Introduction
Osteonecrosis (ON) is a pathological process that
predominantly impairs the femoral head and gradually progresses to
the fracture of the subchondral bone, the collapse of the femoral
head surface and the destruction of the hip joint. Although the
etiology of ON has been attributed to a number of factors,
glucocorticoid (GC) administration is the predisposing causative
factor most commonly associated with the development of ON.
Compromised vascularity and bone ischemia represent the traditional
etiological background of ON. It was then realized that osteoblast
and osteocyte apoptosis, but not compromised vascularity, is the
primary etiology of GC-induced ON (1–12).
In addition, the expression of vascular endothelial growth factor
(VEGF), a key regulator that couples angiogenesis, bone formation
and repair, reduces accompanied by the increase of osteoblasts and
osteocytes apoptosis (13). Also,
the compromised expression of VEGF has been found to impair the
bone regeneration in the necrotic zones of the femoral head
(14). On the basis of the above
considerations, suppression of the apoptosis of the osteoblast and
osteocytes and stimulation of the expression of VEGF could be two
effective attempts in preventing the GC-induced ON of the femoral
head.
Erythropoietin (EPO) is a pleiotropic cytokine
originally identified for its role in erythropoiesis (15). Accumulating evidence has indicated
that erythropoietin exerts anti-apoptotic and tissue-protective
effects in a variety of human diseases, such as myocardial
infraction (16–18), diabetes mellitus (19), spinal cord injury (20–22), ischemia-reperfusion (I/R)-induced
kidney injury (23) and acute
lung injury (24,25). Galeano et al reported that
recombinant human EPO (rhuEPO) may be an effective therapeutic
approach for improving clinical outcomes by enhancing the wound
content of vascular endothelial growth factor (VEGF) following
thermal injury (26). Rezaeian
et al also demonstrated that the pharmacological
manipulation of ischemic musculocutaneous tissue with 3 repetitive
doses of EPO (500 IU/kg) upregulated inducible nitric oxide
synthase (iNOS) and VEGF expression, and reduced apoptotic cell
death and inflammation in the absence of any hematopoietic effect
(27). Additionally, Holstein
et al found that treatment with EPO upregulated the
expression of VEGF during the early phase of bone defect healing,
as shown by immunoblot and immunohistochemistry analyses (28). These data suggest that EPO exerts
tissue protective effects through a VEGF-related pathway.
Therefore, we hypothesized that the administration
of EPO can protect the femoral head from GC-induced ON by
inhibiting apoptosis and increasing the expression of VEGF. We
investigated this hypothesis using rats and a variety of methods,
including histological staining and protein biochemistry. Indeed,
we found that the administration of EPO markedly reduced the
incidence of GC-induced ON in rats. Moreover, EPO suppressed the
apoptosis of osteoblasts and osteocytes and increased the
expression of VEGF.
Materials and methods
Animals
All experimental procedures adhered to the
recommendations of the Experimental Animal Center of Wuhan
University, Wuhan, China and the US Department of Health Guide for
the Care and Use of Laboratory Animals, and were approved by the
Ethics Committee of Wuhan University. A total of 54 male Wistar
rats (10 weeks old) were obtained from the Hubei Provincial Center
for Disease Control and Prevention, Wuhan, China. The rats were
housed in a temperature- and humidity-controlled environment with
unlimited access to food and water and a 12-h light/dark cycle.
Experimental protocols
A total of 54 rats were divided equally into 2
groups: the control and EPO group. A rat model of ON was created by
a sequential drug administration. The animals were administered 2
mg/kg lipopolysaccharide (LPS, from Escherichia coli 055:
B5; Sigma, St. Louis, MO, USA) intravenously on days 0 and 1. On
days 2, 3 and 4, the animals were administered 20 mg/kg
methylprednisolone (MPS; Pfizer Pharmaceutical, Puurs, Belgium)
intramuscularly. The animals in the EPO group were administered 500
U/kg rhuEPO (Shenyang Sunshine Pharmaceutical Co. Ltd., Shenyang,
China) intramuscularly daily from day 0 for 7 days. The final day
of administration was regarded as experimental week 0. The rats in
the control group were not administered EPO. Nine rats in each
group were sacrificed (6 for histological analysis and 3 for
molecular biological analysis) on weeks 0, 2 and 4.
Blood biochemistry
Blood was collected from the inferior vena cava at
the time of sacrifice partially for regular testing and the
remaining blood was centrifuged immediately. The supernatant was
stored as platelet-rich plasma at −80°C. The triglyceride
concentrations in the plasma were measured by using Triglyceride
E-test kit (Nanjing Jiancheng Bioengineering Institute, Nanjing,
China) according to the manufacturer’s instructions. The total
cholesterol levels in the plasma were measured using the
Cholesterol E-test kit (Nanjing Jiancheng Bioengineering Institute)
according to the manufacturer’s instructions.
Histopathological analysis
The proximal femurs were harvested and fixed with
10% formalin-0.1 M phosphate buffer, pH 7.4. After fixing in
formalin, the bone samples were decalcified in 10% EDTA for 2
months. The decalcified bones were then embedded in paraffin and
sectioned at 5 μm for histopathological analysis and
immunohistochemistry. The sections were processed for routine
haematoxylin and eosin staining to evaluate the ON lesions using
well established criteria (29–32). The diagnosis of ON was performed
in a blinded manner by 3 of the authors on the basis of the diffuse
presence of empty lacunae or pyknotic nuclei of osteocytes in the
bone trabeculae, accompanied by surrounding bone marrow cell
necrosis. Rats that had at least 1 ON lesion in the areas examined
were considered as ON+, while those with no ON lesions
were considered as ON−. The incidence of ON was defined
as the numbers of ON+ rats divided by the numbers of
total rats in each group.
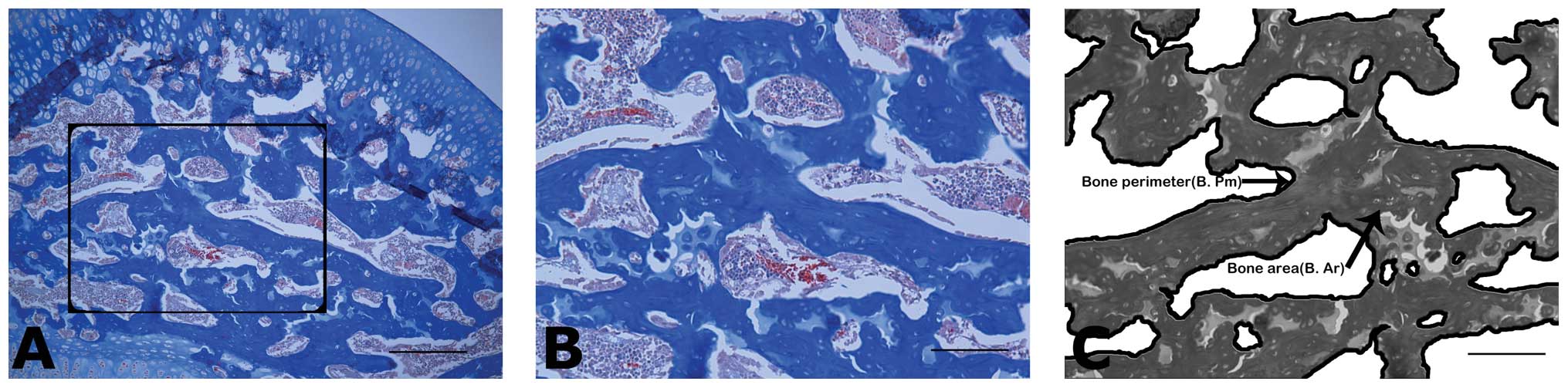
Trabecular bone architecture
Frontal sections (4-μm-thick) of each femoral head
were obtained and stained with Masson’s trichrome to highlight the
microstructure. The trabecular tissue area (T.Ar, mm2),
the trabecular bone area (B.Ar, mm2) and trabecular
perimeter (B.Pm, mm) were quantified for each section in the
central region of the proximal epiphysis; the bone volume fraction
(%) was calculated as the trabecular bone area (BV) divided by the
trabecular tissue area (TV) (Fig.
1). Other architectural properties, including trabecular
thickness (Tb.Th, mm), trabecular number (Tb.N, mm−1)
and trabecular separation (Tb.Sp, mm) were calculated according to
a parallel plate model (33,34).
TUNEL assays
Apoptotic osteoblasts and osteocytes were detected
using the terminal deoxynucleotidyl transferase-mediated dUTP nick
end-labeling (TUNEL) assay, with an In Situ Cell Death Detection
Kit (Roche Diagnostics, Mannheim, Germany), according to the
manufacturer’s instructions. Briefly, following routine
deparaffinization and treatment with H2O2
(3%), the sections were digested with proteinase K (20 μg/ml, pH
7.4, 12 min) at 25°C and incubated with the reaction mixture (1:40,
60 min) at 37°C. Incorporated fluorescein was detected with
horseradish peroxidase following incubation for 30 min at 37°C and
were subsequently dyed with 3,3′-diaminobenzidine (DAB). Brown
nuclei were assessed as positive apoptotic cells. The apoptotic
index (AI) was evaluated for 1 section of 5 randomly selected
high-power fields.
Immunoblot analysis
Femoral heads dissected from the proximal one-third
of the femur neck were powdered in liquid nitrogen by hand milling,
followed by homogenization on ice-cold radioimmunoprecipitation
(RIPA, Beyotime Institute of Biotechnology, Beijing, China) buffer
containing phenylmethylsulfonyl fluoride (PMSF, Beyotime Institute
of Biotechnology) and a cocktail of protease inhibitors (Complete,
EDTA-free; Roche Diagnostics). Following sonication, the samples
were centrifuged twice at 14000 rpm at 4°C for 10 min to remove
cell debris, nuclei and large particulates. The supernatant
containing the cytosolic protein fraction was then collected. A
quarter volume of 5× loading buffer was added and boiled at 95°C
for 5 min then stored at −20°C until electrophoresis. Proteins were
separated by 10% sodium dodecyl sulfate polyacrylamide gel
electrophoresis and transferred onto polyvinylidene difluoride
membranes (Millipore Corp., Bedford, MA, USA). After being blocked
with 2% bovine serum albumin (Roche Diagnostics), the membranes
were incubated at 4°C overnight with rabbit anti-caspase-3 or
anti-VEGF antibody (1:500, Santa Cruz Biotechnology, Santa Cruz,
CA, USA) or rabbit anti-GAPDH antibody (1:500, Santa Cruz
Biotechnology) as primary antibodies, followed by incubation with
peroxidase-conjugated secondary antibodies (1:2000, Jackson
Laboratories, West Grove, PA, USA) at 25°C for 1 h. The proteins on
the membranes were visualized using an ECL plus detection kit
(Amersham Pharmacia Biotech, Buckinghamshire, UK), exposed to Kodak
X-ray film and processed using a scanner. The optical density of
the bands was analyzed using Image-Pro Plus 6.0 software. The
expression of caspase-3 and VEGF was then normalized to GAPDH.
Statistical analysis
Data are presented as the means ± standard error of
the mean (SEM). Statistical analysis was performed using SPSS 13.0
software (SPSS Inc., Chicago, IL, USA). One-way analysis of
variance (ANOVA) with Turkey’s post hoc test was used to examine
differences between groups. Statistical differences were considered
significant when the P-value was <0.05.
Results
EPO administration does not affect body
weight, red blood cell (RBC) count, hemoglobin and hematocrit
levels in rats
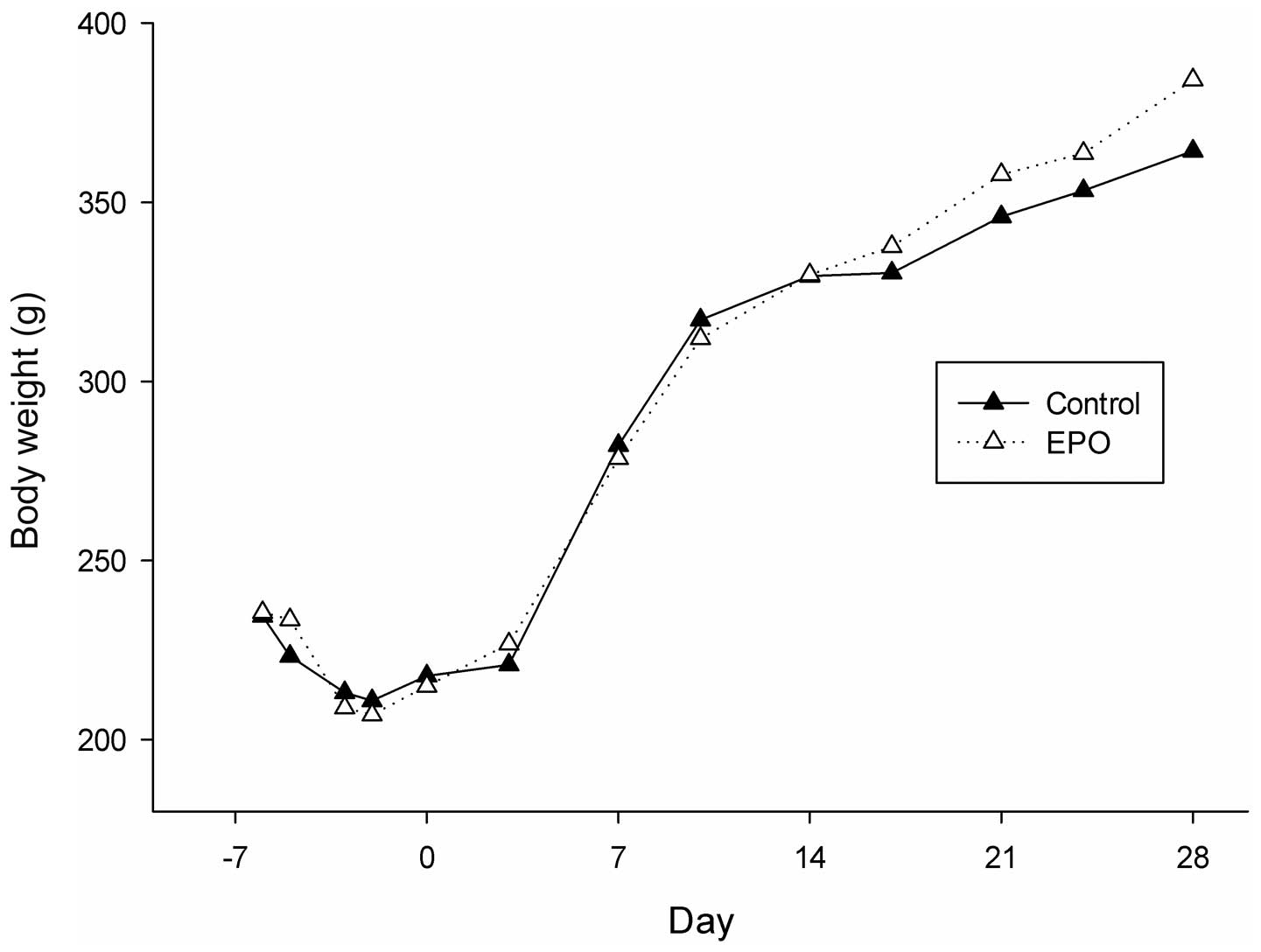
No accidental deaths took place during the
experiment. The body weight of the animals in both groups decreased
slightly during the 1st week (week, −1), and thereafter increased
from weeks 0 to 4. There were no significant differences between
the body weight of the animals in the 2 groups during the whole
experimental period (Fig. 2).
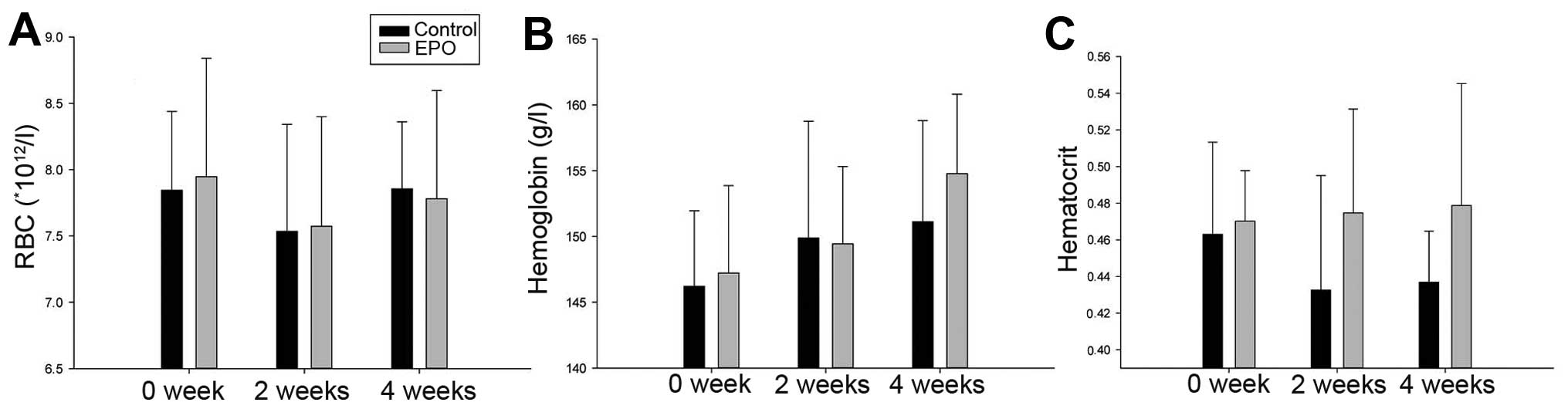
Blood analyses did not reveal a significantly higher RBC count in
the rhuEPO-treated animals when compared with the controls.
Additionally, the levels of hemoglobin and hematocrit displayed a
similar trend (Fig. 3), as the
statistical analysis revealed no significant differences between
the groups. These data indicate that the appropriate dose of EPO
does not affect body weight and blood components in rats.
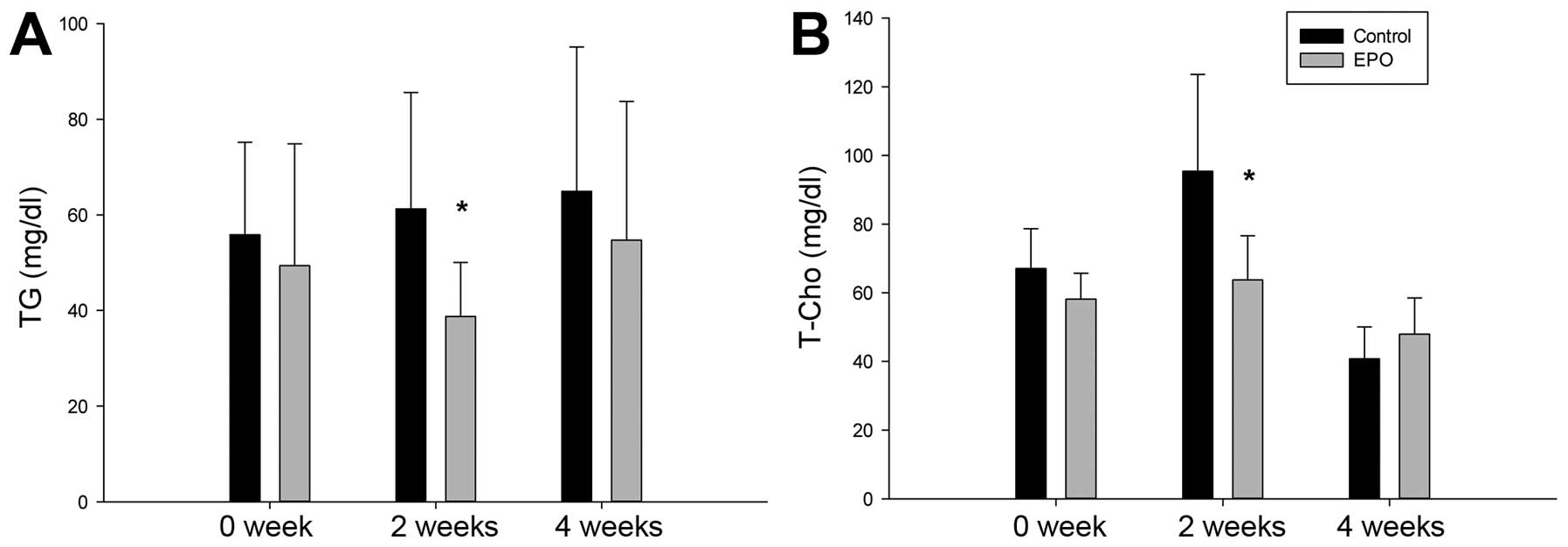
EPO administration decreases the plasma
concentration of triglycerides and total cholesterol in rats
We then examined the concentrations of triglycerides
and total cholesterol in plsma. As shown in Fig. 4, the plasma levels of
triglycerides in the EPO group were lower than those in the control
group. Notably, a significant difference was observed on week 2
(P<0.05). The level of total cholesterol in the plasma in the
EPO group was also significantly lower than that in the control
group on week 2. These results suggest that the appropriate dosage
of EPO reduces the plasma concentration of triglycerides and total
cholesterol during the early stages of ON.
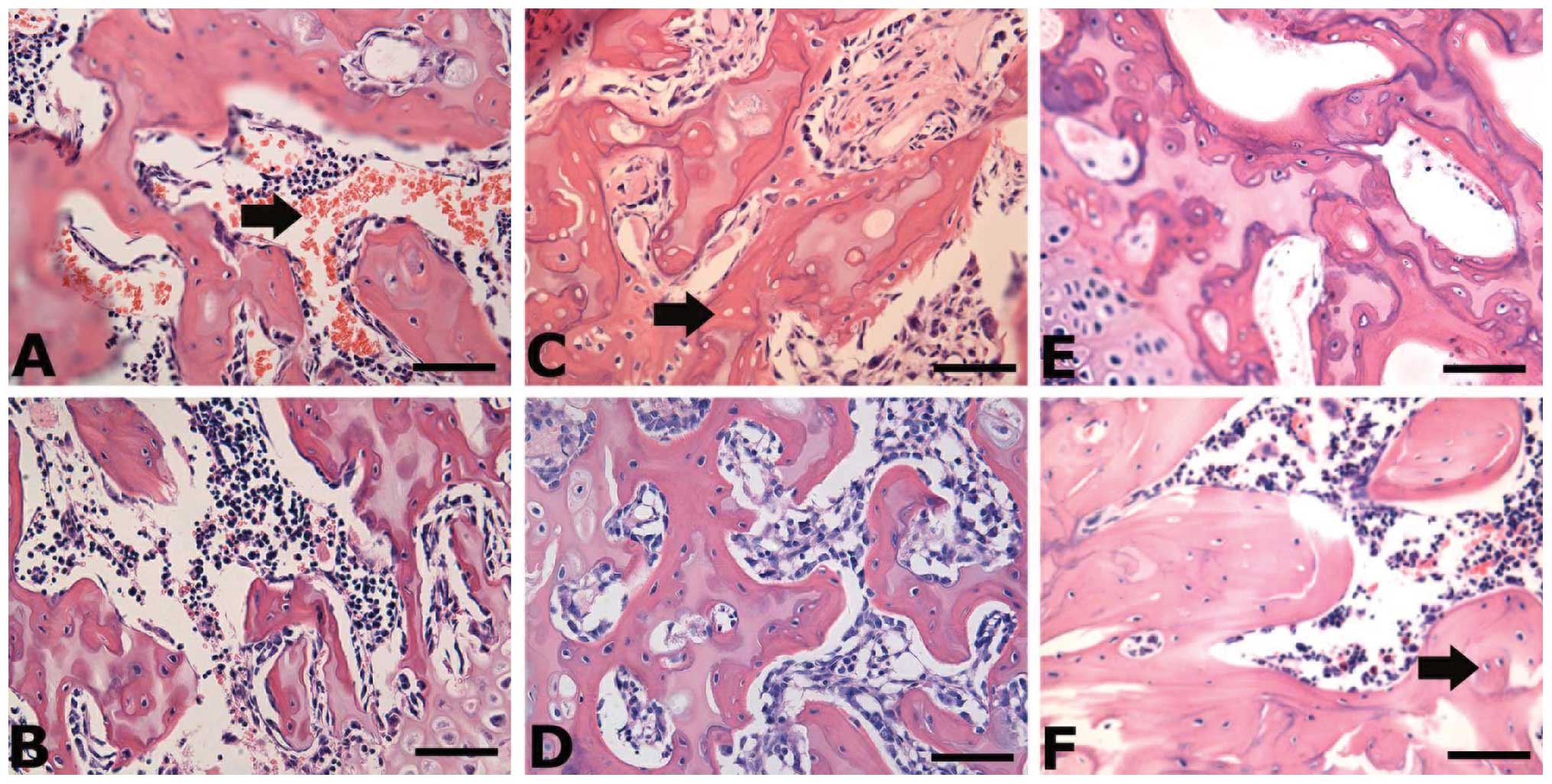
EPO administration improves histological
performance and reduces the incidence of ON in rats
The presence of diffuse and empty lacunae or
pyknotic nuclei of osteocytes in the bone trabeculae, accompanied
by surrounding bone marrow cell necrosis, is defined as ON. To
assess the ON lesions in the control and EPO groups, we performed
haematoxylin and eosin staining to determine the histological
characteristics (Fig. 5). On week
0, the necrotic bone trabeculae showed pyknotic nuclei of
osteocytes and empty lacunae accompanied by the hemorrhage and
necrosis of bone marrow (Fig. 5A and
B). On week 2, the necrotic bone trabeculae also showed
pyknotic nuclei and empty lacunae, while the numbers of empty
lacunae markedly increased (Fig. 5C
and D). Additionally, the fibrous tissue was found to
accumulate in the medullary space (Fig. 5C and D). On week 4, the necrotic
bone trabeculae showed further empty lacunae and the presence of
scar tissue in the medullary space (Fig. 5E and F). Based on the
histopathological characteristics, we determined the incidence of
ON as presented in Table I. In
the control group, the incidence of ON increased with time, whereas
the administration of EPO prevented the occurrence of ON. The total
incidence of ON markedly decreased in the EPO group compared with
the control group (22.2 vs. 66.7%) (Table I), suggesting that the appropriate
dose of rhuEPO can greatly reduce the incidence of
steroid-associated ON in rats.
 | Table IIncidence of osteonecrosis (%). |
Table I
Incidence of osteonecrosis (%).
| Group | Week 0 | Week 2 | Week 4 | Total |
|---|
| Control | 33.3 (2) | 66.7 (4) | 100.0 (6) | 66.7 |
| Erythropoietin | 16.7 (1) | 33.3 (2) | 16.7 (1) | 22.2 |
| χ2
test | 0.4444 | 1.3333 | 8.5714 | 7.2000 |
| P-value | 0.5050 | 0.2482 | 0.0034 | 0.0073 |
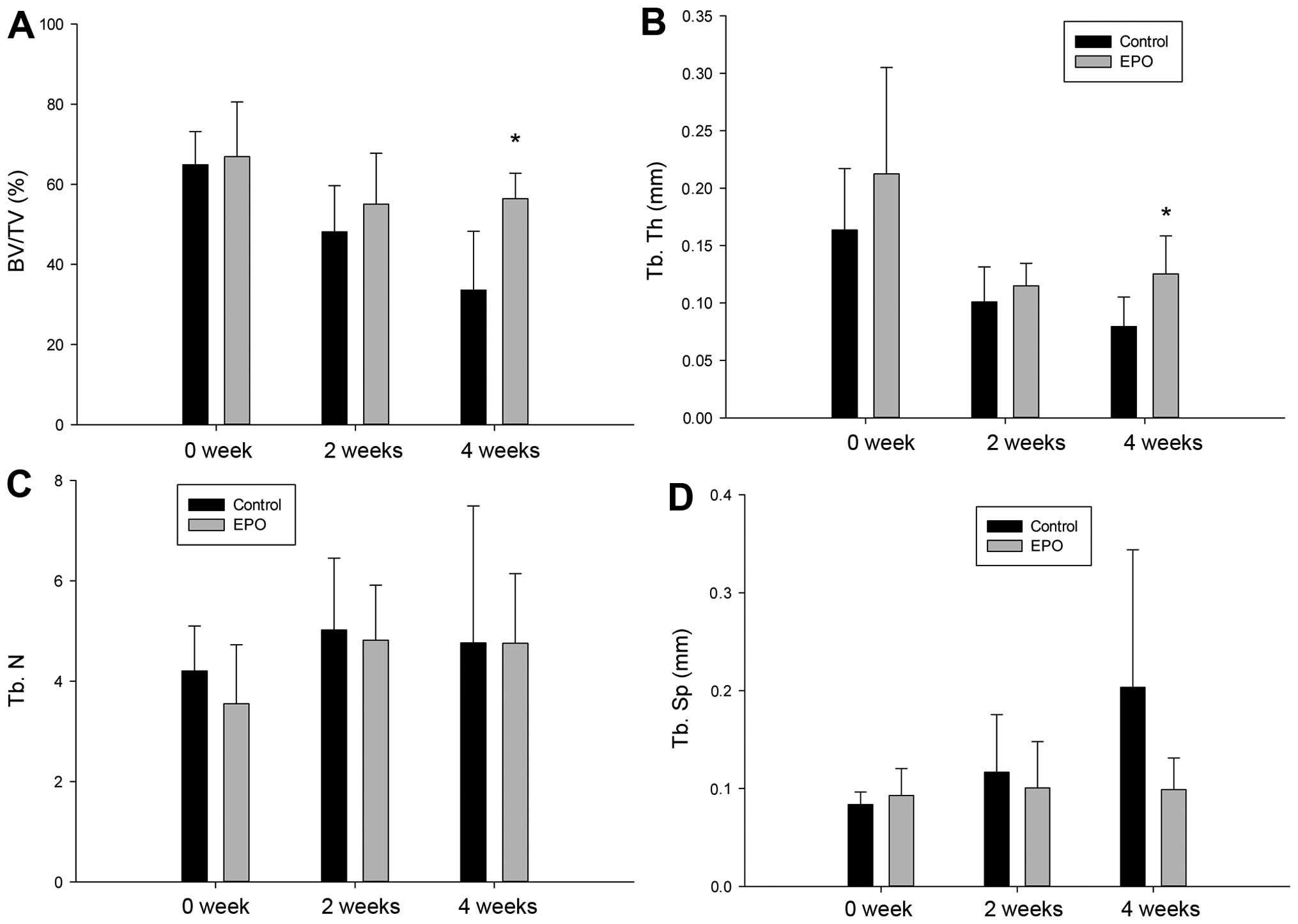
Trabecular bone volume fraction and
trabecular thickness increases upon EPO administration
To assess the microstructural architecture of the
trabecular bone, we introduced 4 indicators, including bone volume
fraction, trabecular thickness, trabecular number and trabecular
separation, as described in Materials and methods. No significant
difference was observed as regards the 4 indicators between the 2
groups on weeks 0 and 2. Of note, the trabecular bone volume
fraction and trabecular thickness in the EPO group increased with
statistical significance on week 4 (P=0.006 and P=0.023; Fig. 6A and B). However, there were no
significant differences observed in trabecular number and
trabecular separation between the 2 groups (Fig. 6C and D).
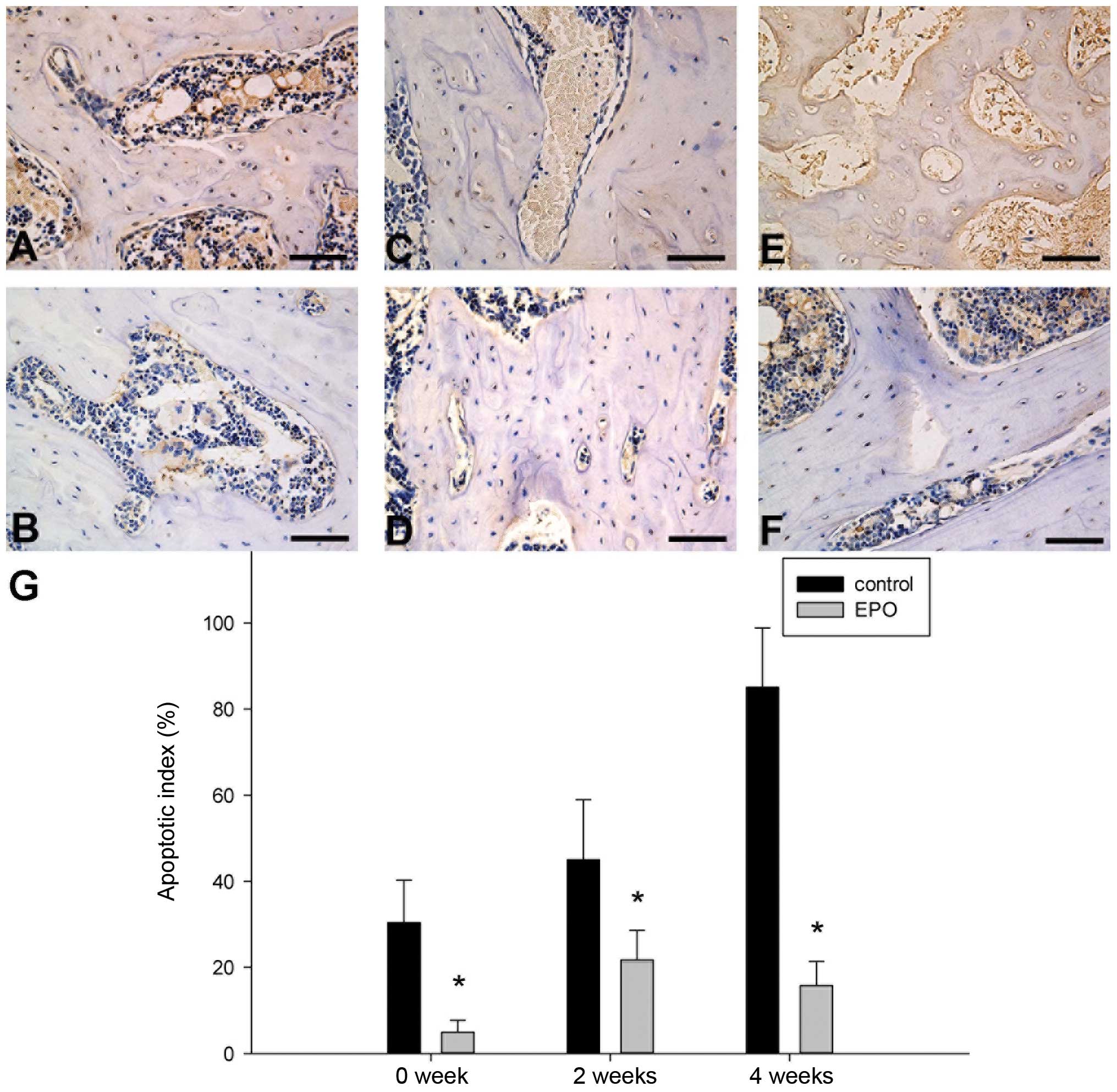
EPO administration inhibits apoptosis in
the ON zone
To determine whether the administration of EPO
affects apoptosis in the trabecular bone of the femoral head, we
conducted TUNEL assays and found that positively stained cells were
detected in the ON zones in both groups, although the number of
TUNEL-positive cells varied at different time points (Fig. 7A–F). Additionally, treatment with
EPO resulted in less apoptotic cells/high-power field, which was
further confirmed by quantitative analysis showing a significantly
lower apoptotic index in the EPO group compared with the control
group at all the 3 time points (P<0.05; Fig. 7G). These data indicate that the
administration of EPO protects the trabecular bone cells from
apoptosis.
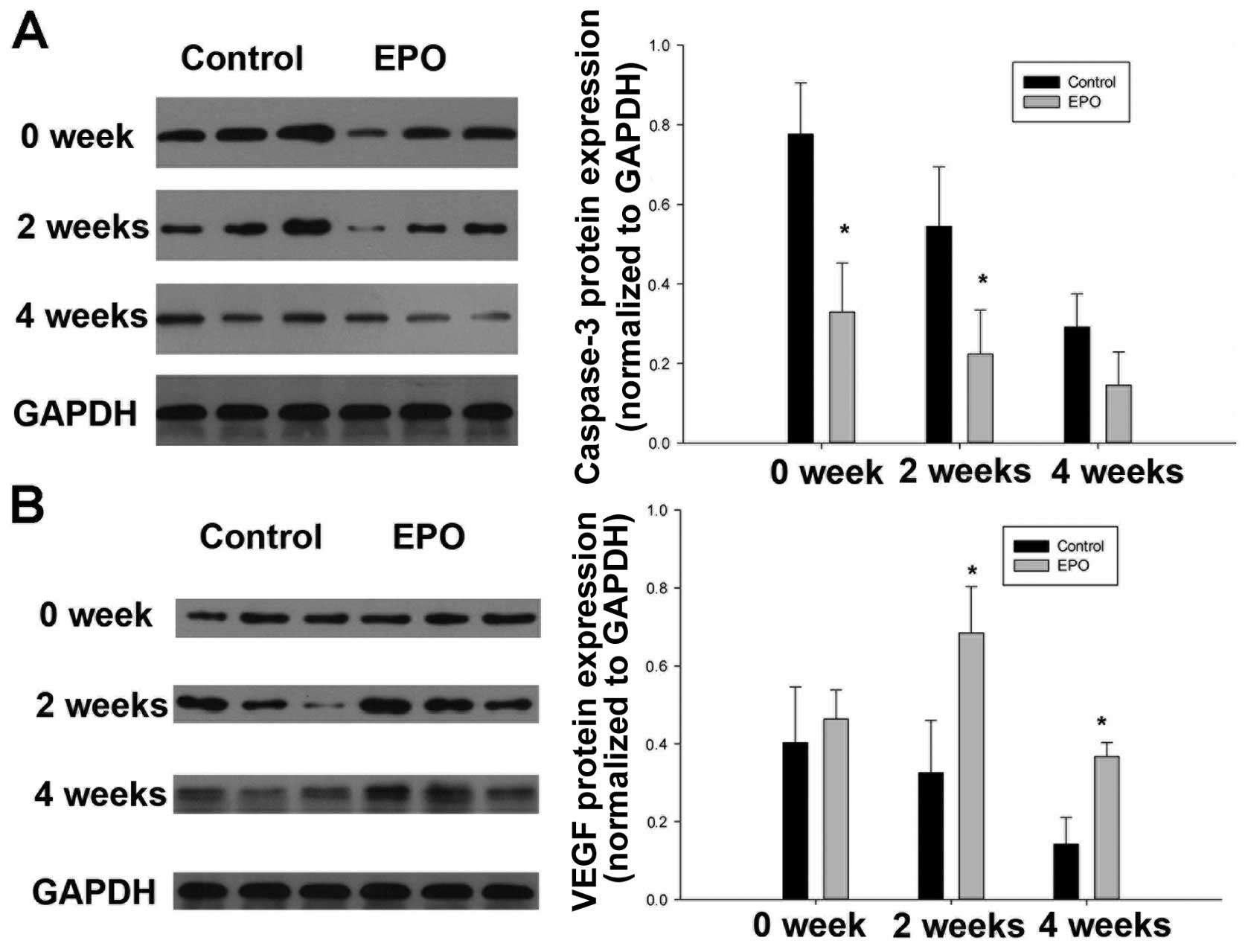
EPO administration reduces caspase-3 and
increases VEGF levels
To further consolidate our previous observations, we
performed immunoblot analysis to assess the expressions of
caspase-3, an indicator of apoptosis, as well as the levels of
VEGF. Indeed, the expression of caspase-3 within the femoral head
markedly decreased in response to EPO treatment (Fig. 8A), while the difference was
significant on week 0 and week 2 (P<0.05). On the contrary, the
expression level of VEGF markedly increased following treatment
with EPO on weeks 2 and 4 (Fig.
8B). These data demonstrate that EPO exerts tissue-protective
effects against ON through 2 mechanisms, the inhibition of
apoptosis and the enhancement of VEGF expression.
Discussion
EPO has been found to initiate tissue-protective
effects, including the prevention of apoptotic cell death (35) and the induction of angiogenesis
and tissue regeneration (36), in
addition to its regulatory function in erythropoiesis (37). These effects may be beneficial to
the pathological process of ON. However, the excessive use of EPO
can boost the hematocrit, which is detrimental for microcirculatory
perfusion in ischemic situations, such as ON, and which causes
polycythemia, a condition with abnormally high levels of RBCs.
Rezaeian et al (27)
reported that the administration of 5 repeated doses of 500 units
of EPO only marginally increased the hematocrit in C57BL/6 mice
after a period of approximately 7 days, whereas the administration
of 5,000 units 5 times significantly increased the hematocrit. It
has been found that a significantly increased hematocrit can
aggravate bone necrosis due to impaired rheology, i.e., decreased
RBC velocity and nutritive perfusion and hyperviscosity, which is
believed to be detrimental despite the anti-apoptotic and
angiogenic effects mediated by EPO (27). Furthermore, a previous study
demonstrated that, to prevent the detrimental effects of EPO, the
treatment of anemia in chronic renal failure usually requires low
doses of EPO between 350 and 400 units or less on a very repetitive
base over several weeks or months (27). Therefore, we decided to administer
7 doses of (500 units/kg/day) of EPO in our study. Blood analyses
did not reveal a significantly higher RBC count in the EPO-treated
animals compared with the untreated controls on weeks 0, 2 and 4
(Fig. 3). The levels of
hemoglobin and hematocrit showed a similar trend (Fig. 3). Additionally, histological
analysis revealed a lower ON index in the EPO group compared iwth
the control group (Figs. 5 and
6). These results illustrate that
the administration of EPO at the appropriate dosage and repetitive
manner exerts beneficial effects rather than harmful effects on the
pathological process of ON. In accordance with the former data, the
apoptotic index of osteoblasts and osteocytes in the EPO group was
greatly reduced compared with the controls (Fig. 7). Moreover, the expression of VEGF
markeldy increased following treatment with EPO (Fig. 8B). In conclusion, the results from
our study suggest that the tissue-protective function of EPO in
GC-induced ON is possibly attributed to the combination of its
anti-apoptotic and VEGF-enhancing effects.
GCs have been reported to elicit the apoptosis of
osteoblasts and osteocytes through direct and indirect mechanisms.
Osteoblasts and osteocytes can be the direct targets of GC action
in vivo and excess levels of steroid hormones directly
induce the apoptosis of these cell types (5). Alternatively, infarction, as well as
oxygen and nutrient deprivation caused by a high-dose
administration of GCs inevitably lead to the apoptosis of
osteoblasts and osteocytes (7).
The activation of caspase-3, a common downstream effector of
apoptotic signaling pathways, including the involvement of direct
and indirect mechanisms, is observed in the GC-induced apoptosis of
osteoblasts and osteocytes (38,39). Consistent with the data from other
studies (40,41), we also demonstrated that GC
enhanced apoptosis, as well as caspase-3 expression in trabecular
bone in a time-dependent manner (Figs. 7 and 8A). However, the elevated levels of
apoptosis and caspase-3 expression were significantly repressed in
response to EPO administration (Figs.
7 and 8A). However, it
remains to be determined whether caspase-3 expression is causative
to the apoptosis observed in the ON zone and whether caspase-3 is a
direct target of EPO. Our results demonstrate that the appropriate
dose of EPO exerts anti-apoptotic effects on GC-induced ON.
A number of of studies have suggested that GC
impairs angiogenesis and osteogenesis by suppressing the production
of VEGF in the femoral head in GC-induced ON (13,42–46). It has been reported that GCs
suppress angiogenesis in fetal metatarsals, as well as
hypoxia-inducible factor-1α transcription and VEGF production in
osteoblasts and osteocytes (13).
Osteoblasts derived from femoral heads have been shown to exhibit a
decrease in VEGF expression within 24 h of incubation with GCs
(43). Furthermore, it is likely
that VEGF and its receptors play vital roles coupling osteogenic
and angiogenic processes in adult bone during remodeling and repair
processes (47). In an in
vivo model of ON, rabbits treated with depomedrol plus
adrenocorticotropic hormone (ACTH)for 1 month displayed fewer signs
of trabecular necrosis and an increased expression of VEGF in
comparison to animals treated with the steroid, depomedrol, alone
(48). In the present study,
immunoblot analysis revealed an elevated VEGF expression in the ON
zone in the group administered EPO (Fig. 8B). Moreover, a higher VEGF
expression was usually accompanied with a better histological
performance and a higher trabecular bone volume fraction of the
femoral head (Figs. 5 and
6). Taken together, the data from
our study suggest that the administration of EPO exerts angiogenic
and osteogenic effects in ON through a VEGF-related mechanism.
In conclusion, the present study demonstrates that
the administration of EPO exerts prominent protective effects
against GC-induced ON of the femoral head in rats by inhibiting the
apoptosis of osteoblasts and osteocytes and increasing the
expression of VEGF. Further investigations of the molecular basis
of EPO-mediated anti-apoptosis and regeneration in ON are required
in order to develop novel strategies for the prevention and
treatment of GC-induced ON.
Acknowledgements
The authors thank Changgeng Xu, Lei Wang, Shuibing
Wang and Xiaolin Wu (Renmin Hospital of Wuhan University, Wuhan,
Hubei, P.R. China) for their assistance in the process of the
present study. This study was supported by the Fundamental Research
Funds for the Central Universities and Independent Research Project
of Wuhan University for Graduate Students (no. 2012302020207) and
the National Natural Science Foundation of China (no.
81301592).
References
|
1
|
Silvestrini G, Ballanti P, Patacchioli FR,
et al: Evaluation of apoptosis and the glucocorticoid receptor in
the cartilage growth plate and metaphyseal bone cells of rats after
high-dose treatment with corticosterone. Bone. 26:33–42. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Weinstein RS, Nicholas RW and Manolagas
SC: Apoptosis of osteocytes in glucocorticoid-induced osteonecrosis
of the hip. J Clin Endocrinol Metab. 85:2907–2912. 2000.PubMed/NCBI
|
|
3
|
Calder JD, Pearse MF and Revell PA: The
extent of osteocyte death in the proximal femur of patients with
osteonecrosis of the femoral head. J Bone Joint Surg Br.
83:419–422. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zalavras C, Shah S, Birnbaum MJ and
Frenkel B: Role of apoptosis in glucocorticoid-induced osteoporosis
and osteonecrosis. Crit Rev Eukaryot Gene Expr. 13:221–235. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
O’Brien CA, Jia D, Plotkin LI, et al:
Glucocorticoids act directly on osteoblasts and osteocytes to
induce their apoptosis and reduce bone formation and strength.
Endocrinology. 145:1835–1841. 2004.PubMed/NCBI
|
|
6
|
Yun SI, Yoon HY, Jeong SY and Chung YS:
Glucocorticoid induces apoptosis of osteoblast cells through the
activation of glycogen synthase kinase 3beta. J Bone Miner Metab.
27:140–148. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kaushik AP, Das A and Cui Q: Osteonecrosis
of the femoral head: an update in year 2012. World J Orthop.
3:49–57. 2012.PubMed/NCBI
|
|
8
|
Moutsatsou P, Kassi E and Papavassiliou
AG: Glucocorticoid receptor signaling in bone cells. Trends Mol
Med. 18:348–359. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kerachian MA, Seguin C and Harvey EJ:
Glucocorticoids in osteonecrosis of the femoral head: a new
understanding of the mechanisms of action. J Steroid Biochem Mol
Biol. 114:121–128. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Weinstein RS: Glucocorticoid-induced
osteonecrosis. Endocrine. 41:183–190. 2012. View Article : Google Scholar
|
|
11
|
Weinstein RS: Glucocorticoid-induced
osteoporosis and osteonecrosis. Endocrinol Metab Clin North Am.
41:595–611. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kerachian MA, Cournoyer D, Harvey EJ, et
al: New insights into the pathogenesis of glucocorticoid-induced
avascular necrosis: microarray analysis of gene expression in a rat
model. Arthritis Res Ther. 12:R1242010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Weinstein RS, Wan C, Liu Q, et al:
Endogenous glucocorticoids decrease skeletal angiogenesis,
vascularity, hydration, and strength in aged mice. Aging Cell.
9:147–161. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gerber HP, Vu TH, Ryan AM, Kowalski J,
Werb Z and Ferrara N: VEGF couples hypertrophic cartilage
remodeling, ossification and angiogenesis during endochondral bone
formation. Nat Med. 5:623–628. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Leist M, Ghezzi P, Grasso G, et al:
Derivatives of erythropoietin that are tissue protective but not
erythropoietic. Science. 305:239–242. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Weng S, Zhu X, Jin Y, Wang T and Huang H:
Protective effect of erythropoietin on myocardial infarction in
rats by inhibition of caspase-12 expression. Exp Ther Med.
2:833–836. 2011.PubMed/NCBI
|
|
17
|
Teixeira M, Rodrigues-Santos P, Garrido P,
et al: Cardiac antiapoptotic and proproliferative effect of
recombinant human erythropoietin in a moderate stage of chronic
renal failure in the rat. J Pharm Bioallied Sci. 4:76–83. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Calvillo L, Latini R, Kajstura J, et al:
Recombinant human erythropoietin protects the myocardium from
ischemia-reperfusion injury and promotes beneficial remodeling.
Proc Natl Acad Sci USA. 100:4802–4806. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Choi D, Schroer SA, Lu SY, et al:
Erythropoietin protects against diabetes through direct effects on
pancreatic beta cells. J Exp Med. 207:2831–2842. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Celik M, Gokmen N, Erbayraktar S, et al:
Erythropoietin prevents motor neuron apoptosis and neurologic
disability in experimental spinal cord ischemic injury. Proc Natl
Acad Sci USA. 99:2258–2263. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gorio A, Gokmen N, Erbayraktar S, et al:
Recombinant human erythropoietin counteracts secondary injury and
markedly enhances neurological recovery from experimental spinal
cord trauma. Proc Natl Acad Sci USA. 99:9450–9455. 2002. View Article : Google Scholar
|
|
22
|
Xiong M, Chen S, Yu H, Liu Z, Zeng Y and
Li F: Neuroprotection of erythropoietin and methylprednisolone
against spinal cord ischemia-reperfusion injury. J Huazhong Univ
Sci Technolog Med Sci. 31:652–656. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Imamura R, Moriyama T, Isaka Y, et al:
Erythropoietin protects the kidneys against ischemia reperfusion
injury by activating hypoxia inducible factor-1alpha.
Transplantation. 83:1371–1379. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
MacRedmond R, Singhera GK and Dorscheid
DR: Erythropoietin inhibits respiratory epithelial cell apoptosis
in a model of acute lung injury. Eur Respir J. 33:1403–1414. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kakavas S, Demestiha T, Vasileiou P and
Xanthos T: Erythropoetin as a novel agent with pleiotropic effects
against acute lung injury. Eur J Clin Pharmacol. 67:1–9. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Galeano M, Altavilla D, Bitto A, et al:
Recombinant human erythropoietin improves angiogenesis and wound
healing in experimental burn wounds. Crit Care Med. 34:1139–1146.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rezaeian F, Wettstein R, Amon M, et al:
Erythropoietin protects critically perfused flap tissue. Ann Surg.
248:919–929. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Holstein JH, Orth M, Scheuer C, et al:
Erythropoietin stimulates bone formation, cell proliferation, and
angiogenesis in a femoral segmental defect model in mice. Bone.
49:1037–1045. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yamamoto T, Irisa T, Sugioka Y and Sueishi
K: Effects of pulse methylprednisolone on bone and marrow tissues:
corticosteroid-induced osteonecrosis in rabbits. Arthritis Rheum.
40:2055–2064. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Qin L, Zhang G, Sheng H, et al: Multiple
bioimaging modalities in evaluation of an experimental
osteonecrosis induced by a combination of lipopolysaccharide and
methylprednisolone. Bone. 39:863–871. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ding S, Peng H, Fang HS, Zhou JL and Wang
Z: Pulsed electromagnetic fields stimulation prevents
steroid-induced osteonecrosis in rats. BMC Musculoskelet Disord.
12:2152011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sugano N, Kubo T, Takaoka K, et al:
Diagnostic criteria for non-traumatic osteonecrosis of the femoral
head. A multicentre study. J Bone Joint Surg Br. 81:590–595. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
McGee-Lawrence ME, Stoll DM, Mantila ER,
Fahrner BK, Carey HV and Donahue SW: Thirteen-lined ground
squirrels (Ictidomys tridecemlineatus) show microstructural
bone loss during hibernation but preserve bone macrostructural
geometry and strength. J Exp Biol. 214:1240–1247. 2011.PubMed/NCBI
|
|
34
|
Parfitt AM, Drezner MK, Glorieux FH, et
al: Bone histomorphometry: standardization of nomenclature,
symbols, and units. Report of the ASBMR Histomorphometry
Nomenclature Committee. J Bone Miner Res. 2:595–610. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yatsiv I, Grigoriadis N, Simeonidou C, et
al: Erythropoietin is neuroprotective, improves functional
recovery, and reduces neuronal apoptosis and inflammation in a
rodent model of experimental closed head injury. FASEB J.
19:1701–1703. 2005.PubMed/NCBI
|
|
36
|
Ribatti D, Presta M, Vacca A, et al: Human
erythropoietin induces a pro-angiogenic phenotype in cultured
endothelial cells and stimulates neovascularization in vivo. Blood.
93:2627–2636. 1999.PubMed/NCBI
|
|
37
|
Erslev A: Humoral regulation of red cell
production. Blood. 8:349–357. 1953.
|
|
38
|
Liu Y, Porta A, Peng X, et al: Prevention
of glucocorticoid-induced apoptosis in osteocytes and osteoblasts
by calbindin-D28k. J Bone Miner Res. 19:479–490. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Thornberry NA and Lazebnik Y: Caspases:
enemies within. Science. 281:1312–1316. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fukuzuka K, Edwards CK III, Clare-Salzler
M, Copeland EM III, Moldawer LL and Mozingo DW:
Glucocorticoid-induced, caspase-dependent organ apoptosis early
after burn injury. Am J Physiol Regul Integr Comp Physiol.
278:R1005–R1018. 2000.PubMed/NCBI
|
|
41
|
Gao YS, Guo SC, Ding H and Zhang CQ:
Caspase-3 may be employed as an early predictor for fracture
induced osteonecrosis of the femoral head in a canine model. Mol
Med Rep. 6:611–614. 2012.PubMed/NCBI
|
|
42
|
Wang G, Zhang CQ, Sun Y, et al: Changes in
femoral head blood supply and vascular endothelial growth factor in
rabbits with steroid-induced osteonecrosis. J Int Med Res.
38:1060–1069. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Varoga D, Drescher W, Pufe M, Groth G and
Pufe T: Differential expression of vascular endothelial growth
factor in glucocorticoid-related osteonecrosis of the femoral head.
Clin Orthop Relat Res. 467:3273–3282. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Weinstein RS: Glucocorticoids, osteocytes,
and skeletal fragility: the role of bone vascularity. Bone.
46:564–570. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Seamon J, Keller T, Saleh J and Cui Q: The
pathogenesis of nontraumatic osteonecrosis. Arthritis.
2012:6017632012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang Y, Wan C, Deng L, et al: The
hypoxia-inducible factor alpha pathway couples angiogenesis to
osteogenesis during skeletal development. J Clin Invest.
117:1616–1626. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Clarkin CE and Gerstenfeld LC: VEGF and
bone cell signalling: an essential vessel for communication? Cell
Biochem Funct. 31:1–11. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zaidi M, Sun L, Robinson LJ, et al: ACTH
protects against glucocorticoid-induced osteonecrosis of bone. Proc
Natl Acad Sci USA. 107:8782–8787. 2010. View Article : Google Scholar : PubMed/NCBI
|