Introduction
The global incidence of non-alcoholic fatty liver
disease (NAFLD) continues to increase, and NAFLD is now recognized
as a hepatic component of metabolic syndrome (1,2).
In this regard, its occurrence is strongly associated with obesity,
insulin resistance, hypertension and dyslipidemia (3). NAFLD covers a spectrum of hepatic
pathologies, ranging from simple steatosis (fatty liver) to
non-alcoholic steatohepatitis (4).
Metformin was introduced into clinical practice in
the 1950s and is widely used as a first-line treatment for patients
with type 2 diabetes (5). The effectiveness of metformin as an
antidiabetic drug is explained by its ability to lower blood
glucose levels by decreasing hepatic gluconeogenesis, stimulating
glucose uptake into muscles, and increasing fatty acid oxidation in
adipose tissue (6). It was
previously demonstrated that some of the beneficial effects of this
drug are related to an insulin-sensitizing effect through the
activation of AMP-activated protein kinase (AMPK) (7). It is now accepted that the
mitochondrial respiratory chain I is the primary target of
metformin (8,9). Recent studies have reported that
metformin is capable of ameliorating NAFLD (10); however, its exact mechanisms of
action remain to be elucidated.
Hepatic steatosis refers to an excess accumulation
of lipids, primarily triglycerides (TG), which is the hallmark of
NAFLD. Lipid droplets (LDs) are spherical organelles composed of a
core of neutral lipids covered by a monolayer of phospholipids and
specific proteins. The excess accumulation of LDs in non-adipose
tissues, such as the liver, coronary arteries or pancreatic islets,
is often associated with fatty liver, coronary atherosclerotic
heart disease and type 2 diabetes, respectively (11,12). Previous studies have identified
LD-associated proteins that are involved in lipid metabolism and
transport, intracellular trafficking, signaling and cytoskeletal
organization (12–14). The most abundant and most well
characterized family of LD-associated proteins is the PAT family,
which is characterized by sequence similarity and its localization
on LDs (15,16). The PAT family includes the
following proteins: perilipin (PLIN)1, PLIN2 [also known as adipose
differentiation related protein (ADRP)], PLIN3 [also known as
tail-interacting protein 47 (TIP47)], PLIN4 (S3-12) and PLIN5 [also
known as lipid storage droplet protein 5 (LSDP5) or OXPAT or
MLDP].
It is well known that ADRP is the major
LD-associated protein expressed in almost all cells, and it is
widely used as a marker of LD. Previous in vitro studies
have demonstrated that ADRP is involved in LD formation by
enhancing the uptake of free fatty acids (FFA), thereby stabilizing
LD particles (17,18). It has been demonstrated that the
absence of ADRP expression reduces LD formation and protects
against the development of fatty liver (19). TIP47 expression is present in the
same cell types as ADRP (20) and
can functionally compensate for it (21). However, the role of ADRP in liver
diseases remains unknown.
In this study, in order to gain a better
understanding of the role of ADRP in the alleviation of hepatic
steatosis by metformin, we examined the effects of metformin in
ob/ob mice and primary hepatocytes. Our results revealed
that metformin prevented the development of hepatic steatosis by
downregulating ADRP expression. These results suggest that ADRP may
be a target of metformin in the treatment of NAFLD, which provides
direct evidence of the mechanisms through which metformin inhibits
hepatic lipid accumulation.
Materials and methods
Chemicals
Metformin (1,1-dimethylbiguanide hydrochloride) and
the peroxisome proliferator-activated receptor (PPAR)α antagonist,
GW6471, were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Anti-PPARα antibody was obtained from Cell Signaling Technology
(Beverly, MA, USA), and the anti-α-tubulin antibody was purchased
from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). The
Cy3-conjugated anti-mouse IgG secondary antibody was purchased from
Invitrogen (Carlsbad, CA, USA). Collagenase type II was obtained
from Sigma-Aldrich. Fatty acid-free bovine serum albumin (BSA) was
purchased from Calbiochem (La Jolla, CA, USA). BODIPY 493/503 was
also purchased from Invitrogen.
Animal husbandry
Adult (aged 8–10 weeks) male ob/ob mice were
generated by mating heterozygous leptin-deficient mice
(ob/+) in a C57BL/6 background. Mice were housed in
individual cages in a temperature-controlled environment with a
12-h light/dark cycle and were fed a standard laboratory chow diet.
In this study, wild-type or ob/ob mice were randomly divided
into 4 treatment groups as follows: group I (n=8), C57BL/6 mice
were gavaged with distilled water; group II: C57BL/6 mice (n=8)
were gavaged with metformin (75 mg/kg/day); group III: ob/ob
mice (n=8) were gavaged with distilled water; and group IV:
ob/ob mice (n=8) were gavaged with metformin (75 mg/kg/day).
All mice were weighed at the beginning of the feeding period and
weekly thereafter until the end of the experimental period, at
which time tissues were collected for further analysis. All animal
experiments were performed in accordance with the Guide for the
Care and Use of Laboratory Animals of the National Institutes of
Health and approved by the Ethics Committee of the Fourth Military
University, Xi’an, China (Permit no: SCXK2007-007). All surgical
procedures were performed under sodium pentobarbital anesthesia. We
ensured that the animals did not suffer unnecessarily at any stage
of this experiment.
Isolation and culture of primary
hepatocytes
Hepatocytes were isolated from male C57BL/6 mice by
in situ digestion of the liver with perfusion of collagenase
type II, as previously described (33). Following perfusion, the livers
were immediately moved to a sterile 10 cm dish for mincing, before
the hepatocytes were dispersed by aspiration with a large-bore
pipette. The hepatocytes were then filtered through a 70-μm cell
strainer (Millipore, Billerica, MA, USA) to remove tissue debris.
After washing twice with cold DMEM and centrifuging at 50 × g for 3
min at 4ºC, an aliquot of freshly isolated hepatocytes was placed
in a hemocytometer and stained with trypan blue to evaluate cell
viability and determine the number of cells. Following isolation,
1×107 cells were plated in 6-cm dishes and incubated at
37ºC in DMEM with 10% FBS, 10 μg·ml−1 penicillin and 100
μg·ml−1 streptomycin for 12–16 h. Prior to treatment
with metformin, fresh DMEM was added to each dish; 1 h after the
addition of metformin, the cells were treated with 200 μM oleate
for 18 h. Duplicate dishes were used for all experiments. Metformin
was used at a final concentration of 500 μM. A control solution
containing ethanol and BSA was similarly prepared and
administered.
Generation and infection of recombinant
adenovirus
The recombinant adenovirus expressing full-length
ADRP (Ad-ADRP) was constructed using the AdEasy-1 System
(Stratagene, La Jolla, CA, USA). The adenovirus carrying green
fluorescent protein (GFP) (Benyuan Zhengyang Gene Technology
Company Ltd., Beijing, China) was used as the control. We generated
recombinant adenoviruses for the knockdown of ADRP (si-ADRP) and
si-scramble as the control. Primary hepatocytes were infected with
Ad-ADRP and Ad-GFP (control virus) or si-ADRP and si-scramble
luciferase (si-control virus) in the presence or absence of
metfromin, using Lipofectamine 2000 (Invitrogen) according to the
manufacturer’s instructions. After 48 h, the cells were rinsed,
collected and total RNA or protein was then extracted.
Oil Red O staining
To detect fat accumulation in the liver and in
primary hepatocytes, staining with Oil Red O was performed
according to a previously described method (33). Briefly, liver cryosections and
hepatocytes were fixed in 4% paraformaldehyde at room temperature
for 30 min, and then dipped in 60% isopropanol for 3 min. After
washing, the slides were immersed in freshly prepared 1% Oil Red O
working solution for 15 min, and then washed in 60% isopropanol
followed by distilled water, and finally mounted using aqueous
mounting medium.
Neutral lipid staining
The hepatocytes were seeded in a 12-well plate and
treated with 500 μM metformin and 200 μM oleate for 18 h. After
being washed twice with phosphate-buffered saline (PBS), the cells
were fixed with 4% paraformaldehyde for 30 min at room temperature.
BODIPY 493/503 (1 μg·ml−1) was used to stain the neutral
lipid in the cells, and the nucleus was stained with Hoechst 33258
(10 μg·ml−1).
Western blot analysis
Protein of the primary hepatocytes and liver was
harvested using cold RIPA buffer (1% NP-40, 50 mM Tris-HCl, 0.1%
SDS, 1% sodium deoxycholate, 150 mM NaCl, pH 7.4), containing
leupeptin (2 μg·ml−1) and sodium orthovanadate (2
μg·ml−1). All mixtures were then centrifuged at 12,000 ×
g at 4ºC for 10 min, and the protein content of the supernatant was
determined using a commercially available kit (Bio-Rad
Laboratories, Hercules, CA,USA), using BSA as the standard.
Equal amounts of protein samples were resolved by
SDS-PAGE, and then transferred onto PVDF membranes (Millipore). The
membranes were immunoblotted with antibodies against PLIN (Abcam,
Cambridge, UK), ADRP, (Abcam) and α-tubulin (Santa Cruz
Biotechnology, Inc.). Antibodies generated in our own laboratory
were used for LSDP5 (monoclonal antibody) and TIP47 (polyclonal
antibody).
Real-time PCR
Total RNA was extracted from the liver samples using
TRIzol reagent (Invitrogen) and cDNA produced using a reverse
transcription kit (Takara, Dalian, China). Quantitative analysis of
the mRNA levels was performed by real-time PCR using a Master Mix
SYBR-Green kit (Takara), according to the manufacturer’s
instructions. The primer sequences for all genes of interest are
listed in Table I. GAPDH was used
as a reference gene in all experiments. Relative abundance of RNA
was calculated from the cycle threshold (Ct) using the
2−ΔΔCt method and expressed in arbitrary units.
 | Table ISequences of primers used for
real-time PCR. |
Table I
Sequences of primers used for
real-time PCR.
| Gene | Sequence
(5′→3′) |
|---|
| GAPDH | F:
AACCCCTTCATTGACCTCAACTAC
R: ATTTGATGTTAGTGGGGTCTCGCT |
| ADRP | F:
GATTGAATTCGCCAGGAAGA
R: TGGCATGTAGTCTGGAGCTG |
| TIP47 | F:
GTGTGGGACAGATGGTGAT
R: AAGTAGTTCTGCTCCTGTCG |
Blood sampling and biochemical
assays
Blood samples were obtained after 4 weeks of
treatment with metformin and stored at 4ºC until use in biochemical
analysis. Serum total cholesterol, TG and blood glucose, as well as
high-density lipoprotein (HDL) and low-density lipoprotein (LDL)
cholesterol levels were measured using automated techniques within
the Department of Clinical Chemistry, the Fourth Military Medical
University.
Lipid extraction and thin layer
chromatography (TLC)
To measure the total TG levels, lipids were
extracted from the tissues and cells using the Folch method, as
previously described (30). In
brief, the dried lipids were reconstituted in chloroform/methanol
(2:1, v/v) and loaded onto a TLC plate (Sigma-Aldrich). The lipids
were separated in a hexane/diethyl ether/acetic acid (70:30:1,
v/v/v) solution. The TLC plates were sprayed with 10%
CuSO4 in 10% phosphoric acid and were developed by
drying in a 120ºC oven. The protein concentration was determined
using the Bio-Rad Protein Assay (Bio-Rad #500-0001) and the amount
of TG was quantified using Bio-Rad Quantity One Software (Bio-Rad
Laboratories).
Statistical analysis
All data are expressed as the means ± standard error
of the mean (SEM), and analyzed using a paired sample two-sided
Student’s t-test for paired samples or one-way ANOVA and Dunnett’s
post-test using SPSS version 13.0 (SPSS Inc., Chicago, IL, USA). A
value of P<0.05 was considered to indicate a statistically
significant difference.
Results
Metformin prevents the development of
fatty liver in ob/ob mice and inhibits lipid accumulation in
primary hepatocytes
To investigate the potential role of metformin in
the prevention of hepatic steatosis, we used ob/ob mice
presenting with hepatomegaly, as previously described (22). After 4 weeks of metformin
treatment (75 mg/kg/day), the body weight, serum TG, LDL
cholesterol and glucose levels in the ob/ob mice decreased
significantly; however, there was no change in the total
cholesterol and HDL cholesterol levels, compared with the control
groups (Table II). Hematoxylin
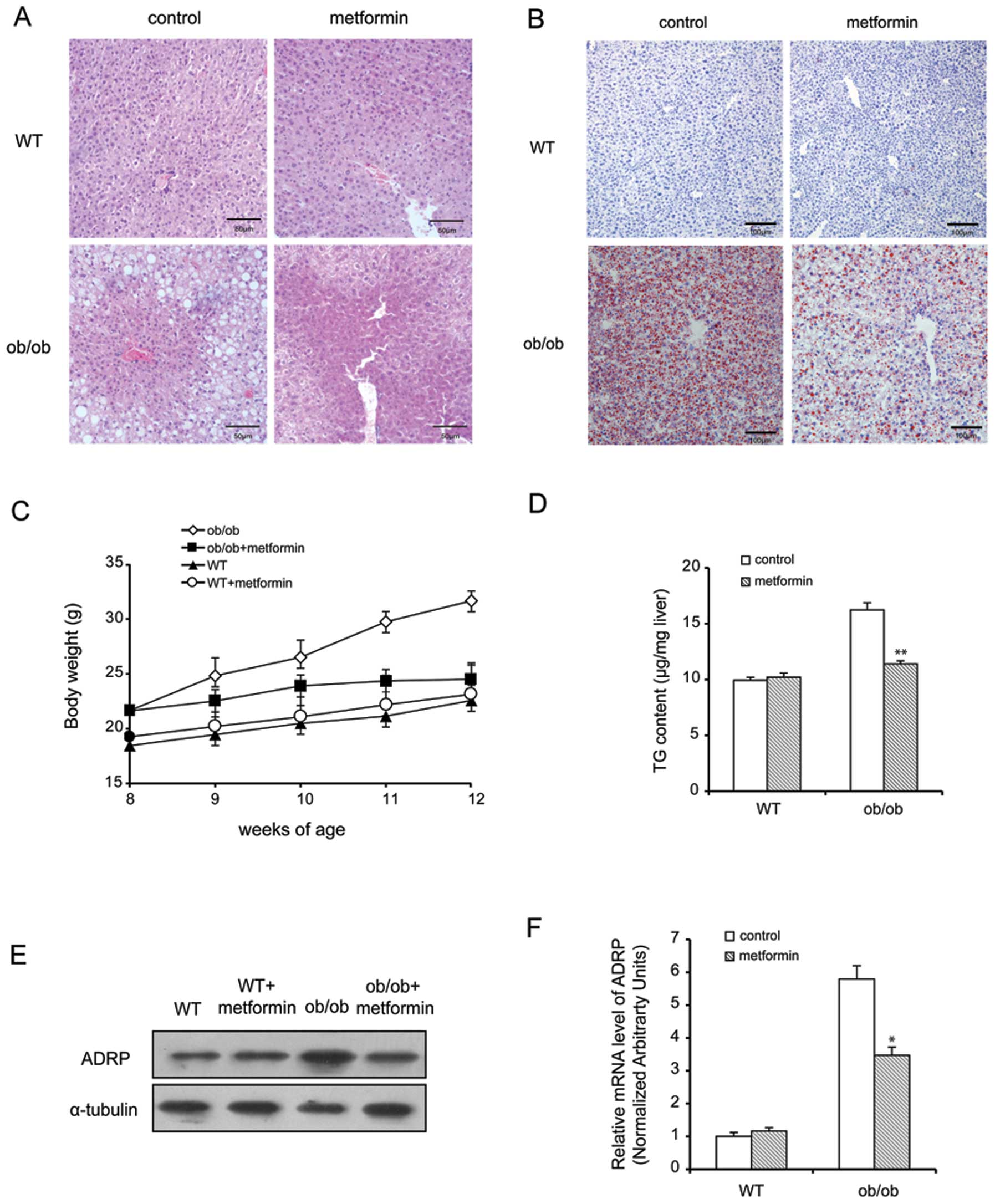
and eosin (H&E) and Oil Red O staining demonstrated that
treatment with metformin reduced the number and size of LDs in the
hepatocytes treated with oleate, and ameliorated fatty liver in the
ob/ob mice (Fig. 1A and
B). Of note, the metformin-treated ob/ob mice failed to
gain additional weight at the beginning or end of the experimental
period, although both groups of C57BL/6 (wild-type) mice gained
weight (Fig. 1C). Lipid
extraction and TLC revealed that the levels of hepatic TG
significantly decreased in the metformin-treated mice (Fig. 1D), supporting our histological
results. To investigate the potential molecular mechanisms
underlying the inhibitory effects of metformin on the development
of liver steatosis, we first explored the effects of metformin on
hepatic ADRP expression by western blot analysis and real-time PCR.
As illustrated in Fig. 1E and F,
following treatment with oleate, the mRNA and protein levels of
hepatic ADRP were increased by almost 6-fold; however, treatment
with metformin significantly reduced the levels of ADRP by 43%
compared with the control group (P<0.05).
 | Table IIEffects of metformin on serum
biochemistry (mM). |
Table II
Effects of metformin on serum
biochemistry (mM).
| Biochemical markers
(mM) | Group I | Group II | Group III | Group IV |
|---|
| Glucose | 6.76±0.75 | 6.51±0.6 |
11.11±1.54b |
5.99±0.13d |
| Triglycerides | 0.55±0.05 | 0.57±0.06 |
0.88±0.08b |
0.6±0.11d |
| Cholesterol | 1.62±0.07 | 1.67±0.14 |
2.12±0.27b | 2.08±0.34 |
| HDL | 1.41±0.43 | 1.33±0.34 |
2.03±0.28a | 2.41±0.54 |
| LDL | 0.25±0.06 | 0.26±0.04 |
0.33±0.07a |
0.29±0.04c |
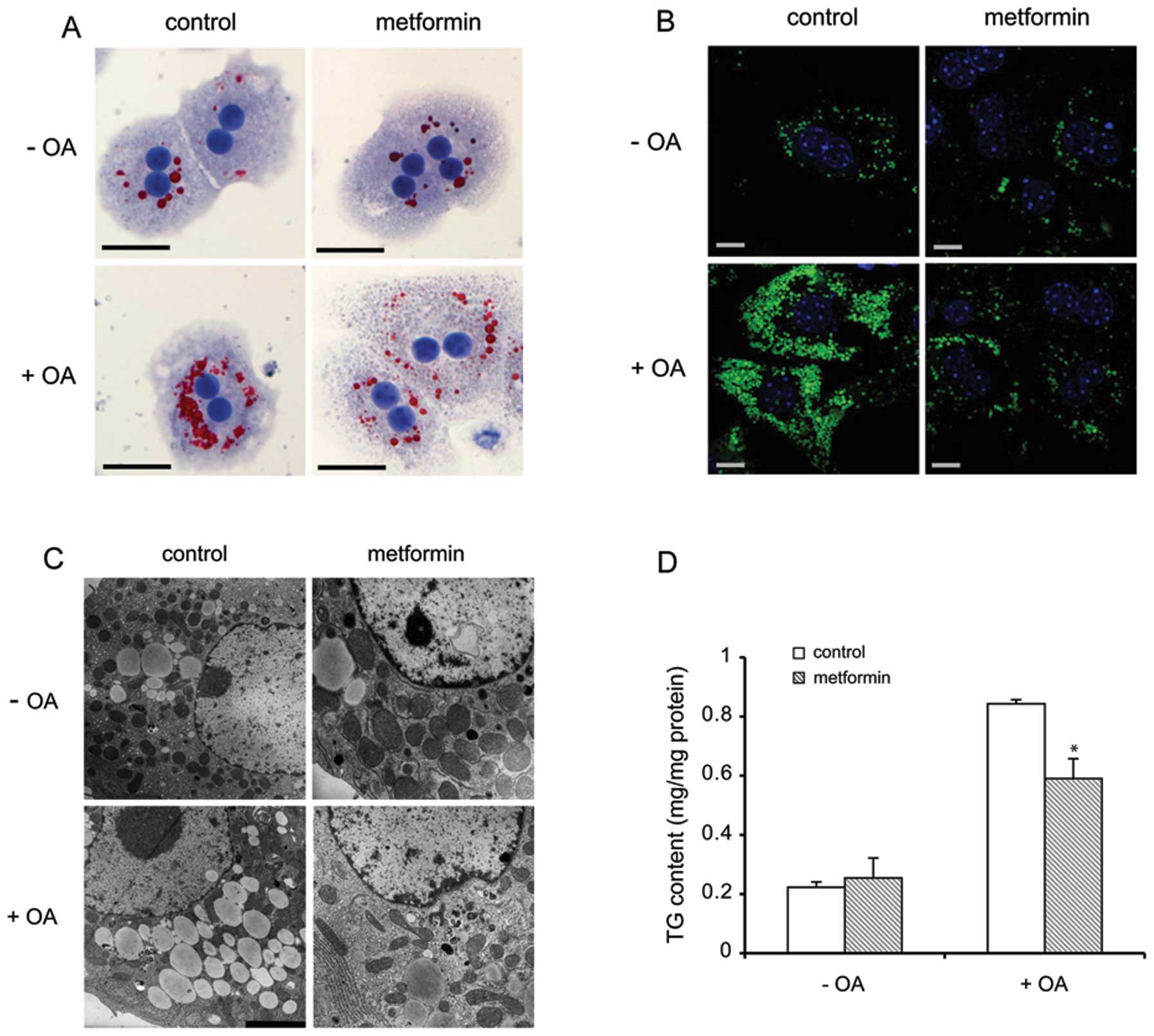
To further evaluate the physiological role of
metformin in the prevention of hepatic steatosis, we employed
primary hepatocytes to confirm our in vivo results using an
in vitro model. Oil Red O, BODIPY 493/503 staining and
electron microscopic analysis revealed that lipid loading
significantly decreased in the metformin-treated hepatocytes,
compared with the control cells (Fig.
2A–C). Furthermore, metformin markedly reduced the TG content
by 33% in the primary hepatocytes, as analyzed by TLC (Fig. 2D).
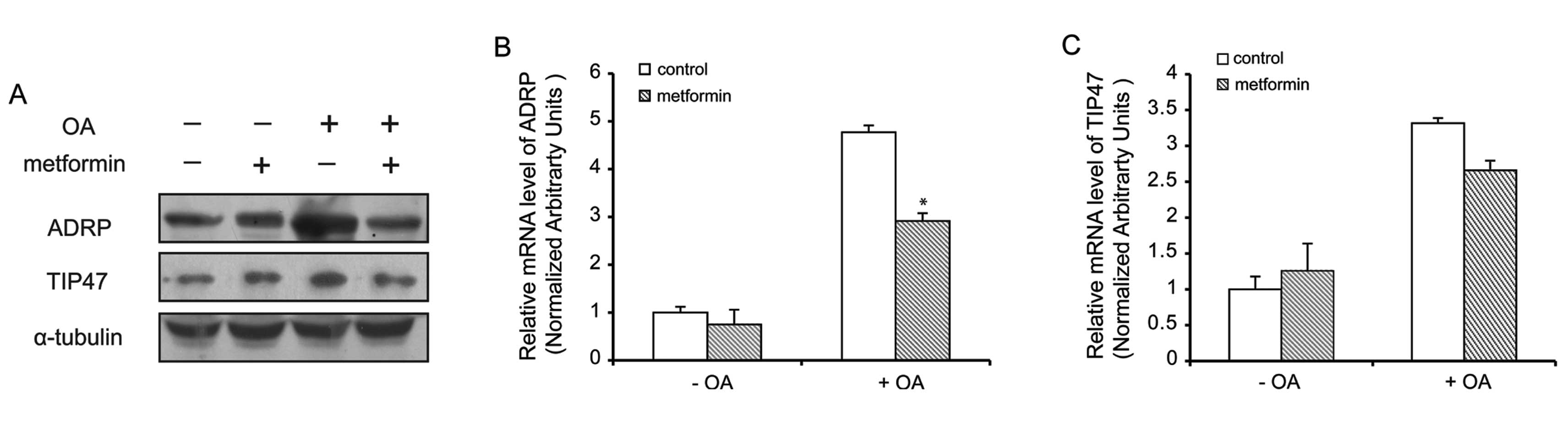
Metformin suppresses the expression of
ADRP
The function of LDs is regulated by their associated
proteins. To confirm whether the effects of metformin are a result
of a decrease in the expression of PAT family proteins, we examined
PAT family protein levels in hepatocytes treated with metformin.
Our western blot analysis data demonstrated that ADRP and TIP47
protein levels were markedly decreased in the oleate-treated
hepatocytes pre-treated with metformin, compared with the control
cells (Fig. 3A). However,
metformin did not inhibit the expression of PLIN, S3-12 and LSDP5
(data not shown). As expected, and as shown in Fig. 3B, metformin decreased the mRNA
level of ADRP by almost 40%, but failed to decrease the expression
of TIP47 (Fig. 3C) and other PAT
proteins (data not shown).
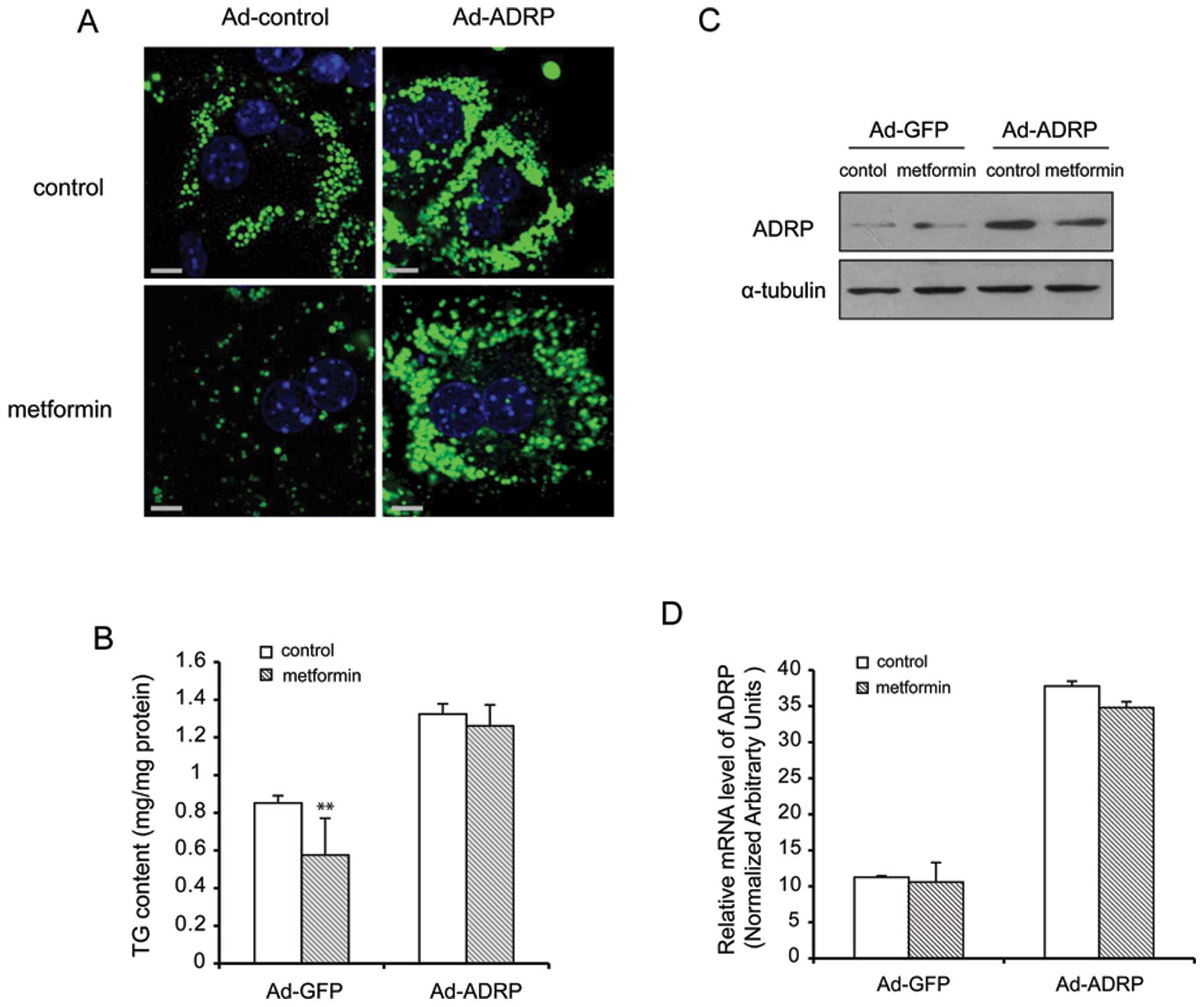
Induction of ADRP overexpression by
Ad-ADRP diminishes the reducing effects of metformin on TG
accumulation
To determine whether the induction of ADRP
overexpression plays an indispensable role in the
metformin-mediated storage of TG, primary hepatocytes were infected
with adenovirus expressing either ADRP or GFP. As shown in Fig. 4A, LDs were stained using BODIPY.
BODIPY fluorescence increased in the cells infected with Ad-ADRP;
however, the fluorescence signal did not decrease in the presence
of metformin. As shown in Fig.
2B, the overexpression of ADRP increased the cellular TG levels
by 40% compared with the Ad-GFP-infected (control adenovirus)
hepatocytes. However, following treatment with metformin, the
amount of TG did not decrease to the levels of the control. The
expression of ADRP was measured by western blot analysis and
real-time PCR (Fig. 4C and D).
The results revealed that after the cells were infected with Ad-
ADRP, the expression of ADRP increased by almost 3.7-fold.
Metformin-mediated lipid accumulation is
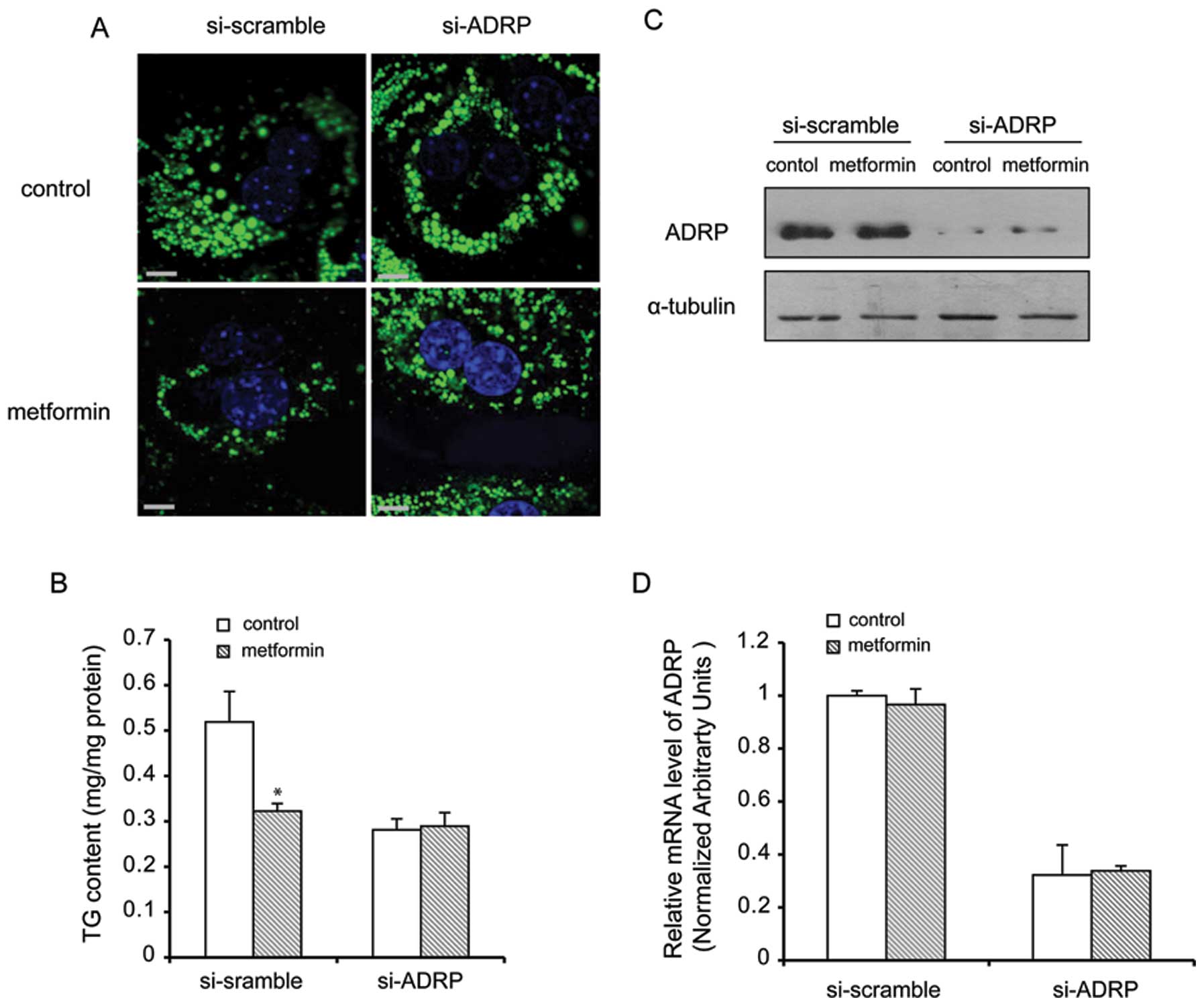
enhanced by the absence of ADRP in primary hepatocytes
To further deterime the effects of metformin on
lipid metabolism, we used an adenovirus-mediated gene silencing
approach. ADRP mRNA was successfully reduced below 70% by si-ADRP
compared with si-scramble (control). Following pre-treatment with
si-ADRP and treatment with metformin, the relative intracellular
lipid content in each group was determined. The results (Fig. 5A) revealed that BODIPY
fluorescence in the cells infected with si-ADRP was less than that
in the cells infected with si-scramble (control) under lipid
loading conditions, suggesting that si-ADRP prevented lipid
accumulation with or without metformin treatment. Pre-treatment
with si-ADRP markedly reduced the TG content in the cells by 37%
when compared with the control group (P<0.05) (Fig. 5B). Western blot analysis of the
cells treated with metformin and pre-treated with si-ADRP revealed
the reduced protein and mRNA expression of ADRP (Fig. 5C and D).
Collectively, the data from overexpression and
silencing experiments indicated that ADRP may repesent a key and
direct cellular target in the metformin treatment of hepatic
steatosis.
Discussion
It has previously been reported that metformin, a
commonly used drug for the treatment of diabetes, is capable of
ameliorating NAFLD (23,24); however, its exact mechanisms of
action remain to be elucidated. The hypothesis that respiratory
chain complex I, but not AMPK, is the primary target of metformin
was recently strengthened by a study demonstrating that the
metabolic effects of this drug are preserved in liver-specific
AMPK-deficient mice (25),
although the implications of this result with respect to NAFLD
remain to be elucidated. In this study, we explored the
physiological functions of metformin in the improvement of hepatic
steatosis and provide a direct mechanistic understanding of the
mechanisms through which metformin alleviates hepatic lipid
accumulation. In agreement with previous studies, we demonstrated
that metformin prevented lipid accumulation and hepatic steatosis
in ob/ob mice, and prevented lipid accumulation in isolated
primary hepatocytes. Furthermore, we observed that the number and
size of LDs and the TG content markedly decreased following
treatment with metformin in vivo and in vitro.
Although an increasing number of studies have
indicated that metformin activates AMPK, the direct role of
metformin in the regulation of hepatic lipid accumulation remains
to be clarified. PAT family proteins are major regulators of
various aspects of lipid metabolism, including the promotion of
lipid storage (26) and the
recruitment of lipases or co-lipases (19,27). ADRP is the major LD protein in all
cells that accumulate lipids either normally or abnormally. ADRP is
considered a reliable and sensitive marker for LDs in steatosis,
and it promotes the incorporation of lipids in LDs at the cost of
reduced lipid export via lipoprotein secretion or reverse transport
(20); by contrast, TIP47
preferentially labels nascent LDs (28) and binds to the LDs in response to
lipid loading (19). ADRP binding
to LDs is ubiquitous in non-adipose LD-containing cells (20,29), and plays important roles in LD
formation and stabilization, as well as lipolysis (30). In the liver, ADRP is upregulated
in association with drug- and diet-induced hepatic steatogenesis
(28,31). Reduced ADRP expression either in
ADRP-deficient mice (19), or in
mice administered ADRP antisense oligonucleotides while on a high
fat diet (32), attenuates
hepatic steatosis. To elucidate the mechanisms of action of
metformin in the improvement of hepatic steatosis, our study
provides novel insight into the association between metformin and
PAT family proteins. Our data demonstrated that the expression of
ADRP was predominant out of the PAT family proteins, and was
markedly decreased following treatment with metformin in the livers
of mice and primary hepatocytes. Thus, we focused on ADRP, which
seems to play the most singificant role in hepatic steatosis, and
the effects of metformin on its expression.
Our study provides evidence that ADRP plays a
crucial role in the prevention of hepatic steatosis by metformin.
ADRP expression was increased in hepatocytes treated with oleate
and decreased following treatment with metformin. Transfection with
Ad-ADRP increased the storage of TG and si-ADRP prevented lipid
accumulation. Of note, the reducing effects of metformin on TG
accumulation were diminished in the hepatocytes infected with
Ad-ADRP. Most importantly, to our knowledge, we identified for the
first time that metformin treatment decreases the expression of
ADRP. These findings suggest that ADRP mediates the prevention of
hepatic steatosis by metformin.
Overall, our data demonstrate that metformin
prevents hepatic steatosis in vivo and in vitro by
inhibiting the expression of LD-associated proteins, namely ADRP.
The downregulation of ADRP enhanced the oxidation of fatty acids
and reduced lipid synthesis, thus preventing lipid accumulation in
the hepatocytes, contributing to the prevention of hepatic
steatosis. In conclusion, our study demosntrates that metformin
exerts an inhibitory effect on ADRP expression; thus, it may be
useful to further establish ADRP as a marker for liver steatosis.
Our data provide further insight into the possible mechanisms
underlying the improvement of liver steatosis following tretment
with metformin.
Acknowledgements
We would like to thank Dr Ri Zhang for providing
technical assistance and critically editing the manuscript. This
study was supported by grants from the National Natural Science
Foundation of China (nos. 81000171 and 81170798) and CBSKL
201103.
References
|
1
|
Rector RS, Thyfault JP, Wei Y and Ibdah
JA: Non-alcoholic fatty liver disease and the metabolic syndrome:
an update. World J Gastroenterol. 14:185–192. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Farrell GC and Larter CZ: Nonalcoholic
fatty liver disease: from steatosis to cirrhosis. Hepatology.
43(Suppl 1): S99–S112. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Marchesini G, Brizi M, Bianchi G,
Tomassetti S, Bugianesi E, Lenzi M, McCullough AJ, Natale S,
Forlani G and Melchionda N: Nonalcoholic fatty liver disease: a
feature of the metabolic syndrome. Diabetes. 50:1844–1850. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Neuschwander-Tetri BA and Caldwell SH:
Nonalcoholic steatohepatitis: summary of an AASLD Single Topic
Conference. Hepatology. 37:1202–1219. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stumvoll M, Nurjhan N, Perriello G, Dailey
G and Gerich JE: Metabolic effects of metformin in
non-insulin-dependent diabetes mellitus. N Engl J Med. 333:550–554.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Suwa M, Egashira T, Nakano H, Sasaki H and
Kumagai S: Metformin increases the PGC-1alpha protein and oxidative
enzyme activities possibly via AMPK phosphorylation in skeletal
muscle in vivo. J Appl Physiol. 101:1685–1692. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stephenne X, Foretz M, Taleux N, van der
Zon GC, Sokal E, Hue L, Viollet B and Guigas B: Metformin activates
AMP-activated protein kinase in primary human hepatocytes by
decreasing cellular energy status. Diabetologia. 54:3101–3110.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hardie DG: Neither LKB1 nor AMPK are the
direct targets of metformin. Gastroenterology. 131:9732006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mazza A, Fruci B, Garinis GA, Giuliano S,
Malaguarnera R and Belfiore A: The role of metformin in the
management of NAFLD. Exp Diabetes Res. 2012:7164042012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Friedman JM: Obesity: causes and control
of excess body fat. Nature. 459:340–342. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ducharme NA and Bickel PE: Lipid droplets
in lipogenesis and lipolysis. Endocrinology. 149:942–949. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thiele C and Spandl J: Cell biology of
lipid droplets. Curr Opin Cell Biol. 20:378–385. 2008. View Article : Google Scholar
|
|
14
|
Zehmer JK, Huang Y, Peng G, Pu J, Anderson
RG and Liu P: A role for lipid droplets in inter-membrane lipid
traffic. Proteomics. 9:914–921. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brasaemle DL: Thematic review series:
adipocyte biology. The perilipin family of structural lipid droplet
proteins: stabilization of lipid droplets and control of lipolysis.
J Lipid Res. 48:2547–2559. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Miura S, Gan JW, Brzostowski J, Parisi MJ,
Schultz CJ, Londos C, Oliver B and Kimmel AR: Functional
conservation for lipid storage droplet association among Perilipin,
ADRP, and TIP47 (PAT)-related proteins in mammals,
Drosophila, and Dictyostelium. J Biol Chem.
277:32253–32257. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gao J and Serrero G: Adipose
differentiation related protein (ADRP) expressed in transfected
COS-7 cells selectively stimulates long chain fatty acid uptake. J
Biol Chem. 274:16825–16830. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Paul A, Chang BH, Li L, Yechoor VK and
Chan L: Deficiency of adipose differentiation-related protein
impairs foam cell formation and protects against atherosclerosis.
Circ Res. 102:1492–1501. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chang BH, Li L, Paul A, Taniguchi S,
Nannegari V, Heird WC and Chan L: Protection against fatty liver
but normal adipogenesis in mice lacking adipose
differentiation-related protein. Mol Cell Biol. 26:1063–1076. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bickel PE, Tansey JT and Welte MA: PAT
proteins, an ancient family of lipid droplet proteins that regulate
cellular lipid stores. Biochim Biophys Acta. 1791:419–440. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sztalryd C, Bell M, Lu X, Mertz P,
Hickenbottom S, Chang BH, Chan L, Kimmel AR and Londos C:
Functional compensation for adipose differentiation-related protein
(ADFP) by Tip47 in an ADFP null embryonic cell line. J Biol Chem.
281:34341–34348. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Anstee QM and Goldin RD: Mouse models in
non-alcoholic fatty liver disease and steatohepatitis research. Int
J Exp Pathol. 87:1–16. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Medina J, Fernandez-Salazar LI,
Garcia-Buey L and Moreno-Otero R: Approach to the pathogenesis and
treatment of nonalcoholic steatohepatitis. Diabetes Care.
27:2057–2066. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gao ZQ, Lu FE, Dong H, Xu LJ, Wang KF and
Zhou X: Study on therapeutic effects of metformin on rat fatty
livers induced by high fat feeding. Zhonghua Gan Zang Bing Za Zhi.
13:101–104. 2005.(In Chinese).
|
|
25
|
Wanders RJ, Ferdinandusse S, Brites P and
Kemp S: Peroxisomes, lipid metabolism and lipotoxicity. Biochim
Biophys Acta. 1801:272–280. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Magnusson B, Asp L, Bostrom P, Ruiz M,
Stillemark-Billton P, Linden D, Boren J and Olofsson SO: Adipocyte
differentiation-related protein promotes fatty acid storage in
cytosolic triglycerides and inhibits secretion of very low-density
lipoproteins. Arterioscler Thromb Vasc Biol. 26:1566–1571. 2006.
View Article : Google Scholar
|
|
27
|
Granneman JG, Moore HP, Mottillo EP and
Zhu Z: Functional interactions between Mldp (LSDP5) and Abhd5 in
the control of intracellular lipid accumulation. J Biol Chem.
284:3049–3057. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Steiner S, Wahl D, Mangold BL, Robison R,
Raymackers J, Meheus L, Anderson NL and Cordier A: Induction of the
adipose differentiation-related protein in liver of
etomoxir-treated rats. Biochem Biophys Res Commun. 218:777–782.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Heid HW, Moll R, Schwetlick I, Rackwitz HR
and Keenan TW: Adipophilin is a specific marker of lipid
accumulation in diverse cell types and diseases. Cell Tissue Res.
294:309–321. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Folch J, Lees M and Sloane Stanley GH: A
simple method for the isolation and purification of total lipides
from animal tissues. J Biol Chem. 226:497–509. 1957.PubMed/NCBI
|
|
31
|
Motomura W, Inoue M, Ohtake T, Takahashi
N, Nagamine M, Tanno S, Kohgo Y and Okumura T: Up-regulation of
ADRP in fatty liver in human and liver steatosis in mice fed with
high fat diet. Biochem Biophys Res Commun. 340:1111–1118. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Imai Y, Varela GM, Jackson MB, Graham MJ,
Crooke RM and Ahima RS: Reduction of hepatosteatosis and lipid
levels by an adipose differentiation-related protein antisense
oligonucleotide. Gastroenterology. 132:1947–1954. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ye J, Li JZ, Liu Y, Li X, Yang T, Ma X, Li
Q, Yao Z and Li: Cideb, an ER- and lipid droplet-associated
protein, mediates VLDL lipidation and maturation by interacting
with apolipoprotein B. Cell Metab. 9:177–190. 2009. View Article : Google Scholar : PubMed/NCBI
|