Introduction
Diabetes affects approximately 170 million
individuals worldwide, and it is anticipated that this number will
double over the next 20 years (1). The diminished production of
proangiogenic growth factors and decreased wound angiogenesis are
thought to contribute to impaired wound repair in diabetic patients
(2), resulting in scarring,
delayed and compromised mechanical wound healing (3). Wound healing is strongly dependent
on the formation of granulation tissue, which in turn intimately
correlates with the induction of new vessel formation. Wound
angiogenesis represents a merged process that relies on the
extracellular matrix in the wound bed, as well as on the migration
and mitogenic stimulation of endothelial cells (4). Therefore, several studies have
investigated whether the stimulation of angiogenesis can promote
diabetic wound healing (5).
Adenoviral gene transfer with proangiogenic growth
factors [particularly vascular endothelial growth factor (VEGF)]
has been shown to accelerate diabetic wound healing, whereas the
topical application with VEGF or platelet-derived growth factor
(PDGF) has shown ambivalent results (6). Our own previous experiments have
demonstrated the potential therapeutic benefits of priming with
proangiogenic factors and cell prior to surgery with a combination
of proangiogenic growth factors for wound healing in normoglycemic
and diabetic mice (7,8). Priming with a combination of
supraphysiological doses of VEGF, fibroblast growth factor (FGF)
and PDGF led to more rapid times to closure, higher vessel
densities and better functional outcomes (8). In addition to proangiogenic growth
factors, endothelial progenitor cells (EPCs) may present a
potential pre-treatment option for diabetic wounds.
Asahara et al identified circulating EPCs as
the key cell type contributing to neovascularization (9), and subsequent studies revealed that
bone marrow (BM)-derived EPCs are essential for the tissue repair
process in ischemia-induced damage of the limbs, kidneys and heart
(10–12). BM-derived EPCs can also be
incorporated into newly formed capillaries in granulation tissue,
thereby promoting neovascularization during wound healing (13,14).
EPCs may be incorporated into newly formed vessels
through multiple steps, including sensing the ischemic signal from
the remote tissue, releasing EPCs from the BM niche into the
circulation, homing circulating EPCs to the target tissues,
integrating EPCs into blood vessels and the in situ
differentiation/maturation of EPCs into mature and functional
endothelial cells (15,16). Tissue ischemia is presumed to be
the strongest stimulus for EPC mobilization from the BM to the
circulation (11,17). Moreover, EPC mobilization can be
augmented by various cytokines, including granulocyte
colony-stimulating factor (G-CSF), granulocyte-macrophage
colony-stimulating factor (GM-CSF), VEGF and placental growth
factor (PGF) (18–21). With regard to the fact that EPCs
provide both, strong autocrine and paracrine proangiogenic effects,
as well as the building material for vessel creation, EPCs may
present a powerful treatment option to improve revascularization
and wound healing in diabetic wounds.
The aim of this study was to eludicate the in
vitro and in vivo effects of pre-treatment with a
combination of proangiogenic growth factors (VEGF, bFGF and PDGF),
a monotherapy with PDGF and with EPCs on the healing of diabetic
incisional wounds.
Materials and methods
EPC isolation and culture
Mononuclear cells (MNCs) were isolated by density
gradient centrifugation with Biocoll (Biochrom KG, Berlin, Germany)
from peripheral human blood as previously described (21). Immediately following isolation,
total MNCs (8×106 cells/ml medium) were plated on 25
cm2 culture flasks coated with human fibronectin (Sigma,
Steinheim, Germany) and maintained in endothelial basal medium
(EBM) supplemented with EGM SingleQuots, 100 ng/ml VEGF and 20%
fetal calf serum (FCS).
In vitro angiogenesis assay
To evaluate the proangiogenic potential of EPCs
in vitro, a commercial HUVEC angiogenesis assay (3D
Angiogenesis Assay; PromoCell, Heidelberg, Germany) was used. The
assay was performed according to the manufacturer’s instructions.
EPCs were added to 8 of the 16 wells (2×106 cells/well,
dissolved in 400 μl EPC culture medium). The remaining 8 wells
served as controls. The sprouting colonies were photo-documented
over a period of 48 h. For the measurement of sprout densities,
areas and lengths, a software program (CellAnalyzer 2.25) was used
as previously described (23).
Animals
Twelve nude mice (obtained from Charles River
Laboratories Inc., Wilmington, MA, USA), 25–28 g, were used for the
in vivo Matrigel assay. Forty-eight female Balb/c mice,
25–33 g, were used for the diabetic wound experiments, which were
obtained from the Central Animal Facilities of the University of
Mainz, Mainz, Germany. All mice were allowed to acclimate for 14
days prior to the treatment and were housed in an approved animal
care facility with 12-h light cycles. Food and water were provided
ad libitum. The care of the animals was consistent with the
legal guidelines and all experiments were conducted after obtaining
approval from the local animal welfare authorities (licence no. 1.5
177-07/041-2). Animals were kept in individual cages during the
experiment in order to avoid biting and interference with the
wounds. Soft tissue paper was used instead of conventional bedding
in order to avoid wound irritation or contamination.
In vivo Matrigel assay
Twelve nude mice were divided randomly into 2 groups
(6 mice in each group). The mice were anesthetized with an
intraperitoneal injection of avertin [1.5 ml/100 g body weight
(BW), 2,2,2-tribromoethanol; Sigma-Aldrich, Munich, Germany] and a
total of 500 μl of Matrigel Basement Membrane Matrix (BD
Biosciences, Franklin Lakes, NJ, USA) containing 100 ng/ml VEGF
(PeproTech, Rocky Hill, NJ, USA) and 100 U/ml of heparin (Sigma,
St. Louis, MO, USA) was injected subcutaneously in the dorsal
midline of the 12 mice, using a 26-gauge needle. Group I mice were
administered a retro-orbital, intravenous injection of EPCs
(2×106 cells), dissolved in 100 μl phosphate-buffered
saline (PBS) per animal. Group II mice were administered a
retro-orbital, intravenous injection of 100 μl PBS as the controls.
After 2 weeks, 5 mice from each group were sacrificed by cervical
dislocation and the Matrigel plugs were removed and prepared for
histological analysis and immunochemistry. The remaining 2 mice (1
from each group) were used for microvascular corrosion casting. A
previous study demonstrated that 2 weeks are a sufficient time
period to allow measurable angiogenesis in the implanted Matrigel
plugs (22).
Model of streptozotocin (STZ)-induced
diabetes
To assess the effects of priming on diabetic mice,
hyperglycaemia was induced by intraperitoneal injections (24) of 150 mg/kg BW STZ (Sigma-Aldrich,
Taufkirchen, Germany). The injection of STZ was repeated twice
every other day with a concentration of 50 mg/kg BW. Blood glucose
levels were determined weekly and mice that did not show
hyperglycaemia ≥250 mg/dl at the time of surgery were excluded from
the study.
Proangiogenic priming
Forty-eight Balb/c mice with STZ-induced diabetes
were divided randomly into 4 groups (12 mice in each group). Prior
to the proangiogenic priming, the dorsal skin of the mice was
shaved and depilated. Subsequently, 7, 5 and 3 days prior to
surgery, group 1 was pre-treated with a mixture of VEGF (35.0 μg;
R&D Systems, Minneapolis, MN, USA), bFGF (2.5 μg; R&D
Systems) and PDGF (3.5 μg; R&D Systems). Group 2 was
pre-treated with PDGF (3.5 μg; R&D Systems) and group 3 with an
aliquot of two million EPCs. Proangiogenic growth factors and EPCs
were particulary dissolved in 0.2 ml saline solution (B. Braun
Melsungen AG, Melsungen, Germany). The control group (group 4 was
pre-treated with 0.2 ml pure saline solution. The injections were
administered subcutaneously 1.0 cm paramedian under the dorsal skin
of the mice after skin disinfection with 70% ethanol.
Surgical procedure
The mice were anesthetized with an intraperitoneal
injection of avertin (1.5 ml/100 g BW, 2,2,2-tribromoethanol;
Sigma-Aldrich, Munich, Germany). Immediately prior to surgery, an
area of 15×15 mm was shaved again using disposable shavers followed
by skin disinfections with 70% ethanol. Under sterile conditions,
15-mm-long full-thickness incisional skin wounds were set in the
midline of the lumbar dorsal skin of the 48 mice and closed by 4
single button sutures using absorbable suture material (Vicryl 4-0;
Ethicon, Hamburg, Germany).
Tissue sampling
In vivo Matrigel assay
The central parts of the removed Matrigel plugs were
used for histological analysis [hematoxylin and eosin (H&E)]
and immunochemistry (CD31) according to standard protocols. The
CD31 stained sections were photographed (Leica MZ12) and discretely
analyzed by a Weibel Grid analysis system for exact quantification
(25). Microvessel density (MVD)
was expressed as the number of microvessels (MV) per square
millimeter. The microvessel area (MVA) was expressed as the
percentage area of all microvessels in a section proportional to
the section Matrigel-area and microvessel size (MVS) was expressed
as the mean size of the vessels. Additionally, 1 mouse from the
control group and 1 mouse from the EPC-treated group was used for
microvascular corrosion casting as previously described (26).
Model STZ-induced diabetes
Animals in all groups were sacrificed on day 14 and
21 after surgery. The central parts of the wound area were used for
the maximum tensile strength measurements, and the adjacent parts
for histological analysis and immunochemistry. Five-micrometer-thin
sections of paraffin-embedded specimens were stained with H&E
according to standard protocols. H&E-stained sections were used
to assess tissue layer thicknesses of the epidermis and dermis,
scar thickness and the progress of remodeling with image analyzing
software Diskus 4.80 (Hilgers, Königswinter, Germany).
Immunohistochemical staining of endothelial cells was performed
using a monoclonal antibody against CD31 (BD Biosciences
Pharmingen, Heidelberg, Germany). Antibody binding was visualized
via a 3-step staining procedure using a biotinylated polyclonal
anti rat IgG secondary antibody (DakoCytomation GmbH, Hamburg,
Germany) and the streptavidin horseradish peroxidase reaction
together with the DAB detection system. Vessel densities and
lymphatic densities were assessed using a Weibel grid, as
previously described (25) and
expressed as a percentage of the surface area.
Tensile strength measurements
Test strips were punched out from the harvested
wound vertically to the craniocaudal axis on day 24 post-surgery.
The test strips had a defined hour glass form with 3-mm width at
the narrowest part constituting a pre-determined breaking point.
The design of the hour glass form was based on material testing
standards. Test strips without the hour glass form would inevitably
tear at the wedge grips, where the tissue is already bruised. The
breaking strength test device consisted of 2 opposing gripping jaws
which fixed the tissue strip. The electric motor driven gripping
jaws were moved apart with a constant strain rate of 0.5 mm/sec
under displacement control. Time, force and displacement were
recorded for stretching up until failure. A position encoder
(WA300) was used to register the stretching distance; a force
transducer (S2, maximum value 150 N) was used to quantify the power
applied to the tissue strip. The endpoint was the breaking strength
in Newton (kg·m/sec2). The resulting values were
recorded by a multiple channel PC measuring device [Spider 8,
Hottinger Baldwin Messtechnik GmbH (HBM), Darmstadt, Germany] and
plotted as a force-deflection curve (software, Catman 4.5, all from
HBM). The maximum breaking strength was determined from the
stress-strain curve.
Statistical analysis
Statistical analysis was based on measurements in at
least 34 different mice. The unpaired Student’s t-test for samples
of unequal variances was used to calculate statistical
significance. The data are expressed as the means ± one standard
deviation. The significance level for the sample distribution was
defined as p<0.05.
Results
In vitro angiogenesis assay
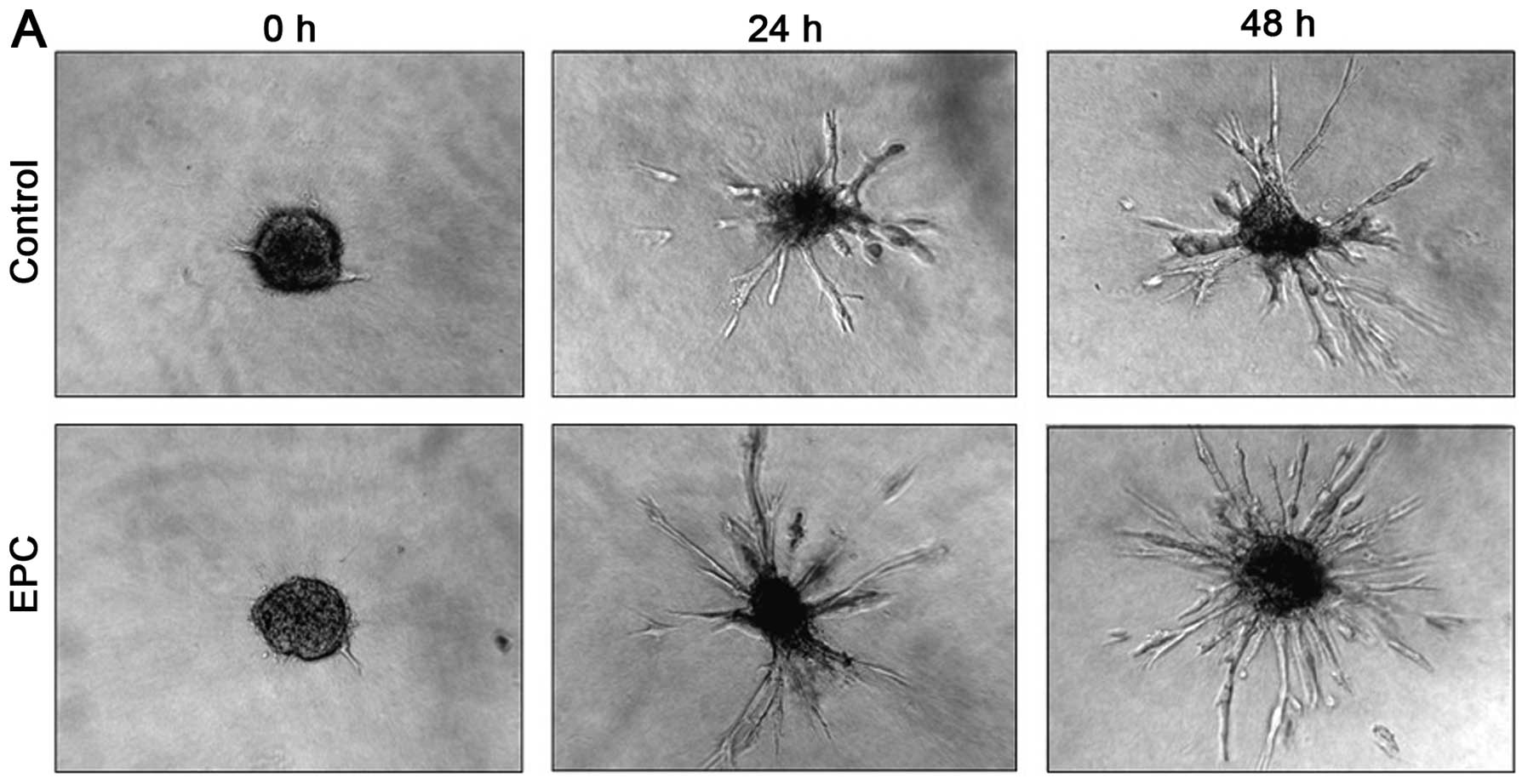
Fig. 1A shows
exemplary images of HUVEC sprouts directly after EPC incubation and
after 24 and 48 h. The control group presented a dense, radial
sprouting out of the initial cell spheroid. In comparison to the
control group (p0 h=0.0479 mm2, p24
h=0.0848 mm2, p48 h=0.1034
mm2), incubation with EPCs demonstrated significant
increased sprouting areas (p0 h=0.0438 mm2,
p24 h=0.1523 mm2, p48 h=0.1899
mm2) (p<0.001) (Fig.
1B).
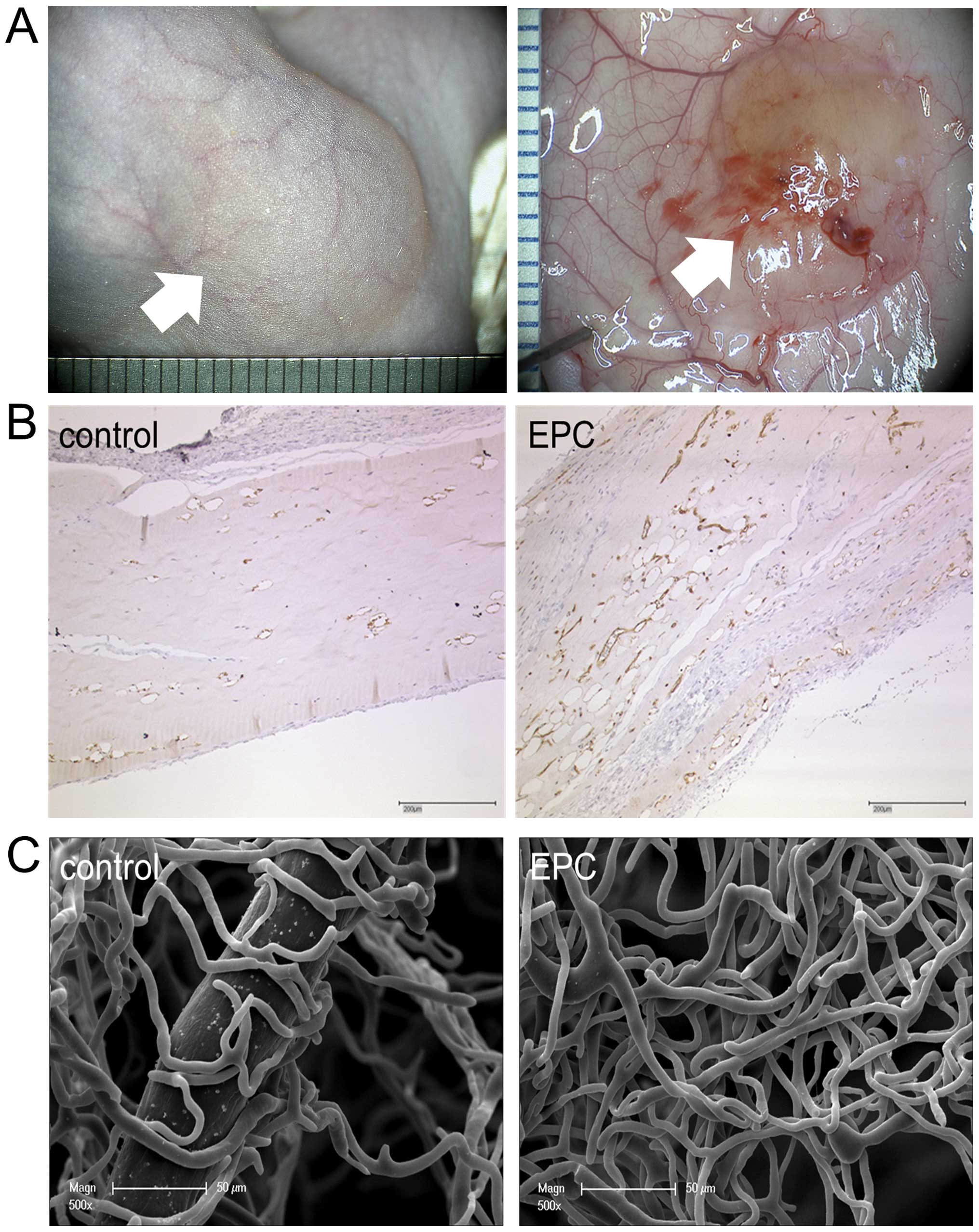
In vivo Matrigel assay
After the explantation of the Matrigel plug
(Fig. 2A), in the
anti-CD31-stained Matrigel sections (Fig. 2B) and in the microvascular
corrosion casts (Fig. 2C), a
higher vascular density in the EPC-treated plugs compared to the
controls was observed. Morphometrical analysis revealed that the
EPC application increased the MVD significantly up to 135.1±32.2
MV/mm2 (p<0.001) compared to the control group with a
median of 64.9±5.0 MV/mm2. The EPC-pre-treated animals
showed an MVA of 5.4±1.6%, whereas the control group showed an MVA
of 2.0±0.1%. The EPC application increased the MVS significantly up
to a median MVS of 402.3±30.8 μm2 (p<0.0001), whereas
the control group had a median MVS of 309.1±18.1
μm2.
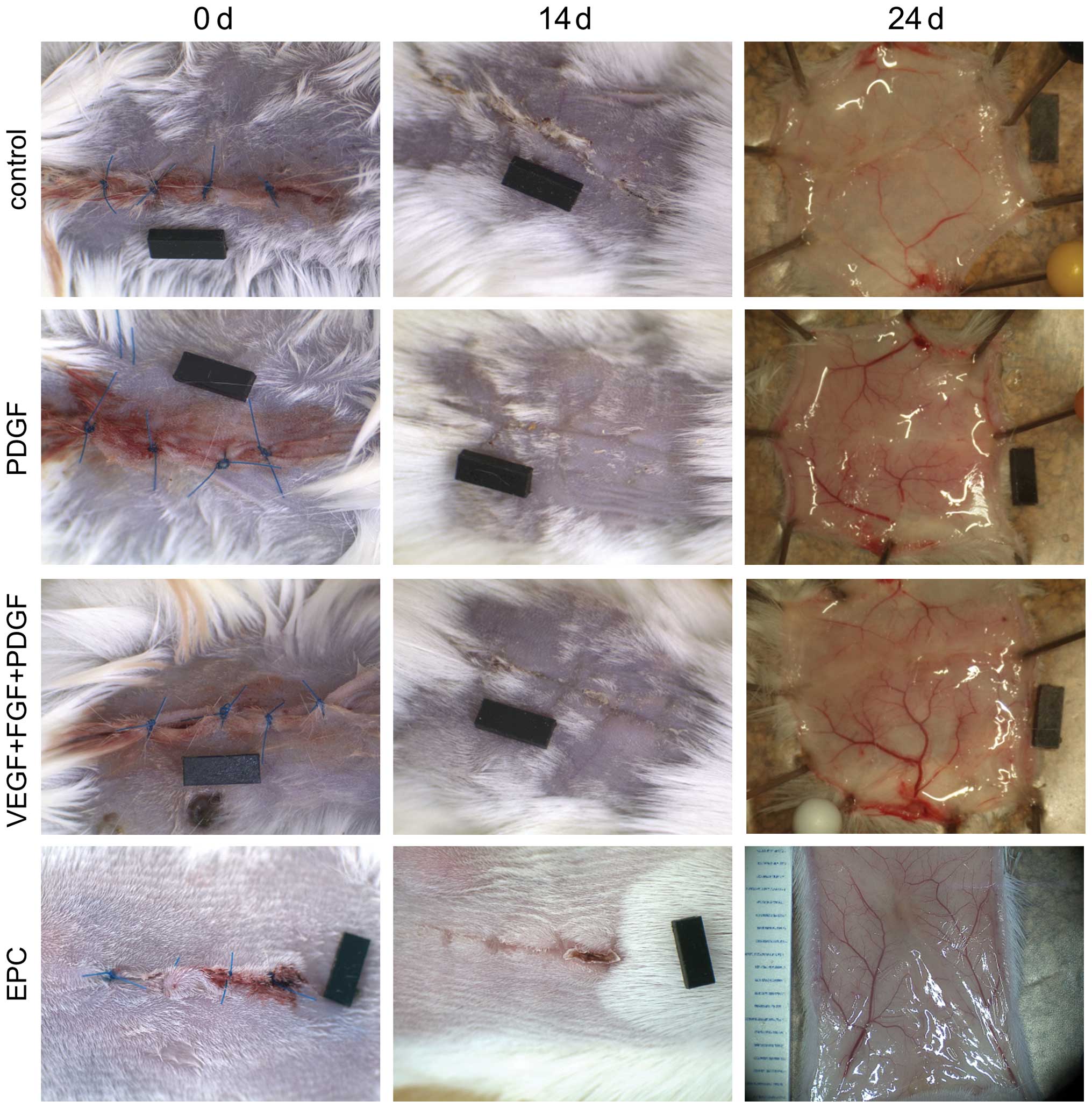
Gross appearance
As shown in Fig.
3, the wounds of all the animals were correctly adapted on day
14 and 24 after surgery. Macroscopically, a more rapid wound
closure was observed in the treated animals. The higher vessel
densities of the groups pre-treated with proangiogenic growth
factors were evident on day 24 after harvesting.
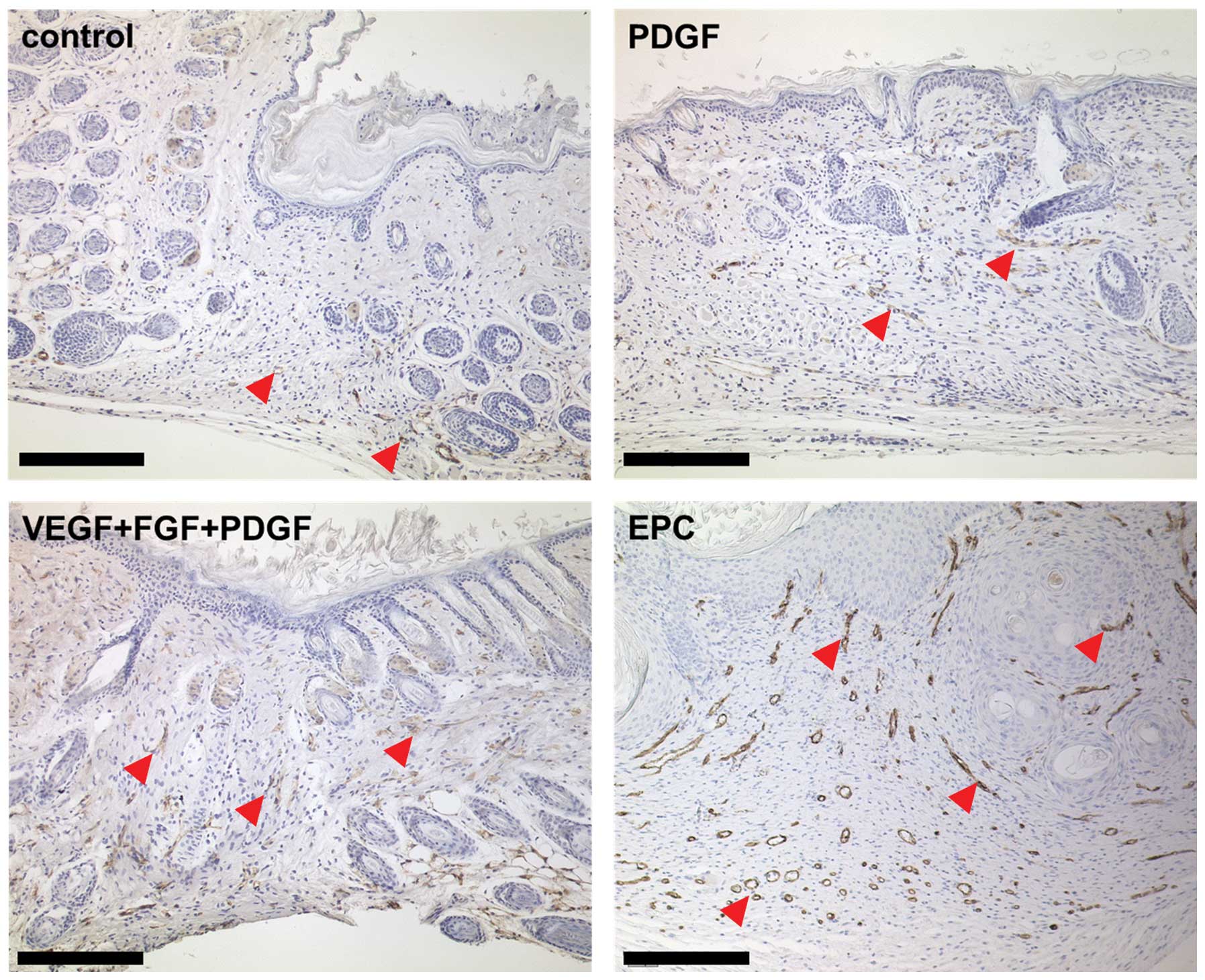
Vessel density
The vessel densities were determined in the wounds
after sacrificing the animals on day 14 and 24 after surgery.
Assessment of the local vessel expression revealed that vessel
allocation accumulated in particular in the subcutis and panniculus
carnosus (Fig. 4). The harvested
tissue of the animals primed with a combination of proangiogenic
growth factors showed a higher cell density that invaded the dermis
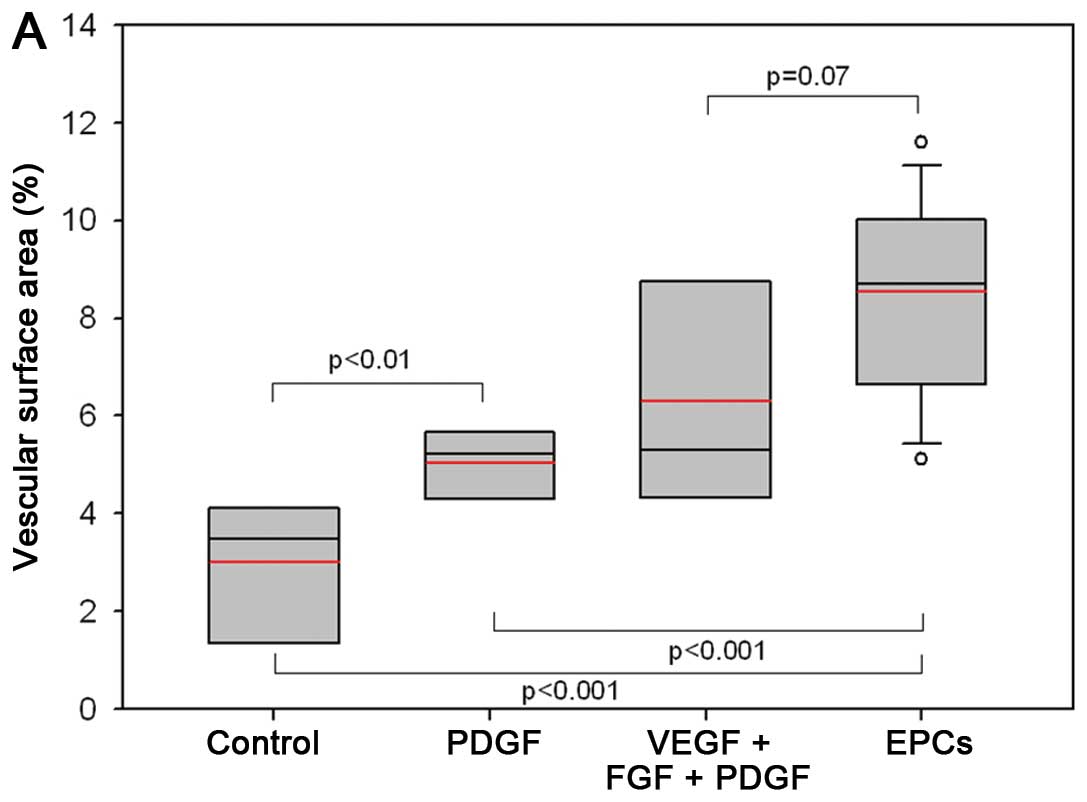
(Fig. 4). Fig. 5A shows that priming resulted in
significantly higher percentual vessel densities 14 days after
wounding: priming yielded >2-fold higher vessel surface areas in
the groups primed with a combination of proangiogenic growth
factors and EPCs vs. the controls (mean, 4.5 vs. 2.8% vascular
surface area; p<0.01). Following complete wound closure and
harvesting, vessel densities were assessed separately in the wound
ground, the former wound margin and in the adjacent unwounded
tissue. Fig. 5B illustrates that
the differences in vessel densities occurred on day 24
post-surgery. The animals primed with a combination of
proangiogenic growth factors (VEGF + FGF + PDGF) yielded vessel
densities of 11.5±3.5% (mean), the EPC-pre-treated mice densities
of 10.0±1.4%, whereas the PDGF-primed mice (6.3±3.2%) and the
controls (4.0±2.3%) revealed significant lower vessel densities
(p<0.05). Nonetheless, the highest values were observed in the
pre-treated animals.
Maximum tensile strength
Functional tensile strength testing revealed a
certain advantage of the group pre-treated with proangiogenic
growth factors in comparison to the sham-treated control group;
however, significant differences were not observed (Fig. 6). The mean values of the animals
primed with a combination of proangiogenic growth factors wer
0.58±0.28 N. The PDGF-monotherapy group showed the highest values
(0.70±0.33 N) and the control animals showed a value of 0.43±0.23
N. The animals pre-treated with EPCs revealed a mean tensile
strength of 0.65±0.24 N.
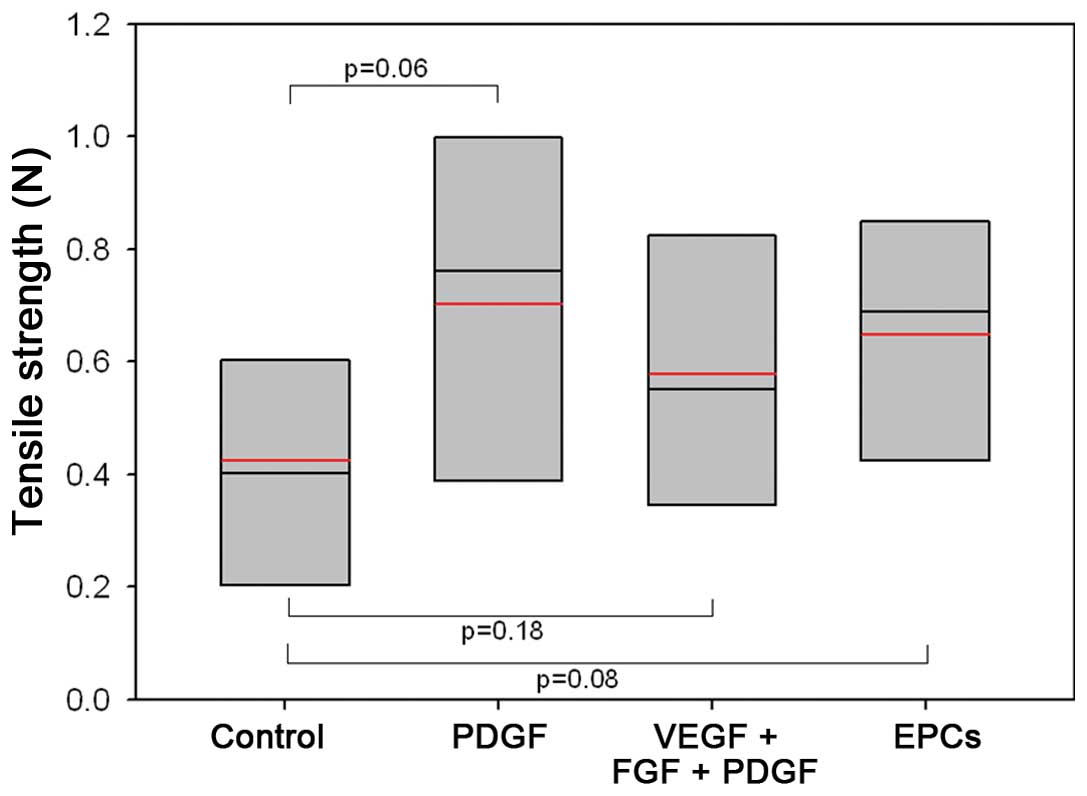 | Figure 6Comparison of tensile strength values
obtained on day 24 after surgery. Mice received either control
treatment (0.2 ml isotonic saline solution), PDGF monotherapy (3.5
μg), combination treatment (35.0 μg VEGF, 2.5 μg bFGF, and 3.5 μg
PDGF), or endothelial progenitor cells (EPCs) (2 million cells) on
days 3, 5 and 7 prior to surgery. Box-whisker plot showing the
median, 10th, 25th, 75th, and 90th percentile and mean (red). |
Discussion
This study demonstrates that priming with
proangiogenic growth factors and EPCs enhances incisional wound
healing, as defined by a more rapid wound re-epithelialization,
higher wound vascularization and higher tensile strength. In
particular, the assessment of time-to-closure and functional
outcome revealed an advantage for the groups primed with EPCs in
comparison to the control animals. Therefore, the findings in this
study allow the presumption that local EPC pre-treatment may be a
novel and clinically applicable strategy for enhancing wound
healing in diabetic wounds.
A number of studies have reviewed the therapeutic
implication of different proangiogenic growth factors or EPCs. In
general, the mode of application (topical, systemic, or priming)
and the dosage seem to represent variables with the highest impact
in terms of boosting angiogenesis in wound healing (27). Whereas topically applied growth
factors have been shown to be ineffective (28), the direct application of
angiogenic factors, such as VEGF, FGF and PDGF (8), has shown improved angiogenesis and
increased functional quality. Sander et al demonstrated the
positive impact of systemic transplantation of EPCs in mouse ear
model (29).
Cell-based therapy is a promising therapeutic option
for treating patients with diabetic, non-healing wounds. Of various
different types of stem or progenitor cells, the EPC is a type of
cell that has been moved from experimental models to clinical
trials. A few properties, such as its endogenous, BM-derived
characteristics, the ability to home to sites of pathological
entities and relative stability in terms of lineage specification
in culture, which allows genetic and epigenetic manipulation, make
EPCs an ideal cell candidate to be tested in cell-based therapeutic
applications for ischemic disorders, such as diabetes. Several
mechanisms are impaired in diabetes, the level of circulating EPCs
in diabetic wounds is decreased and mobilization is reduced
(30,31). Thus, a combination of therapeutic
approaches to target individual impairments and to correct
diabetes-related EPC deficits will likely synergize and may lead to
a more-successful treatment outcome for diabetic wounds.
Taken together, the results from the present study
demonstrate that priming with proangiogenic growth factors and EPCs
results in more rapid wound closure, higher vessel density and a
better functional outcome. Future experiments are planned which
will examine the effects of a combination of EPCs and proangiogenic
growth factors and compare the effects of proangiogenic EPC priming
with gene therapeutic approaches.
Acknowledgements
The authors acknowledge the skillful technical
assistance of Mrs. Kerstin Bahr.
References
|
1
|
Brem H and Tomic-Canic M: Cellular and
molecular basis of wound healing in diabetes. J Clin Invest.
117:1219–1222. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Galiano RD, Tepper OM, Pelo CR, et al:
Topical vascular endothelial growth factor accelerates diabetic
wound healing through increased angiogenesis and by mobilizing and
recruiting bone marrow-derived cells. Am J Pathol. 164:1935–1947.
2004. View Article : Google Scholar
|
|
3
|
Falanga V: Wound healing and its
impairment in the diabetic foot. Lancet. 366:1736–1743. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Singer AJ and Clark RA: Cutaneous wound
healing. N Engl J Med. 341:738–746. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Velazquez OC: Angiogenesis and
vasculogenesis: inducing the growth of new blood vessels and wound
healing by stimulation of bone marrow-derived progenitor cell
mobilization and homing. J Vasc Surg. 45(Suppl A): A39–A47. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chan RK, Liu PH, Pietramaggiori G, et al:
Effect of recombinant platelet-derived growth factor (Regranex) on
wound closure in genetically diabetic mice. J Burn Care Res.
27:202–205. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ackermann M, Wolloscheck T, Wellmann A, et
al: Priming with a combination of proangiogenic growth factors
improves wound healing in normoglycemic mice. Int J Mol Med.
27:647–653. 2011.PubMed/NCBI
|
|
8
|
Ackermann M, Wolloscheck T, Wellmann A, et
al: Priming with a combination of proangiogenic growth factors
Enhances wound healing in streptozotocin-induced diabetes in mice.
Eur Surg Res. 47:81–89. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Asahara T, Murohara T, Sullivan A, et al:
Isolation of putative progenitor endothelial cells for
angiogenesis. Science. 275:964–967. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kawamoto A, Gwon HC, Iwaguro H, et al:
Therapeutic potential of ex vivo expanded endothelial progenitor
cells for myocardial ischemia. Circulation. 103:634–637. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rafii S and Lyden D: Therapeutic stem and
progenitor cell transplantation for organ vascularization and
regeneration. Nat Med. 9:702–712. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Patschan D, Krupincza K, Patschan S, et
al: Dynamics of mobilization and homing of endothelial progenitor
cells after acute renal ischemia: modulation by ischemic
preconditioning. Am J Physiol Renal Physiol. 291:F176–F185. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Galiano RD, Tepper OM, Pelo CR, et al:
Topical vascular endothelial growth factor accelerates diabetic
wound healing through increased angiogenesis and by mobilizing and
recruiting bone marrow-derived cells. Am J Pathol. 164:1935–1947.
2004. View Article : Google Scholar
|
|
14
|
Sivan-Loukianova E, Awad OA, et al:
CD34+blood cells accelerate vascularization and healing
of diabetic mouse skin wounds. J Vasc Res. 40:368–377. 2003.
|
|
15
|
Rafii S and Lyden D: Therapeutic stem and
progenitor cell transplantation for organ vascularization and
regeneration. Nat Med. 9:702–712. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Urbich C and Dimmeler S: Endothelial
progenitor cells: characterization and role in vascular biology.
Circ Res. 95:343–353. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yoon CH, Hur J, Oh IY, et al:
Intercellular adhesion molecule-1 is upregulated in ischemic
muscle, which mediates trafficking of endothelial progenitor cells.
Arterioscler Thromb Vasc Biol. 26:1066–1072. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Asahara T, Takahashi T, Masuda H, et al:
VEGF contributes to postnatal neovascularization by mobilizing bone
marrow-derived endothelial progenitor cells. EMBO J. 18:3964–3972.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shi Q, Bhattacharya V, Hong-De Wu M and
Sauvage LR: Utilizing granulocyte colony-stimulating factor to
enhance vascular graft endothelialization from circulating blood
cells. Ann Vasc Surg. 16:314–320. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Takahashi T, Kalka C, Masuda H, et al:
Ischemia- and cytokine-induced mobilization of bone marrow-derived
endothelial progenitor cells for neovascularization. Nat Med.
5:434–438. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Luttun A, Tjwa M and Carmeliet P:
Placental growth factor (PlGF) and its receptor Flt-1 (VEGFR-1):
novel therapeutic targets for angiogenic disorders. Ann NY Acad
Sci. 979:80–93. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ziebart T, Yoon CH, Trepels T, et al:
Sustained persistence of transplanted proangiogenic cells
contributes to neovascularization and cardiac function after
ischemia. Circ Res. 103:1327–1334. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ziebart T, Pabst A, Klein MO, et al:
Bisphosphonates: restrictions for vasculogenesis and angiogenesis:
inhibition of cell function of endothelial progenitor cells and
mature endothelial cells in vitro. Clin Oral Investig. 15:105–111.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lenzen S: The mechanisms of alloxan- and
streptozotocin-induced diabetes. Diabetologia. 51:216–226. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Weibel ER, Kistler GS and Scherle WF:
Practical stereological methods for morphometric cytology. J Cell
Biol. 30:23–38. 1966. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Erba P, Miele LF, Adini A, et al: A
morphometric study of mechanotransductively induced dermal
neovascularization. Plast Reconstr Surg. 128:288e–299e. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hendrickx B, Den Hondt M, Verdonck K,
Vranckx JJ and Luttun A: Cell and Gene Transfer Strategies for
Vascularization During Skin Wound Healing. Emerging Trends in Cell
and Gene Therapy. Danquah MK and Mahato RI: Humana Press; New York,
NY: pp. 637–695. 2013, View Article : Google Scholar
|
|
28
|
Smiell JM, Wieman TJ, Steed DL, et al:
Efficacy and safety of becaplermin (recombinant human
platelet-derived growth factor-BB) in patients with nonhealing,
lower extremity diabetic ulcers: a combined analysis of four
randomized studies. Wound Repair Regen. 7:335–346. 1999. View Article : Google Scholar
|
|
29
|
Sander AL, Jakob H, Henrich D, et al:
Systemic transplantation of progenitor cells accelerates wound
epithelialization and neovascularization in the hairless mouse ear
wound model. J Surg Res. 165:165–170. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gallagher KA, Liu ZJ, Xiao M, et al:
Diabetic impairments in NO-mediated endothelial progenitor cell
mobilization and homing are reversed by hyperoxia and SDF-1 alpha.
J Clin Invest. 117:1249–1259. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu ZJ and Velazquez OC: Hyperoxia,
endothelial progenitor cell mobilization, and diabetic wound
healing. Antioxid Redox Signal. 10:1869–1882. 2008. View Article : Google Scholar : PubMed/NCBI
|