Introduction
Along with the increasing average life-span of
humans over the past decades, human society is becoming more
concerned about the malfunctions associated with aging (1). Senile dementia is one of the most
formidable consequences of aging, including Alzheimer’s disease
(AD), which accounts for >50% of senile dementia (2). AD is characterized by neuronal loss
and the presence of extracellular senile plaques mainly constituted
by amyloid-β peptide (Aβ). Bu-Shen-Yi-Zhi prescription (BSYZ) is a
traditional Chinese compound prescription which is commonly used in
China for the treatment of AD. Due to its complex components, it is
difficult to conduct studies on its curative mechanisms.
Traditional methods for screening drug components involve a great
amount of effort with high development expenses and limited
economic return. Furthermore, a usually overlooked impediment to
drug development is the lack of objective high-throughput screening
methods for assessing drug efficacy. In this study, we investigated
the effects of different effective components of BSYZ compound on
Aβ1-42-induced apoptosis in PC12 cells. By applying real-time
cellular analysis (RTCA) for screening the main active ingredients
of BSYZ, we found that RTCA technology with biological and
pharmacological relevance amenable for high-throughput screening,
is a novel tool that can by applied for high-throughput screening.
The Bu-Shen-Yi-Zhi compound contains Fructus Cnidii (FC),
Panax ginseng, Cortex Moutan, Polygonum
multiflorum, Fructus lycii and Ligustrum lucidum
(Ait. Patent no. ZL 200610112916.1).
Materials and methods
Real-time cell electronic sensing
(RT-CES) system
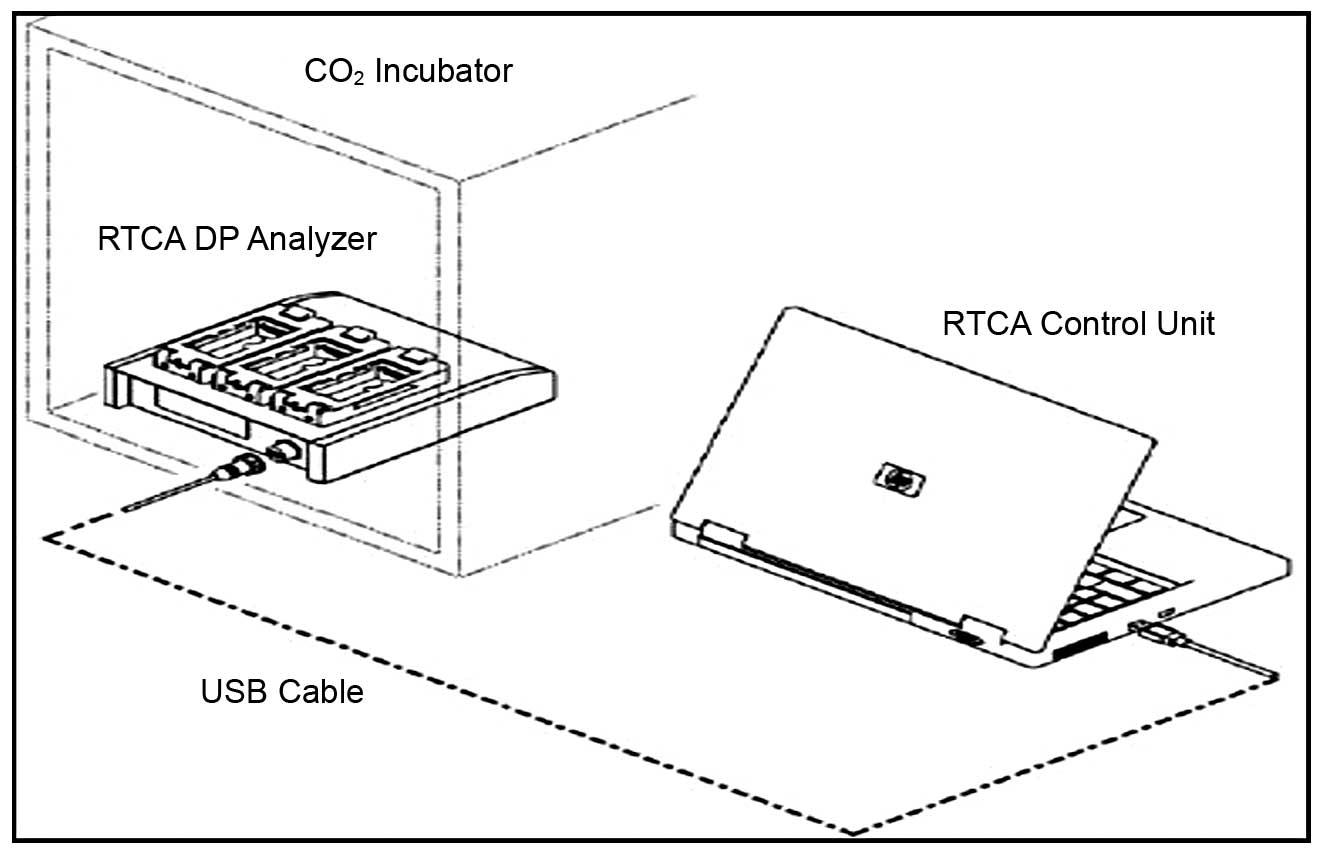
The RT-CES system (RTCA; Roche Applied Science,
Mannheim, Germany) used in this study consists of single-use
E-plates inserted into an RTCA single-plate (SP) station which is
located within the incubator. It is comprised of three components:
an electronic sensor analyzer, a device station and a 16-well strip
(Fig. 1). Giaever and Keese first
described a technique for measuring fluctuations in impedance when
a population of cells grow on the surface of electrodes (3,4).
We utilized a similar technique to measure changes in electrical
impedance as cells attach and spread in a culture dish covered with
a gold microelectrode array that covers approximately 80% of the
area on the bottom of a well. Under the RT-CES software control,
the sensor analyzer can automatically select wells to be measured
and continuously conduct measurements on wells.
Cell culture
The PC12 cells were obtained from the Shanghai
Institutes for Biological Sciences, PC12 cells were grown in DMEM
medium containing 10% heat-inactivated horse serum and 5% FBS at
37°C in a humidified atmosphere of 5% CO2/95% air.
Deployment of RT-CES in cell culture
The xCELLigence system used in this study consists
of single-use E-plates inserted into an RTCA single-plate station
which is located within the incubator. All steps were performed
under sterile conditions. The cells were passaged 1 day before the
experiments until they reached 60–80% confluence. The cells were
then trypsinized by the addition of 0.05% Trypsin/0.02% EDTA
solution at room temperature or 37°C for 1–2 min. Trypsinization
was terminated by the addition of 10% FBS-containing medium.
Subsequently, 100 μl cell suspension of 5×104 cells/well
were added to the CIM-Plate 16, and the CIM-Plate 16 was loaded
onto the RTCA DP Analyzer inside the incubator for measurements to
be taken.
Preparation of BSYZ extracts
BSYZ prescription compound was chopped and extracted
with distilled water at 80°C for 2 h. This procedure was repeated
three times. After filtering, the mixture was concentrated under
reduced pressure using a rotary evaporator to afford 50 g (1 g/ml)
of the crude water extract (WE). The BSYZ compound was extracted
with ethanol (85% v/v) for 2 h with occasional mechanical shaking,
and the extraction process was repeated, and the mixture was then
concentrated under reduced pressure using a rotary evaporator to
afford 50 g (2 g/ml) of the crude ethanol extract (EE).
Eight samples (numbered 1–8) were extracted from the
former two extractions using petroleum ether, dichloromethane,
ethyl acetate and n-butanol in this order. The crude extract was
concentrated under a vacuum and evaporated to dryness to yield a
brown, sticky fraction (F1, F2, F3, F4, F5, F6, F7 and F8; 5 g/ml)
(5). The crude extract and its
fractions were stored in a refrigerator until use.
Preparation of aged Aβ1-42
Aβ1-42 was solubilized in DMEM at 1 mm, incubated in
a capped vial at 37°C for 4 days to form aggregates (6), and stored at 20°C until further use.
For the aging procedure, the stock solutions were diluted to the
desired concentration (200, 100, 50, 25 and 12.5 μM) immediately
prior to use and added to the culture medium.
Toxicity test of Aβ1-42
The PC12 cells were cultivated in 16-well plates at
a density of 5×104 cells/well. After adhesion for 24 h,
the medium was replaced with serum-free medium at 200 μl per well.
Aβ1-42 was added at the indicated concentrations, unless otherwise
specified. The cells were first stabilized at 37°C for 24 h with
culture medium. Thereafter, the culture medium was replaced with
fresh serum-free DMEM with or without various concentrations of
Aβ1-42 (final concentrations: 12.5, 25, 50, 100 and 200 μM) for 24
h.
Toxicity test of BSYZ extracts and
fractions
The PC12 cells were seeded on a 16-well plate at a
density of 5×104 cells/well. After adhesion for 24 h,
the medium was replaced with serum-free medium at 200 μl per well.
The culture medium was replaced with fresh serum-free DMEM with or
without various concentrations of BSYZ extracts and fractions
(final concentrations: 1.5625, 3.125, 6.25, 12.5, 25, 50 and 100
μg/ml) for 24 h. There were eight fractions: 100 mg/ml stock
solution was prepared by the addition of 10 mg crude extract into
0.1 ml DMSO and dissolving by an ultrasonic dissolving instrument
(ETUS). The working solution was then prepared by diluting the
stock solution with serum-free DMEM.
Effect of BSYZ extracts against the
damaging effects of Aβ1-42
The PC12 cells were seeded onto a 16-well plate at a
density of 5×104 cells/well. After complete adhesion for
24 h, the medium was replaced with serum-free medium at 200 μl per
well. BSYZ extracts were added at the indicated concentrations. The
cultivation was continued for an additional 24 h. Subsequently, 50
μM of Aβ1-42 were added to the cells followed by incubation for
another 24 h. The control cells were treated in a similar manner
without the addition of BSYZ extracts and Aβ1-42 to the serum-free
culture medium. The cultivation was continued.
Statistical analysis
Parametric values were compared by the two-tailed,
unequal variance Student’s t-test using a minimum of four replicate
wells. The mean absolute percentage error (MAPE) was calculated as
the average of the absolute value of the percentage difference
between the test and reference well CI values over the duration of
the experiment.
Results
Cell quantification and monitoring
proliferation
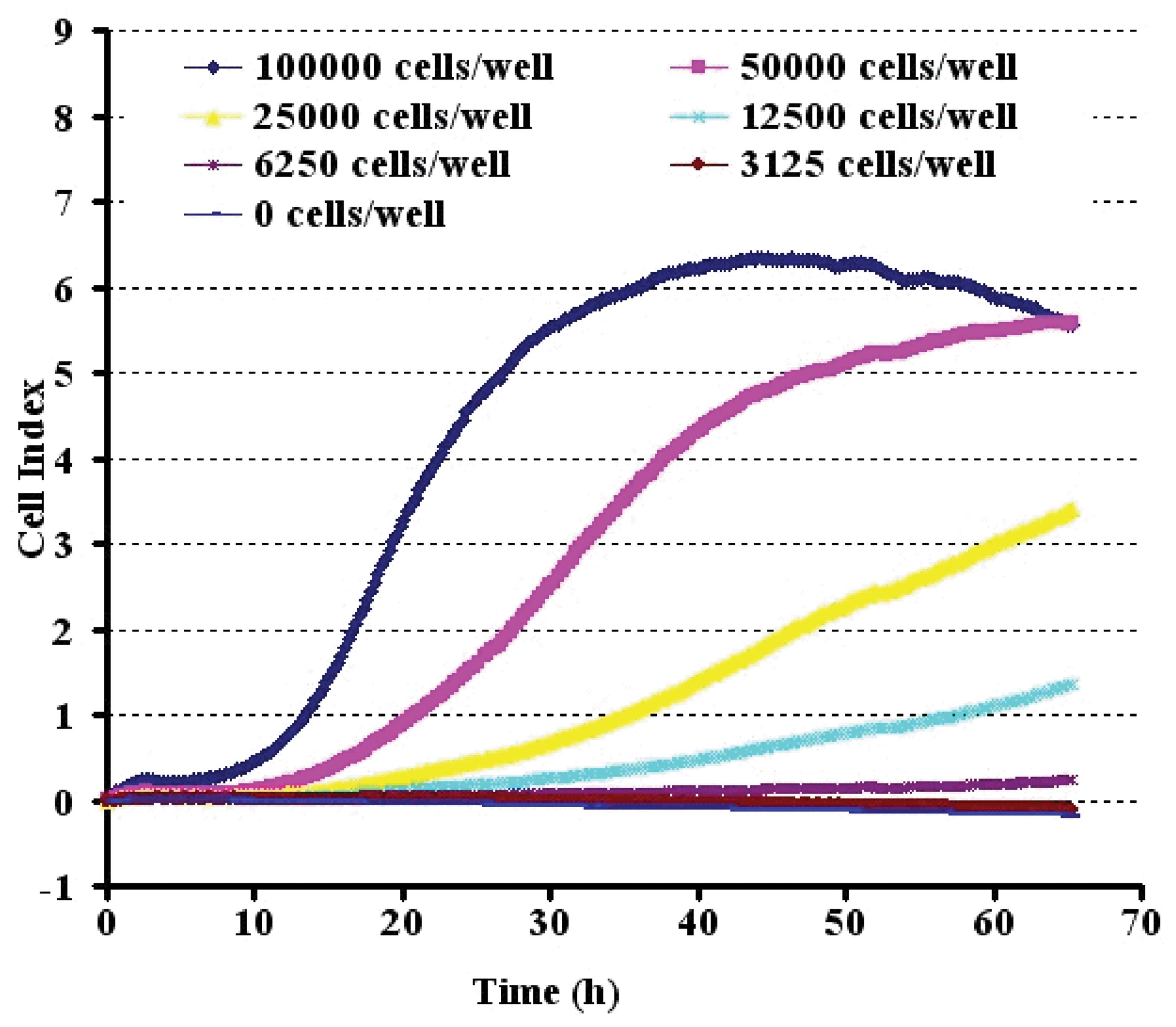
The CI values determined on the RT-CES system were
linearly correlated with the PC12 cell numbers over a range of
3,125 to 100,000 cells (Fig. 2).
To evaluate the precision in the RT-CES assay, the CI values for
each cell number were measured by three replicates of appropriate
cell numbers. The cells were seeded at 50,000 cells/well onto an
E-Plate, which was the optimal inoculation density.
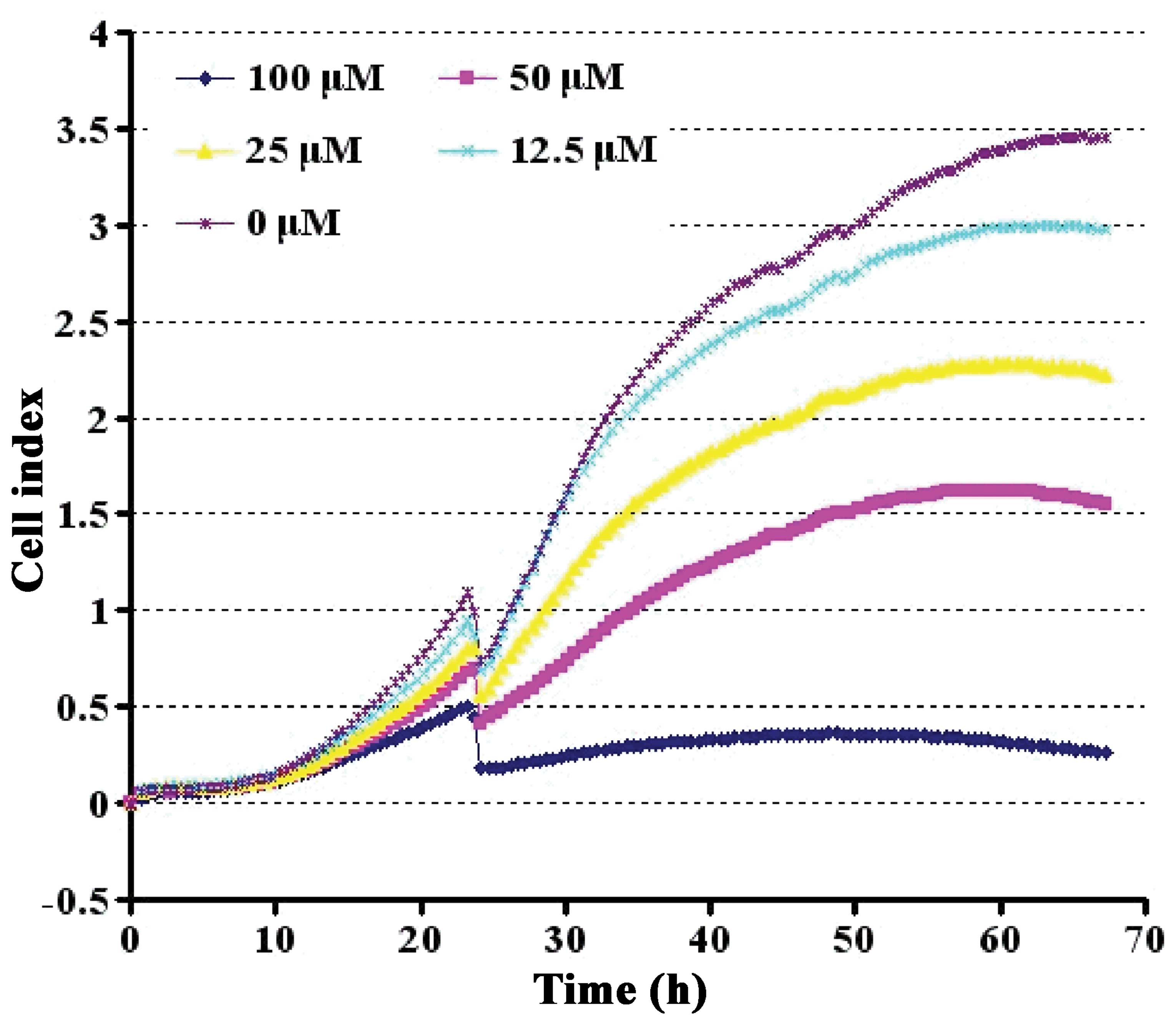
Effect of Aβ1-42 on cell viability
To determine whether the CI value obtained on the
RT-CES system quantitatively correlated with the PC12 cell numbers,
the PC12 cells were titrated and grown on the sensor devices,
resulting in device impedance signals and were accurately measured
by the RT-CES system. Aβ1-42 suppressed cell viability in a
dose-dependent manner. During the initial 48 h, cell growth was
differentially stimulated. However, a prominent suppressive effect
appeared at 60 h. At the concentration of 50 μM, Aβ1-42 reduced the
viability of the PC12 cells to approximately 50% (Fig. 3).
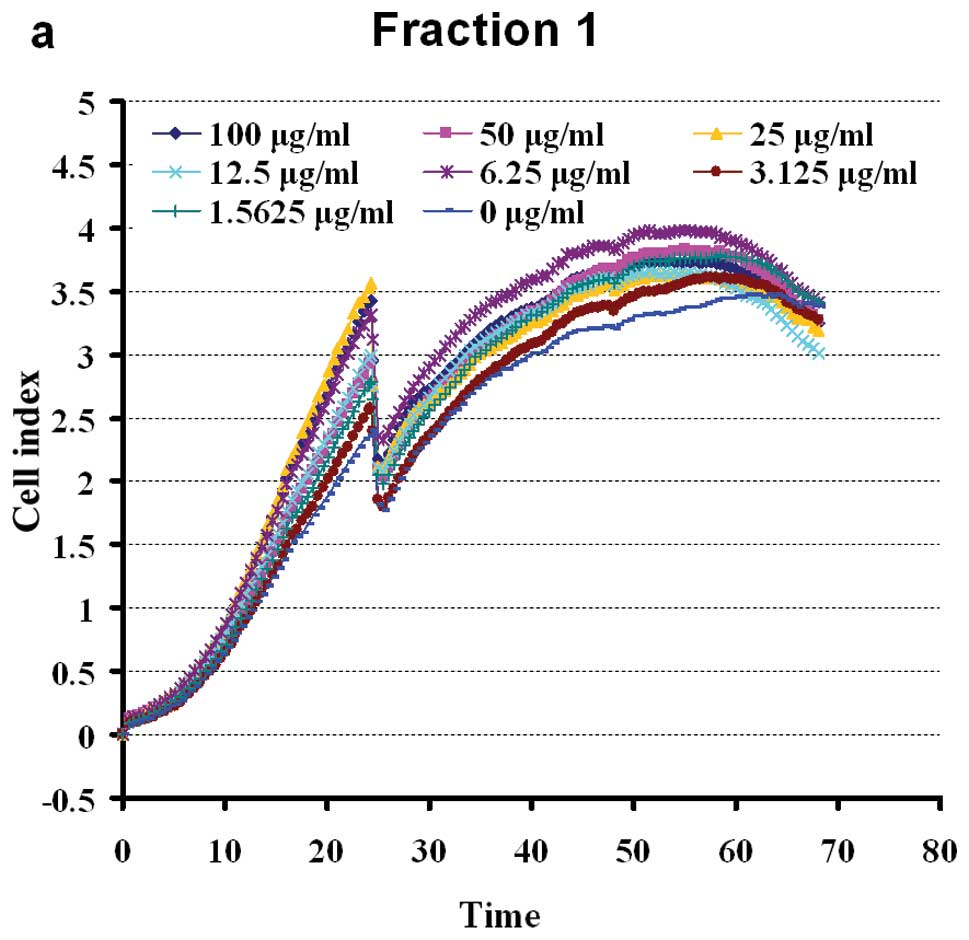
Effect of BSYZ extracts on cell
viability
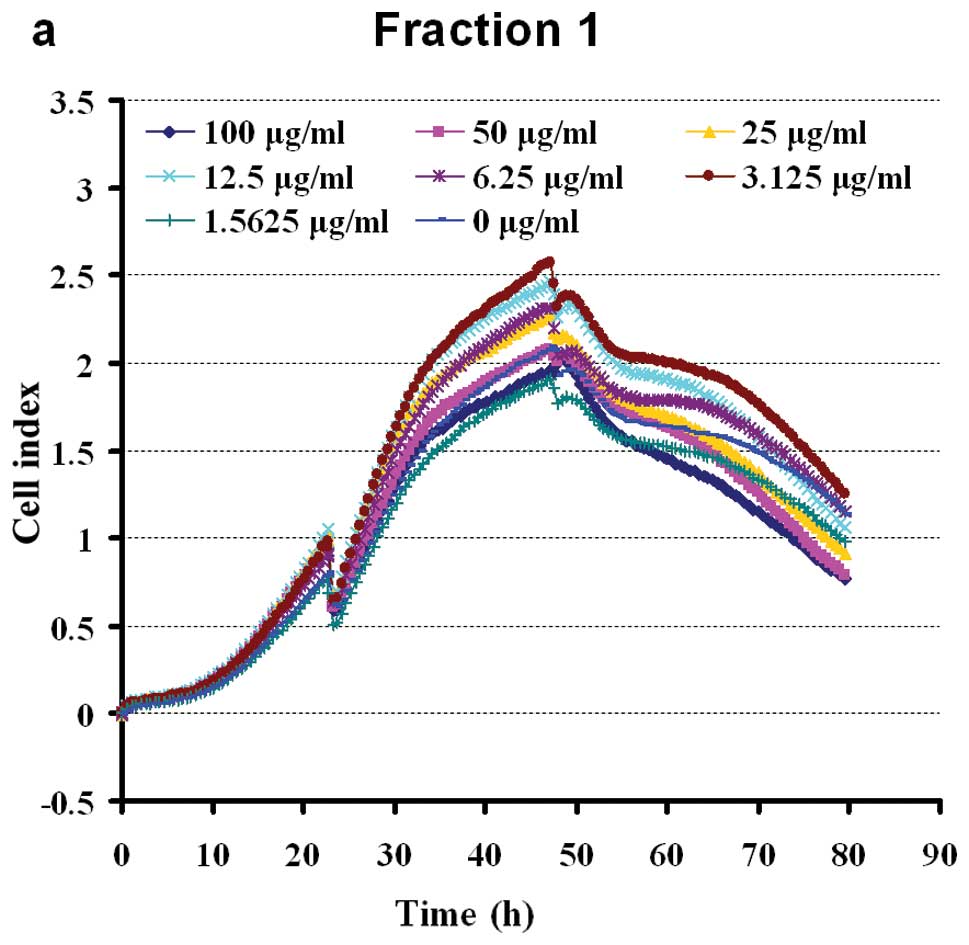
Extracts of eight fractions of BSYZ stimulated the
proliferation of PC12 cells in a dose-dependent manner. The cells
were shown to grow steadily within the dose range of 100-1.5625
μg/ml (apart from, F2). Doses of 100 μg/ml of F4 and F5 exerted
slight inhibitory effect on cell viability (Fig. 4). The results revealed a
non-dose-dependent effect on the proliferation of PC12 cells by WE
petroleum ether, dichloromethane, ethylacetate and n-butanol
fractions in early extraction. At a dose of 100-3.125 μg/ml, F3
enhanced cell viability. The aqueous fraction, F3, was the most
effective of the four aqueous fractions, inducing a growth rate of
16.18–60.18%. The effects of the crude ethanol extracts (F6–F7)
were significant at 100-1.5625 μg/ml (Fig. 4). The n-butanol fraction, F8, was
the least effective of the four ethanol fractions, enhancing the
viability of the PC12 cells to approximately 0–40.14%. The ethyl
acetate fraction (F7) had intermediate activity, inducing
significant (P<0.05) proliferation of PC12 cells at a dose of
1.5625 μg/ml (44.71%). The chloroform and ethyl acetate fractions
(F3, F6 and F7) possessed marked PC12 cell stimulating activity, as
shown in Fig. 4.
Effect of BSYZ extracts on Aβ1-42-induced
cytotoxicity
Following incubation for 48 h, the extracts of eight
fractions of BSYZ completely reversed the suppressive effect of
Aβ1-42 on cell viability (Fig.
5). The results revealed a non-dose-dependent effect on the
proliferation of PC12 cells by WE and petroleum ether,
dichloromethane ethyl acetate and n-butanol fractions. PC12 cells
were pre-treated with BSYZ extracts for 24 h prior to incubation
with or without 50 μM Aβ1-42 for another 24 h. At a dose of
100-3.125 μg/ml, F3 enhanced cell viability. The growth rate was
15.18–50.8%. The effect of the crude ethanol extract (F6 and F7)
was significant at a dose of 100-3.125 μg/ml (Fig. 5) as compared to the
vehicle-treated control group. Under the same conditions,
pre-treatment of the cells for 24 h with F6 and F7 at
concentrations of 100 and 3.125 μg/ml markedly enhanced cell
proliferation (47% and 54%). Although 50 μg/ml of the BSYZ extracts
was able to reduce Aβ1-42-induced cell death, no statistically
significant differences were observed when compared with the
Aβ1-42-treated control group. As shown in Fig. 5, incubation of the PC12 cells with
1.5625 μg/ml for 24 h markedly decreased the cell viability as
compared to the control group. The effects of all the fractions on
cell proliferation were evaluated to further investigate the
protective effects of BSYZ extracts.
Discussion
PC12 is a cell line derived obtained from a
pheochromocytoma of the rat adrenal medulla. PC12 cells have the
same characteristics as neuroendocrine cells and have the ability
to passage. Therefore, PC12 cells are widely used in
neurophysiological and neuropharmacological studies. Previous
studies have demonstrated that amyloid fibrils induce PC12 cell
death through apoptosis (7,8).
Our study first demonstrated the neuroprotective effects of BSYZ
extracts on Aβ1-42-damaged PC12 cells, a typical model of AD in a
culture system evidenced by increased cell viability and decreased
cell apoptosis. In recent decades, attention has been paid to
finding natural compounds with advantages of having anti-apoptotic
activity and low toxicity for use as neuroprotective agents
(9,10). Eight fractions, major active
components isolated from BSYZ, have been commonly used as a safe
and effective medication ingredient in China for acute
kidney-reinforcing for centuries (11–15). Over the past few years, BSYZ has
been reported to exert neuroprotective effects in vivo and
in vitro (16–20).
As previously described, the E-plates contain 16
wells in a standard microtiter plate format, with up to 16 wells
being monitored at any one time. The ease of experimentation
enables the simultaneous monitoring of different fractions or
developmental stages on the same plate. The RTCA unit that we used
was the original single-plate xCELLigence model (RTCA SP
instrument), allowing for the testing of additional samples with
PC12 cells per well. These larger scale applications may be adapted
to incorporate robotic handling for screening Chinese herbal
compounds for their efficacy.
We firstly established the optimum growth of PC12
cells on the microelectrodes in the microwells and evaluated the
linear correlation between the cell index and cell numbers without
particle treatment in order to develop a quantitative measurement
of cell response to particles. PC12 cells have previously been
shown to be more sensitive to Aβ1-42. As shown in Fig. 2, the sensing curves of cell index
versus incubation time, demonstrating typical cell growth curves of
PC12 cells with initial seeding cell numbers approximately 50,000
cells per well under optimized conditions for the RT-CES
experiments. At time zero, no cells are attached to the
microelectrodes, thus the cell index is zero. With increasing
numbers of cells attaching to the microelectrodes over time, the
cell index increased. During the log phase of the cell growth,
Aβ1-42 suppressed PC12 cell viability in a dose-dependent manner.
As shown in Fig. 3, approximately
half of the viability suppression was achieved with treatment at a
dose of 50 mM Aβ1-42; hence, the 50% lethal dose value of Aβ1-42
was estimated to be 50 μM. This dose was thus selected for use in
the following experiments. BSYZ extracts, at a dose within the
range of 50-1.5625 μg/ml, had significant effects on cell
viability, stimulating PC12 cell proliferation. At doses >50
μg/ml, the cell viability was slightly suppressed (Fig. 4). As shown in Fig. 5, BSYZ exerted a non-dose-dependent
on the proliferation of PC12 cells. The effects of the drug were
inconsistent with those shown in Fig.
4.
The approach applied in this study has the
advantages of easy handling, high sensitivity, real-time monitoring
and is thus useful for screening drugs. This approach provides
information not only on the onset time a drug or chemical acts on
cells, but also information on cell physiology. Thus, we expect to
identify the cytotoxicities at different stages for various BSYZ
extracts concentrations. In order to determine the status of PC12
cells upon co-incubation with Chinese herbal compounds, the cell
cycle and cell apoptosis were examined using the RT-CES system. The
time phase of the cell cycle and cell apoptosis analysis reported
in our study focused on 64 samples of crude extract of BSYZ
(Figs. 4 and 5). Although the RT-CES system can be
used for the detection of previously unexpected effects, it is a
rather unspecific method and the conclusions about the nature
and/or extent of an observed effect can be drawn if the relevant
molecular mechanisms associated with the tested samples are
investigated simultaneously. In conclusion, we present a novel use
of a RTCA device (xCELLigence) that can simply and objectively
assess the effectiveness of anti-AD drugs in real-time by measuring
motility in a high-throughput, reproducible manner with minimal
effort and training required.
Acknowledgements
The authors would like to thank Professor Ying-jie
Hu (Guangzhou University of Chinese Medicine) for providing the
BSYZ extracts. This study was supported by the National Natural
Science Foundation of China (no. 81273817), the Doctoral Fund of
Education Ministry of China (nos. 20114425110007 and
20124425120016), Guangdong Provincial Major Science and Technology
for Special Program of China (no. 2012A080202017), Guangdong
Provincial Department of Science and Technology Foundation of China
(no. 2010A030100009), Guangdong Provincial Natural Science
Foundation of China (no. S2012040006514), the Scientific and
Technical Innovation Project of Guangdong Provincial Education
Department of China (no. 2012KJCX0032), ands the Characteristic Key
Discipline Construction Fund of Chinese Internal Medicine of
Guangzhou University of Chinese Medicine.
References
|
1
|
Li XL, Wang de S, Zhao BQ, Li Q, et al:
Effects of Chinese herbal medicine fuzhisan on aged rats. Exp
Gerontol. 43:853–858. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Holliday R: The urgency of research on
ageing. Bioessays. 18:89–90. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Giaever I and Keese CR: Monitoring
fibroblast behavior in tissue culture with an applied electric
field. Proc Natl Acad Sci USA. 81:3761–3764. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Giaever I and Keese CR: Micromotion of
mammalian cells measured electrically. Proc Natl Acad Sci USA.
88:7896–7900. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ifeoma O, Samuel O, Itohan AM and Adeola
SO: Isolation, fractionation and evaluation of the antiplasmodial
properties of Phyllanthus niruri resident in its chloroform
fraction. Asian Pac J Trop Med. 6:169–175. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Feng Z and Zhang JT: Melatonin reduces
amyloid beta-induced apoptosis in pheochromocytoma (PC12) cells. J
Pineal Res. 37:257–266. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thorn DC, Meehan S, Sunde M, et al:
Amyloid fibril formation by bovine milk kappa-casein and its
inhibition by the molecular chaperones alphaS-and beta-casein.
Biochemistry. 44:17027–17036. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Onoue S, Ohshima K, Endo K, Yajima T and
Kashimoto K: PACAP protects neuronal PC12 cells from the
cytotoxicity of human prion protein fragment 106–126. FEBS Lett.
522:65–70. 2002.PubMed/NCBI
|
|
9
|
Xing QL, Chen JW and Ma RQ: Protective
effect of panaxatriol saponins on cerebral ischemia. J Sun Yat-sen
Univ (Medical Sciences). 29:705–710. 2008.(In Chinese).
|
|
10
|
Yang HJ and Wei CY: The clinical approach
of ginseng chemical composition. Renshen Yanjiu. 2:4–5. 1999.(In
Chinese).
|
|
11
|
Tan RJ: Tan RJ: The research progress on
pharmacological effects of Polygonum multiflorum. Anthology
of Medicine. 1:145–148. 2000.(In Chinese).
|
|
12
|
Li GS, Tian JW, Feng FH and Yang JQ:
Protective effect of ginsenoside Rb3 from rat cortex mitochondrial
injuries due to cerebral ischemia. Chin J New Drug. 15:518–522.
2006.(In Chinese).
|
|
13
|
Dai CF and Yang XY: The study on recent
development of Fructus Ligustri Lucidi. Shanxi J Tradit Chin
Med. 1:44–46. 1999.(In Chinese).
|
|
14
|
Cheng SY, Chen YB and Wang Q: The
experimental research on protective effect of Bu-Shen-Yi-Zhi
compound in Aβ25–35 injured PC12 cells. J N Chin Med. 5:102–104.
2010.(In Chinese).
|
|
15
|
Fang CW: The study on Harvesting and
production processing method of Cortex Moutan. J Chin Med
Mat. 2:82–83. 2000.(In Chinese).
|
|
16
|
Qiao ZL, Guo L and Li F: The effects of
Bu-Shen-Yi-Zhi compound on central neurotransmitter in Alzheimer’s
disease model rats. Chin Arch Tradit Chin Med. 12:2565–2568.
2009.(In Chinese).
|
|
17
|
Li FJ: The medical food aplication of
barbary wolfberry fruit. Lishizhen Med Materia Medica Res.
4:901999.(In Chinese).
|
|
18
|
Zhong ZG, Liu MC, Lai SL, Gao J and Cheng
SY: The effects of Bu-Shen-Yi-Zhi compound on central
neurotransmitter in Alzheimer’s disease model rats. Chin J Clin
Rehabil. 44:167–170. 2005.
|
|
19
|
Cheng SY, Zhong ZG, Liu MC and Lai SL: The
experimental research on protective effect of Bu-Shen-Yi-Zhi
compound in AD cell model. Chin Med Res. 4:15–18. 2003.(In
Chinese).
|
|
20
|
Zhang KH, Lai SL, Wang Q, Cheng SY and
Chen YB: The effects of Bu-Shen-Yi-Zhi compound on Space
exploration learning and memory function in AD animal model.
Chinese Traditional and Herbal Drugs. 12:40–42. 2002.(In
Chinese).
|