood interleukin-1β production. Ann Neurol.
57:741–745. 2005.
|
|
11
|
Tabet N: Acetylcholinesterase inhibitors
for Alzheimer’s disease: anti-inflammatories in acetylcholine
clothing! Age Ageing. 35:336–338. 2006.
|
|
12
|
Nizri E, Hamra-Amitay Y, Sicsic C, Lavon I
and Brenner T: Anti-inflammatory properties of cholinergic
up-regulation: A new role for acetylcholinesterase inhibitors.
Neuropharmacology. 50:540–547. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brenner T, Nizri E, Irony-Tur-Sinai M,
Hamra-Amitay Y and Wirguin I: Acetylcholinesterase inhibitors and
cholinergic modulation in Myasthenia Gravis and neuroinflammation.
J Neuroimmunol. 201–202:121–127. 2008.PubMed/NCBI
|
|
14
|
Ruan Q, Liu F, Gao Z, et al: The
anti-inflamm-aging and hepatoprotective effects of huperzine A in
D-galactose-treated rats. Mech Ageing Dev. 134:89–97. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hong-Li S, Lei L, Lei S, et al:
Cardioprotective effects and underlying mechanisms of oxymatrine
against ischemic myocardial injuries of rats. Phytother Res.
22:985–989. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ming X, Tongshen W, Delin W and Ronghua Z:
Cardioprotective effect of the compound yangshen granule in rat
models with acute myocardial infarction. Evid Based Complement
Alternat Med. 2012:7171232012.PubMed/NCBI
|
|
17
|
Katus HA, Remppis A, Scheffold T,
Diederich KW and Kuebler W: Intracellular compartmentation of
cardiac troponin T and its release kinetics in patients with
reperfused and nonreperfused myocardial infarction. Am J Cardiol.
67:1360–1367. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamada J, Yoshimura S, Yamakawa H, et al:
Cell permeable ROS scavengers, Tiron and Tempol, rescue PC12 cell
death caused by pyrogallol or hypoxia/reoxygenation. Neurosci Res.
45:1–8. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Y, Bao Y, Jiang B, et al: Catalpol
protects primary cultured astrocytes from in vitro ischemia-induced
damage. Int J Dev Neurosci. 26:309–317. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Manabat C, Han BH, Wendland M, et al:
Reperfusion differentially induces caspase-3 activation in ischemic
core and penumbra after stroke in immature brain. Stroke.
34:207–213. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu X, Gu J, Fan Y, Shi H and Jiang M:
Baicalin attenuates acute myocardial infarction of rats via
mediating the mitogen-activated protein kinase pathway. Biol Pharm
Bull. 36:988–994. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu W, Liu Q and Zhu S: Carvacrol protects
against acute myocardial infarction of rats via anti-oxidative and
anti-apoptotic pathways. Biol Pharm Bull. 36:579–584. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Frantz S, Fraccarollo D, Wagner H, et al:
Sustained activation of nuclear factor kappa B and activator
protein 1 in chronic heart failure. Cardiovasc Res. 57:749–756.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wong SC, Fukuchi M, Melnyk P, Rodger I and
Giaid A: Induction of cyclooxygenase-2 and activation of nuclear
factor-kappaB in myocardium of patients with congestive heart
failure. Circulation. 98:100–103. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yoshiyama M, Omura T, Takeuchi K, et al:
Angiotensin blockade inhibits increased JNKs, AP-1 and NF-kappa B
DNA-binding activities in myocardial infarcted rats. J Mol Cell
Cardiol. 33:799–810. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang ZF, Wang J, Zhang HY and Tang XC:
Huperzine A exhibits anti-inflammatory and neuroprotective effects
in a rat model of transient focal cerebral ischemia. J Neurochem.
106:1594–1603. 2008. View Article : Google Scholar : PubMed/NCBI
|