Introduction
As the leading cause of death in economically
developed countries and the second leading cause of death in
developing countries, cancer is a major public health concern
worldwide (1). Atkin et al
have reported that colorectal cancer is the third most common
cancer worldwide and has a high mortality rate (2). Grady and Carethers have confirmed
that colorectal cancer developed as a consequence of the
accumulation of genetic alterations, such as gene mutation and gene
amplification, and epigenetic alterations, including aberrant DNA
methylation and chromatin modification that is able to transform
colonic epithelial cells into colonic adenocarcinoma cells
(3). Due to the high mortality,
there is a need to investigate the pathogenesis and molecular
mechanism of colorectal cancer.
During the last 15 years, the focus has been on
recognition of the ‘serrated neoplastic pathway’ and has led to a
paradigm shift in our understanding of the molecular basis of
colorectal cancer and significant changes in clinical practice
(4). The changes that have
occurred in the DNA sequence of the genomes of cancer cells result
in the development of various types of cancer (5) and multiple gene expression patterns
are altered during the evolution of normal cells to cancer cells.
Furthermore, genome-wide analysis of the gene expression has been
largely used to identify important genes of human cancers (6). The gene expression profile has been
previously characterized in various types of human cancer,
including prostate, colorectal and epithelial ovarian cancer
(6–8).
In addition, it has been reported that genetically
altered core pathways and regulatory processes become evident once
the coding regions of the genome are analyzed in depth, while
dysregulation of these core pathways and processes through mutation
can explain the major features of tumorigenesis (7). The development of cancer depends on
the abnormal activation of signal transduction pathways that
control the growth and survival of cells (8). Therefore, various signaling pathways
are altered in the pathogenesis of cancer. Activation of the
signaling pathway of hypoxia inducible factor (HIF) is crucial in
the progression of physiological development and tumor growth
(9). Activation of the Wnt
signaling pathway promotes neoplastic transformation in humans
(8). Other signaling pathways
such as gefitinib-sensitizing EGFR, β-catenin-Tcf, and p53 have
also been reported to be dysregulated in cancer (12–14).
By binding to specific DNA sequences within the
promoter regions of target genes, transcription factors (TFs) are
able to regulate DNA expression (10). Findings of previous studies
identified several cancer-related TFs, such as TMPRSS2 and
ETS in prostate cancer (11). KLF4 and KLF5 affect
proliferation, apoptosis and invasion in esophageal cancer cells by
regulating a number of genes (12). NF-κB has an impact on the
development and progression of cancer by affecting cell
proliferation, migration, and apoptosis (13).
The transcriptome profile of human colorectal
adenomas has been previously characterized (14), however, the molecular mechanism
involved remains to be determined. Galectin-3 is a human galectin
(galactose-binding lectin) family member and is expressed by many
types of cells. The concentration of galectin-3 is increased to
almost 31-fold in the blood circulation of colorectal cancer
patients and the increased concentration of circulating galectin-3
correlates closely with cancer progression and metastasis (15). Recently, we revealed that
galectin-3, at concentrations similar to those found in the
circulation of cancer patients, interacts with mucin protein MUC1,
promoting cancer metastasis (16,17). As the Galectin-3 protein is
encoded by the LGALS3 gene, the possibility that the
LGALS3-related network likely represents a fundamental mechanism in
promoting colon cancer metastasis was examined. In the present
study, differentially expressed genes (DEGs) between colorectal
cancer and normal cells were identified and functional analyses
were subsequently performed. The TFs were then predicted and a
LGALS3-related protein-protein interaction (PPI) network was
constructed. Based on this bioinformatics information, the roles of
LGALS3 and signaling pathways were analyzed in the pathogenesis of
colorectal cancer.
Materials and methods
Affymetrix microarray data
The Affymetrix microarray data were accessible at
the National Center for Biotechnology Information Gene Expression
Omnibus data repository (http://www.ncbi.nlm.nih.gov/geo/) using the series
accession number GSE8671 (14).
In total, 32 adenomas and 32 normal colonic epitheliums were
collected based on the GPL570 (HG-U133-Plus-2) Affymetrix Human
Genome U133 plus 2.0 Array. The original data were converted into
expression measures and normalized by the robust multiarray average
(RMA) algorithm (18).
Identification and gene ontology analysis
of DEGs
The DEGs were identified by using Significant
Analysis of Microarray (SAM) with |logFC| >1.5 and a false
discovery rate (FDR)<0.05 (δ=1) (19). GO analysis (20) was performed on the top 500
upregulated and 500 downregulated DEGs using DAVID (Database for
Annotation, Visualization, and Integrated Discovery) (21). The biological process with
P<0.05 considered statistically significant were screened in the
present study.
Signaling pathway impact analysis
The signaling pathway impact analysis (SPIA) was
performed to predict the signaling pathways that the DEGs would
likely impact. SPIA combines the evidence obtained from the
classical enrichment analysis with a novel type of evidence, which
measures the actual perturbation on a given pathway under a given
condition (22). In SPIA, pG
combines enrichment pNDE and perturbation pPERT, and is then
adjusted to pGFdr. In the present study, pGFdr<0.05 was set as a
threshold.
Predication of transcription factors
TFatS (www.tfacts.org) was used as a
bioinformatics tool to evaluate the transcription factor target
genes among the list of regulated genes (23). The top 500 upregulated and 500
downregulated genes were mapped to TfactS to identify target genes
with p<0.05, q<0.05, E<0.05 and FDR<0.05. In addition,
the Fisher’s exact test was used to examine whether the
transcription factor was activated or suppressed.
Protein-protein interaction (PPI) network
for LGALS3
LGALS3 was submitted to STRING database to predict
the potential interacted proteins. STRING (www.//string.embl.de) is a database of predicted
functional associations between proteins (24). STRING database produces a score to
estimate the accuracy of each pairwise association from 0 to 1. In
the present study, the PPIs were screened with score >0.7. The
PPI network was subsequently visualized using Cyoscape software
(25).
Results
Identification and GO analysis of
DEGs
Based on SAM analysis, a total of 6,593 upregulated
and 5,897 downregulated DEGs were identified. Subsequently, the GO
analysis was performed to the top 500 upregulated and 500
downregulated genes, respectively (Table IA and B). The results showed that
41 downregulated DEGs, including CLDN8 and CLDN23, were enriched in
cell adhesion (P=2.23E-06) (Table
IA). The upregulated DEGs which included KIF23,
PRC1, TTK, AURKA, AURKB, PTTG1,
and RUVBL1 were mainly enriched in the terms associated with
cell cycle, such as the mitotic cell cycle (P=3.74E-34) and cell
cycle process (P=3.49E-29) (Table
IB).
 | Table IThe enriched GO terms. |
Table I
The enriched GO terms.
| A, The top 10 GO
terms of the top 500 upregulated DEGs |
|---|
|
|---|
| Category | Term | Count | Genes | P-value |
|---|
| GO:0000278 | Mitotic cell
cycle | 67 | KIF23, PRC1,
TTK | 3.74E-34 |
| GO:0022402 | Cell cycle
process | 75 | AURKA, AURKB,
PTTG1 | 3.49E-29 |
| GO:0000280 | Nuclear
division | 49 | KIF23, AURKA,
PTTG1 | 4.99E-29 |
| GO:0007067 | Mitosis | 49 | KIF23, AURKA,
PTTG1 | 4.99E-29 |
| GO:0000087 | M phase of mitotic
cell cycle | 49 | KIF23, AURKA,
PTTG1 | 1.19E-28 |
| GO:0022403 | Cell cycle
phase | 64 | KIF23, PRC1,
TTK | 1.65E-28 |
| GO:0048285 | Organelle
fission | 49 | KIF23, PTTG1,
AURKA | 3.43E-28 |
| GO:0007049 | Cell cycle | 85 | KIF23, PCR1,
CDK2 | 3.01E-27 |
| GO:0000279 | M phase | 65 | PCR1, KIF23,
AURKA | 5.30E-27 |
| GO:0051301 | Cell division | 46 | PRC1, KIF23,
CDK1 | 1.49E-20 |
|
| B, The enriched
terms of the top 500 downregulated DEGs |
|
| Category | Term | Count | Genes | P-value |
|
| GO:0007155 | Cell adhesion | 41 | CLDN8, CLDN23 | 2.23E-06 |
| GO:0022610 | Biological
adhesion | 41 | CLDN8, CLDN23 | 2.27E-06 |
| GO:0007584 | Response to
nutrient | 14 | BMP2, A2M | 7.14E-05 |
KEGG pathways analysis
Based on SPIA analysis, a total of 21 KEGG signaling
pathways were screened to determine whether they were dysregulated
in colorectal cancer (Table II).
Then the cell cycle (pGFdr=3.00E-04), p53 signaling pathway
(pGFdr=8.82E-03), and NF-κB signaling pathway (pGFdr=3.77E-02),
which significantly correlated with cancer were selected for
subsequent investigation. In detail, cyclin-dependent kinase genes,
such as CDK1, CDK2, CDK4, CDK6 and
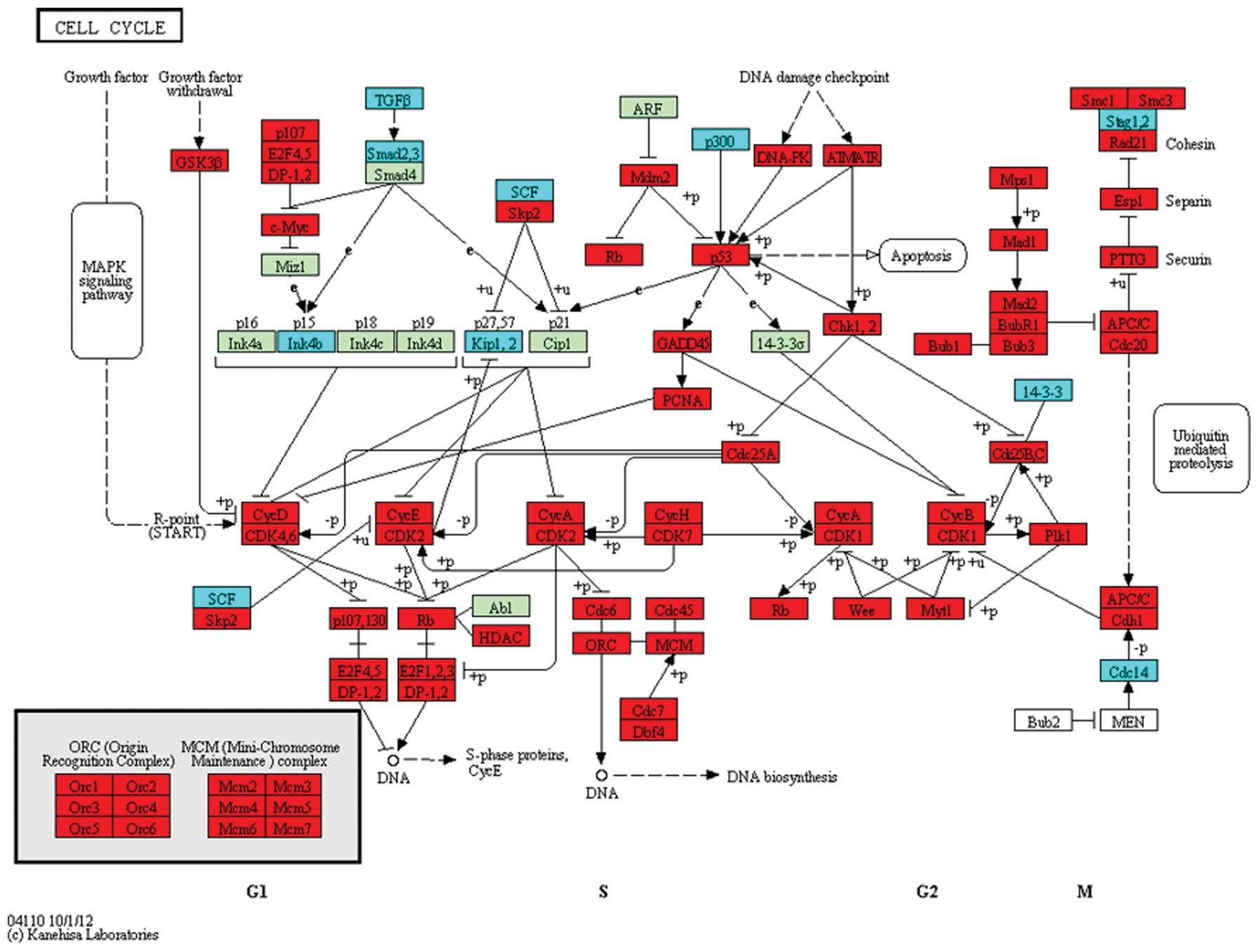
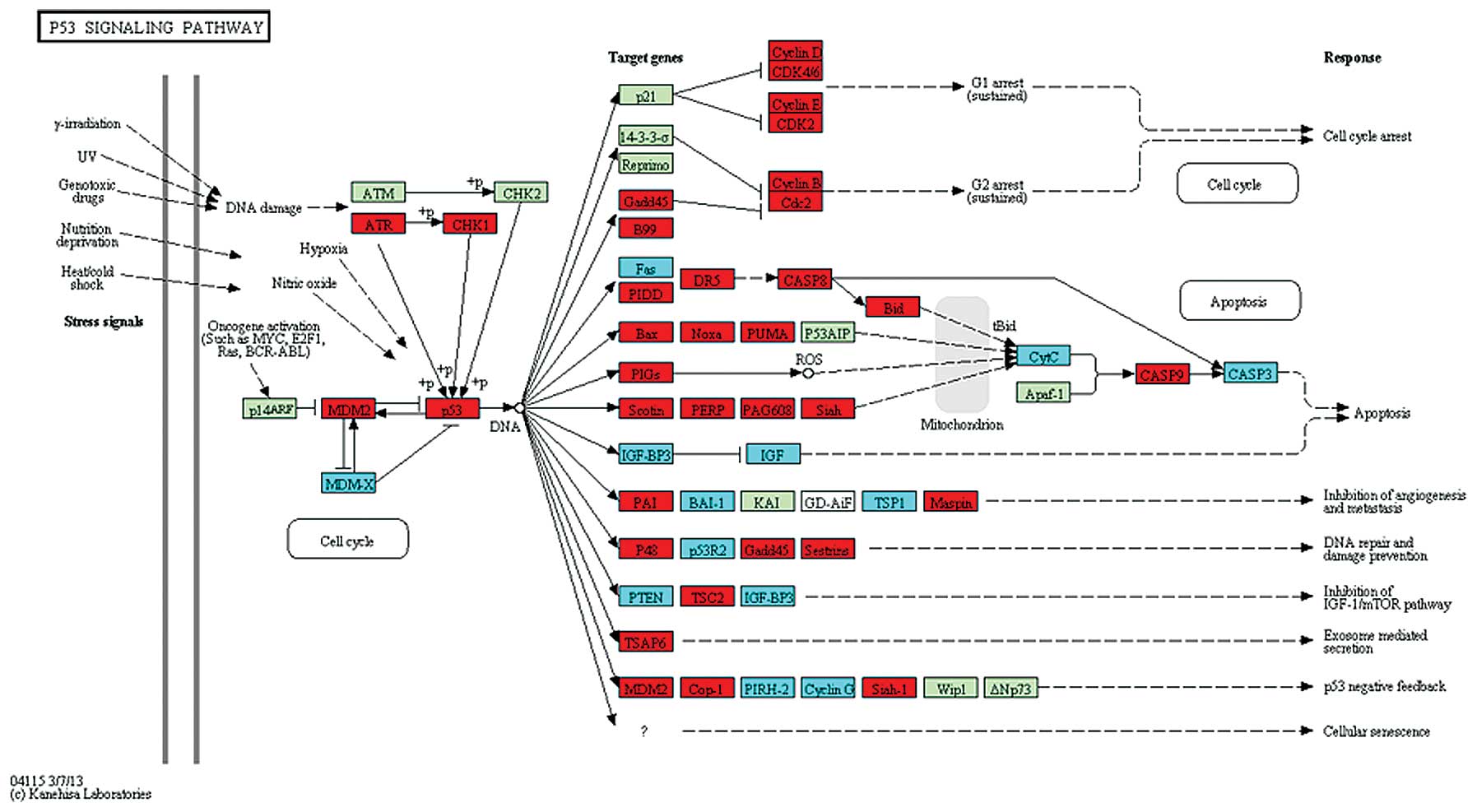
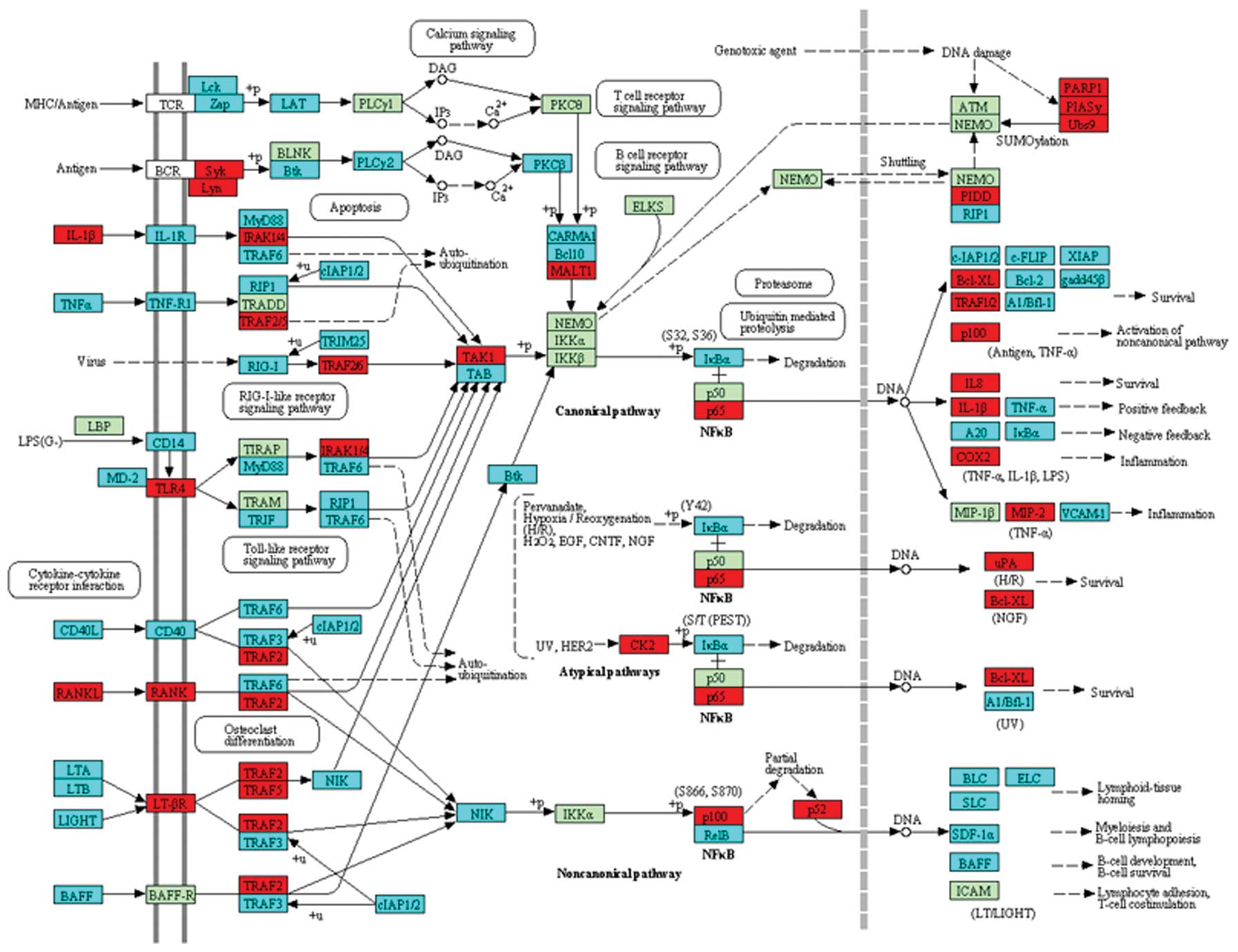
CDK7 were upregulated in the cell cycle pathway (Fig. 1). In the p53 signaling pathway,
ATR and p53 were upregulated (Fig. 2), while in the NF-κB pathway,
TRAFs were significantly differentially expressed (Fig. 3).
 | Table IIThe 21 pathways identified based on
signaling pathway impact analysis (pGFdr<0.05). |
Table II
The 21 pathways identified based on
signaling pathway impact analysis (pGFdr<0.05).
| Pathway | Count | Genes | pGFdr |
|---|
| RNA transport | 125 | XPOT, NCBP1,
DDX20 | 9.08E-09 |
| HTLV-1
infection | 195 | NRP1, SLC2A1,
TGFB3 | 3.51E-05 |
| Natural killer
cell-mediated cytotoxicity | 87 | NFNT5, PPP3CB,
TNFSF10 | 3.00E-04 |
| Cell cycle | 97 | CDK1, CDK2,
MCM2 | 3.00E-04 |
| Epstein-Barr virus
infection | 150 | CR2, HLA-DRA,
CD38 | 1.44E-03 |
| Fanconi anemia
pathway | 43 | FANCM, FANCI,
FANCF | 1.83E-03 |
| Antigen processing
and presentation | 54 | CD74, HLA-DMA,
NFYA | 2.35E-03 |
| Chemokine signaling
pathway | 132 | CXCR6, CCR1,
CXCR3 | 8.80E-03 |
| Staphylococcus
aureus infection | 37 | CFD, FCGR2B,
HLA-DMA | 8.80E-03 |
| P53 signaling
pathway | 56 | P53, ATR, CDK2 | 8.82E-03 |
| Fc γ R-mediated
phagocytosis | 66 | FCGR2B, HCK,
LYN | 1.11E-02 |
| Pathways in
cancer | 236 | CASP3, CTNNB1,
WNT2 | 2.29E-02 |
| Protein processing
in endoplasmic reticulum | 124 | MAPK9, SEC61B,
VCP | 2.29E-02 |
| RNA
degradation | 57 | EN01, TTC37,
EXOSC9 | 2.29E-02 |
| Oocyte meiosis | 86 | CDK1, MAD2L1,
CCNB2 | 2.35E-02 |
| Focal adhesion | 139 | ITGB3, ITGA8,
FLNA | 2.39E-02 |
| Systemic lupus
erythematosus | 66 | FCGR2B, C5,
TNF | 2.39E-02 |
| Gap junction | 64 | CSNK1D, PRKCB,
GNAI3 | 2.39E-02 |
| NF-κB signaling
pathway | 70 | TRAF5, BCL2L1,
BCL2 | 3.77E-02 |
| Lysosome | 94 | TCTRG1, ATP6VOA2,
CTCS | 3.85E-02 |
| T-cell receptor
signaling pathway | 83 | CDK4, TNF,
CSF2 | 4.43E-02 |
Regulation of DEGs by transcription
factors
TFactS analysis was performed to determine changes
in transcription factor activity based on upregulated and
downregulated genes in colorectal cancer (Table III). The results showed that
MYC and TCF7L2 were activated in colorectal cancer. A
total of 26 target genes of MYC were identified, including 24
upregulated and 2 downregulated genes, while for TCF7L2, 8 target
genes were upregulated and 2 genes were downregulated. Of note,
TCF7L2 was activated by MYC. Additionally, 9 target
genes of FOXO3 were downregulated and 1 gene was
upregulated.
 | Table IIIResults of the TfactS analysis. |
Table III
Results of the TfactS analysis.
| Gene name | TF | Regulation
type | Differential
expression type |
|---|
| ID1 | FOXO3 | Down | Up |
| TNFSF10 | FOXO3 | Up | Down |
| KLF4 | FOXO3 | Up | Down |
| BTG1 | FOXO3 | Up | Down |
| PINK1 | FOXO3 | Up | Down |
| SFRP1 | FOXO3 | Up | Down |
| BCL2L11 | FOXO3 | Up | Down |
| HPGD | FOXO3 | Up | Down |
| CDKN2B | FOXO3 | Up | Down |
| CITED2 | FOXO3 | Up | Down |
| MYC | MYC | Down | Up |
| DUSP1 | MYC | Down | Down |
| CDKN2B | MYC | Down | Down |
| PCNA | MYC | Up | Up |
| RFC2 | MYC | Up | Up |
| RCC1 | MYC | Up | Up |
| NOP56 | MYC | Up | Up |
| NME1 | MYC | Up | Up |
| CCT6A | MYC | Up | Up |
| C1QBP | MYC | Up | Up |
| NPM1 | MYC | Up | Up |
| CCNB1 | MYC | Up | Up |
| CDK4 | MYC | Up | Up |
| ODC1 | MYC | Up | Up |
| CKS2 | MYC | Up | Up |
| CCNA2 | MYC | Up | Up |
| SNRPB | MYC | Up | Up |
| PPAT | MYC | Up | Up |
| APEX1 | MYC | Up | Up |
| MIF | MYC | Up | Up |
| H2AFZ | MYC | Up | Up |
| TRAP1 | MYC | Up | Up |
| MTHFD1 | MYC | Up | Up |
| TP53 | MYC | Up | Up |
| TYMS | MYC | Up | Up |
| UBE2C | MYC | Up | Up |
| CCT3 | MYC | Up | Up |
| CASP7 | TCF7L2 | Down | Down |
| MXD1 | TCF7L2 | Down | Down |
| MYC | TCF7L2 | Up | Up |
| ENC1 | TCF7L2 | Up | Up |
| MMP7 | TCF7L2 | Up | Up |
| MMP1 | TCF7L2 | Up | Up |
| AXIN2 | TCF7L2 | Up | Up |
| PTTG1 | TCF7L2 | Up | Up |
| CD44 | TCF7L2 | Up | Up |
| SP5 | TCF7L2 | Up | Up |
| SGK1 | TCF7L2 | Up | Down |
| CAPN2 | TCF7L2 | Up | Down |
| TAGLN | TCF7L2 | Up | Down |
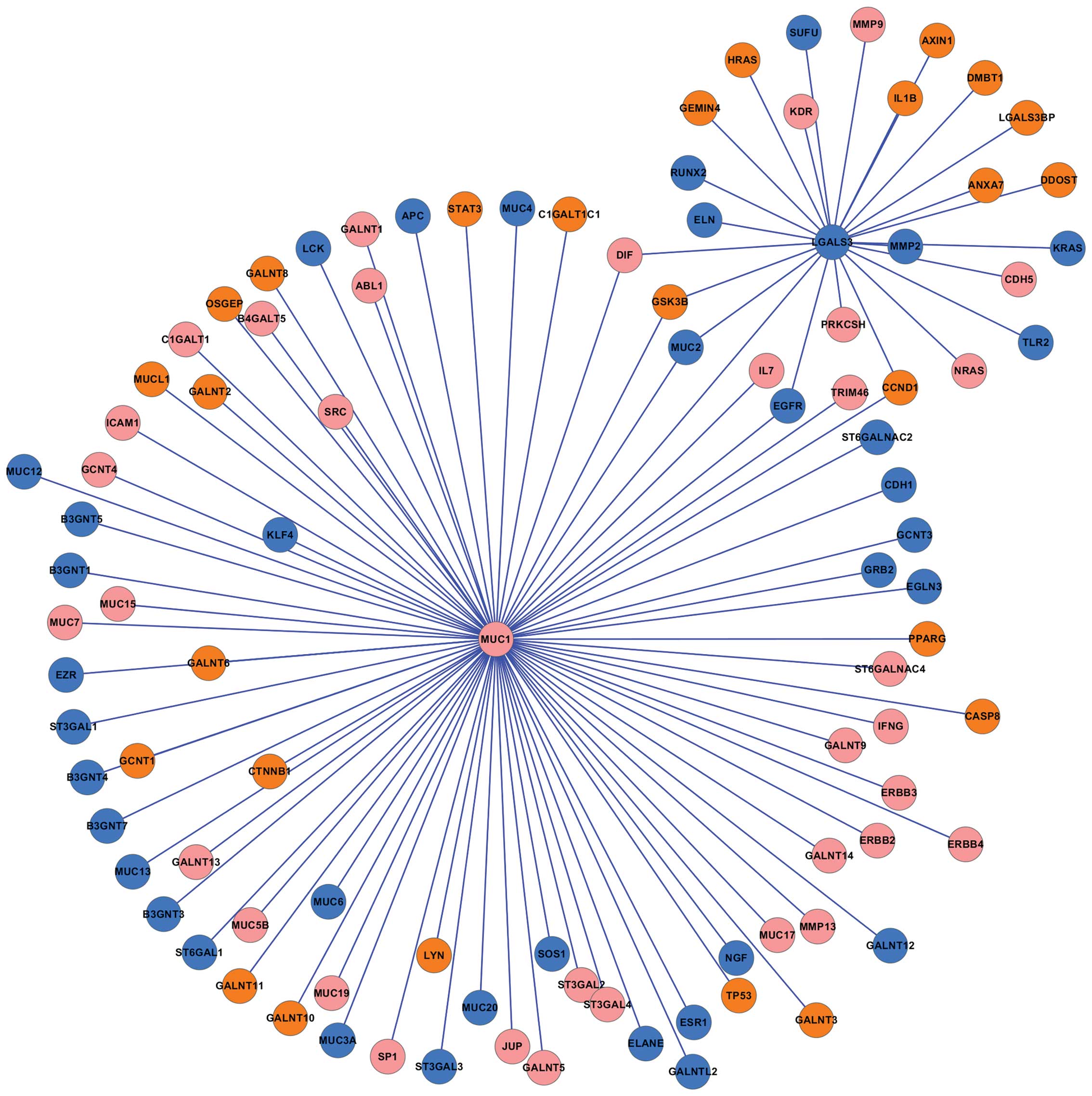
The LGALS3 PPI network
Tumor metastasis is the primary cause of mortality
in patients with cancer (26).
LGALS3, a member of a family of β-galactoside-binding lectins, has
been found to promote tumor metastasis (22,23). To investigate the function of
LGALS3 in colorectal cancer, the LGALS3-related PPI network was
constructed (Fig. 4). The results
predicated that 8 proteins (SUFU, RUNX2, ELN, MUC2, EGFR, TLR2,
KRAS, and MMP2) which were encoded by downregulated genes
interacted with LGALS3, while 10 proteins (HRAS, GEMIN4, GSK3B,
CCND1, ANXA7, DDOST, LGALS3BP, DMBT1, IL1B, and AXIN1) encoded by
upregulated genes interacted with LGALS3. In addition, no
significant changes in the expression levels of MMP9,
KDR, DIF, PRKCSH, NRAS, and CDH5
were observed, however, the proteins encoded by these genes
interacted with LGALS3.
Discussion
Colorectal cancer is the third most common type of
cancer worldwide and has a high mortality rate (2). Although a number of studies have
been conducted, the underlying mechanism of colorectal cancer
remains to be clarified. In this study, the DEGs were identified
between colorectal cancer and normal samples and their functions
were predicted by GO analysis. The pathways which these DEGs
dysregulated and the TFs were identified. A LGALS3-related PPI
network was also established. Our findings provide a new angle for
the prediction of the pathogenesis of colorectal cancer.
The GO enrichment analysis revealed that the
upregulated genes were mainly enriched in cell proliferation
processes, including mitotic cell cycle, cell cycle progression,
nuclear division and cell division of tumor. The oncogene
AURKA, enriched in the cell cycle, is an important protein
that regulates G2 transit into M during mitosis (27). In addition, AURKA is
associated with abnormal chromosome segregation, aneuploidy and
predisposition (28). Previously,
it was suggested that pituitary tumor transforming gene 1
(PTTG1) is an oncogene (29). The expression levels of
RUVBL1 and RUVBL2 were increased in different types
of cancer and interacted with oncogenic factors, including
β-catenin and c-Myc to regulate their function (30). These upregulated genes led to
abnormal cell accumulation in order to accelerate the process of
colorectal cancer.
The downregulated genes, including CLDN8 and CLDN23,
in colorectal cancer were significantly enriched in the cell
adhesion biological process. Claudins, major components of the
strands, promote cell-cell adhesion (31). CLDN8 codes for tight junction
proteins expressed in distal nephron epithelium, and it is
considered a candidate marker for distinguishing chromophobe renal
cell carcinoma from other types of renal cancer (32). In addition, CLDN23 gene,
frequently downregulated in intestinal-type gastric cancer, is a
novel member of CLAUDIN gene family (33). Findings of the present study are
consistent with those of previous studies.
The role of the signaling pathway in cancer
pathogenesis has been previously investigated (34). Alterations in cyclin-dependent
kinase (CDK) activity often leads to cell cycle defects in tumor
growth (35). In the present
study, CDK2, CDK4 and CDK6 were enriched in the cell cycle pathway.
This result indicates that these DEGs are important in the
development of colorectal cancer by dyregulating the cell cycle
pathway. Previously, it has been shown that one of the most
prominent regulators disrupted in cancer is the tumor suppressor,
p53 (36). TRAF (TNF
receptor-associated factor) family member-associated NF-κB
activator is a negative regulator of osteoclastogenesis and bone
formation (37). NF-κB is one of
the best-characterized transcription factors involved in the
regulation of immune responses and inflammation (38,39). It has been previously suggested
that inhibition of the NF-κB signaling pathway presents a notable
therapeutic potential for the diagnosis of cancer (40). Results of this study have shown
that genes enriched in the cell cycle, p53 signaling pathway and
NF-κB signaling pathway were differentially expressed in colorectal
cancer.
The list of transcription factors in most human
cancer cells is limited and these factors usually serve as targets
for anticancer drugs development (41). NF-κB has been used as a target for
cancer drug development which induces drug resistance by changing
MDR1 expression in cancer cells (18,27). Transcription activation mediated
by HIF-1α and STAT serve as targets for cancer drug development
(29,30). In this study, we have shown that
the transcription factors of MYC, TCF7L2, and
FOXO3 were regulators of some DEGs. MYC was activated
in colorectal cancer and the overexpression pattern was identified
as a downstream step at the end of the Wnt/APC/β-catenin signaling
pathways is crucial in human cancer (42,43). The TCF7L2 gene has been
shown to be involved in renal cell carcinoma metastasis (44). Members of the FOXO transcription
family were involved in several cell processes, including
apoptosis, stress resistance, metabolism, cell cycle, and DNA
repair (45,46). These findings are contributory to
the development of cancer treatment.
Current investigations have focused on the molecular
mechanism of tumor formation and metastasis (47). The expression of LGALS3 is
associated with neoplastic transformation and the differentiation
of monocytes into macrophages. The present study result suggest
that LGALS3 may be involved in colorectal cancer progression by
interacting with upregulated and downregulated genes. Due to the
LGALS3-related genes being mainly differentially expressed, LGALS3
is important in the development of colorectal cancer. The
predicated network of the metastatic factor LGALS3 may
facilitate understanding of the mechanism of tumor cell metastasis
to provide a therapeutic target in cancer treatment.
In conclusion, findings of the present study have
demonstrated that, LGALS3, cell cycle, p53 signaling pathway and
NF-κB signaling pathway are crucial in the development of
colorectal cancer. Additionally, several genes that are potential
candidate targets for colorectal cancer therapy have been
identified. However, more studies with regard to other signaling
pathway and key cancer-related proteins should be conducted in
order to reveal the underlying molecular mechanism of colorectal
cancer.
Acknowledgements
This study was funded by the Chongqing Natural
Science Foundation (CSTC, 2011BB5120).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Atkin WS, Edwards R, Kralj-Hans I, et al:
Once-only flexible sigmoidoscopy screening in prevention of
colorectal cancer: a multicentre randomised controlled trial.
Lancet. 375:1624–1633. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Grady WM and Carethers JM: Genomic and
epigenetic instability in colorectal cancer pathogenesis.
Gastroenterology. 135:1079–1099. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Leggett B and Whitehall V: Role of the
serrated pathway in colorectal cancer pathogenesis.
Gastroenterology. 138:2088–2100. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stratton MR, Campbell PJ and Futreal PA:
The cancer genome. Nature. 458:719–724. 2009. View Article : Google Scholar
|
|
6
|
Lanza G, Ferracin M, Gafa R, et al:
mRNA/microRNA gene expression profile in microsatellite unstable
colorectal cancer. Mol Cancer. 6:542007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jones S, Zhang X, Parsons DW, et al: Core
signaling pathways in human pancreatic cancers revealed by global
genomic analyses. Science. 321:1801–1806. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lustig B and Behrens J: The Wnt signaling
pathway and its role in tumor development. J Cancer Res Clin Oncol.
129:199–221. 2003.PubMed/NCBI
|
|
9
|
Maxwell PH, Pugh CW and Ratcliffe PJ:
Activation of the HIF pathway in cancer. Curr Opin Genet Dev.
11:293–299. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sankpal UT, Goodison S, Abdelrahim M and
Basha R: Targeting SP1 transcription factor in prostate cancer
therapy. Med Chem. 7:518–525. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tomlins SA, Rhodes DR, Perner S, et al:
Recurrent fusion of TMPRSS2 and ETS transcription factor genes in
prostate cancer. Science. 310:644–648. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang Y, Goldstein BG, Chao HH and Katz JP:
KLF4 and KLF5 regulate proliferation, apoptosis and invasion in
esophageal cancer cells. Cancer Biol Ther. 4:1216–1221. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dolcet X, Llobet D, Pallares J and
Matias-Guiu X: NF-κB in development and progression of human
cancer. Virchows Arch. 446:475–482. 2005.
|
|
14
|
Sabates-Bellver J, Van Der Flier LG, De
Palo M, et al: Transcriptome profile of human colorectal adenomas.
Mol Cancer Res. 5:1263–1275. 2007. View Article : Google Scholar
|
|
15
|
Bresalier RS, Mazurek N, Sternberg LR, et
al: Metastasis of human colon cancer is altered by modifying
expression of the β-galactoside-binding protein galectin 3.
Gastroenterology. 115:287–296. 1998.PubMed/NCBI
|
|
16
|
Zhao Q, Guo X, Nash GB, et al: Circulating
galectin-3 promotes metastasis by modifying MUC1 localization on
cancer cell surface. Cancer Res. 69:6799–6806. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao Q, Barclay M, Hilkens J, et al:
Research interaction between circulating galectin-3 and
cancer-associated MUC1 enhances tumour cell homotypic aggregation
and prevents anoikis. Mol Cancer. 9:1542010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Irizarry RA, Hobbs B, Collin F, et al:
Exploration, normalization, and summaries of high density
oligonucleotide array probe level data. Biostatistics. 4:249–264.
2003. View Article : Google Scholar
|
|
19
|
Tusher VG, Tibshirani R and Chu G:
Significance analysis of microarrays applied to the ionizing
radiation response. Proc Natl Acad Sci USA. 98:5116–5121. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Harris M, Clark J, Ireland A, et al: The
gene ontology (GO) database and informatics resource. Nucleic Acids
Res. 32:D258–D261. 2004.PubMed/NCBI
|
|
21
|
Huang da W, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2008.PubMed/NCBI
|
|
22
|
Tarca AL, Draghici S, Khatri P, et al: A
novel signaling pathway impact analysis. Bioinformatics. 25:75–82.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Essaghir A, Toffalini F, Knoops L, Kallin
A, van Helden J and Demoulin JB: Transcription factor regulation
can be accurately predicted from the presence of target gene
signatures in microarray gene expression data. Nucleic Acids Res.
38:e1202010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
von Mering C, Huynen M, Jaeggi D, Schmidt
S, Bork P and Snel B: STRING: a database of predicted functional
associations between proteins. Nucleic Acids Res. 31:258–261.
2003.PubMed/NCBI
|
|
25
|
Kohl M, Wiese S and Warscheid B:
Cytoscape: Software for visualization and analysis of biological
networks. Data Mining in Proteomics. Hamacher M, Eisenacher M and
Stephan C: Humana Press; pp. 291–303. 2011, PubMed/NCBI
|
|
26
|
Steeg PS: Tumor metastasis: mechanistic
insights and clinical challenges. Nat Med. 12:895–904. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cox DG, Hankinson SE and Hunter DJ:
Polymorphisms of the AURKA (STK15/Aurora Kinase) gene and breast
cancer risk (United States). Cancer Causes Control. 17:81–83. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Couch FJ, Sinilnikova O, Vierkant RA, et
al: AURKA F31I polymorphism and breast cancer risk in BRCA1 and
BRCA2 mutation carriers: a consortium of investigators of modifiers
of BRCA1/2 study. Cancer Epidemiol Biomarkers Prev. 16:1416–1421.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhu X, Mao Z, Na Y, Guo Y, Wang X and Xin
D: Significance of pituitary tumor transforming gene 1 (PTTG1) in
prostate cancer. Anticancer Res. 26:1253–1259. 2006.PubMed/NCBI
|
|
30
|
Gorynia S, Bandeiras TM, Pinho FG, et al:
Structural and functional insights into a dodecameric molecular
machine - the RuvBL1/RuvBL2 complex. J Struct Biol. 176:279–291.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Carattino MD, Prakasam HS, Ruiz WG, et al:
Bladder filling and voiding affect umbrella cell tight junction
organization and function. Am J Physiol Renal Physiol. Jul
24–2013.(Epub ahead of print).
|
|
32
|
Osunkoya AO, Cohen C, Lawson D, Picken MM,
Amin MB and Young AN: Claudin-7 and claudin-8: immunohistochemical
markers for the differential diagnosis of chromophobe renal cell
carcinoma and renal oncocytoma. Hum Pathol. 40:206–210. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Katoh M and Katoh M: CLDN23 gene,
frequently down-regulated in intestinal-type gastric cancer, is a
novel member of CLAUDIN gene family. Int J Mol Med. 11:683–689.
2003.PubMed/NCBI
|
|
34
|
Vogelstein B and Kinzler KW: Cancer genes
and the pathways they control. Nat Med. 10:789–799. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Malumbres M and Barbacid M: Cell cycle,
CDKs and cancer: a changing paradigm. Nat Rev Cancer. 9:153–166.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sherr CJ: Cancer cell cycles. Science.
274:1672–1677. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Maruyama K, Kawagoe T, Kondo T, Akira S
and Takeuchi O: TRAF family member-associated NF-κB activator
(TANK) is a negative regulator of osteoclastogenesis and bone
formation. J Biol Chem. 287:29114–29124. 2012.
|
|
38
|
O’neill LA and Kaltschmidt C: NF-κB: a
crucial transcription factor for glial and neuronal cell function.
Trends Neurosci. 20:252–258. 1997.
|
|
39
|
Barnes PJ: Nuclear factor-κB. Int J
Biochem Cell Biol. 29:867–870. 1997.
|
|
40
|
Scartozzi M, Bearzi I, Pierantoni C, et
al: Nuclear factor-κB tumor expression predicts response and
survival in irinotecan-refractory metastatic colorectal cancer
treated with cetuximab-irinotecan therapy. J Clin Oncol.
25:3930–3935. 2007.
|
|
41
|
Darnell JE Jr: Transcription factors as
targets for cancer therapy. Nat Rev Cancer. 2:740–749. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bièche I, Laurendeau I, Tozlu S, et al:
Quantitation of MYC gene expression in sporadic breast tumors with
a real-time reverse transcription-PCR assay. Cancer Res.
59:2759–2765. 1999.
|
|
43
|
Le Floch N, Rivat C, De Wever O, et al:
The proinvasive activity of Wnt-2 is mediated through a
noncanonical Wnt pathway coupled to GSK-3β and c-Jun/AP-1
signaling. FASEB J. 19:144–146. 2005.PubMed/NCBI
|
|
44
|
Kojima T, Shimazui T, Horie R, et al:
FOXO1 and TCF7L2 genes involved in metastasis and poor prognosis in
clear cell renal cell carcinoma. Genes Chromosomes Cancer.
49:379–389. 2010.PubMed/NCBI
|
|
45
|
Arden KC: Multiple roles of FOXO
transcription factors in mammalian cells point to multiple roles in
cancer. Exp Gerontol. 41:709–717. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Roy S, Srivastava R and Shankar S:
Inhibition of PI3K/AKT and MAPK/ERK pathways causes activation of
FOXO transcription factor, leading to cell cycle arrest and
apoptosis in pancreatic cancer. J Mol Signal. 5:102010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
John A and Tuszynski G: The role of matrix
metalloproteinases in tumor angiogenesis and tumor metastasis.
Pathol Oncol Res. 7:14–23. 2001. View Article : Google Scholar : PubMed/NCBI
|