Introduction
Bone metabolism is strictly regulated by osteoblasts
and osteoclasts, which are responsible for bone formation and bone
resorption, respectively (1).
These functional cells are considered to affect one another via
humoral factors as well as by direct cell-to-cell interaction. It
has been firmly established that osteoblasts also play a crucial
role in the regulation of bone resorption through receptor
activator of nuclear factor-κB ligand (RANKL) expression in
response to a variety of bone resorptive stimuli (2). The resorption of preexisting bone by
osteoclasts and the formation of new bone by osteoblasts, bone
remodeling, is a strictly coordinated process to maintain adequate
bone mass. The disorder of bone remodeling causes metabolic bone
diseases such as osteoporosis and fracture healing distress. In the
process of bone remodeling, it is generally recognized that
numerous humoral factors including cytokines and growth factors
play pivotal roles (3).
Bone morphogenetic proteins (BMPs) are
multifunctional cytokines and belong to the transforming growth
factor-β (TGF-β) superfamily (4).
It is well known that BMPs expressed in bone are essential for
skeletal development and bone remodeling (5). The effects of BMPs on osteoblasts
are exerted through Smad (Smad1/5/8)-dependent signaling and
Smad-independent signaling such as the mitogen-activated protein
(MAP) kinase family (4,6). Moreover, vascular endothelial growth
factor (VEGF) is currently recognized to play critical roles in
effective coupling of angiogenesis and osteogenesis (7). Among bone cells, the osteoblast
lineage is considered as a major source of VEGF (7). In addition, the receptors for VEGF
are expressed both on osteoblasts and osteoclasts (7). The production of VEGF by osteoblasts
is reportedly modulated by a wide range of stimulations including
hormonal, mechanical and environmental influences, suggesting that
VEGF synthesis by osteoblasts plays a crucial role for the control
of angiogenesis in bone via an autocrine and/or paracrine mechanism
(7). We previously demonstrated
that BMP-4 stimulates VEGF synthesis through activation of p70 S6
kinase in osteoblast-like MC3T3-E1 cells (8,9).
It has been firmly established that polyphenolic
compounds in foods such as vegetables and fruits confer beneficial
effects on human being. It has been reported that flavonoids, among
the polyphenolic compounds, possess antioxidative,
anti-inflammatory and anti-tumor effects (10,11). Resveratrol, a natural polyphenol
found abundantly in the skins of red grapes and red wine, has
recently received increased attention as a means to improve health
and prolong life (12,13). In mammalian cells, the effects of
resveratrol are recognized to be dependent upon SIRT1, known as a
longevity gene, improving the function of cells and organs via
activation of the nicotinamide adenine dinucleotide
(NAD+)-dependent deacetylase (14). Regarding the effects of
resveratrol on bone, it has been shown that resveratrol promotes
osteoblast differentiation (15,16). However, the exact mechanisms
underlying the effects of resveratrol on bone metabolism have not
yet been clarified.
In the present study, we investigated the effect of
resveratrol on BMP-4-stimulated VEGF synthesis in osteoblast-like
MC3T3-E1 cells and the related mechanisms. We demonstrated that
resveratrol suppresses BMP-4-stimulated VEGF synthesis via
inhibition of p70 S6 kinase in these cells.
Materials and methods
Materials
Resveratrol and SRT1720 were obtained from
Calbiochem-Novabiochem Co. (La Jolla, CA, USA). BMP-4 and mouse
VEGF enzyme-linked immunosorbent assay (ELISA) kits were obtained
from R&D Systems, Inc. (Minneapolis, MN, USA). Phospho-specific
Smad1/5/8 antibodies, phospho-specific p70 S6 kinase antibodies and
p70 S6 kinase antibodies were obtained from Cell Signaling
Technology, Inc. (Beverly, MA, USA). Glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) antibodies were obtained from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). An ECL Western blotting
detection system was obtained from GE Healthcare (Buckinghamshire,
UK). TRIzol reagent was purchased from Invitrogen, Co. (Carlsbad,
CA, USA). FastStart DNA Master SYBR-Green I was purchased from
Roche Diagnostics (Mannheim, Germany). Omniscript Reverse
Transcriptase kit was purchased from Qiagen, Inc. (Hilden,
Germany). Other materials and chemicals were obtained from
commercial sources. Resveratrol and SRT1720 were dissolved in
dimethyl sulfoxide. The maximum concentration of dimethyl sulfoxide
was 0.1%, which did not affect either the assay for VEGF or western
blot analysis.
Cell culture
Cloned osteoblast-like MC3T3-E1 cells, which were
derived from newborn mouse calvaria (17), were maintained as previously
described (18). Briefly, the
cells were cultured in α-minimum essential medium (α-MEM)
containing 10% fetal calf serum (FCS) at 37°C in a humidified
atmosphere of 5% CO2/95% air. The cells were seeded into
35-mm (5×104 cells/dish) or 90-mm (2×105
cells/dish) diameter dishes in α-MEM containing 10% FCS. After 5
days, the medium was replaced with α-MEM containing 0.3% FCS. The
cells were used for experiments after 48 h.
Assay for VEGF
The cultured cells were pretreated with various
doses of resveratrol, SRT1720 or vehicle for 60 min, and then
stimulated with 70 ng/ml of BMP-4 or vehicle in 1 ml of α-MEM
containing 0.3% FCS for the indicated periods. The conditioned
medium was collected, and VEGF in the medium was then measured by
mouse VEGF ELISA kits according to the manufacturer’s protocol.
Real-time RT-PCR
The cultured cells were pretreated with 50 μM of
resveratrol, 10 μM of SRT1720 or vehicle for 60 min, and then
stimulated with 70 ng/ml of BMP-4 or vehicle in α-MEM containing
0.3% FCS for 24 h. Total RNA was isolated and transcribed into
complementary DNA using TRIzol reagent and the Omniscript Reverse
Transcriptase kit, respectively. Real-time RT-PCR was performed
using a LightCycler system in capillaries and FastStart DNA Master
SYBR-Green I provided with the kit. Sense and antisense primers for
mouse VEGF or GAPDH mRNA were purchased from Takara Bio, Inc.
(Tokyo, Japan) (primer set ID: MA039013). The amplified products
were determined by melting curve analysis and agarose
electrophoresis. VEGF mRNA levels were normalized with those of
GAPDH mRNA.
Western blot analysis
The cultured cells were stimulated with BMP-4 for
the indicated periods. When indicated, the cells were pretreated
with resveratrol or SRT1720 for 60 min. The cells were washed twice
with phosphate-buffered saline and then lysed, homogenized and
sonicated in a lysis buffer containing 62.5 mM Tris/HCl, pH 6.8, 2%
sodium dodecyl sulfate (SDS), 50 mM dithiothreitol and 10%
glycerol. SDS-polyacrylamide gel electrophoresis (PAGE) was
performed by the method described by Laemmli (19) on 10% polyacrylamide gels. Western
blot analysis was performed as described previously (20) by using phospho-specific Smad1/5/8
antibodies, phospho-specific p70 S6 kinase antibodies, p70 S6
kinase antibodies or GAPDH antibodies, with peroxidase-labeled
antibodies raised in goat against rabbit IgG being used as
secondary antibodies. The peroxidase activity on the PVDF membrane
was visualized on X-ray film by means of the ECL western blotting
detection system.
Statistical analysis
The data were analyzed by ANOVA followed by
Bonferroni method for multiple comparisons between pairs, and
values of P<0.05 were considered to indicate statistically
significant results. All data are presented as the mean ± standard
error of the mean (SEM) of triplicate determinations from three
independent cell preparations.
Results
Effect of resveratrol on BMP-4-stimulated
VEGF release in MC3T3-E1 cells
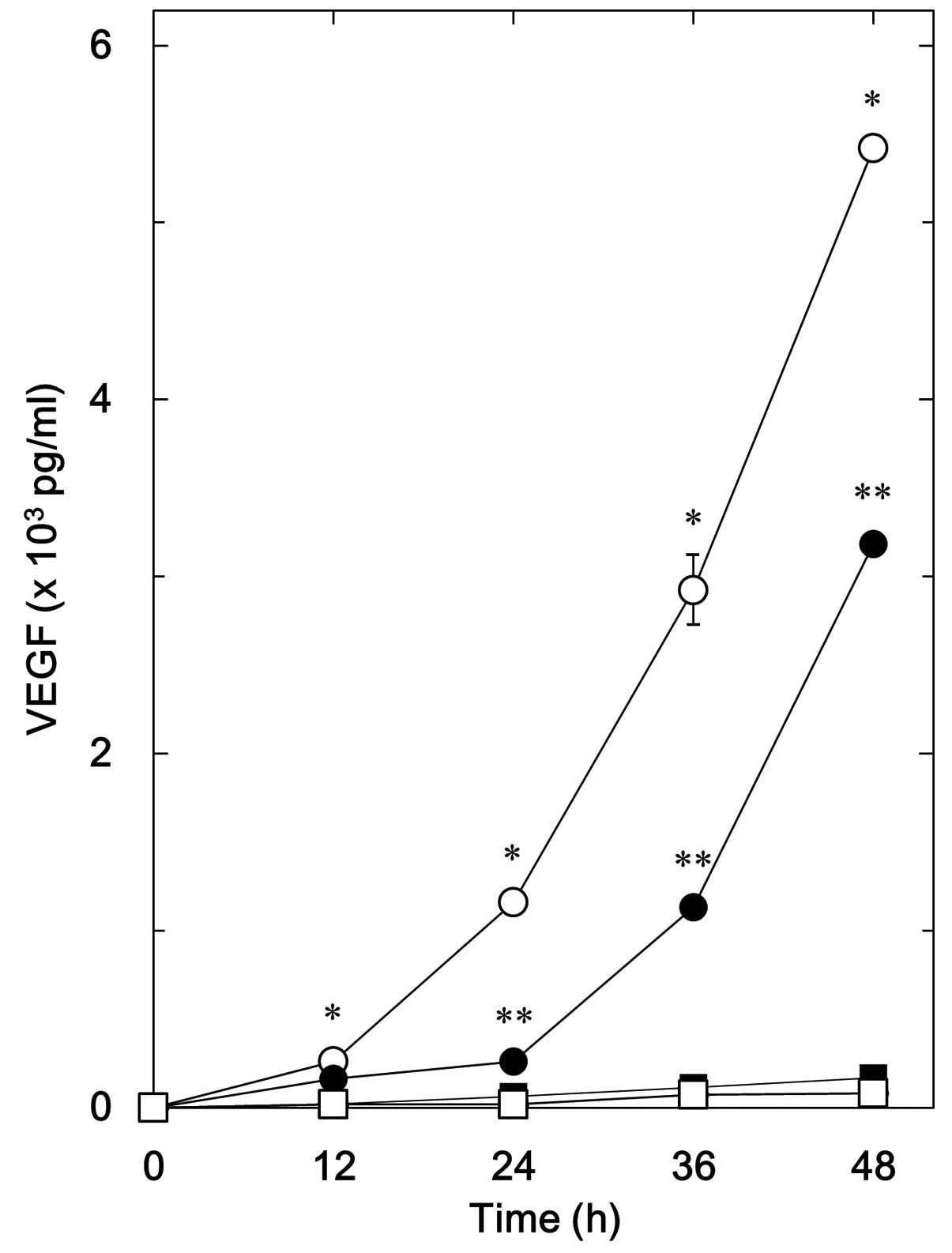
We previously demonstrated that BMP-4 stimulates the
synthesis of VEGF in osteoblast-like MC3T3-E1 cells (8). We first examined the effect of
resveratrol on BMP-4-stimulated VEGF release. Resveratrol, which
alone had little effect on VEGF levels, significantly suppressed
BMP-4-stimulated VEGF release in MC3T3-E1 cells (Fig. 1). The inhibitory effect of
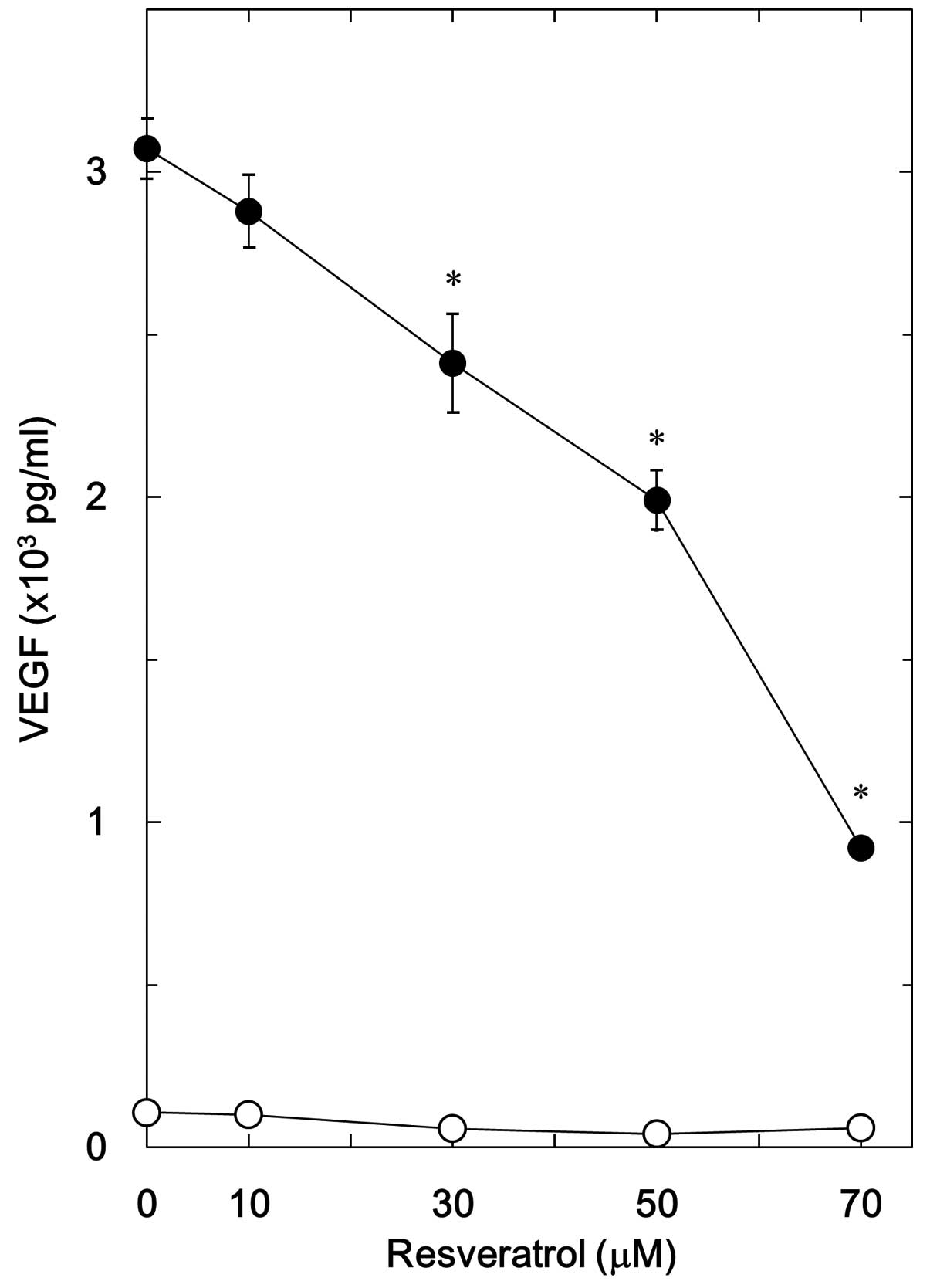
resveratrol on VEGF synthesis was dose-dependent in the range
between 10 and 70 μM (Fig. 2).
Resveratrol (70 μM) caused an ~70% decrease in the BMP-4-mediated
effect.
Effect of resveratrol on BMP-4-induced
expression levels of VEGF mRNA in MC3T3-E1 cells
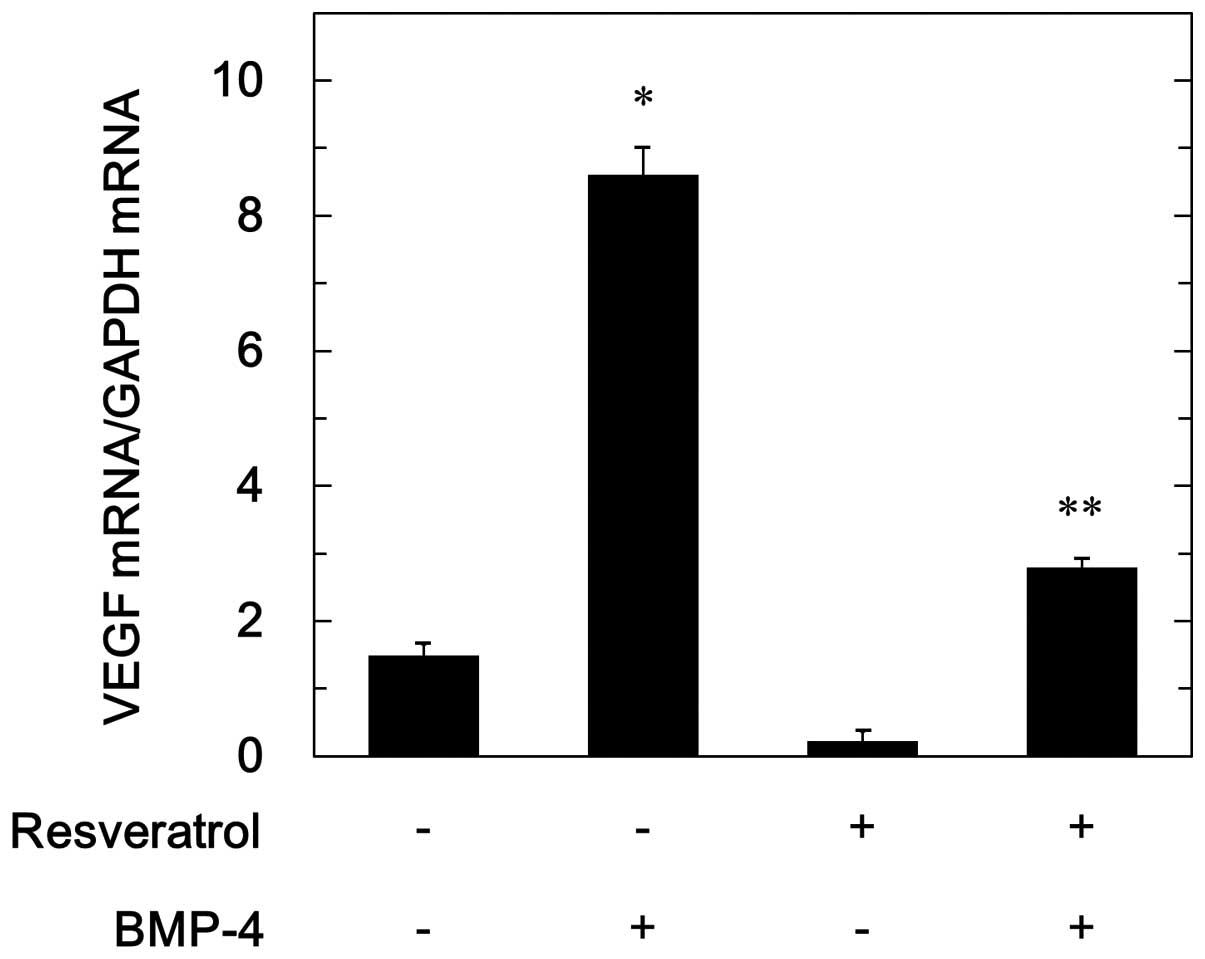
To investigate whether the suppressive effect of
resveratrol on BMP-4-stimulated VEGF release is mediated through a
transcriptional event, we next examined the effect of resveratrol
on BMP-4-induced VEGF mRNA expression. Resveratrol significantly
reduced the VEGF mRNA expression levels induced by BMP-4 (Fig. 3).
Effects of SRT1720 on the release of VEGF
and the mRNA expression stimulated by BMP-4 in MC3T3-E1 cells
SRT1720 is also known as an activator of SIRT1 as
well as resveratrol, and the potencies are estimated to be
~1,000-fold greater than resveratrol (21,22). Next, we investigated the effect of
SRT1720 on BMP-4-stimulated VEGF synthesis in MC3T3-E1 cells.
SRT1720, which alone had little effect on VEGF release,
significantly suppressed the BMP-4-stimulated VEGF release
(Table I). In addition, SRT1720,
which alone did not affect the VEGF mRNA expression levels,
significantly reduced the expression levels of VEGF mRNA induced by
BMP-4 (Table II).
 | Table IEffect of SRT1720 on BMP-4-stimulated
VEGF release in MC3T3-E1 cells. |
Table I
Effect of SRT1720 on BMP-4-stimulated
VEGF release in MC3T3-E1 cells.
| SRT1720 | BMP-4 | VEGF (pg/ml) |
|---|
| − | − | 36.9±1.2 |
| − | + | 2577.0±202.1a |
| + | − | 32.4±2.2 |
| + | + | 1124.4±46.1b |
 | Table IIEffect of SRT1720 on BMP-4-induced
expression levels of VEGF mRNA in MC3T3-E1 cells. |
Table II
Effect of SRT1720 on BMP-4-induced
expression levels of VEGF mRNA in MC3T3-E1 cells.
| SRT1720 | BMP-4 | VEGF mRNA/GAPDH
mRNA |
|---|
| − | − | 1.49±0.30 |
| − | + | 8.60±0.71a |
| + | − | 1.51±0.26 |
| + | + |
5.94±0.12b |
Effects of resveratrol or SRT1720 on the
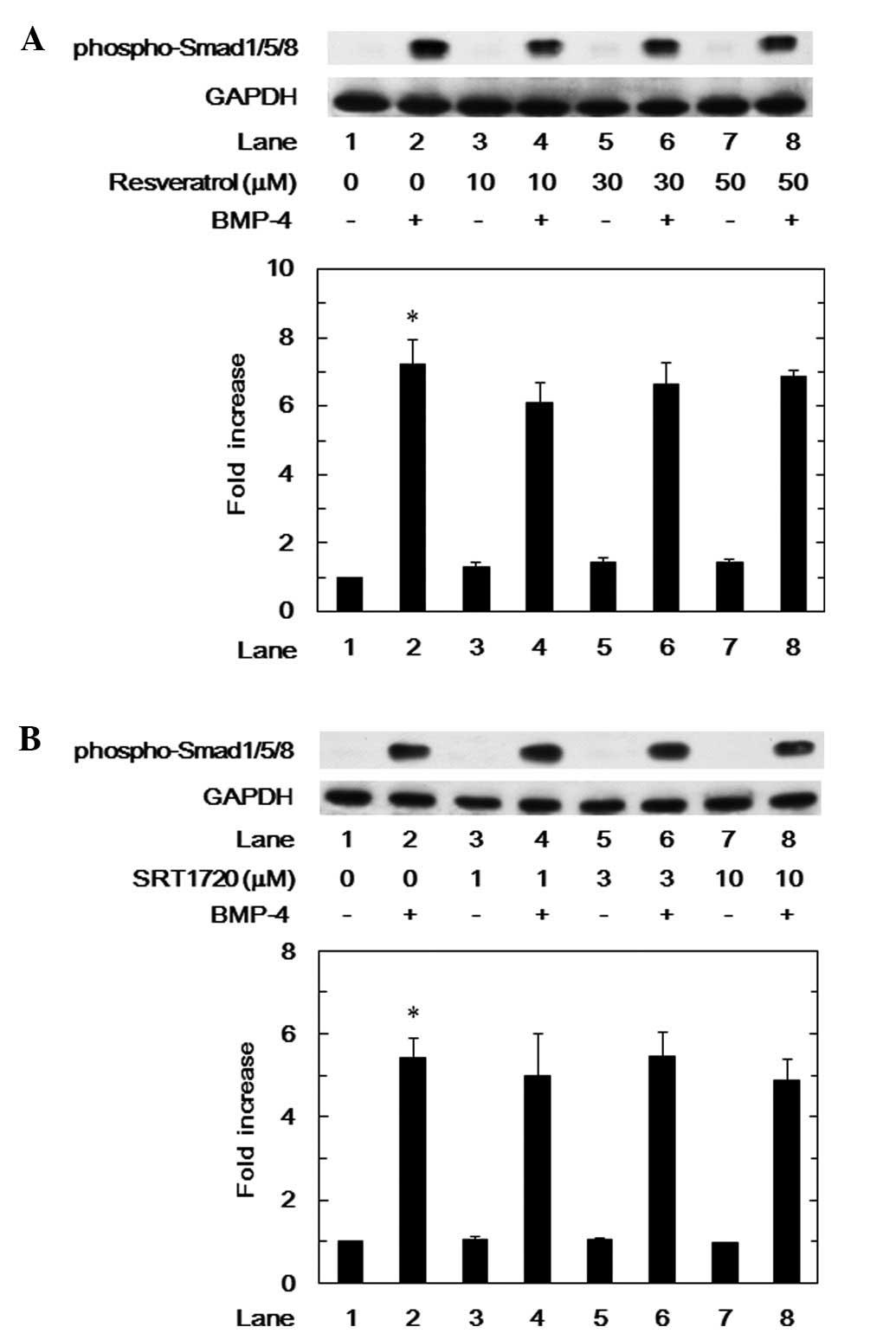
BMP-4-induced phosphorylation of Smad1/5/8 in MC3T3-E1 cells
It has been previously established that the effects
of BMPs are exerted through the intracellular signaling of Smad
proteins such as Smad1, Smad5 and Smad8 (4). In order to clarify whether the
inhibitory effect of resveratrol on the BMP-4-stimulated VEGF
synthesis is mediated by the modification of Smad1/5/8 activation
in MC3T3-E1 cells, we examined the effect of resveratrol on the
BMP-4-induced phosphorylation of Smad1/5/8. Resveratrol, which
alone had little effect on the phosphorylation levels of Smad1/5/8,
failed to affect the levels induced by BMP-4 at a dose up to 50 μM
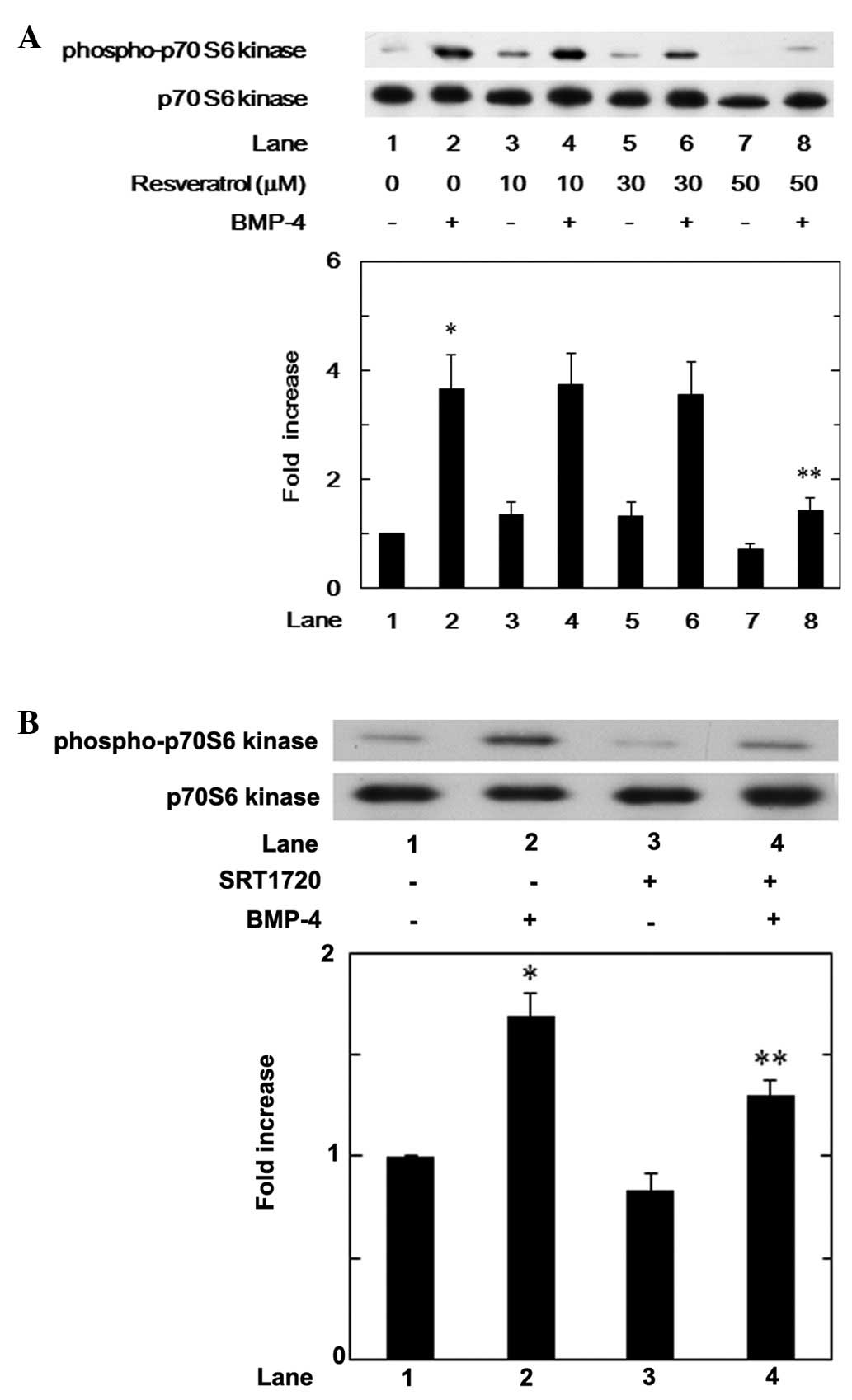
(Fig. 4A). In addition, SRT1720
did not affect the Smad1/5/8 phosphorylation levels at a dose up to
10 μM (Fig. 4B).
Effects of resveratrol or SRT1720 on the
BMP-4-induced phosphorylation of p70 S6 kinase in MC3T3-E1
cells
We previously demonstrated that BMP-4 stimulates
VEGF synthesis through activation of p70 S6 kinase in
osteoblast-like MC3T3-E1 cells (8). In order to elucidate whether the
suppressive effect of resveratrol on BMP-4-stimulated VEGF
synthesis is mediated by the modulation of p70 S6 kinase activation
in MC3T3-E1 cells, we examined the effect of resveratrol on the
BMP-4-induced phosphorylation of p70 S6 kinase. Resveratrol
significantly attenuated the phosphorylation of p70 S6 kinase
induced by BMP-4 in a dose-dependent manner between 10 and 50 μM
(Fig. 5A). Furthermore, SRT1720,
which alone barely affected the phosphorylation of p70 S6 kinase,
also suppressed the p70 S6 kinase phosphorylation in these cells
(Fig. 5B).
Discussion
In the present study, we demonstrated that
resveratrol, a polyphenolic flavonoid enriched in the skins of red
grapes or red wine (12,13), significantly suppressed
BMP-4-stimulated VEGF release in osteoblast-like MC3T3-E1 cells.
Resveratrol reportedly functions at least in part via activation of
SIRT1, which is known as one of the longevity genes (21). We also found that SRT1720
suppressed BMP-4-stimulated VEGF release in these cells. Therefore,
the suppressive effect of resveratrol on BMP-4-induced VEGF
synthesis in osteoblasts appears to be a SIRT1-dependent event. In
addition, we demonstrated that both resveratrol and SRT1720
markedly decreased the BMP-4-induced expression levels of VEGF
mRNA. Based on our findings, it is probable that the inhibitory
effect of resveratrol on BMP-4-induced VEGF release is mediated
through transcriptional events. To the best of our knowledge, this
is the first report to demonstrate the suppression of VEGF
synthesis by resveratrol in osteoblasts.
It is well known that Smad proteins are central
mediators of the intracellular signaling system of the TGF-β
superfamily such as TGF-β and BMPs (23). Regarding BMP signaling, BMPs
employ the activation of Smad1/5/8 as receptor-regulated Smads
(23). Thus, we investigated
whether Smad1/5/8 are involved in the inhibitory effects of
resveratrol or SRT1720 on BMP-4-stimulated VEGF synthesis in
osteoblast-like MC3T3-E1 cells. However, neither resveratrol nor
SRT1720 affected the BMP-4-induced phosphorylation levels of
Smad1/5/8. Therefore, it seems unlikely that the suppressive effect
of resveratrol on VEGF synthesis stimulated by BMP-4 is due to the
modulation of Smad1/5/8-mediating signaling. Moreover, accumulating
evidence indicates that the TGF-β superfamily exerts their effects
on a variety of biological functions via Smad-independent signaling
(24). We previously reported
that activation of p70 S6 kinase positively regulates
BMP-4-stimulated VEGF synthesis in osteoblast-like MC3T3-E1 cells
(8). Thus, we next investigated
whether resveratrol or SRT1720 affects the activation of p70 S6
kinase upregulated by BMP-4 in MC3T3-E1 cells. We found that the
phosphorylation levels of p70 S6 kinase induced by BMP-4 were
significantly attenuated by both resveratrol and SRT1720. Based on
our findings, it is likely that the suppression of BMP-4-stimulated
VEGF synthesis by resveratrol through SIRT-1 activation is mediated
by the modulation of p70 S6 kinase in osteoblast-like MC3T3-E1
cells.
Resveratrol is a natural polyphenol abundantly found
in grape skins and red wine and shows numerous favorable effects on
the health of humans through antioxidation, anti-aging and
anti-stress (12,13). It has been reported that
resveratrol prevents various types of cancers such as colon
carcinoma, and attenuates the progression of Alzheimer’s disease,
in addition to protection against obesity and its associated
diseases (25–27). Recent studies have also linked
resveratrol to a prolonged lifespan in humans and other species
(28,29). It has been shown that BMP
signaling is required for both bone development and angiogenesis
(30). During bone development
and fracture healing, BMPs not only increase bone formation, but
also enhance angiogenesis through regulation of the expression of
VEGF. Moreover, modifications of VEGF expression were reportedly
observed in osteoporosis in vivo (7). Both the reduction in VEGF expression
in the tibial metaphysis and the contrasting increases in VEGF
expression related to vascularization in the periosteum have been
recognized in osteoporotic rat models (7), suggesting the complicated mechanism
of the pathogenesis. Thus, our present findings, clearly
demonstrating the reduction of BMP-4-induced VEGF synthesis by
resveratrol in osteoblasts-like MC3T3-E1 cells, provides novel
insight underlying the favorable effects of polyphenols on the
health of humans particularly on elder individuals. Further
investigation is necessary to clarify the detailed mechanisms of
resveratrol underlying the VEGF synthesis in osteoblasts.
In conclusion, our findings strongly suggest that
resveratrol attenuates BMP-4-stimulated VEGF synthesis through
suppression of the activation of p70 S6 kinase in osteoblasts, and
that the inhibitory effect is at least in part mediated by SIRT1
activation.
Acknowledgements
We are grateful to Yumiko Kurokawa for her skillful
technical assistance. This investigation was supported in part by a
Grant-in-Aid for Scientific Research (19591042) from the Ministry
of Education, Science, Sports and Culture of Japan, the Foundation
for Growth Science, and the Research Funding for Longevity Sciences
(22-4, 23-9) from the National Center for Geriatrics and
Gerontology (NCGG), Japan.
References
|
1
|
Karsenty G and Wagner EF: Reaching a
genetic and molecular understanding of skeletal development. Dev
Cell. 2:389–406. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Boyce BF and Xing L: Functions of
RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem
Biophys. 473:139–146. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Parfitt AM: Targeted and nontargeted bone
remodeling: relationship to basic multicellular unit origination
and progression. Bone. 30:5–7. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Miyazono K, Maeda S and Imamura T: BMP
receptor signaling: transcriptional targets, regulation of signals,
and signaling cross-talk. Cytokine Growth Factor Rev. 16:251–263.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Krause C, de Gorter DJJ, Karperien M and
ten Dijke P: Signal transduction cascades controlling osteoblast
differentiation. Primer on the Metabolic Bone Diseases and
Disorders of Mineral Metabolism. Rosen CJ: 7th edition. American
Society for Bone and Mineral Research; Washington: pp. 10–16. 2008,
View Article : Google Scholar
|
|
6
|
Yamaguchi K, Nagai S, Ninomiya-Tsuji J,
Nishita M, Tamai K, Irie K, Ueno N, Nishida E, Shibuya H and
Matsumoto K: XIAP, a cellular member of the inhibitor of apoptosis
protein family, links the receptors to TAB1-TAK1 in the BMP
signaling pathway. EMBO J. 18:179–187. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Clarkin CE and Gerstenfeld LC: VEGF and
bone cell signalling: an essential vessel for communication? Cell
Biochem Funct. 31:1–11. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kozawa O, Matsuno H and Uematsu T:
Involvement of p70 S6 kinase in bone morphogenetic protein
signaling: vascular endothelial growth factor synthesis by bone
morphogenetic protein-4 in osteoblasts. J Cell Biochem. 81:430–436.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tokuda H, Hatakeyama D, Shibata T,
Akamatsu S, Oiso Y and Kozawa O: p38 MAP kinase regulates
BMP-4-stimulated VEGF synthesis via p70 S6 kinase in osteoblasts.
Am J Physiol Endcrinol Metab. 284:E1202–E1209. 2003.PubMed/NCBI
|
|
10
|
Jankun J, Selman SH, Swiercz R and
Skrzypczak-Jankun E: Why drinking green tea could prevent cancer.
Nature. 387:5611997. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Harborne JB and Williams CA: Advances in
flavonoid research since 1992. Phytochemistry. 55:481–504. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Blander G and Guarente L: The Sir2 family
of protein deacetylases. Annu Rev Biochem. 73:417–435. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Koo SH and Montminy M: In vino veritas: a
tale of two sirt1s? Cell. 127:1091–1093. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Baur JA, Pearson KJ, Price NL, Jamieson
HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K,
Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M,
Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D,
Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R and Sinclair
DA: Resveratrol improves health and survival of mice on a
high-calorie diet. Nature. 444:337–342. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mizutani K, Ikeda K, Kawai Y and Yamori Y:
Resveratrol stimulates the proliferation and differentiation of
osteoblastic MC3T3-E1 cells. Biochem Biophys Res Commun.
253:859–863. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kupisiewicz K, Boissy P, Abdallah BM,
Hansen FD, Erben RG, Savouret JF, Søe K, Andersen TL, Plesner T and
Delaisse JM: Potential of resveratrol analogues as antagonists of
osteoclasts and promoters of osteoblasts. Calcif Tissue Int.
87:437–449. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sudo H, Kodama HA, Amagai Y, Yamamoto S
and Kasai S: In vitro differentiation and calcification in a new
clonal osteogenic cell line derived from newborn mouse calvaria. J
Cell Biol. 96:191–198. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kozawa O, Tokuda H, Miwa M, Kotoyori J and
Oiso Y: Cross-talk regulation between cyclic AMP production and
phosphoinositide hydrolysis induced by prostaglandin E2
in osteoblast-like cells. Exp Cell Res. 198:130–134. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Laemmli UK: Cleavage of structural
proteins during the assembly of the head of bacteriophage T4.
Nature. 227:680–685. 1970. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kato K, Ito H, Hasegawa K, Inaguma Y,
Kozawa O and Asano T: Modulation of the stress-induced synthesis of
hsp27 and αB-crystallin by cyclic AMP in C6 rat glioma cells. J
Neurochem. 66:946–950. 1996.
|
|
21
|
Bradamante S, Barenghi L and Villa A:
Cardiovascular protective effects of resveratrol. Cardiovasc Drug
Rev. 22:169–188. 2004. View Article : Google Scholar
|
|
22
|
Milne JC, Lambert PD, Schenk S, Carney DP,
Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, Bemis JE, Xie
R, Disch JS, Ng PY, Nunes JJ, Lynch AV, Yang H, Galonek H,
Israelian K, Choy W, Iffland A, Lavu S, Medvedik O, Sinclair DA,
Olefsky JM, Jirousek MR, Elliott PJ and Westphal CH: Small molecule
activators of SIRT1 as therapeutics for the treatment of type 2
diabetes. Nature. 450:712–716. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Miyazawa K, Shinozaki M, Hara T, Furuya T
and Miyazono K: Two major Smad pathways in TGF-β superfamily
signalling. Genes Cells. 7:1191–1204. 2002.
|
|
24
|
Moustakas A and Heldin CH: Non-Smad
TGF-beta signals. J Cell Sci. 118:3573–3584. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kundu JK and Surh YJ: Cancer
chemopreventive and therapeutic potential of resveratrol:
mechanistic perspectives. Cancer Lett. 269:243–261. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ramassamy C: Emerging role of polyphenolic
compounds in the treatment of neurodegenerative diseases: a review
of their intracellular targets. Eur J Pharmacol. 545:51–64. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li F, Gong Q, Dong H and Shi J:
Resveratrol, a neuroprotective supplement for Alzheimer’s disease.
Curr Pharm Des. 18:27–33. 2012.
|
|
28
|
Howitz KT, Bitterman KJ, Cohen HY, Lamming
DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL,
Scherer B and Sinclair DA: Small molecule activators of sirtuins
extend Saccharomyces cerevisiae lifespan. Nature.
425:191–196. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wood JG, Rogina B, Lavu S, Howitz K,
Helfand SL, Tatar M and Sinclair D: Sirtuin activators mimic
caloric restriction and delay ageing in metazoans. Nature.
430:686–689. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang F, Qiu T, Wu X, Wan C, Shi W, Wang
Y, Chen JG, Wan M, Clemens TL and Cao X: Sustained BMP signaling in
osteoblasts stimulates bone formation by promoting angiogenesis and
osteoblast differentiation. J Bone Miner Res. 24:1224–1233. 2009.
View Article : Google Scholar : PubMed/NCBI
|