Introduction
Chitosan oligosaccharides (COS), consisting of
D-glucosamine linked via β-1,4-glycosides, are derived from
chitosan by chemical or enzymatic hydrolysis (1). It has been reported that COS exhibit
various biological activities, such as antitumor (2) and antioxidant properties (3), owing to their low molecular weight,
high absorption, solubility and biocompatibility (4). In addition, recent studies have paid
more attention to the regulatory role of COS in inflammatory
responses (5,6).
Lipopolysaccharide (LPS), the major component of the
outer membrane of Gram-negative bacteria, can act as an endotoxin
and initiates serious inflammatory responses in vitro and
in vivo (7,8) by upregulating the expression of
adhesion molecules and cytokines, such as E-selectin, intercellular
adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1
(VCAM-1) and tumor necrosis factor-α (TNF-α) (9,10).
Endothelial cells are the target of inflammatory responses and
thereby, of a number of severe disorders, including sepsis and
multiple organ dysfunctions that occur via LPS stimulation
(11). In this study, we detected
the expression of the adhesion molecules, E-selectin and ICAM-1 in
LPS-treated porcine iliac artery endothelial cells (PIECs), since
the upregulation of adhesion molecules and the increased secretion
of cytokines and chemokines are known to occur during endothelial
cell activation (12–14).
The evolutionary conserved family of
mitogen-activated protein kinases (MAPKs) includes extracellular
p38 MAPK, extracellular regulated protein kinase (ERK) and c-Jun
N-terminal kinase (JNK) (15).
Numerous environmental stresses, such as osmotic shock, ultraviolet
irradiation, as well as LPS and pro-inflammatory cytokines, have
been confirmed to activate MAPK signaling cascades in a variety of
cell lines (11,16). In addition, nuclear factor-κB
(NF-κB) and activator protein-1 (AP-1) have been identified as
major downstream targets of MAPK signaling pathways, regulating,
upon activation, a number of proteins, including cytokines and
adhesion molecules (8,17,18).
Accumulating evidence suggests that COS can
attenuate inflammatory responses caused by stimuli, such as
endotoxins, bacteria and cytokines (19,20). However, the molecular mechanisms
by which COS exert these effects in LPS-stimulated endothelial
cells have not yet been fully elucidated. Whether the MAPK and/or
NF-κB signaling pathways are involved in the protective effects of
COS in LPS-induced inflammatory responses in endothelial cells
remains largely unknown. Therefore, the aim of the present study
was to establish the in vitro roles of COS in LPS-induced
inflammation in endothelial cells and to elucidate the underlying
molecular mechanisms.
Materials and methods
Chemicals and reagents
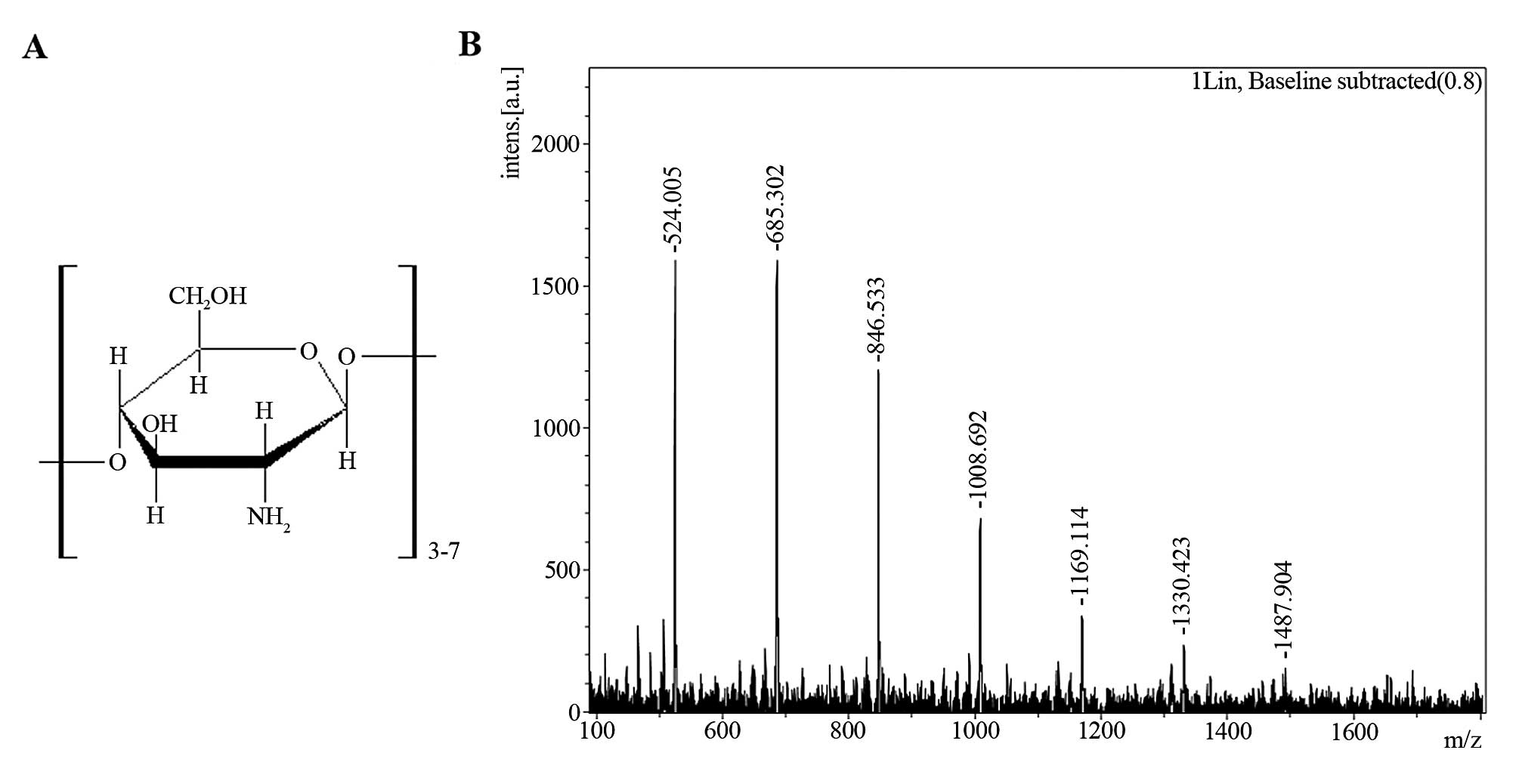
COS were prepared in our laboratory (degree of
deacetylation, >95%) according to a previously described method
(21). Matrix-assisted laser
desorption/ionization (MALDI) - time-of-flight (TOF) mass
spectrometry analysis indicated that the polymerization degree of
the prepared COS was 3–7 (Fig.
1), as described in our previous study (4). The weight percentages of COS with
degrees of polymerization of 3–7 were 3.7, 16.1, 28.8, 37.2 and
14.2%, respectively, as previously described (22). LPS extract (from Escherichia
coli serotype 055:B5),
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
and the proteasome inhibitor, MG132, were purchased from
Sigma-Aldrich (St. Louis, MO, USA). The p38 MAPK inhibitor,
SB203580, was purchased from Tocris Bioscience (Bristol, UK). The
ERK1/2 inhibitor, PD98059, RPMI-1640 medium and fetal bovine serum
(FBS) were obtained from Gibco/Invitrogen (Grand Island, NY, USA).
Mouse anti-phospho-ERK (Thr202/Tyr204) monoclonal antibody, rabbit
anti-ERK polyclonal antibody and Hoechst 33258 were purchased from
Beyotime Institute of Biotechnology (Jiangsu, China). Anti-GAPDH
polyclonal antibody, goat anti-rabbit IgG-HRP, goat anti-mouse
IgG-HRP and goat anti-rabbit IgG-FITC were obtained from Santa Cruz
Biotechnology (Santa Cruz, CA, USA). Rabbit anti-phospho-p38
(Thr180/Tyr182), as well as antibodies against p38, NF-κB p65, IKKa
and histone H3 were purchased from Cell Signaling Technology
(Beverly, MA, USA).
Cell culture
PIECs were obtained from the Shanghai Institute of
Cell Biology, Chinese Academy of Sciences (Shanghai, China) and
were grown in RPMI-1640 medium containing 10% FBS, 2 mM
L-glutamine, 100 μg/ml streptomycin and 100 U/ml penicillin. Cells
were incubated at 37°C in a humidified atmosphere of 5%
CO2. In all the experiments, cells from passages 3–10
were used; they were grown up to 70–80% confluence prior to
treatment with the different agents. The PIECs were pre-treated
with the vehicle [phosphate-buffered saline (PBS), pH 7.4] or COS
(50–200 mg/ml) in 1% FBS medium for 24 h. They were then washed
with PBS twice and exposed to 1 mg/ml LPS in 1% FBS medium for
different periods of time.
Reverse transcription polymerase chain
reaction (RT-PCR)
Changes in the mRNA levels of E-selectin and ICAM-1
were assessed by RT-PCR. Total RNA was extracted from the treated
cells using TRIzol reagent (Takara Biotechnology, Dalian, China)
according to the manufacturer’s instructions: The following primers
were used for amplification: E-selectin forward, 5′-AAG CAA AGC AAC
GAG GAC-3′ and reverse, 5′-ACA GGT GAA GTG GCA GGT-3′; ICAM-1
forward, 5′-AAA CAC CAT CAT ACC CAA AGG-3′ and reverse, 5′-TGC CAC
GAC AAG TTA GCC-3′; and GAPDH forward, 5′-TTC CAC GGC ACA GTC AA-3′
and reverse, 5′-GCA GGT CAG GTC CAC AA-3′. The thermal cycling
program was as follows: initial denaturation for 5 min at 94°C,
followed by 35 cycles of 30 sec at 94°C, 30 sec at 52°C and 30 sec
at 72°C. The amplified PCR products were electrophoresed on a 1.5%
agarose gel containing 1 mg/ml ethidium bromide, visualized and
photographed on a UV transilluminator (UVP, LLC BioImaging Systems,
Upland, CA, USA).
Western blot analysis
PIECs (2×106) were directly lysed after
treatment with a buffer containing 20 mmol/l Tris-HCl at pH 7.5,
150 mmol/l NaCl, 1 mmol/l Na2EDTA, 1 mmol/l EGTA, 1%
Triton, 2.5 mmol/l sodium pyrophosphate, 1 mmol/l
β-glycerophosphate, 1 mmol/l Na3VO4 and 1
mg/ml leupeptin, to which 1 mmol/l phenylmethanesulfonylfluoride
(PMSF) was added before use. Nuclear and cytoplasmic fractions were
separated using a nuclear and cytoplasmic protein extraction kit
(Beyotime, Jiangsu, China). The concentration of the protein
samples was measured using a bicinchoninic acid protein assay kit
(Solarbio, Beijing, China). All samples were stored at −80°C until
further analysis. Equal amounts of protein (30 mg) were separated
by SDS-PAGE (8–12%) gel electrophoresis and electroblotted onto a
0.45-mm polyvinylidene fluoride membrane. The membrane was blocked
by incubation with 5% skim milk in Tris-buffered saline with 0.1%
Tween-20 (TBST) for 1 h at room temperature, and incubated with
primary antibodies overnight at 4°C. After 3 washes in TBST, the
membrane was incubated with HRP-/FITC-conjugated secondary
antibodies for 1 h at room temperature. Respective proteins were
detected using an enhanced chemoluminescence (ECL) assay kit and
chemiluminescent signals were detected on an X-ray film.
Densitometric analysis was performed using the PDI ImageWare system
(Bio-Rad, Hercules, CA, USA).
MTT assay for cell viability
The viability of the PIECs was assessed by MTT
assay. PIECs were plated into 96-well plates ( 5×103
cells/well) and incubated overnight with 150 ml of RPMI-1640
solution supplemented with 10% FBS. The cells were treated with 150
ml of the vehicle or COS (50–800 μg/ml) in RPMI-1640 with 1% FBS.
Following incubation for 24 h, the PIECs were washed with PBS and
incubated with MTT (1 mg/ml, final concentration) for a further 3
h. The MTT solution was aspirated and 100 ml of DMSO were added to
solubilize the formazan crystals that formed inside the cells. The
absorbance was measured at 490-nm wavelength. The viability of the
PIECs in each well was expressed as a percentage relative to the
vehicle-treated group.
Monocyte cell adhesion assay
In order to monitor cell tracking, the PIECs were
seeded into 24-well culture plates (1×105 cells/well)
and were pre-treated with COS for 24 h and then stimulated with LPS
(1 mg/ml) for 4 h. U937 monocytes were incubated in RPMI-1640
medium containing 10% FBS and with 10 mmol/l of the fluorescent
dye, BCECF-AM, for 1 h in the dark at 37°C. After washing twice
with PBS, 5×104 cells/well were incubated with PIECs for
1 h in the dark at 37°C. Non-adherent U937 cells were removed by
washing gently with PBS. Adherent U937 cells were visualized under
a FluoView microscope (Olympus, Japan) and in a fluorescence EM
microplate reader (Gemini, USA) equipped with 488-nm excitation and
510-nm emission filters. Quantified fluorescence intensities were
expressed as fold ratios relative to the vehicle-treated group.
Immunocytochemistry
PIECs at a density of 4×104 cells were
cultured on glass coverslips. Following treatment, the cells were
washed with ice-cold PBS and fixed in 4% formaldehyde/PBS for 30
min at room temperature, then incubated with 0.3% Triton X-100/PBS
for 10 min. After washing, the coverslips were blocked for 1 h at
room temperature in 10% goat serum/PBS and then incubated with a
1:200 dilution of anti-p65 antibody in 10% goat serum/PBS for 1 h
at 37°C. After washing, the coverslips were incubated with a 1:200
dilution of FITC-conjugated goat anti-rabbit IgG in 10% goat
serum/PBS for 45 min at room temperature. After washing, a 1:1,000
dilution of 10 mg/ml Hoechst 33258 was used to counterstain the
nuclei for 10 min. Finally, the coverslips were washed with PBS and
mounted with aqueous mounting medium. Fluorescence signals were
analyzed using a FluoView microscope.
Statistical analysis
Data are reported as the means ± SD (n=3–10). An
unpaired Student’s t-test was used to assess the significance of
the differences between groups. A one-way ANOVA was used to compare
the differences among 3 or more groups followed by Bonferroni
multiple comparison tests, where applicable. A value of P<0.05
was considered to indicate a statistically significant
difference.
Results
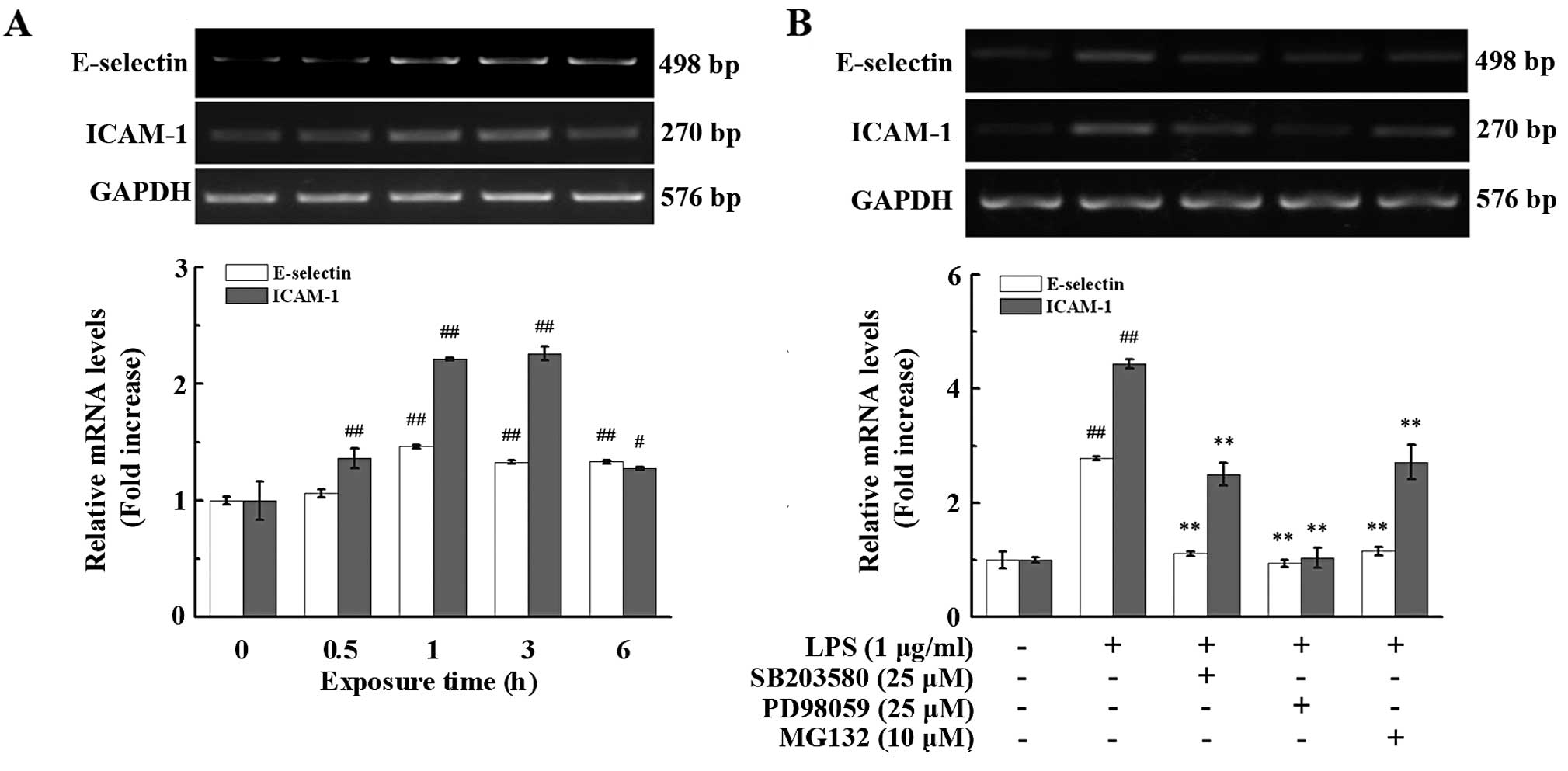
LPS induces the mRNA expression of
E-selectin and ICAM-1 in endothelial cells
LPS is known to induce critical inflammation by
promoting the expression of cell adhesion molecules (23,24). Thus, in this study, we first
determined the mRNA expression of E-selectin and ICAM-1 in the
PIECs exposed to LPS for various periods of time. Treatment with
LPS (1 μg/ml) induced the mRNA expression of E-selectin and ICAM-1
in a time-dependent manner (Fig.
2A). Treatment with LPS for 1 h markedly enhanced E-selectin
and ICAM-1 mRNA expression. The increase in the expression of
E-selectin (132.93±1.51%, P<0.01) and ICAM-1 (225.80±5.91%,
P<0.01) reached a peak at 3 h and then ceased and declined after
6 h of treatment (Fig. 2A).
Inhibition of MAPK and NF-κB pathways
suppresses the mRNA expression of E-selectin and ICAM-1 in
endothelial cells
We then examined the involvement of NF-κB, a
transcription factor that plays a crucial role in inflammation,
immunity, cell proliferation and apoptosis (25–28). NF-κB affected the LPS-induced
E-selectin and ICAM-1 expression. The PIECs were pre-treated with
the proteasome inhibitor, MG132 (10 mmol/l), which blocks the
activation of NF-κB by preventing the degradation of IκB for 1 h
prior to LPS challenge for 3 h. As depicted in Fig. 2B, the inhibition of the activation
of NF-κB significantly suppressed the mRNA expression of E-selectin
and ICAM-1, which was reduced by 41.65±2.58% (P<0.01) and
61.26±6.67% (P<0.01), respectively, compared with the
LPS-treated group.
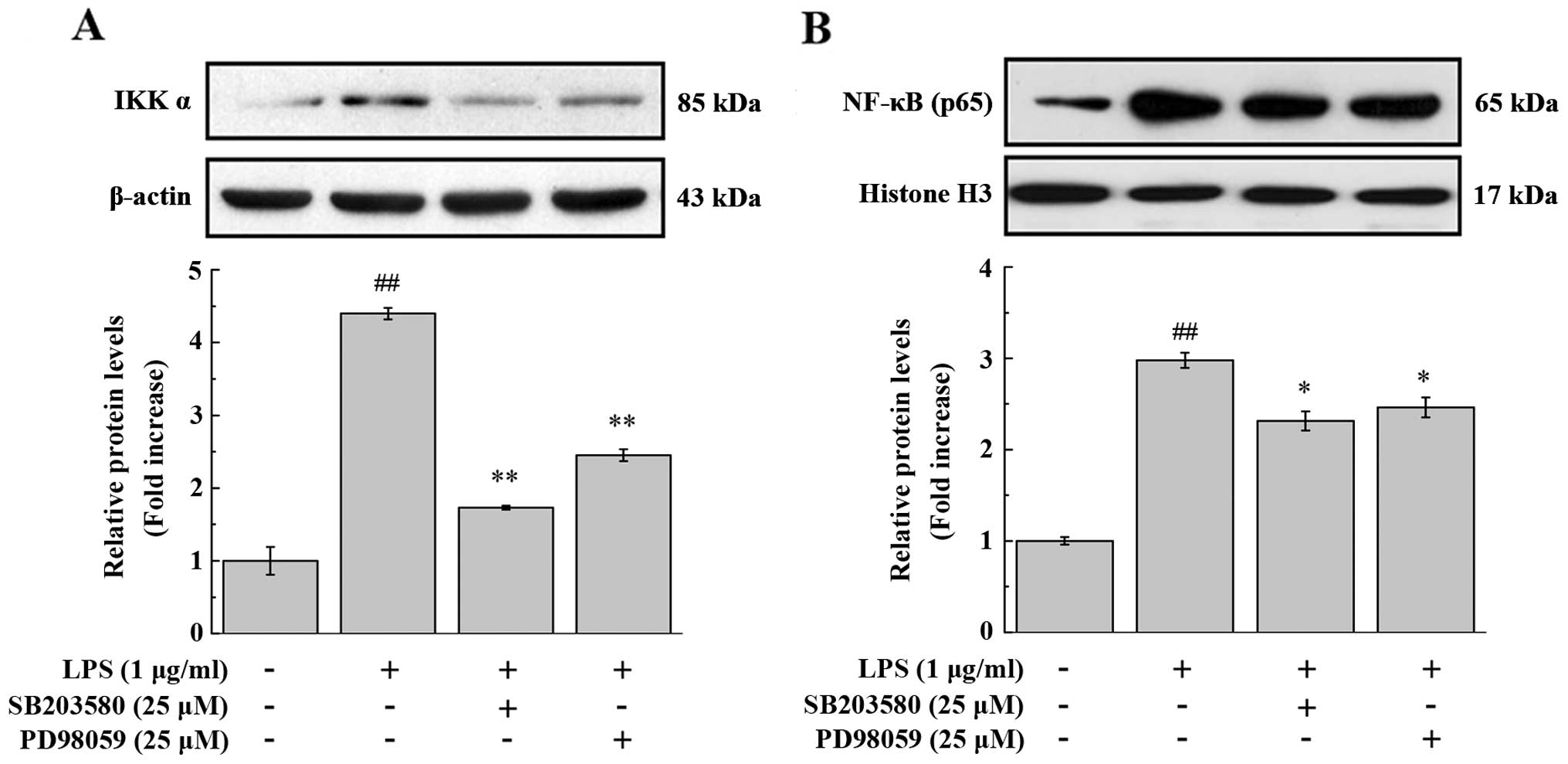
We then determined whether MAPK and LPS are involved
in the activation of NF-κB and in subsequent molecular adhesion.
NF-κB is sequestered by IκB in the cytoplasm under normal
conditions, whereas upon stimulation, IKK phosphorylates IκB,
inducing its degradation via the ubiquitination pathway and
allowing free NF-κB dimers (most commonly, the p50/p65 dimer) to
enter the nucleus (29,30). As shown in Fig. 3, LPS caused a marked elevation of
the IKKα protein level in the cytoplasm, as well as of the NF-κB
p65 protein level in the nuclear fraction of PIECs. The inhibitors
of p38 MAPK (SB203480, 25 mmol/l) and ERK1/2 (PD98059, 25 mmol/l)
significantly attenuated these effects. The activation of p38
MAPK/ERK1/2 accelerates the translocation of the NF-κB dimer into
the nucleus, where it binds to the promoter or enhancer regions of
NF-κB-regulated genes to modulate gene transcription (31). In agreement with this, the
inhibition of p38 MAPK and ERK1/2 significantly reduced the mRNA
expression of E-selectin and ICAM-1 in the PIECs (Fig. 2B). Compared to LPS stimulation
alone (assigned a 100% level), the mRNA levels of E-selectin were
decreased to 39.96±1.38% (P<0.01) and 33.81±2.35% (P<0.01) by
pre-treatment with p38 MAPK (SB203480) and ERK1/2 (PD98059)
inhibitors, respectively, whereas the mRNA levels of ICAM-1 were
reduced to 56.36±4.52% (P<0.01), 23.44±3.91% (P<0.01),
respectively. Taken together, these data demonstrate that the p38
MAPK/ERK1/2 and NF-κB signaling pathways regulate the LPS-induced
mRNA expression of E-selectin and ICAM-1 in the PIECs.
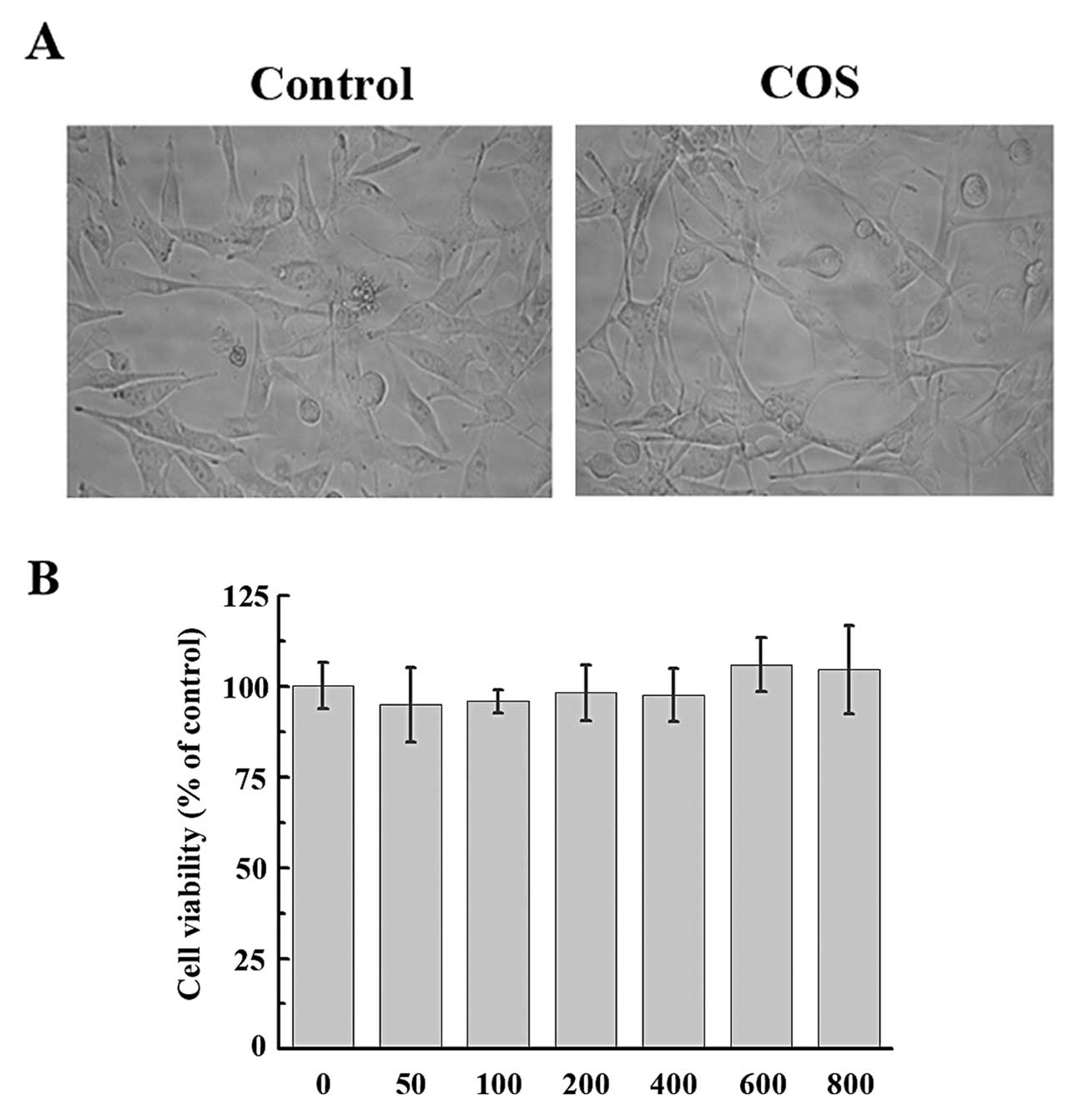
COS do not affect cell viability
The effect of COS on the viability of PIECs was
evaluated by MTT assay. PIECs were treated with various
concentrations of COS (50, 100, 200, 400, 600 and 800 μg/ml). As
shown in Fig. 4, neither
concentration of COS had any effect on cell viability (P=0.158;
P=0.243; P=0.596; P=0.483; P=0.127; P=0.234, respectively). Based
on these results, we selected the concentration of 50–200 μg/ml COS
for further experiments.
COS inhibit the mRNA expression of
E-selectin and ICAM-1 and reduce the adhesion of monocytes to
endothelial cells
A few studies have provided evidence for the
potentially beneficial effects of COS on oxidative and inflammatory
damage, which occur via the improvement of the redox imbalance and
limitation of cytokine production (32,33). Moreover, under certain
pathological conditions, endothelial cells are responsive and allow
the migration of dendritic immune cells (34). In this study, we investigated the
anti-inflammatory effects of COS on LPS-stimulated endothelial
cells by treating PIECs with various concentrations of COS (50, 100
and 200 μg/ml) for 24 h and subsequently incubating the cells with
LPS for 3 h. Treatment with COS reduced the mRNA expression of
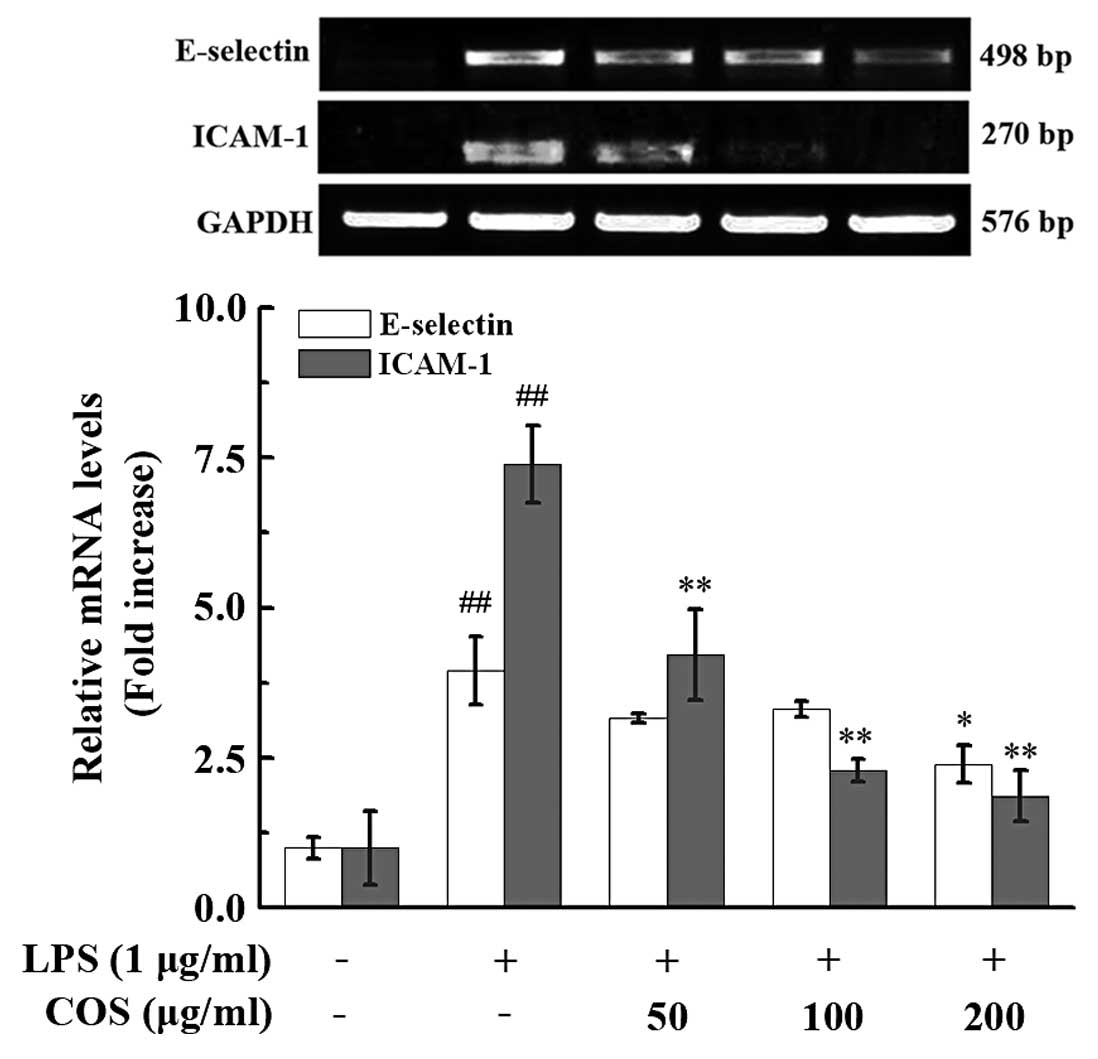
ICAM-1 and E-selectin (Fig. 5).
This reduction was dose-dependent and significant for ICAM-1, and
significant for E-selectin only with pre-treatment with 200 μg/ml
COS; at this concentration, the E-selectin and ICAM-1 mRNA
expression levels were decreased by 38.14±7.29% (P<0.01) and
70.29±12.86% (P<0.01), respectively, compared with the
LPS-treated group (Fig. 5).
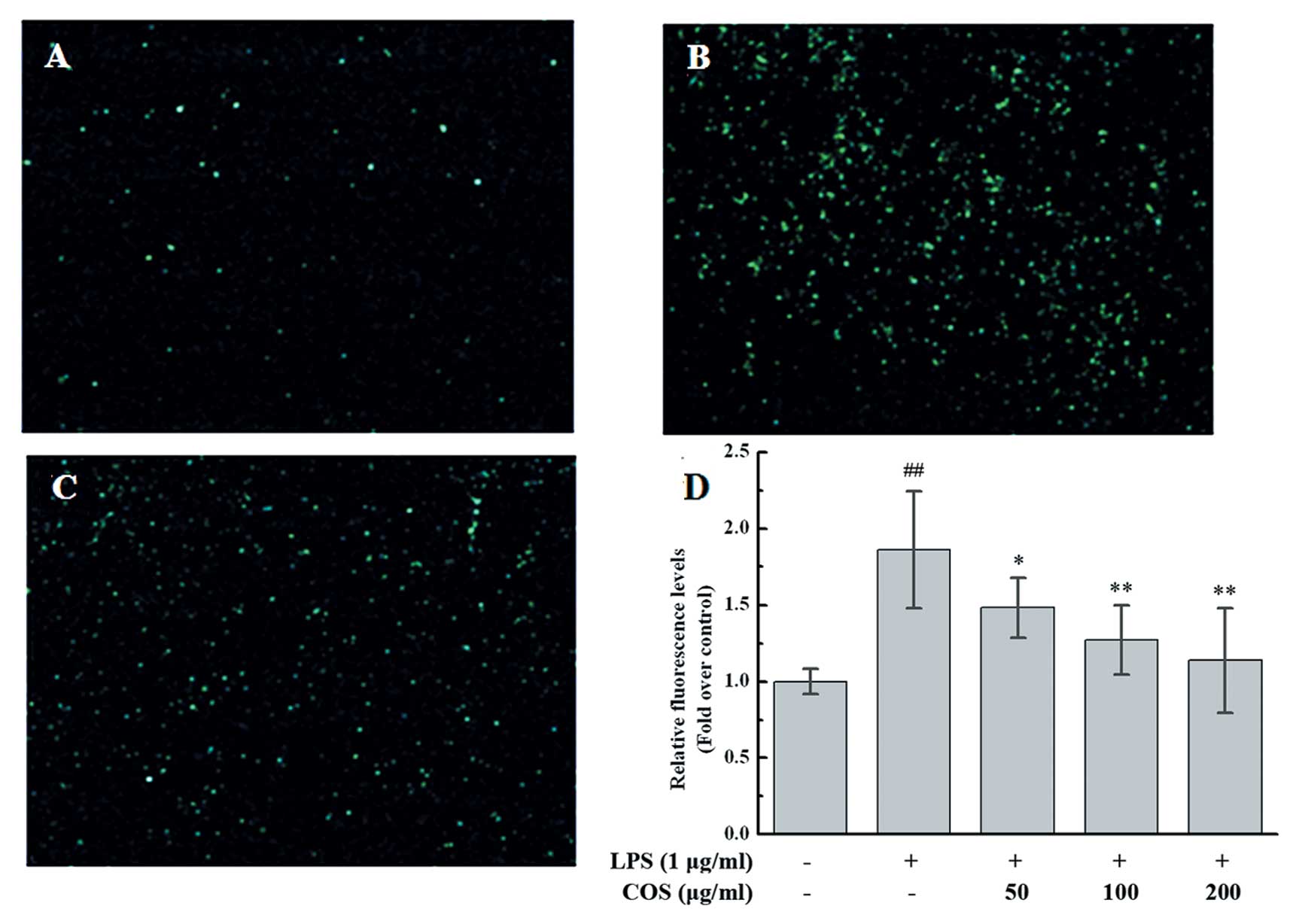
Since COS inhibited the expression of adhesion
molecules induced by LPS in the PIECs, we hypothesized that COS may
attenuate monocyte adhesion to endothelial cells in LPS-induced
inflammatory response. To confirm this, we assayed the adhesion of
fluorescence-labeled U937 cells to LPS-treated PIECs. In this
assay, LPS induced the adhesion of monocytes to endothelial cells,
whereas COS inhibited this adhesion (Fig. 6A–C). Further confirmation of this
result came from the quantification of the fluorescent intensity of
labeled monocytes that adhered to PIECs; the latter were
pre-treated with various concentrations of COS for 24 h, followed
by LPS stimulation. As expected, COS reduced the LPS-induced
monocyte adhesion to PIECs in a dose-dependent manner (50 mg/ml,
79.69±10.54%, P<0.05; 100 mg/ml, 68.27±12.22%, P<0.01; 200
mg/ml, 61.14±18.39%, P<0.01 vs. the LPS-treated group) (Fig. 6D).
COS inhibits the LPS-induced acvtivation
of the MAPK signaling pathway in endothelial cells
Based on our data, COS appears to play a protective
role in LPS-induced inflammation in PIECs through the inhibition of
the expression of the adhesion molecules, E-selectin and ICAM-1,
whereas their upregulation by LPS is mediated by the p38
MAPK/ERK1/2 and NF-κB pathways. Thus, we investigated whether the
inhibitory effects of COS are mediated through the MAPK signaling
pathway.
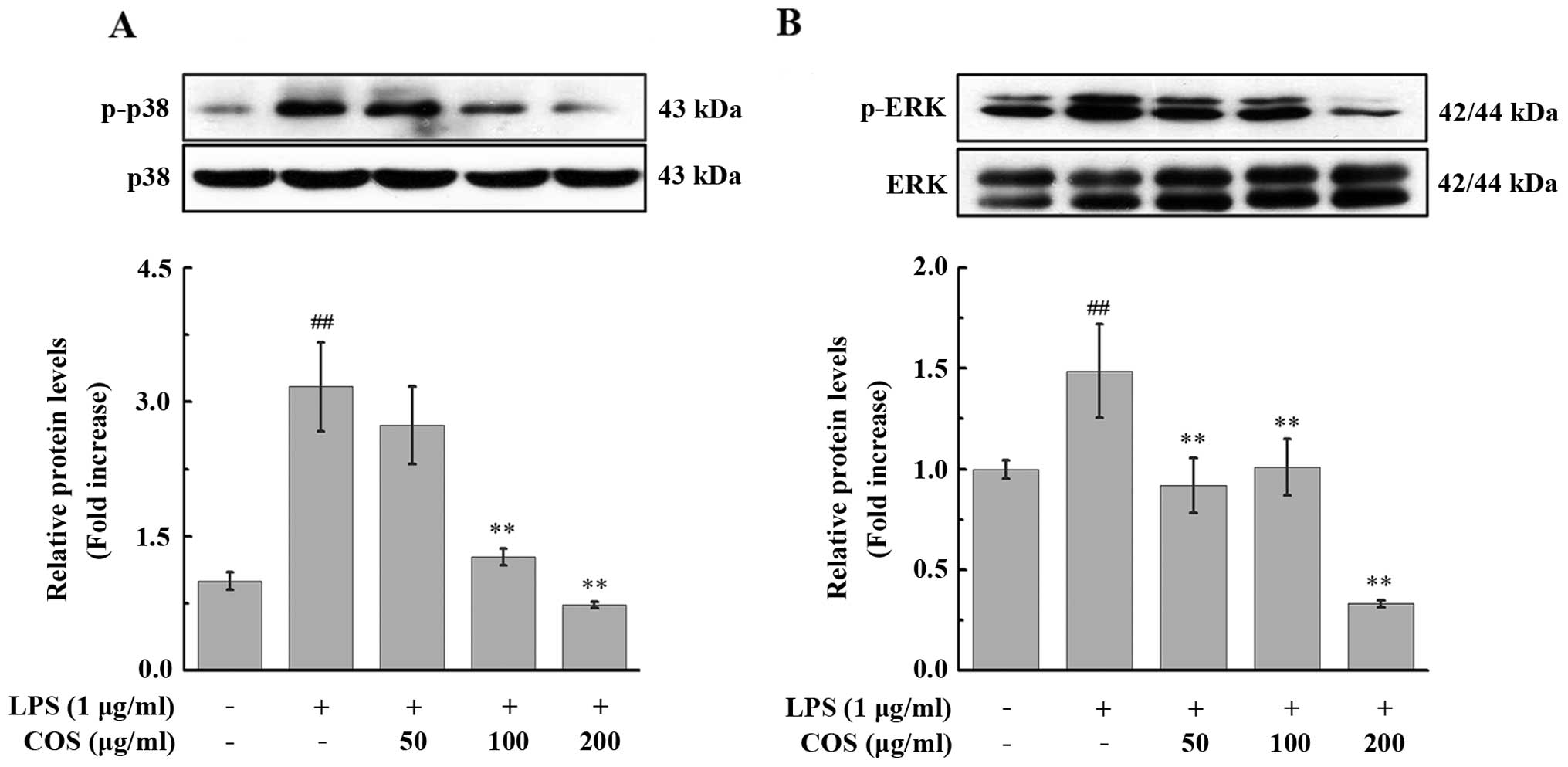
The PIECs were pre-treated with various
concentrations of COS for 24 h prior to exposure to 1 μg/ml LPS for
15 min and phosphorylated p38 MAPK and ERK1/2 were detected in the
PIECs by western blot analysis. As shown in Fig. 7, the levels of phosphorylated p38
MAPK and ERK1/2 were increased upon treatment with LPS. By
contrast, COS treatment significantly inhibited the phosphorylation
of p38 MAPK and ERK1/2; this effect was dose-dependent for p38
MAPK. The maximal inhibitory effect of COS on the phosphorylation
of p38 MAPK and ERK1/2 was observed at 200 μg/ml, where the levels
of the phosphorylated forms of these proteins decreased to
23.15±3.72% (P<0.01) and 22.36±2.87% (P<0.01) of the
LPS-stimulated group, respectively (Fig. 7).
COS suppresses the translocation of NF-κB
induced by LPS in endothelial cells
Our results revealed that the LPS-induced NF-κB
translocation is dependent on the MAPK signaling pathway. We then
determined whether COS inhibits NF-κB activation by inhibiting the
activation of the p38 MAPK/ERK1/2 signaling pathway in PIECs.
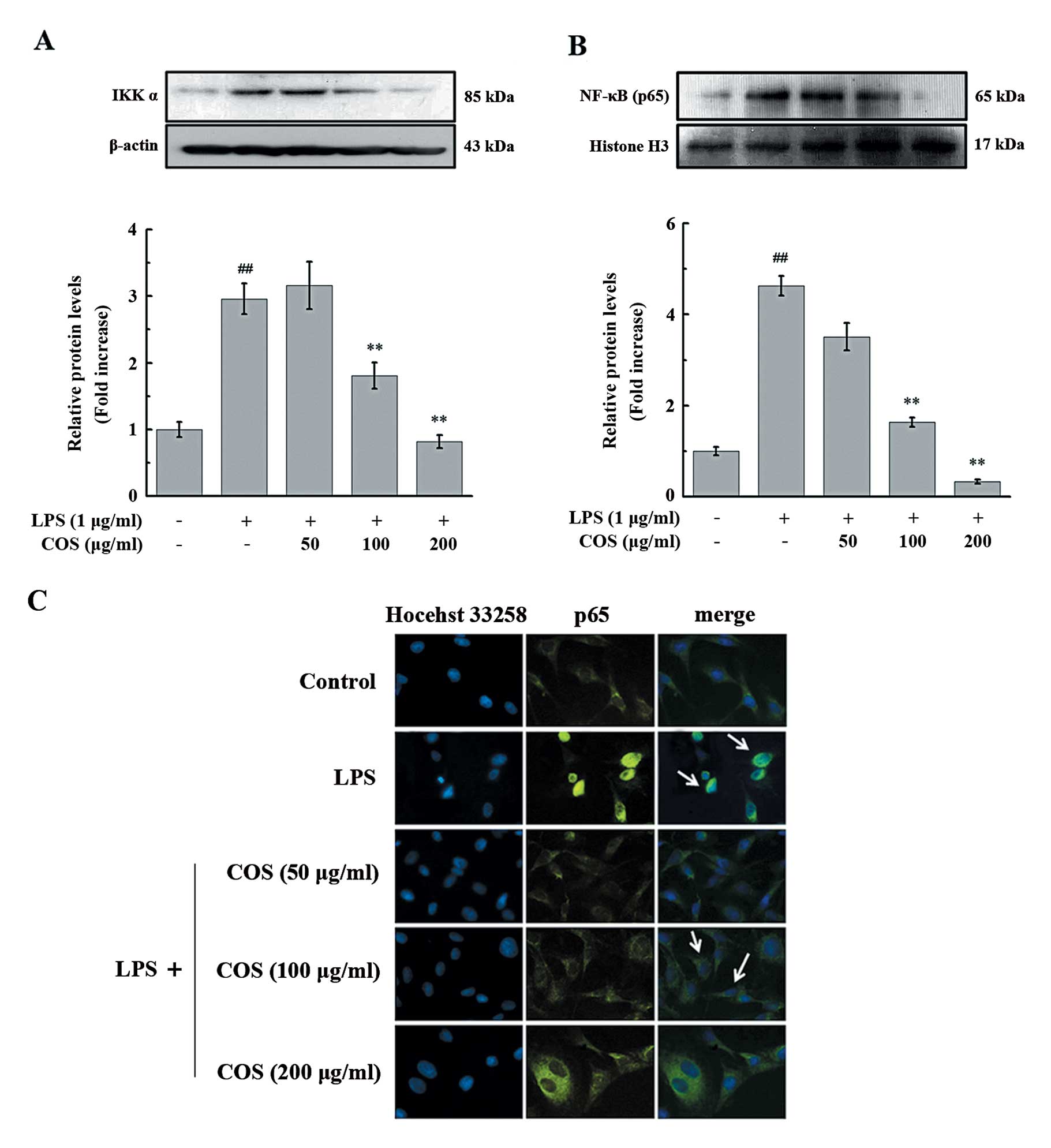
Treatment with LPS for 1 h increased the protein level of IKKα and
promoted NF-κB p65 nuclear translocation; these effects were
significantly and dose-dependently attenuated by COS pre-treatment
(Fig. 8). To confirm the
inhibitory effects of COS on LPS-induced NF-κB activation in the
PIECs, NF-κB p65 localization was determined by
immunocytochemistry. As shown in Fig.
8C, treatment with LPS induced the translocation of NF-κB p65
into the nucleus, an effect that was considerably attenuated upon
pre-treatment with COS.
Discussion
In the present study, we demonstrate that COS
suppresses the LPS-induced expression of adhesion molecules in
endothelial cells by inhibiting the activation of the MAPK and
NF-κB signaling pathways and the consequent adhesion of monocytes
to endothelial cells. Our results demonstrated that LPS induced the
mRNA expression of E-selectin and ICAM-1 by activating the p38
MAPK/ERK1/2 and NF-κB signaling pathways in PIECs. In line with
these observations, the inhibtion of LPS-induced p38 MAPK/ERK1/2
and NF-κB signaling by COS resulted in a decrease in the mRNA
levels of E-selectin and ICAM-1 in PIECs.
One of the major findings of the present study is
that COS significantly inhibited the LPS-induced mRNA expression of
E-selectin and ICAM-1 in a dose-dependent manner, but had no effect
on PIEC viability. The binding of circulating leukocytes to the
microvascular endothelium is the initial event in leukocyte
emigration and extravasation (35). The binding is mediated by
endothelial ligands, such as E-selectin at the initial stages and
ICAM-1 at subsequent stages (23,36). The high expression of the adhesion
molecules, E-selectin, ICAM-1 and VCAM-1, can promote endothelial
cell migration and leukocyte adhesion to endothelial cells
(37), which initiates the
interaction between endothelial cells and leukocytes following
tissue damage and is coupled with the induction of a variety of
immune responses (23,24). In our study, COS significantly
reduced monocyte (U973 cell) adhesion to PIECs, which was induced
by LPS. This result highlights the protective role of COS in
LPS-induced inflammatory response in endothelial cells.
We also demonstrated that COS inhibited MAPK
phosphorylation and NF-κB translocation, which was induced by LPS
in PIECs. Consistent with previous reports demonstrating that the
tyrosine phosphorylation of p38 MAPK and ERK is involved in signal
transduction occurring in LPS-stimulated endothelial cells
(23,38), and further mediates cellular
responses through the NF-κB, AP-1 and activating transcription
factor 2 (ATF2) transcription factors (29). We found that the inhibition of p38
MAPK/ERK1/2 signaling suppressed the LPS-induced NF-κB nuclear
translocation in PIECs, leading to the reduced expression of
E-selectin and ICAM-1; these results are in line with those of
other studies, showing that the expression of E-selectin and ICAM-1
is associated with NF-κB activation, since the NF-κB upstream
promoter region contains binding sites for these two adhesion
molecules (10,11,30). Again in accordance with these
reports, we found that COS inhibited the LPS-induced
phosphorylation of p38 MAPK and ERK1/2 in PIECs, resulting in the
observed dose-dependent suppression of the translocation of NF-κB
into the nucleus. The latter can explain the inhibitory effects of
COS on the mRNA expression of E-selectin and ICAM-1, which also
showed dose-dependence.
In conclusion, our results demonstrate that COS
exerts its anti-inflammatory effects by inhibiting the acvitation
of the LPS-induced p38 MAPK/ERK1/2 and NF-κB signaling pathways,
and consequently, the mRNA levels of E-selectin and ICAM-1, as well
as monocyte adhesion to endothelial cells in vitro. Thus,
COS may represent a promising therapeutic agent for the prevention
of inflammatory responses in systemic diseases.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (nos. 31072065 and 31100589),
and partly by The Twelfth Five Years National Science and
Technology Support Plan Project (2011BAD26B02-3) and grant no.
2012AA021501 from the National High Technology Research and
Development Program (863) of China.
References
|
1
|
Mourya VK, Inamdar NN and Choudhari YM:
Chitooligosaccharides: synthesis, characterization and
applications. Polym Sci Ser A. 53:583–612. 2011. View Article : Google Scholar
|
|
2
|
Xu WH, Jiang CQ, Kong XY, Liang Y, Rong M
and Liu WS: Chitooligosaccharides and N-acetyl-D-glucosamine
stimulate peripheral blood mononuclear cell-mediated antitumor
immune responses. Mol Med Rep. 6:385–390. 2012.PubMed/NCBI
|
|
3
|
Liu HT, He JL, Li WM, et al: Chitosan
oligosaccharides protect human umbilical vein endothelial cells
from hydrogen peroxide-induced apoptosis. Carbohydr Polym.
80:1062–1071. 2010. View Article : Google Scholar
|
|
4
|
Zhang JN, Dang YB, Li S, et al: The
effects of chitosan oligosaccharide on the activation of murine
spleen CD11c(+) dendritic cells via toll-like receptor 4. Carbohydr
Polym. 83:1075–1081. 2011.
|
|
5
|
Yousef M, Pichyangkura R, Soodvilai S,
Chatsudthipong V and Muanprasat C: Chitosan oligosaccharide as
potential therapy of inflammatory bowel disease: therapeutic
efficacy and possible mechanisms of action. Pharmacol Res.
66:66–79. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ji Q, Deng J, Yu X, Xu Q, Wu H and Pan J:
Modulation of pro-inflammatory mediators in LPS-stimulated human
periodontal ligament cells by chitosan and quaternized chitosan.
Carbohydr Polym. 92:824–829. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chu X, Ci X, Wei M, et al: Licochalcone A
inhibits lipopolysaccharide-induced inflammatory response in vitro
and in vivo. J Agric Food Chem. 60:3947–3954. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen G, Li J, Ochani M, et al: Bacterial
endotoxin stimulates macrophages to release HMGB1 partly through
CD14- and TNF-dependent mechanisms. J Leukoc Biol. 76:994–1001.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hu Y, Chen X, Duan H, Hu Y and Mu X:
Chinese herbal medicinal ingredients inhibit secretion of IL-6,
IL-8, E-selectin and TXB(2) in LPS-induced rat intestinal
microvascular endothelial cells. Immunopharmacol Immunotoxicol.
31:550–555. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zou LY, Yang SL, Champattanachai V, et al:
Glucosamine improves cardiac function following trauma-hemorrhage
by increased protein O-GlcNAcylation and attenuation of NF-κB
signaling. Am J Physiol Heart Circ Physiol. 296:H515–H523.
2009.PubMed/NCBI
|
|
11
|
Yan WS, Zhao KS, Jiang Y, et al: Role of
p38 MAPK in ICAM-1 expression of vascular endothelial cells induced
by lipopolysaccharide. Shock. 17:433–438. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tanigawa N, Hagiwara M, Tada H, et al:
Acacetin inhibits expression of E-selectin on endothelial cells
through regulation of the MAP kinase signaling pathway and
activation of NF-κB. Immunopharmacol Immunotoxicol. 35:471–477.
2013.PubMed/NCBI
|
|
13
|
del Pina-Canseco MS, Paez-Arenas A, Masso
F, et al: Protein C activation peptide inhibits the expression of
ICAM-1, VCAM-1, and interleukin-8 induced by TNF-α in human dermal
microvascular endothelial cells. Folia Histochem Cytobiol.
50:407–413. 2012.PubMed/NCBI
|
|
14
|
Shreeniwas R, Koga S, Karakurum M, et al:
Hypoxia-mediated induction of endothelial cell interleukin-1 alpha.
An autocrine mechanism promoting expression of leukocyte adhesion
molecules on the vessel surface. J Clin Invest. 90:2333–2339. 1992.
View Article : Google Scholar
|
|
15
|
Qiao Y, Ruan YY, Xiong CN, et al: Chitosan
oligosaccharides suppressant LPS binding to TLR4/MD-2 receptor
complex. Carbohydr Polym. 82:405–411. 2010. View Article : Google Scholar
|
|
16
|
Yomogida S, Hua J, Sakamoto K and Nagaoka
I: Glucosamine suppresses interleukin-8 production and ICAM-1
expression by TNF-α-stimulated human colonic epithelial HT-29
cells. Int J Mol Med. 22:205–211. 2008.PubMed/NCBI
|
|
17
|
Egorina EM, Sovershaev TA, Hansen JB and
Sovershaev MA: BMP-2 inhibits TF expression in human monocytes by
shutting down MAPK signaling and AP-1 transcriptional activity.
Thromb Res. 129:E106–E111. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hippenstiel S, Soeth S, Kellas B, et al:
Rho proteins and the p38-MAPK pathway are important mediators for
LPS-induced interleukin-8 expression in human endothelial cells.
Blood. 95:3044–3051. 2000.PubMed/NCBI
|
|
19
|
Bahar B, O’Doherty JV, Maher S, McMorrow J
and Sweeney T: Chitooligosaccharide elicits acute inflammatory
cytokine response through AP-1 pathway in human intestinal
epithelial-like (Caco-2) cells. Mol Immunol. 51:283–291. 2012.
View Article : Google Scholar
|
|
20
|
Simunek J, Brandysova V, Koppova I and
Simunek J Jr: The antimicrobial action of chitosan, low molar mass
chitosan, and chitooligosaccharides on human colonic bacteria.
Folia Microbiol (Praha). 57:341–345. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang H, Du Y, Yu X, Mitsutomi M and Aiba
S: Preparation of chitooligosaccharides from chitosan by a complex
enzyme. Carbohydr Res. 320:257–260. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dou J, Tan C, Du Y, Bai X, Wang K and Ma
X: Effects of chitooligosaccharides on rabbit neutrophils in vitro.
Carbohydr Polym. 69:209–213. 2007. View Article : Google Scholar
|
|
23
|
Shan Y, Lin N, Yang X, et al:
Sulphoraphane inhibited the expressions of intercellular adhesion
molecule-1 and vascular cell adhesion molecule-1 through
MyD88-dependent toll-like receptor-4 pathway in cultured
endothelial cells. Nutr Metab Cardiovasc Dis. 22:215–222. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lush CW, Cepinskas G and Kvietys PR: LPS
tolerance in human endothelial cells: reduced PMN adhesion,
E-selectin expression, and NF-kappa B mobilization. Am J Physiol
Heart Circ Physiol. 278:H853–H861. 2000.PubMed/NCBI
|
|
25
|
Junkins RD, MacNeil AJ, Wu Z, McCormick C
and Lin TJ: Regulator of calcineurin 1 suppresses inflammation
during respiratory tract infections. J Immunol. 190:5178–5186.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Viatour P, Merville MP, Bours V and
Chariot A: Phosphorylation of NF-kappaB and IkappaB proteins:
implications in cancer and inflammation. Trends Biochem Sci.
30:43–52. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Luco S, Delmas O, Vidalain PO, Tangy F,
Weil R and Bourhy H: RelAp43, a member of the NF-κB family involved
in innate immune response against Lyssavirus infection. PLoS
Pathog. 8:e10030602012.PubMed/NCBI
|
|
28
|
Wu B, Yao H, Wang S and Xu R: DAPK1
modulates a curcumin-induced G2/M arrest and apoptosis by
regulating STAT3, NF-κB, and caspase-3 activation. Biochem Biophys
Res Commun. 434:75–80. 2013.PubMed/NCBI
|
|
29
|
Park EJ, Cheenpracha S, Chang LC and
Pezzuto JM: Suppression of cyclooxygenase-2 and inducible nitric
oxide synthase expression by epimuqubilin A via IKK/IκB/NF-κB
pathways in lipopolysaccharide-stimulated RAW 264.7 cells.
Phytochem Lett. 4:426–431. 2011.PubMed/NCBI
|
|
30
|
O’Connell MA, Bennett BL, Mercurio F,
Manning AM and Mackman N: Role of IKK1 and IKK2 in
lipopolysaccharide signaling in human monocytic cells. J Biol Chem.
273:30410–30414. 1998.PubMed/NCBI
|
|
31
|
Murayama R, Kobayashi M, Takeshita A, et
al: MAPKs, activator protein-1 and nuclear factor-kappa B mediate
production of interleukin-1 beta-stimulated cytokines,
prostaglandin E-2 and MMP-1 in human periodontal ligament cells. J
Periodont Res. 46:568–575. 2011.
|
|
32
|
Liu HT, Li WM, Xu G, et al: Chitosan
oligosaccharides attenuate hydrogen peroxide-induced stress injury
in human umbilical vein endothelial cells. Pharmacol Res.
59:167–175. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vo TS, Kong CS and Kim SK: Inhibitory
effects of chitooligosaccharides on degranulation and cytokine
generation in rat basophilic leukemia RBL-2H3 cells. Carbohydr
Polym. 84:649–655. 2011. View Article : Google Scholar
|
|
34
|
Bianchi G, D’Amico G, Varone L, Sozzani S,
Mantovani A and Allavena P: In vitro studies on the trafficking of
dendritic cells through endothelial cells and extra-cellular
matrix. Dev Immunol. 7:143–153. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ogawa H, Rafiee P, Heidemann J, et al:
Mechanisms of endotoxin tolerance in human intestinal microvascular
endothelial cells. J Immunol. 170:5956–5964. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Albelda SM, Smith CW and Ward PA: Adhesion
molecules and inflammatory injury. FASEB J. 8:504–512.
1994.PubMed/NCBI
|
|
37
|
Ju Y, Hua J, Sakamoto K, Ogawa H and
Nagaoka I: Modulation of TNF-α-induced endothelial cell activation
by glucosamine, a naturally occurring amino monosaccharide. Int J
Mol Med. 22:809–815. 2008.
|
|
38
|
Binion DG, Heidemann J, Li MS, Nelson VM,
Otterson MF and Rafiee P: Vascular cell adhesion molecule-1
expression in human intestinal microvascular endothelial cells is
regulated by PI 3-kinase/Akt/MAPK/NF-kappa B: inhibitory role of
curcumin. Am J Physiol Gastrointest Liver Physiol. 297:G259–G268.
2009. View Article : Google Scholar : PubMed/NCBI
|