Introduction
Esophageal squamous cell carcinoma (ESCC) is one of
the most lethal types of cancer worldwide owing to its extremely
aggressive nature and poor survival rate (1). The early diagnosis of esophageal
cancer is difficult, therefore, once diagnosed, most of the
patients lack the optimal time required for surgical treatment.
Comprehensive treatment based on chemotherapy is regarded as the
first-line treatment for patients with unresectable or metastatic
ESCC (2). However, tumor cells
often develop resistance to chemotherapeutic agents during the
treatment (3). For this reason,
it is crucial to identify new therapeutic strategies or adjuvant
drugs. A promising possibility is to use dietary agents that can
increase tumor cell sensitivity to drugs (4).
MG132, which acts as a blocker in
ubiquitin-proteasome pathway, is involved in >80% of
intracellular protein degradation (5). Recently, it was shown that the
intrinsic resistance to apoptosis is an important mechanism by
which cancer cells can escape therapeutic control (6). Stoll et al provided
convincing evidence to suggest an essential role for MG132 in
apoptosis (7). Findings of
previous studies have indicated that MG132 enhances
cisplatin-induced apoptosis in various types of tumor cells
(8–10). However, the molecular mechanisms
of MG132-related lethality in esophageal squamous cancer cells are
not fully defined.
In this study, we initially investigated the
antitumor activity of proteasome inhibitor MG132 in vitro
and in vivo. Effects of MG132 on enhancing the anticancer
functions of cisplatin were then investigated in human esophageal
cancer EC9706 cells in relation to apoptosis and cell signaling
events.
Materials and methods
Cell culture
EC9706, EC109, EC1 and TE-1 cells were obtained from
the Open Key Clinical Medical Experimental Laboratory Institute of
Henan Province (Henan, China). The cells were cultured in RPMI-1640
medium (Life Technologies, Grand Island, NY, USA) supplemented with
10% FBS (Sijiqing Biological Engineering Materials Co., Ltd.,
Hangzhou, China), 100 U/ml penicillin and 100 μg/ml streptomycin in
a humidified atmosphere (5% CO2) at 37ºC with medium
changes every two days. Cells in the logarithmic growth phase were
used in this study.
Cell viability assay
Cell proliferation was assessed using a cell
counting kit (CCK-8) (Dojindo Laboratories, Kumamoto, Japan),
following the manufacturer’s instructions. Cells
(5×105/ml) were seeded in 96-well plates with 200 μl in
each well, and added to the culture medium containing agents of
different concentrations (1, 5 and 10 μM) or control PBS with 100
μl in each well, each concentration for parallel 4-wells after
adherence. Following culture, the medium was removed and 100 μl
fresh medium containing 10 μl CCK-8 was added to each well. The
cells were then incubated at 37ºC for 4 h. The optical density
values were determined in at least triplicate against a reagent
blank at a test wavelength of 490 nm and a reference wavelength of
630 nm. Cell viability was calculated as a percentage as follows:
(Atreated/Acontrol) ×100.
Xenograft tumor growth
Female athymic nude mice (5- to 6-weeks old) were
inoculated intraperitoneally (i.p.) with 7×106 EC9706
cells, after which mice received injections with vehicle or MG132
(10 mg/kg, i.p.) for 25 days starting 5 days after the injection of
EC9706 cells. Twenty nude mice were randomly divided into two
groups (n=10 per group). Mice were fed with antioxidant-free
AIN-76A special diet for a week before starting the experiment.
Apoptosis analysis
Adherent cells were digested into suspension of
single cells with EDTA-free trypsin and then washed twice with cold
PBS. To assess apoptosis, EC9706 cells were stained by Annexin
V-FITC/PI using an Annexin V-FITC Apoptosis Detection kit according
to the manufacturer’s instructions. The presence of fluorescent
images was immediately verified microscopically. Flow cytometry was
used to quantify the level of cell apoptosis.
Western blotting
Cells were collected and lysed for 20 min in cold
buffer. Cell extracts were collected and centrifuged at 12,000 rpm
for 5 min. Proteins (20 μg) from whole cell lysates were boiled for
5 min in SDS buffer, resolved by 12% SDS-PAGE, then
electrotransferred to nitrocellulose membranes by semi-dry
transfer, blocked overnight at 4ºC and incubated for 1 h with
primary antibodies at the recommended concentrations for caspase-8,
caspase-3, NF-κB and β-actin. The membranes were then incubated
with horseradish peroxidase-conjugated secondary antibodies
(anti-rabbit or anti-mouse IgG). Blots were developed using
enhanced chemiluminescence and visualized on Kodak X-omat LS film.
Densitometry was performed with Kodak ID image analyses
software.
Statistical analysis
Experiments were performed in triplicate and
quantitative results were expressed as the mean ± standard
deviation (SD). Statistical analysis was performed using the
statistical program SPSS 13.0. Data were compared using standard
ANOVA methodology for repeated measurement, followed by the
Student’s t-test. P<0.05 was considered to indicate statistical
significance.
Results
Proteasome inhibitor MG132 suppressed the
proliferation of EC9706 cells in a dose- and time-dependent
manner
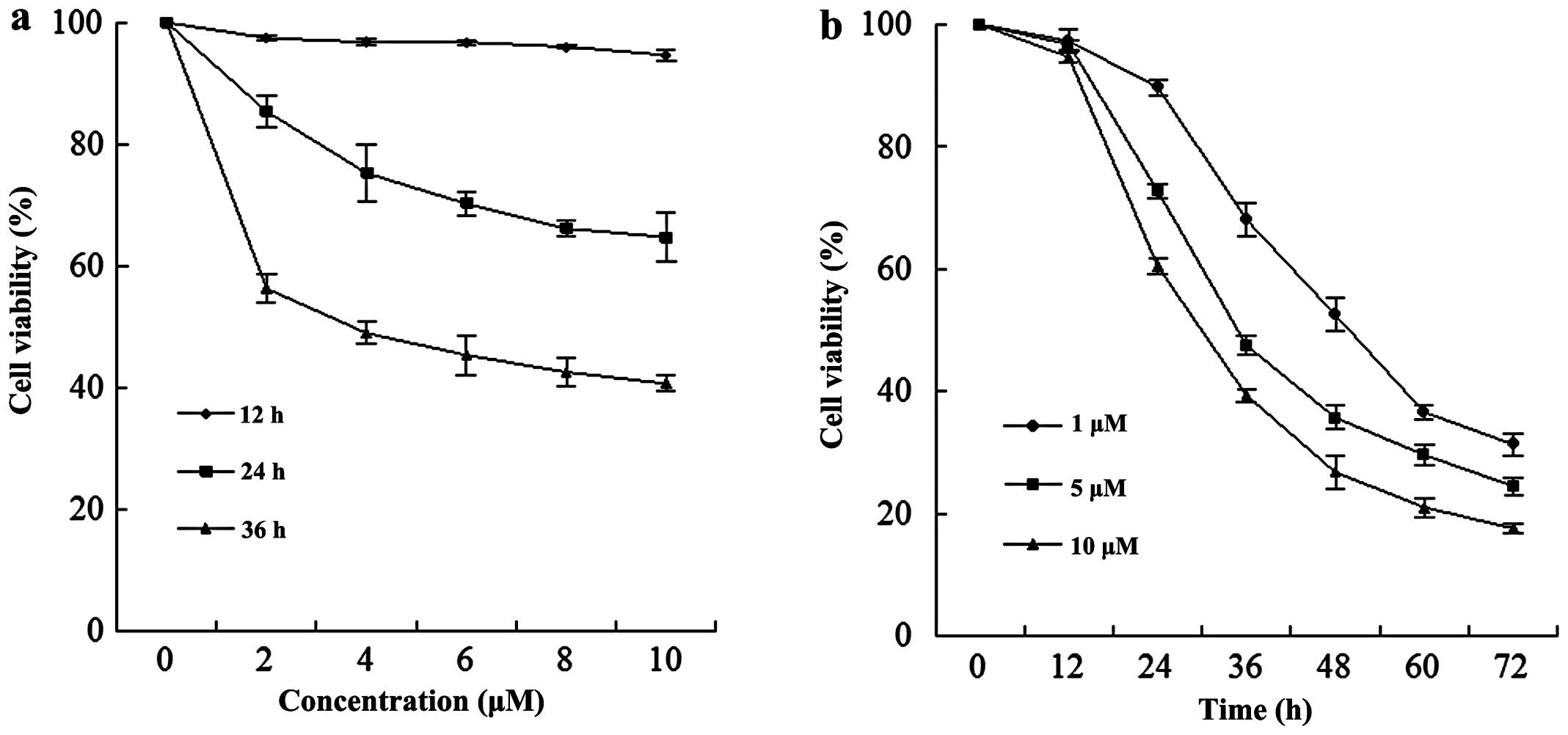
A dose-dependent study of EC9706 cells exposed to
various concentrations of MG132 for 12, 24 and 36 h is shown in
Fig. 1a; the modest degrees of
growth inhibition were noted at a concentration of 2 μM, which
increased substantially at a concentration of 4 μM. These events
were significantly increased at a concentration of 10 μM. A
time-course study of cells exposed to MG132 revealed a significant
increase in cell viability as early as 24 h, and reached
near-maximal levels after 60 h (Fig.
1b).
MG132 had similar antitumor effects on
multiple human esophageal squamous cancer cells
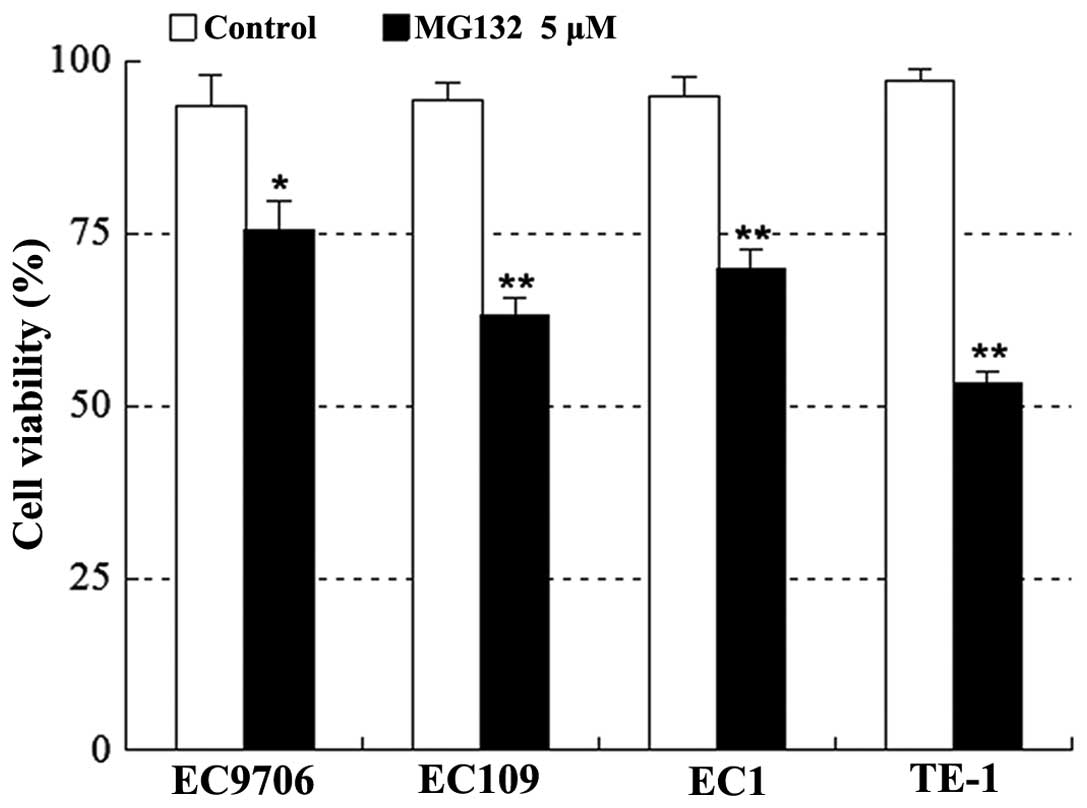
Various human esophageal squamous cancer cells,
including EC9706, EC109, EC1 and TE-1, were exposed to 5 μM MG132
for 24 h, after which apoptosis was determined by CCK-8 assay. As
shown in Fig. 2, treatment with
MG132 resulted in a marked decrease in cell viability in the four
cell lines.
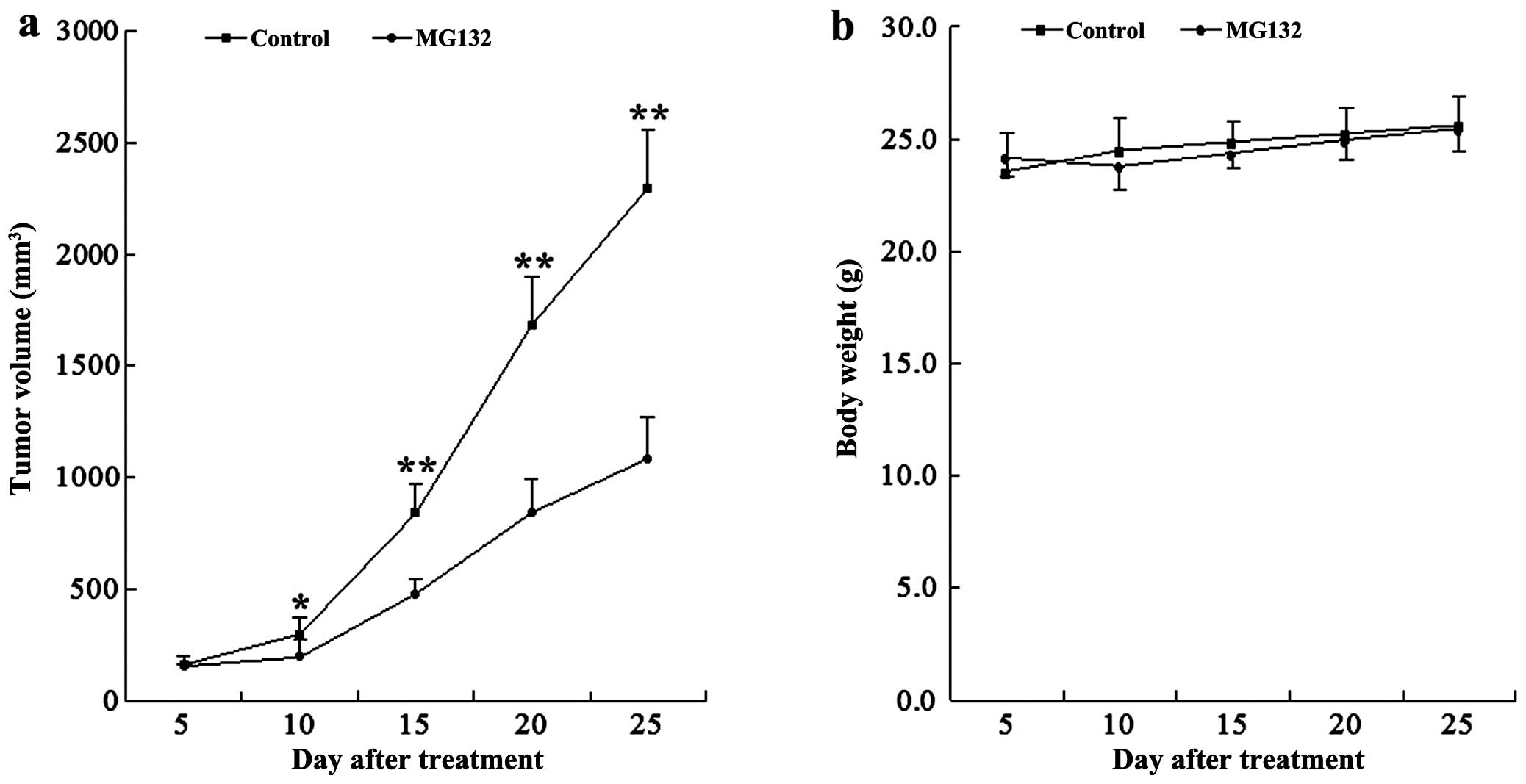
MG132 inhibited tumor growth in EC9706
xenograft animal model
To assess whether our in vitro observations
could be translated into an animal model system, athymic nude mice
were inoculated i.p. with EC9706 cells, after which mice received
injections with vehicle or MG132 (10 mg/kg, i.p.) for 25 days
starting 5 days after the injection. As shown in Fig. 3a, treatment with MG132 resulted in
a modest, but significant suppression of tumor growth 10 days
following drug exposure (P<0.05 vs. vehicle control). These
events became more apparent 15, 20 and 25 days after drug exposure
(P<0.01 between MG132 treatment and vehicle control). By
contrast, no statistically significant change in body weight was
noted when compared with the vehicle control and MG132 regimen
(Fig. 3b). Moreover, the mice of
MG132 group did not exhibit any other signs of toxicity such as
agitation, impaired movement, posture, indigestion, diarrhea or
areas of redness. These results indicated that MG132 administration
significantly inhibited tumor growth of the EC9706 xenograft
without causing toxicity to the mice.
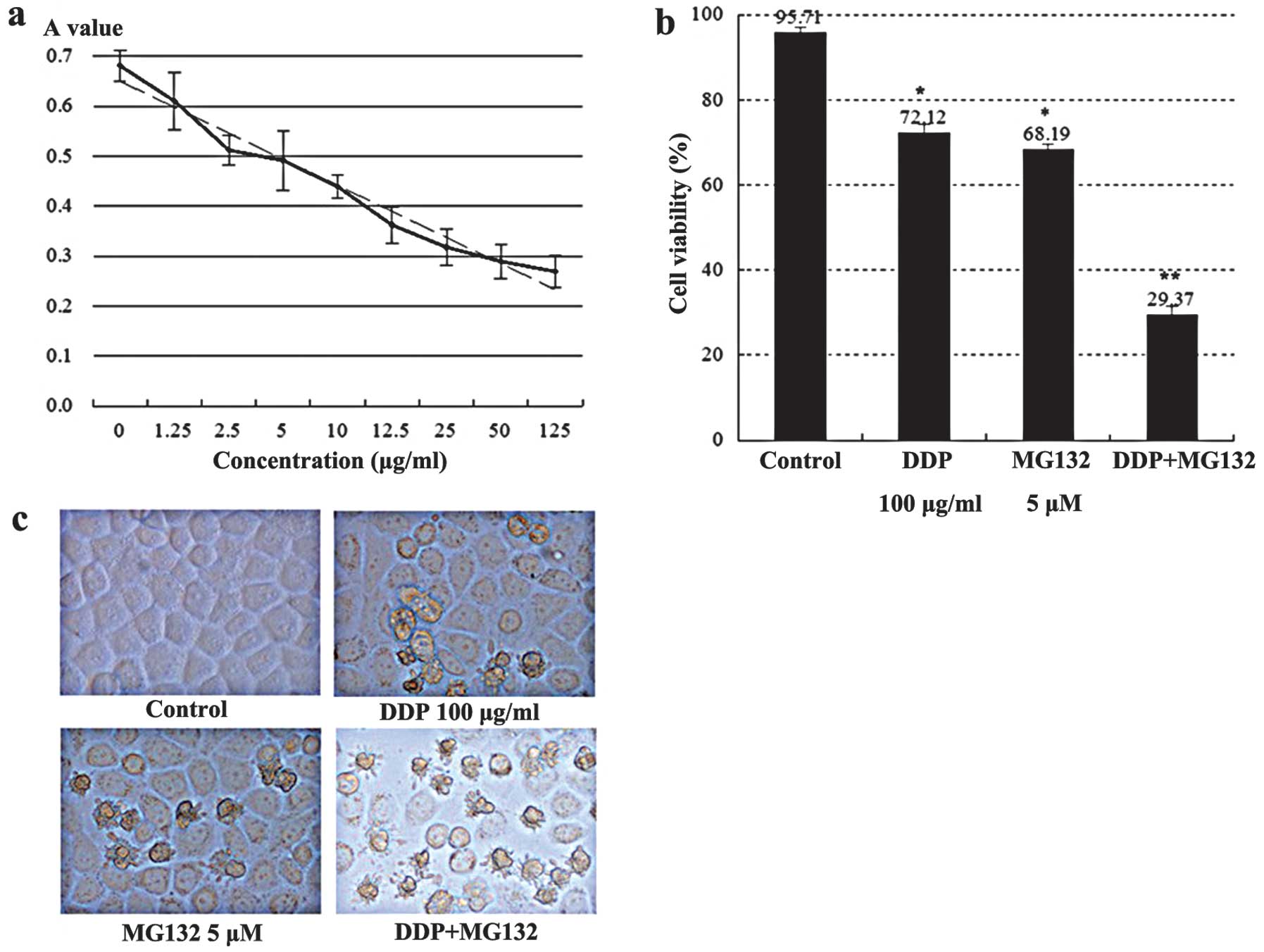
Cell viability and morphological changes
of EC9706 cells
Exposure of cells to a series of concentrations of
cisplatin for 24 h resulted in a significant dose-inhibition effect
between the different groups (P<0.05). There was a linear
relationship between cisplatin concentration and the A value
(Fig. 4a), where the correlation
coefficient was r=−0.023 (P<0.001) and the linear regression
equation was A value=0.735–0.0018 × cisplatin concentration
(μg/ml). The proliferation inhibitory rate of cisplatin on EC9706
cells was 25% when the drug concentration was 100 μg/ml. Then, 100
μg/ml was selected as cisplatin concentration in the follow-up
studies.
Addition of 5 μM MG132 for 24 h resulted in a marked
decrease in cell viability in the combined group as compared with
the individual agents (P<0.01) (Fig. 4b). The results obtained suggested
that the combined use of DDP and MG132 had stronger cytotoxicity
than the single agent.
Normal EC9706 cells were polygonal in shape with a
high refractive index and large cell volumes. The cells were
stretched tightly and adhered to the wall, and had a
cobblestone-like appearance. In the MG132 5 μM and/or DDP 100 μg/ml
group, some cells decreased into round shapes, with a reduced
refractive index. The cells were detached from the wall and floated
in culture medium. A significant increase was observed in these
events in the combined group of MG132 (5 μM) and DDP (100 μg/ml)
(Fig. 4c).
Effect of DDP and MG132 used individually
or in combination on EC9706 cell apoptosis
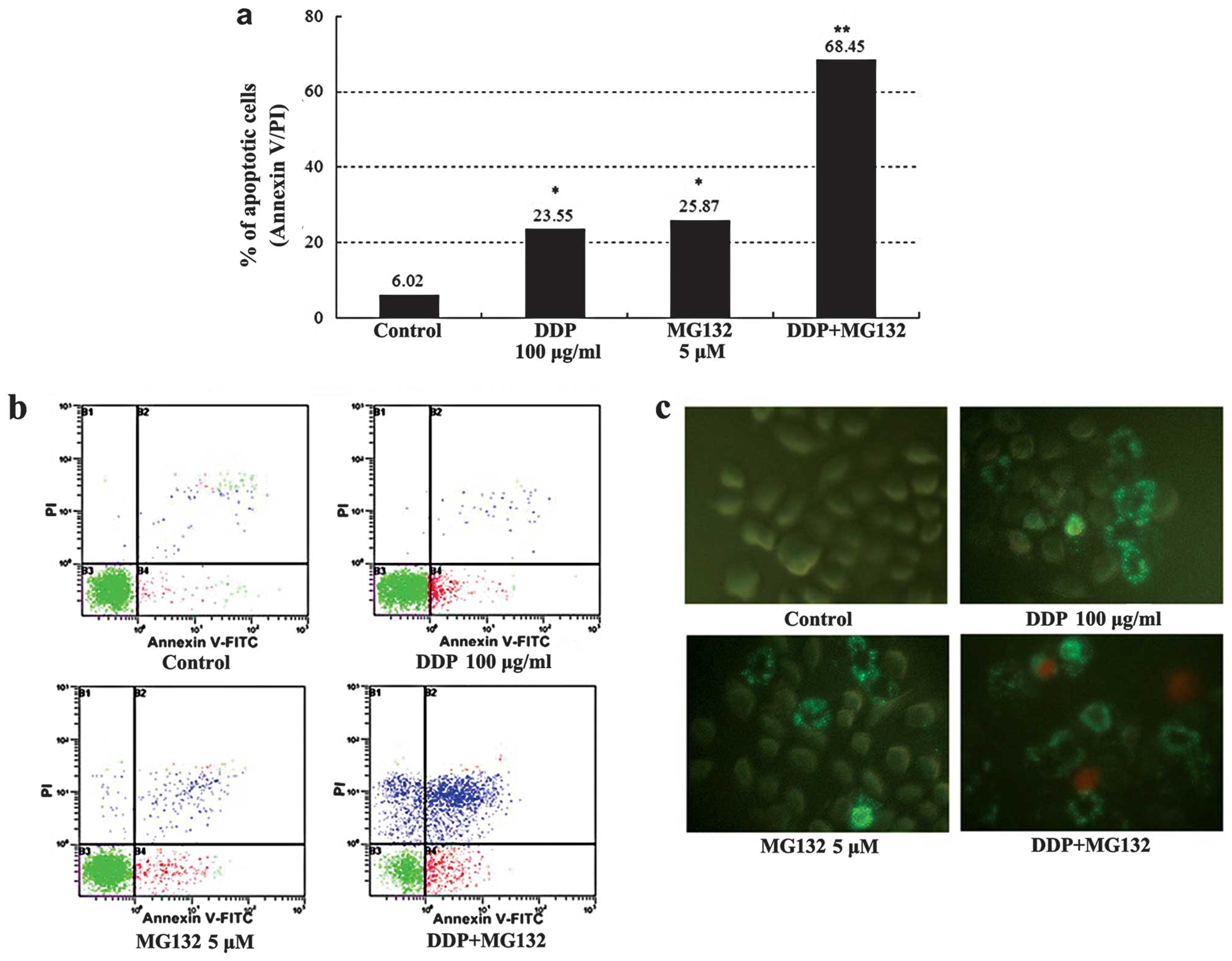
Counts of apoptotic cells detected by flow cytometry
are shown in Fig. 5a and b: The
apoptotic percentage of cells for the DDP + MG132 group was much
higher than that in the blank control and individual groups
(P<0.01). The addition of MG132, increased the cisplatin-induced
apoptosis rate from 23 to 68%. Annexin V-FITC and PI staining was
used to estimate the extent of cell apoptosis. Observed under a
fluorescent microscope, Annexin V-FITC+ cells appeared
as bright apple green on the cell membrane, whereas PI+
cells had different intensities of yellow red throughout the
cytoplasm. Annexin V-FITC+ cells were rarely observed in
the control group, while many positive cells were visible in the
MG132 group, DDP group and, particularly the DDP + MG132 group
(Fig. 5c). The results obtained
suggested that MG132 is able to enhance cisplatin-induced apoptosis
in esophageal cancer cells.
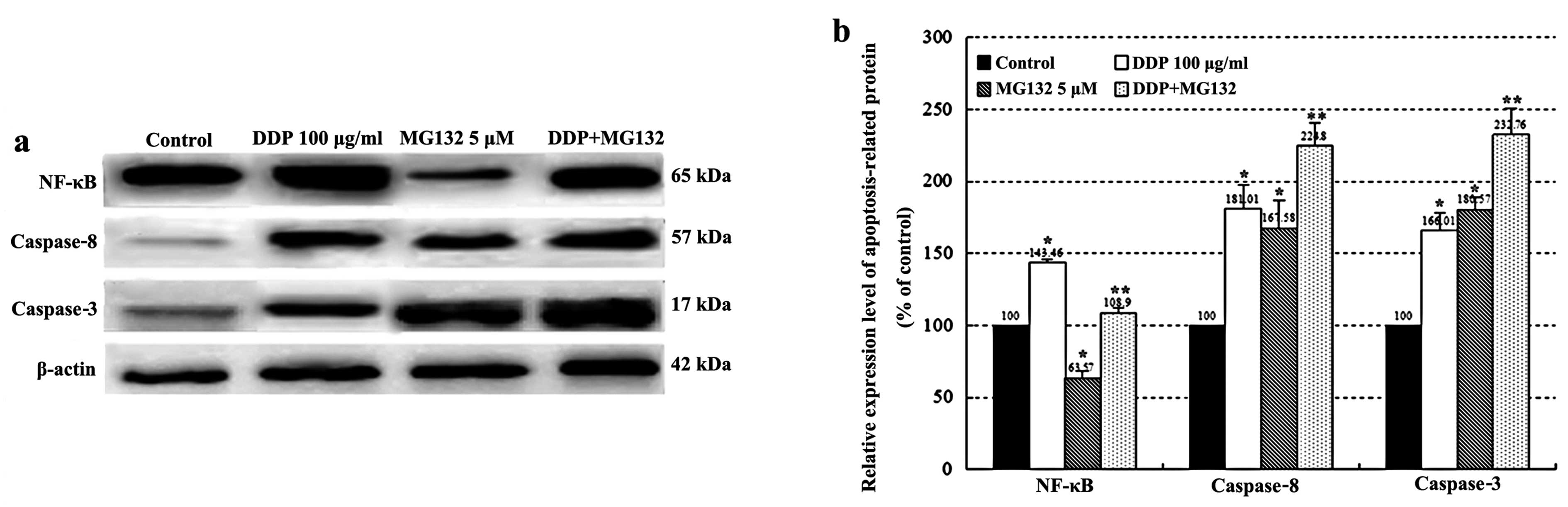
Expression of NF-κB, caspase-8 and
-3
As shown in Fig.
6, activation of caspase-8 and -3, and NF-κB was determined by
western blotting after 24 h of DDP treatment. The expression of
caspase-8, -3 and NF-κB was upregulated in the DDP group as
compared with the control group. In addition, a combination of DDP
and MG132 treatment increased the expression levels of caspase-8
and -3 when compared with DDP or MG132 alone (P<0.05).
Conversely, MG132 partially counteracted the upregulation of NF-κB
in the combined group compared with the DDP group (P<0.01).
Discussion
Comprehensive treatment based on chemotherapy is
regarded as the first-line treatment for patients with unresectable
or metastatic ESCC (11).
However, chemoresistance is common among patients with ESCC
(12–14). Therefore, it is crucial to
identify new therapeutic strategies or adjuvant drugs. A promising
possibility is to use dietary agents that potentially increase
tumor cell sensitivity to drugs.
As a natural triterpene proteasome inhibitor
extracted from a Chinese medicinal plant, MG132 has been
investigated in several cancer cells in relation to apoptosis and
cell signaling events (15,16). In the present study, we have shown
that MG132 suppressed the growth of esophageal cancer cells in
culture as well as in the animal model. The experimental results
in vitro demonstrated that MG132 inhibited the proliferation
in EC9706 cells in a dose- and time-dependent manner, with the
concentration increasing from 0 to 10 μM and the survival rate
decreasing from 100 to 18.43% after 36 h (Fig. 1). MG132 also markedly induced cell
death in multiple human esophageal cancer cell types, including
EC9706, EC109, EC1 and TE-1 cell lines. The results from in
vivo studies about xenograft model showed that MG132 exhibited
significant inhibitory effects on the growth of esophageal cancer
xenograft.
In order to justify whether or not the application
of MG132 could improve the sensitivity to chemotherapy drugs in
esophageal cancer cells, the cells were divided into the blank
control, 100 μg/ml DDP, 5 μM MG132, 100 μg/ml DDP and 5 μM MG132
groups. To elucidate the potential mechanism underlying the
tumor-suppressive function of combined DDP and MG132 treatment, we
examined the proliferation and apoptosis of esophageal cancer
EC9706 cells. Exposure of cells to cisplatin (DDP) combined with
MG132 resulted in a marked increase in the cell cytotoxicity of
esophageal cancer cells as compared with the single agent (Fig. 4b). This result was confirmed by
the Annexin V-FITC apoptosis detection assay, which showed that the
combined DDP and MG132 treatment induced more apoptosis in tumor
cells than in DDP treatment alone (68.45±2.58 vs. 23.5±1.23%;
P<0.01, Fig. 5). Our findings
provide convincing evidence that the combined treatment of DDP and
proteasome inhibitor MG132 exerts a synergistic apoptotic effect as
compared with each agent alone.
There are two pathways in apoptosis: the cell
surface death receptor pathway and the mitochondria-initiated
pathway (17,18). In the cell surface receptor
pathway, activation of caspases following their recruitment to the
death-inducing signaling complex is the critical event that
transmits the death signal (19–21). Apoptosis requires a cascade of
complex biochemical events that are performed with the
participation of a family of cysteine proteases known as caspases
(22). Specifically, caspase-8
and -3 have been viewed as the essential regulators in apoptosis
cascade (23,24). NF-κB is a ubiquitous transcription
factor that plays a key role in basic processes such as the
regulation of cell proliferation and apoptosis (25–27). To identify the mechanism by which
proteasome inhibitor MG132 potentiates cisplatin-induced apoptosis
in human esophageal squamous cancer cells, we investigated changes
of the expression levels of caspase-8, -3 and NF-κB after treatment
with DDP and MG132 individually or in combination. The results of
our study suggest that MG132 significantly enhanced
cisplatin-induced apoptosis in association with the activation of
caspase-3 and -8. These events were accompanied by the
downregulation of the NF-κB pathway which plays a key role in cell
apoptosis (Fig. 6). Activation of
the NF-κB pathway resulting in reduced susceptibility to cisplatin
in esophageal cancer cells may play an important role in drug
resistance induced by cisplatin. MG132 can significantly enhance
the sensitivity of esophageal cancer cells to cisplatin and
effectively improve the rate of cell apoptosis by inhibiting the
activation of NF-κB, potentiating the expression levels of
apoptosis-related protein caspase-8 and -3.
In summary, these findings indicate that proteasome
inhibitor MG132 may promote cisplatin-induced apoptosis by
inhibiting the activation of NF-κB and upregulating the expression
levels of caspase-3 and -8. MG132 may be used alone or in
combination with other therapeutic agents to treat ESCC. However,
our findings require further investigations to clarify the
molecular mechanisms underlying the results of the present study.
Findings of this study have proivded a novel and promising
therapeutic strategy which is likely to benefit the clinical
treatment of patients with ESCC.
Acknowledgements
We would like to thank the staff in the Open Key
Clinical Medical Experimental Laboratory Institute of Henan
Province, the Pharmacogenomics Laboratory of Henan Province and the
Institute of Molecular Cancer Surgery of Zhengzhou University for
their supporting. We also would like to thank Dr Duan Guangcai,
Zhengzhou University, for assistance with statistical analysis.
References
|
1
|
van Hagen P, Hulshof MC, van Lanschot JJ,
et al: Preoperative chemoradiotherapy for esophageal or junctional
cancer. N Engl J Med. 366:2074–2084. 2012.
|
|
2
|
Tomblyn MB, Goldman BH, Thomas CR Jr, et
al: Cetuximab plus cisplatin, irinotecan, and thoracic radiotherapy
as definitive treatment for locally advanced, unresectable
esophageal cancer: a phase-II study of the SWOG (S0414). J Thorac
Oncol. 7:906–912. 2012. View Article : Google Scholar
|
|
3
|
Murtaza M, Dawson SJ, Tsui DW, et al:
Non-invasive analysis of acquired resistance to cancer therapy by
sequencing of plasma DNA. Nature. 497:108–112. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gao D, Hu J, Zhang X, Gao C and Hong J:
Effect of hOGG1 over-expression on cisplatin resistance in
esophageal squamous carcinoma cells. Cancer Biother Radiopharm.
28:433–440. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Khedgikar V, Kushwaha P, Gautam J, et al:
Withaferin A: a proteasomal inhibitor promotes healing after injury
and exerts anabolic effect on osteoporotic bone. Cell Death Dis.
4:e7782013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li X, Huang T, Jiang G, Gong W, Qian H and
Zou C: Proteasome inhibitor MG132 enhances TRAIL-induced apoptosis
and inhibits invasion of human osteosarcoma OS732 cells. Biochem
Biophys Res Commun. 439:179–186. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stoll SJ, Pitt SC and Chen H: Follicular
thyroid cancer cell growth inhibition by proteosome inhibitor
MG132. J Surg Res. 156:39–44. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Duraj J, Pastorek M, Vitkovska J, et al:
Proteasome inhibition leads to altered signaling in the proteome of
cisplatin-resistant human ovarian carcinoma cell line. Neoplasma.
60:627–634. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fribley AM, Evenchik B, Zeng Q, et al:
Proteasome inhibitor PS-341 induces apoptosis in
cisplatin-resistant squamous cell carcinoma cells by induction of
Noxa. J Biol Chem. 281:31440–31447. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fribley A, Zeng Q and Wang CY: Proteasome
inhibitor PS-341 induces apoptosis through induction of endoplasmic
reticulum stress-reactive oxygen species in head and neck squamous
cell carcinoma cells. Mol Cell Biol. 24:9695–9704. 2004. View Article : Google Scholar
|
|
11
|
He YF, Ji CS, Hu B, et al: A phase II
study of paclitaxel and nedaplatin as front-line chemotherapy in
Chinese patients with metastatic esophageal squamous cell
carcinoma. World J Gastroenterol. 19:5910–5916. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kurosu T, Nagao T, Wu N, Oshikawa G and
Miura O: Inhibition of the PI3K/Akt/GSK3 pathway downstream of
BCR/ABL, Jak2-V617F, or FLT3-ITD downregulates DNA damage-induced
Chk1 activation as well as G2/M arrest and prominently enhances
induction of apoptosis. PLoS One. 8:e794782013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen WW, Lin CC, Huang TC, Cheng AL, Yeh
KH and Hsu CH: Prognostic factors of metastatic or recurrent
esophageal squamous cell carcinoma in patients receiving three-drug
combination chemotherapy. Anticancer Res. 33:4123–4128.
2013.PubMed/NCBI
|
|
14
|
Shi Y, Qin R, Wang ZK and Dai GH:
Nanoparticle albumin-bound paclitaxel combined with cisplatin as
the first-line treatment for metastatic esophageal squamous cell
carcinoma. Onco Targets Ther. 6:585–591. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tu Y, Chen C, Pan J, Xu J, Zhou ZG and
Wang CY: The Ubiquitin Proteasome Pathway (UPP) in the regulation
of cell cycle control and DNA damage repair and its implication in
tumorigenesis. Int J Clin Exp Pathol. 5:726–738. 2012.PubMed/NCBI
|
|
16
|
Yang H, Landis-Piwowar K, Chan TH and Dou
QP: Green tea polyphenols as proteasome inhibitors: implication in
chemoprevention. Curr Cancer Drug Targets. 11:296–306. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kandilis AN, Karidis NP, Kouraklis G,
Patsouris E, Vasileiou I and Theocharis S: Proteasome inhibitors:
possible novel therapeutic strategy for ischemia-reperfusion
injury? Expert Opin Investig Drugs. 23:67–80. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Flemming A: Therapeutics: opening the door
to a new class of proteasome inhibitors. Nat Rev Cancer. 12:52011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen FZ and Zhao XK: Ubiquitin-proteasome
pathway and prostate cancer. Onkologie. 36:592–596. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gu JJ, Hernandez-Ilizaliturri FJ, Kaufman
GP, et al: The novel proteasome inhibitor carfilzomib induces cell
cycle arrest, apoptosis and potentiates the anti-tumour activity of
chemotherapy in rituximab-resistant lymphoma. Br J Haematol.
162:657–669. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hui B, Shi YH, Ding ZB, et al: Proteasome
inhibitor interacts synergistically with autophagy inhibitor to
suppress proliferation and induce apoptosis in hepatocellular
carcinoma. Cancer. 118:5560–5571. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Altmann A, Markert A, Askoxylakis V, et
al: Antitumor effects of proteasome inhibition in anaplastic
thyroid carcinoma. J Nucl Med. 53:1764–1771. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rastogi N and Mishra DP: Therapeutic
targeting of cancer cell cycle using proteasome inhibitors. Cell
Div. 7:262012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
da Cunha FM, Demasi M and Kowaltowski AJ:
Aging and calorie restriction modulate yeast redox state, oxidized
protein removal, and the ubiquitin-proteasome system. Free Radic
Biol Med. 51:664–670. 2011.PubMed/NCBI
|
|
25
|
Chitra S, Nalini G and Rajasekhar G: The
ubiquitin proteasome system and efficacy of proteasome inhibitors
in diseases. Int J Rheum Dis. 15:249–260. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
de Almagro MC and Vucic D: The inhibitor
of apoptosis (IAP) proteins are critical regulators of signaling
pathways and targets for anti-cancer therapy. Exp Oncol.
34:200–211. 2012.PubMed/NCBI
|
|
27
|
Napetschnig J and Wu H: Molecular basis of
NF-κB signaling. Annu Rev Biophys. 42:443–468. 2013.
|