Introduction
Invasive cervical cancer (ICC) is one of the most
common types of cancer affecting women worldwide, in both incidence
and mortality (1,2). There is a long developmental process
between the stages of cervical intraepithelial neoplasia (CIN) to
cervical cancer. Early screening and appropriate treatment may
prevent the development of CIN; some lesions can be cured
completely, and other minor lesions will soon return to normal
without treatment (3). Since the
introduction of the Papanicolaou test (Pap smear) decades ago, the
mortality and morbidity rates for patients with invasive cervical
cancer have reduced greatly, particularly in developed countries
(4–9). The achievement of Pap smear testing
for public health is significant. However, there are still 500,000
cases of cervical cancer and 275,000 cervical cancer deaths among
women worldwide, and the incidence rates in developing countries
are particularly high (10). The
sensitivity of the Pap smear varies substantially in areas with
different screening infrastructures (11). In several meta-analyses of the
accuracy of Pap smears, the sensitivity ranged from approximately
50 to 80%, but can be as low as 20% (12–14), which limits the efficacy of cancer
detection (15). Over the past
decade, the liquid-based Pap test has been introduced to overcome
the drawbacks of the conventional Pap smear and has resulted in the
reduction of limited and unsatisfactory specimens and in an
improvement in the adequacy and detection rates for squamous
intraepithelial lesions (16).
Due to sampling error, there are still some discrepancies in the
interpretation between the cytological and histological diagnoses
of the simultaneously sampled smears and biopsy specimens.
Laboratory and epidemiological research has
suggested a strong association between HPV infection and cervical
cancer (17). However, the
discrepancy between high rates of HPV infection and low rates of
cervical cancer development among women suggests that additional
genetic events are necessary for the progression to a malignant
phenotype. Chromosomal instability at a numerical or structural
level is a hallmark of malignant tumors (18). Indeed, recurrent patterns of
chromosomal alterations with specific imbalances that are important
for tumor initiation and progression have been revealed in cervical
cancer (19). Deletion,
duplication and amplification of various genomic regions have been
demonstrated in cervical cancer by comparative genomic
hybridization and fluorescence in situ hybridization (FISH)
methods (20–23). A number of studies have
demonstrated frequent gain or loss of several specific chromosomal
regions in cervical cancer, e.g., gain of 3q, 5p, 8q, 11q, 17q and
20q and loss of 3p, 4p, 5q and 18q (21,23–28). At present, the gene(s) involved in
these regions and their biologic functions in this disease have not
yet been fully identified.
A proto-oncogene can be abnormally activated and
turned into an oncogene; gene amplification is the dominant
mechanism for oncogene activation in solid tumors (29). The aberrant activation and/or
overexpression of different oncogenes are associated with the
development and progression of human tumors (30). MYC genes are key regulators of
cell proliferation, and the enhanced expression of MYC genes
promotes unrestricted proliferation and contributes to the genesis
of most human tumors (31). The
amplification and overexpression of the MYC gene have been detected
in both cell lines and cervical cancers (32,33). Studies from the US NIH in 2005 for
cervical cancer demonstrated that the process of cervical cell
variation into cervical cancer is always accompanied with the
amplification of the long arm of chromosome 3 (34,35). The most important genes involved
are human telomerase RNA component (hTERC). Sokolova et al
(36) assessed biopsy specimens
showing high-grade dysplasia and cancer with FISH probes to 35
unique loci and identified 2 loci, the 3q26.3 region (comprising
the hTERC gene) and the 8q24 region (comprising the c-MYC gene),
which showed the highest frequency of copy number gains in
high-grade dysplasia and cancer. These loci are frequently altered
in cervical cancer tumorigenesis (21,22,28,37). Thus, they may be useful markers
for the detection of cervical dysplasia and carcinoma.
The FISH technique, which provides an accurate
quantification of the signals (2)
is relatively simple to operate, has good reproducibility,
stability, and has the advantages of good sensitivity and
specificity; it does not require cell culture and be used in
interphase cells; FISH is easily adaptable to clinical
detection.
In this study, the FISH technique was used to detect
the amplification of hTERC and c-MYC in cervical epithelial
exfoliated cells. Combined with the liquid-based cytology test
results of these cases, we assessed the significance of the
amplification of hTERC and c-MYC in cervical cancer screening.
Materials and methods
Cytology specimens
Specimens with an abnormal cytological diagnosis
[atypical squamous cells of undetermined significance (ASCUS),
atypical squamous cells - cannot exclude high grade squamous
intraepithelial lesion (HSIL; ASC-H), low grade squamous
intraepithelial lesion (LSIL), HSIL and squamous cell carcinoma
(SCC)] or a suspicious diagnosis as described in accordance with
the TBS system were included in this study if they had a
corresponding biopsy diagnosis [the ThinPrep Cytology Test (TCT)
was used for cytological diagnosis]. Cytology specimens with a
cytological diagnosis of ‘normal’ were also included, but not all
of these patient specimens had a corresponding biopsy according to
the standard practice of care. We collected exfoliated cells from
171 patients. The distribution of specimens based on the cytology
and biopsy classification was as follows: normal uterine cervix
specimens with normal cytology and no biopsy available as controls
(n=40), benign lesions of the uterine cervix, including squamous
metaplasia, chronic cervicitis and atypia of repair, which had a
normal to HSIL cytology (n=24), CIN1 cases which had a normal to
HSIL cytology (n=26), CIN2 cases which had a normal to HSIL
cytology (n=29), and CIN3 or carcinoma in situ cases which
had a normal to suspicious carcinoma cytology (n=36). Invasive
cervical SCC cases which had a cytology of HSIL to suspicious SCC
(ICC), n=16.
Specimens were obtained from the First Affiliated
Hospital of Chongqing Medical University (Chongqing, China) between
December 2010 and July 2011. Adenocarcinoma specimens were not
included. The age of the patients ranged between 22 and 66 years.
Exclusion criteria included pregnancy, menstrual period, chronic or
acute systemic viral infections, a history of cervical neoplasia,
an immunocompromised state, the presence of other cancers, a
history of surgery to the uterine cervix, persons had been
undergone colposcopic biopsy or cases that had been treated.
A cervical brush (a special Pap Brush; Beijing TCT
Medical Technology Company, Ltd., Beijing, China) was used to
collect the cervical epithelial exfoliated cells following the
manufacturer’s instructions. The exfoliated cells were preserved in
phosphate-buffered saline at 4°C until FISH examination. All the
patients were diagnosed and had their specimens banked at the First
Affiliated Hospital of Chongqing Medical University. Patients who
had low- and high-grade lesions identified by cytology underwent
colposcopic cervical biopsy and that sometimes included subsequent
conization or major surgery. The final diagnosis was made by
tissue-proven pathology rather than cytology, apart from the
controls. Controls were recruited from healthy women who underwent
routine Pap screening. Informed consent was obtained from all
patients and control subjects. The Institutional Review Board of
the Peking Union Medical College Hospital as the lead hospital of
the project approved this study.
Probe set formulation
The FISH detection kit, including the locus-specific
probes 3q26.3 and 8q24 were manufactured using a standard labeling
procedure by the Beijing Gold Bodhisattva Ka Medical Technology
Co., Ltd, Beijing, China.
Probe group 1: GLP TERC/CSP3. The chromosome probe
3q26.3 (hTERC) was labeled with the Spectrum Red fluorophore. The
sequence of the 3q26.3 probe was 5′-CUAACCCUAAC-3′. The CSP3 probe
was labeled with the Spectrum Green fluorophore, whose sequence is
protected. The CSP3 probe hybridized with the 3p11.1-q11.1 region
of the centromere in chromosome 3, was used as the control probe.
There were 2 red and 2 aquamarine hybridization signals in each
interphase nucleus of cells which had no amplification of the hTERC
gene. At least 2 aquamarine signals and >2 red signals should be
detected in each interphase nucleus of cells which had an
amplification of the hTERC gene.
Probe group 2: GLP-c-MYC. The probe 8q24 (c-MYC) was
labeled with the Spectrum Red fluorophore. The sequence of the 8q24
probe is protected. There were two red hybridization signals in
each interphase nucleus of cells which had no amplification of the
c-MYC gene. More than 2 red signals should be detected in each
interphase nucleus of cells which had an amplification of the c-MYC
gene.
Fish procedures
FISH detected the hTERC and c-MYC genes in cervical
epithelial exfoliated cells following the instructions of the FISH
detection kit. Approximately 5–10 ml preservation solution
containing the exfoliated cells were placed into a suitable
centrifuge tube. Following centrifuging at 1,300 rpm for 10 min and
removing the supernatant, the exfoliated cells were supplemeted
with 3 ml collagenase B; the suspended cells were then separated,
followed by incubation in 37°C water for 20–30 min, centrifugation
at 1,300 rpm for 10 min, removing the supernatant again, the
addition of 5 ml 37°C water, separating the suspended cells,
incubation in 37°C water for 20 min, the addition of 2 ml
stationary liquid, centrifugation at 1,300 rpm for 10 min, removing
supernatant, the addition of 5 ml stationary liquid, centrifugation
at 1,300 rpm for 10 min, and repeating the section as described
above, and finally removing the supernatant, followed by the
addition of appropriate stationary liquid, separating the suspended
cells, and dropping to the glass slides followed by air drying
overnight at room temperature. The following day, the slides were
soaked twice in 2X SSC (PH 7.0) at room temperature for 5 min and
then soaked in 100 mmol/l HCl at room temperature for 10 min. The
slides were then incubated in pepsin (approximately 0.02 mg/ml in
10 mmol/l HCl) at 37°C for 10 min. Mild pepsin digestion was
performed to increase the penetration of the probes. The slides
were then soaked twice in 2X SSC at room temperature for 5 min,
fixed in 1% neutral-buffered formalin at room temperature for 5
min, dehydrated in an ethanol series of 70, 85 and 100% for 3 min
in each solution and air-dried. The probe mixture was then applied,
and the slides were coverslipped and sealed with rubber cement. The
slides with probe mix were co-denatured at 77°C for 5 min, followed
by hybridizing at 42°C for 16–18 h.
Following hybridization and the removal of the
coverslips, the slides were washed in 3 bottles of 2X SSC/50%
formamide solution for 10 min in each bottle, in 2X SSC for 10 min,
in 0.1% Nonidet P-40/2X SSC for 5 min, in 70% ethanol for 3 min.
Following stringent washing with a series of solution, the slides
followed by air drying were applied with the anti-fade solution
containing the nuclear counterstain 4,6-diamidino-2-phenylindole
(DAPI), and the slides were coverslipped. After being placed back
in a black box for 10–20 min, the slides were observed under a
fluorescence microscope (Olympus).
Signal scoring and evaluation
Each sample had 2 slides and each slide was detected
by FISH with 1 probe group. All the slides were analyzed under a
fluorescence microscope using ×10, ×40 and ×100 magnification
equipped with the corresponding wavelength filter, a CCD camera and
an image-capturing and analyzing system. A magnification of ×10 to
was used to find the cell region in slide; ×40 magnification was
used to scan the entire hybrid area and observe the quality of the
specimens, and ×100 magnification was used for the evaluation of
signal scoring. In most cases, the entire surface area of each
slide was analyzed. However, in cases involving a large number of
cells per slide, only the first 100 cells were analyzed. In these
cases, the estimated number of cells on the entire slide was
extrapolated from the percentage of surface area enumerated after
enumerating 100 cells. The exact copy number of the signals per
nucleus was recorded, and at least 100 nuclei/sample were analyzed.
Only satisfactory samples with ≥3 copies/nucleus of the given genes
in >75% of the counted cells were considered to contain gene
amplification.
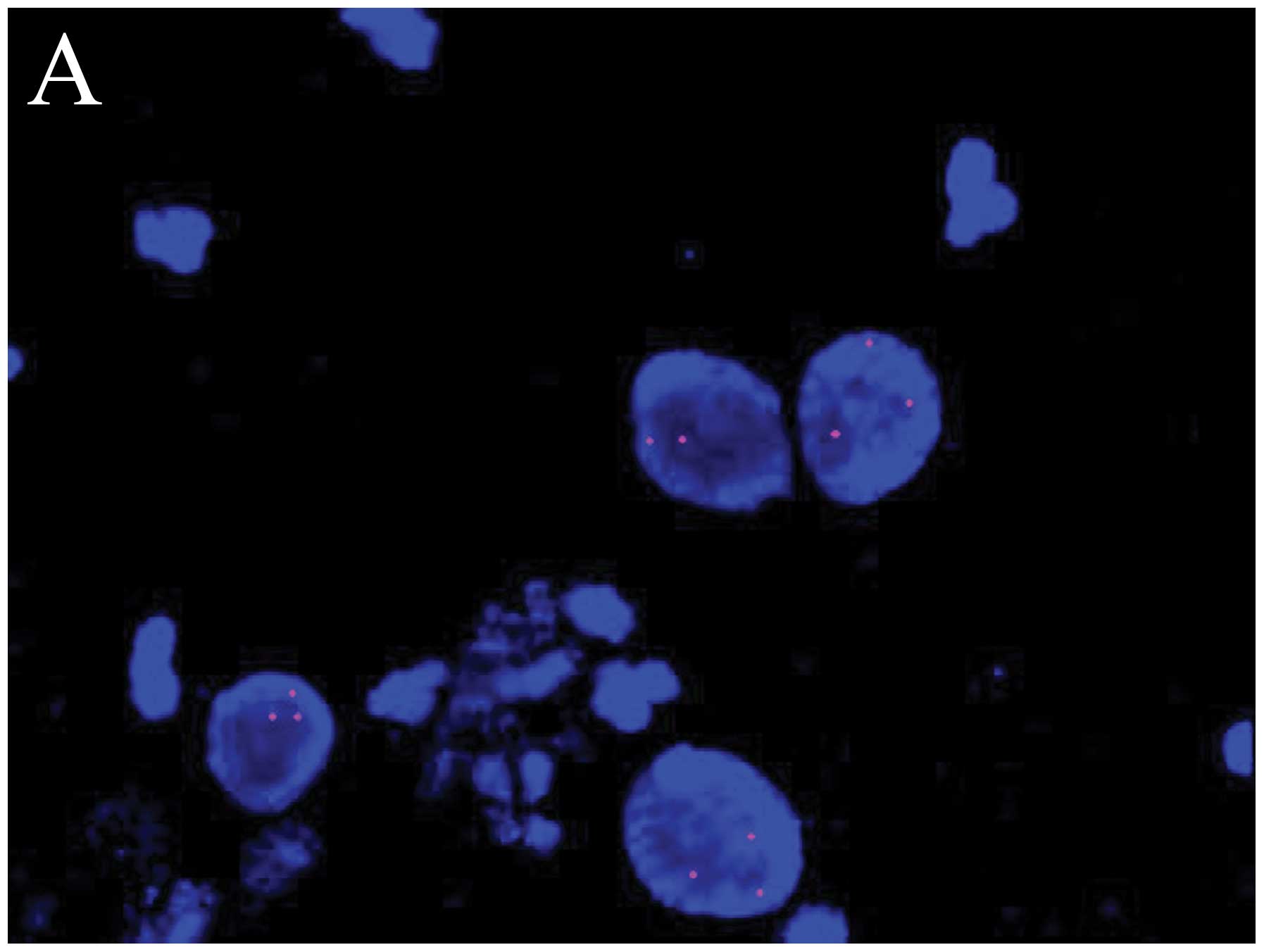
The images in Fig.
1 consist of chromosome probe GLP TERC/CSP3 visualized red and
aquamarine hybridization signals, and chromosome probe GLP-c-MYC
visualized red hybridization signals. A cell has no amplification
of the hTERC gene with only 2 red and 2 aquamarine hybridization
signals in each interphase nucleus (Fig. 1D). A cell has amplification of the
hTERC gene with at least 2 aquamarine signals and >2 red
hybridization signals in each interphase nucleus (Fig. 1B). A cell has no amplification of
the c-MYC gene with only 2 red hybridization signals in each
interphase nucleus (Fig. 1C). A
cell has amplification of the c-MYC gene with >2 red
hybridization signals in each interphase nucleus (Fig. 1A).
Twenty cases of the control group were first
examined by FISH. One hundred nuclei/sample were analyzed, and the
percentage of cells that had amplification of the hTERC or c-MYC
gene in the enumerated cells was counted and used to establish
threshold values as follows: threshold = mean (M) + 3 × standard
deviation (SD). If the proportion of the detection was more than
the threshold, then the sample was evaluated as positive; if it was
less than the threshold was negative; if it was equal to the
threshold value then the number of cells observed needed to be
increased. In this study, the threshold value was 5 for
enumeration.
Data analysis
The enumeration results were analyzed using SAS9.2
statistical software for rank sum test and MedCalc 12.3.0
statistical software for the receiver operating characteristic
(ROC) curve analyses. P-values <0.05 were considered to indicate
statistically significant differences.
Results
FISH was successively performed on 171 cases. The
distribution of the specimens based on the cytology and biopsy
classification is shown in Table
I.
 | Table IDistribution of specimens based on the
cytology and biopsy classification. |
Table I
Distribution of specimens based on the
cytology and biopsy classification.
| | Biopsy | |
|---|
| |
| |
|---|
| Cytology | Normal | Benign lesion | CIN1 | CIN2 | CIN3 | ICC | Total |
|---|
| NILM | 40 | 8 | 4 | 2 | 1 | 0 | 55 |
| ASCUS | 0 | 3 | 9 | 2 | 3 | 0 | 17 |
| ASC-H | 0 | 4 | 3 | 2 | 2 | 0 | 11 |
| LSIL | 0 | 6 | 4 | 5 | 0 | 0 | 15 |
| HSIL | 0 | 3 | 5 | 18 | 28 | 10 | 64 |
| SCC | 0 | 0 | 1 | 0 | 2 | 6 | 9 |
| Total | 40 | 24 | 26 | 29 | 36 | 16 | 171 |
Amplification of hTERC and c-MYC
The results of FISH analysis on 171 cases were
sorted as positive or negative statistical results using the
threshold (gene detection result ≥5) (Table II). For 2 genes (hTERC and
c-MYC), there was a trend toward increasing amplification with the
increasing severity of the cervical squamous lesions. Amplification
was detected for the hTERC gene in 0 of the 40 controls (0%), 6 of
the 24 cervicitis (25%), 6 of the 26 CIN1 (23.08%), 15 of the 29
CIN2 (51.72%), 26 of the 36 CIN3 (72.22%) and in 14 of the 16 ICC
samples (87.50%) (Table II).
Furthermore, as shown in Table
II, amplification for the c-MYC gene was detected in 0 of the
40 controls (0%), 4 of the 24 cervicitis (16.67%), 5 of the 26 CIN1
(19.23%), 11 of the 29 CIN2 (37.93%), 22 of the 36 CIN3 (61.11%)
and in 14 of the 16 ICC (87.50%) samples. hTERC was more frequently
amplified than c-MYC. When a case had an amplification of the c-MYC
gene, it generally had an amplification of the hTERC gene.
 | Table IIDetection of hTERC and c-MYC in 6
groups [n (%)]. |
Table II
Detection of hTERC and c-MYC in 6
groups [n (%)].
| Group | N | hTERC | c-MYC |
Double-positive |
|---|
| Normal | 40 | 0 (0) | 0 (0) | 0 (0) |
| Benign lesion | 24 | 6 (25.00) | 4 (16.67) | 4 (16.67) |
| CIN1 | 26 | 6 (23.08) | 5 (19.23) | 5 (19.23) |
| CIN2 | 29 | 15 (51.72) | 11 (37.93) | 11 (37.93) |
| CIN3 | 36 | 26 (72.22) | 22 (61.11) | 22 (61.11) |
| ICC | 16 | 14 (87.50) | 14 (87.50) | 14 (87.50) |
In the enumerated cells of each sample, the
percentage of cells with an abnormal amplification of the hTERC and
c-MYC genes is termed as the abnormal amplification rate for short.
The respective abnormal amplification rates of the above 2 genes in
different histological lesions were subjected to the rank-sum test.
Multiple comparisons were performed using the Kruskal-Wallis
rank-sum test. The results revealed that multiple comparisons of
the abnormal amplification rates of the hTERC and c-MYC genes in
the different histological lesions all had statistical significance
(P<0.001) (Table III).
Therefore, a pairwise comparison was conducted using the
Mann-Whitney rank-sum test. The hTERC probe revealed significant
differences of several groups except between benign lesions and
CIN1, CIN2 and CIN3. Similarly, the c-MYC probe exhibited
significant differences of several groups except between benign
lesions and CIN1, CIN2 and CIN3, CIN3 and cervical cancer.
 | Table IIIComparison of the amplification rate
for hTERC and c-MYC in the different groups (%, M). |
Table III
Comparison of the amplification rate
for hTERC and c-MYC in the different groups (%, M).
| Group | N | hTERC | c-MYC |
|---|
| Normal | 40 | 1 | 1 |
| Benign lesion | 24 | 1 | 2 |
| CIN1 | 26 | 2 | 2.5 |
| CIN2 | 29 | 8 | 4 |
| CIN3 | 36 | 20 | 17 |
| ICC | 16 | 49.5 | 52 |
| P-value | | <0.001 | <0.001 |
Associations between gene amplification
and clinical diagnosis
On the basis of the percentage of cells that had
gene amplification for the chromosome probe markers, 3q26.3 (hTERC)
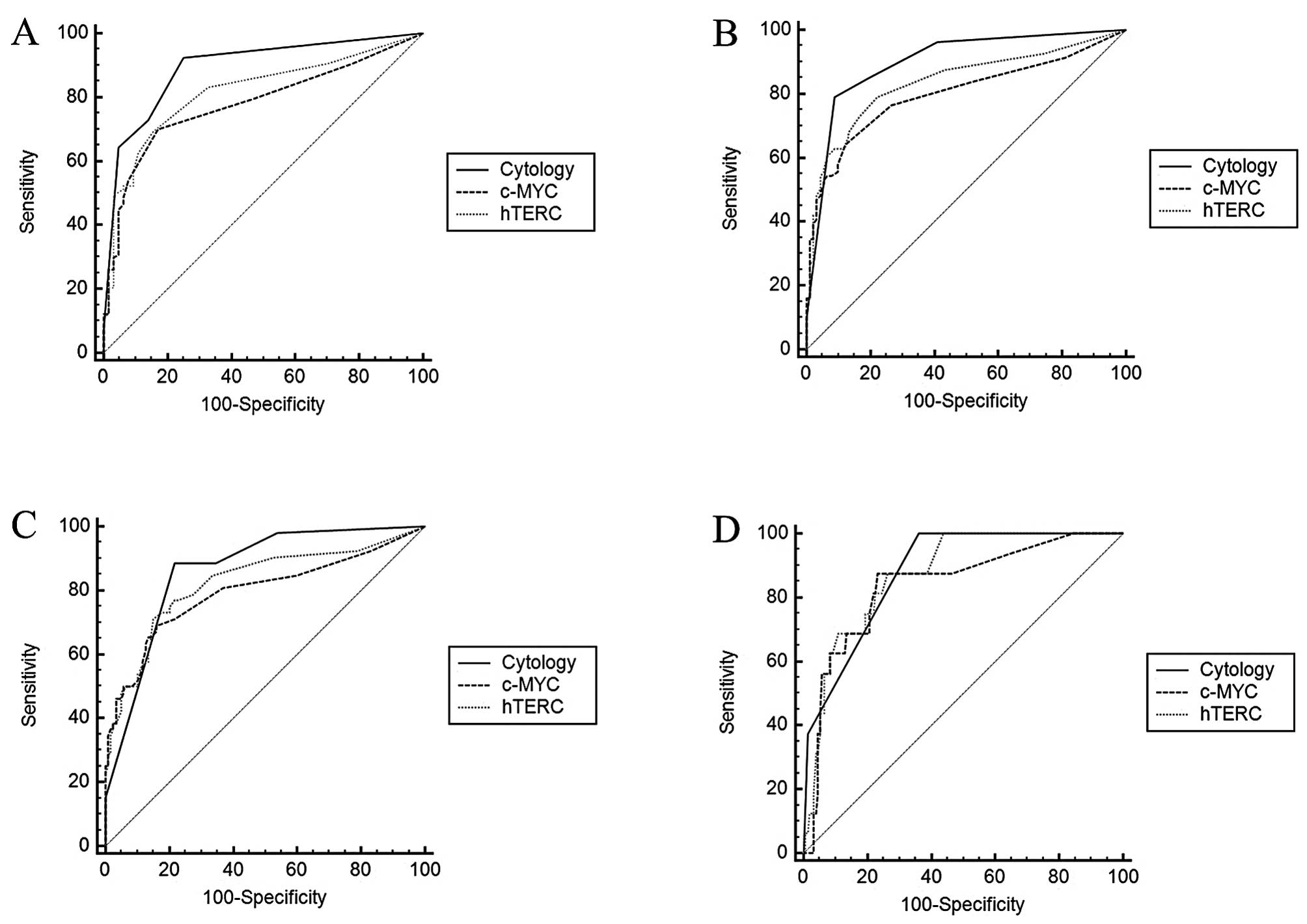
or 8q24 (c-MYC), in the enumerated cells per sample, ROC curves
were generated to confirm the accuracy of diagnosis of each mean
(Fig. 2). Sensitivity and
specificity were computed for each mean at the best. The area under
the ROC curve (AUC) of each grade was calculated for the diagnosis
of a certain CIN type or worse with certain critical value
(Tables IV–VI). According to the AUC values
calculated, the comparison of hTERC, c-MYC and cytology in
discriminating certain a CIN type or worse is shown in Table VII. Cytological diagnosis
involved digitization as follows. negative for intraepithelial or
malignant (NILM) cervical cytology, 0; ASCUS/ASC-H, 1; LSIL, 2;
HSIL, 3; and SCC, 4.
 | Table IVComparison of hTERC detection for the
screening of different cervical lesions. |
Table IV
Comparison of hTERC detection for the
screening of different cervical lesions.
| Screening
level | Critical value
(%) | SEN | SPE | AUC | 95% CI | P-value |
|---|
| >Benign
lesion | >2 | 69.16 | 84.37 | 0.817 | 0.751–0.872 | <0.0001 |
| >4 | 67.90 | 86.67 | | | |
| >CIN1 | >2 | 79.01 | 77.78 | 0.838 | 0.774–0.889 | <0.0001 |
| >4 | 57.01 | 90.62 | | | |
| >CIN2 | >14 | 71.15 | 84.87 | 0.825 | 0.759–0.878 | <0.0001 |
| >CIN3 | >14 | 87.50 | 73.55 | 0.873 | 0.813–0.919 | <0.0001 |
 | Table VIComparison of cytological detection
for the screening of different cervical lesions. |
Table VI
Comparison of cytological detection
for the screening of different cervical lesions.
| Screening
level | Critical value | SEN | SPE | AUC | 95% CI | P-value |
|---|
| >Benign
lesion | >0 | 92.52 | 75.00 | 0.894 | 0.838–0.936 | <0.0001 |
| >CIN1 | >2 | 79.01 | 91.11 | 0.900 | 0.845–0.941 | <0.0001 |
| >CIN2 | >2 | 88.46 | 78.15 | 0.863 | 0.802–0.911 | <0.0001 |
| >CIN3 | >2 | 100.00 | 63.87 | 0.881 | 0.822–0.925 | <0.0001 |
 | Table VIIComparison of hTERC, c-MYC and
cytology in discriminating between the different types [or worse
(>)] of cervical intraepithelial neoplasia. |
Table VII
Comparison of hTERC, c-MYC and
cytology in discriminating between the different types [or worse
(>)] of cervical intraepithelial neoplasia.
| P-value |
|---|
|
|
|---|
| Comparison | >BEN | >CIN1 | >CIN2 | >CIN3 |
|---|
| Cytology and
c-MYC | 0.0015 | 0.0050 | 0.0994 | 0.4259 |
| Cytology and
hTERC | 0.0210 | 0.0520 | 0.3023 | 0.8491 |
| c-MYC and
hTERC | 0.2035 | 0.1698 | 0.2973 | 0.4314 |
The performance of combined testing also was
calculated. According to AUC values calculated, the current results
indicated that the combined detection of the hTERC and c-MYC genes
in cervical epithelial exfoliated cells by the FISH technique could
better discriminate between the CIN1 and worse group (Table VIII). Their sensitivity and
specificity are shown in Table
VIII in detail. ROC curve analysis demonstrated that the
sensitivity and specificity for detecting CIN2+ lesions (CIN2 or
worse) were 57.01 and 90.62, respectively for testing for hTERC
alone; 58.02 and 90, respectively for testing for c-MYC alone; and
60.49 and 92.22, respectively for the combined testing of hTERC and
c-MYC.
 | Table VIIIComparison of estimated SEN, SPE with
hTERC, c-MYC and cytology in discriminating between different types
of cervical intraepithelial neoplasia lesions. |
Table VIII
Comparison of estimated SEN, SPE with
hTERC, c-MYC and cytology in discriminating between different types
of cervical intraepithelial neoplasia lesions.
| SEN | SPE | AUC | 95% CI |
|---|
| hTERC | 57.01 | 90.62 | 0.838 | 0.774–0.889 |
| c-MYC | 58.02 | 90.00 | 0.799 | 0.732–0.857 |
| Cytology | 79.01 | 91.11 | 0.900 | 0.845–0.941 |
| hTERC and
c-MYC | 60.49 | 92.22 | 0.764 | 0.693–0.825 |
| hTERC and
cytology | 65.43 | 86.67 | 0.760 | 0.689–0.822 |
| c-MYC and
cytology | 58.02 | 92.22 | 0.751 | 0.679–0.814 |
| hTERC and c-MYC and
cytology | 58.02 | 92.22 | 0.751 | 0.679–0.814 |
Discussion
Cervical cancer is the principal cause of mortality
due to cancer in women worldwide. The 5-year survival rate ranges
from 15–80%, depending on the extent of the disease (38). Novel predictive markers may
increase survival rates by improving the treatment of patients at
high risk for cancer. Pre-invasive disease can be detected by
cervical cytology. All currently available cytology technologies
rely on the visual analysis of exfoliated cells from the uterine
cervix. Improvement of conventional cytological screening has been
proposed by the introduction of molecular-based markers applied to
liquid-based cytology (LBC), the suspension of cells collected from
the cervix (39).
The distribution of specimens based on the
cytological and histological classification varied: some cases had
normal cytology and normal biopsy, some cases had ASCUS cytology
and CIN2/3 biopsy, some cases had LSIL cytology and normal biopsy,
some cases had LSIL cytology and CIN1 biopsy, some cases had LSIL
cytology and CIN2/3 biopsy, and some cases had HSIL cytology and
CIN2/3 biopsy, and so on. Not every abnormal cytology case had
pathological abnormality. For example, the CIN2 group contained
cases from normal to HSIL cytology in this study.
The development of cervical carcinoma is closely
associated with HPV infection. However, other genetic alterations
also play an important role (17,19,36,42). Previous studies have shown that
the detection of telomerase activity in the cervix may provide
information on cervical carcinogenesis and may be a marker to
monitor CIN transition (2,21,23,25,36).
A systematic review was performed to evaluate the telomerase test
(telomerase repeated amplification protocol) for the diagnosis of
cervical lesions and compare it to paraffin-embedded sections as
the diagnostic standard (40).
Data suggest that telomerase may activate an early event in
cervical carcinogenesis that may be associated with the initiation
and progression of cervical lesions (40). Invasive cervical carcinomas almost
invariably carry extra copies of the chromosome arm 3q, resulting
in a gain of the human telomerase gene (TERC). This provides the
rationale for the development of a multicolor FISH probe set as a
diagnostic tool for the direct detection of TERC gains in Pap
smears. The study by Heselmeyer-Haddad et al (35) showed that CIN2 and CIN3 lesions
can be distinguished from normal samples, ASCUS and CIN1, with a
sensitivity and specificity exceeding 90%, independent of the
cytomorphological assessment; a 3q gain is required for the
transition from CIN1/CIN2 to CIN3 and it predicts progression. The
sensitivity of the research for predicting the progression from
CIN1/CIN2 to CIN3 was 100% and the specificity, i.e., the
prediction of regression, was 70%. Thus the detection of 3q gain
and amplification of TERC in routinely collected Pap smears can
assist in identifying low-grade lesions with a high progression
risk and in decreasing false-negative cytological screenings. The
study by Li et al (41)
showed that the amplification of hTERC was 6.06% in normal or cases
of inflammation, 10.00% in CIN1, 66.67% in CIN2, 72.50% in CIN3,
and 100.00% in carcinoma cases, with a significant difference
between the low- (≤CIN1 or ≤ low-grade squamous intraepithelial
lesion) and high-grade (≥CIN2 or ≥ atypical squamous cells in which
high-grade squamous intraepithelial lesion cannot be excluded)
cervical lesions (P<0.001); the hTERC amplification rate was
consistent with the abnormal rates of cytological and histological
diagnoses. Thus, using FISH to detect the amplification of hTERC
may be a useful and specific screening method in cervical cancer
and precancerous lesions. Our study indicated that the
amplification of hTERC was 9.38% in normal or cases of
inflammation, 23.08% in CIN1, 51.72% in CIN2, 72.22% in CIN3, and
87.50% in carcinoma cases. ROC curve analysis demonstrated that the
AUC values of hTERC gene detection for screening of cervical cancer
of different grades were >0.8, suggesting that excellent
diagnostic results were obtained with this indicator. When the
abnormal amplification rate of the hTERC gene was >4, cervical
lesions of ≤CIN1 and ≥CIN2 were better distinguished (sensitivity,
57.01%; specificity, 90.62%); when the abnormal amplification rate
of the hTERC gene was >14, cervical lesions of ≥CIN2 were better
diagnosed (sensitivity, 71.15%; specificity, 84.87%).
Zhang et al (2) analyzed copy number alterations of
several oncogene loci in a panel of 84 cervical tumors. c-MYC at
8q24 was included. The amplification of c-MYC was detected in 25%
of tumors, using interphase FISH. The increased protein expression
of c-MYC was observed in tumors with the corresponding gene
amplification. c-MYC may have critical biological impact on the
development and progression of carcinoma of the uterine cervix. In
an internal study, Sokolova et al (36) assessed FISH probes to the 3q26 and
the 8q24 regions on a new set of 100 biopsy cases. Experimentation
revealed that the 3q26 and the 8q24 regions had the highest
frequency of copy number gains in samples with high-grade dysplasia
and cancer. Specifically, the 3q26 probe was positive in 100% of
cancer specimens, 90% of CIN3 specimens, 78% of CIN2 specimens, 26%
of CIN1 specimens, and 0% of normal specimens. The 8q24 probe was
positive in 100, 95, 96, 26 and 5% of cancer, CIN3, CIN2, CIN1 and
normal cases, respectively. The increasing trend was in agreement
with our findings. Our study indicated that the amplification of
c-MYC was 6.25% in normal or cases of inflammation, 19.23% in CIN1,
37.93% in CIN2, 61.11% in CIN3 and 87.50% in carcinoma cases. ROC
curve analysis demonstrated that the AUC value of c-MYC gene
detection for the screening of cervical lesions ≥CIN3 was >0.8
and the AUC values for screening of cervical lesions of other
grades were >0.7, indicating the moderate diagnostic results of
this indicator. When the abnormal amplification rate of c-MYC was
>10, cervical lesions ≥CIN3 were better diagnosed (sensitivity,
87.50%; specificity, 76.77%). Furthermore, our study revealed that
using FISH to detect the amplification of hTERC had a bigger AUC
for the diagnoses of different cervical lesions than the detection
of c-MYC.
In the current study, the screening results of
cervical lesions by hTERC gene detection, c-MYC gene detection, and
cytological diagnosis alone were compared. For the screening of
cervical lesions above the grade of benign lesions, cytological
diagnosis was superior to the gene detection with significant
differences in the diagnostic results from the 2 gene detection
methods. For the screening of cervical lesions >CIN1, the
greatest AUC value was obtained in cytological diagnosis, followed
by hTERC detection; however, there were no statistically
significant differences (P>0.05) observed in the screening
results between hTERC gene detection and cytological diagnosis,
whereas the screening results of c-MYC detection and cytological
diagnosis were significantly different (P<0.05). For the
screening of cervical lesions >CIN2, the detection of hTERC and
c-MYC genes and cytological diagnosis had similar screening results
with no statistically significant differences (P>0.05).
In addition, the screening results of cervical
lesions by a combination of hTERC gene detection, c-MYC gene
detection, and/or cytological diagnosis were examined. For cervical
lesions ≥CIN1, the combined detection of hTERC and c-MYC genes
improved the specificity, but decreased the sensitivity of
screening; likewise, the combination of the detection of 2 genes
and the cytological method improved the specificity, but markedly
decreased the sensitivity of screening. For cervical lesions ≥CIN2,
the combined detection of hTERC and c-MYC genes increased both the
specificity and sensitivity of screening. For cervical lesions
≥CIN3 and cervical cancer, different combinations of the detection
of 2 genes and the cytological method did not markedly improve the
specificity or sensitivity of screening.
In the present study, there were a significant
number of cases with marked discrepancies in the interpretation
between the cytological and histological diagnoses of the
simultaneously sampled Pap smears and biopsy specimens. These
discrepancies between the cytological and histological diagnoses
are likely to be attributed to sampling error rather than
interpretation error. Although the amplification of hTERC or/and
c-MYC results in the discrepant cases also showed discordance
between the liquid-based cytology Pap smears and biopsy specimens,
our data reveals that using FISH to detect the amplification of
hTERC or/and c-MYC on cervical epithelial exfoliated cells may be
as a useful and specific screening method in pre-cancerous lesions
and may be helpful in arbitrating some diagnostic disagreements,
although obtaining additional biopsies or Pap smears may eliminate
these discrepancies.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, et
al: Global cancer statistics. CA Cancer J Clin. 61:69–90. 2011.
View Article : Google Scholar
|
|
2
|
Zhang A, Månér S, Betz R, Angström T,
Stendahl U, Bergman F, Zetterberg A and Wallin KL: Genetic
alterations in cervical carcinomas: frequent low-level
amplifications of oncogenes are associated with human
papillomavirus infection. Int J Cancer. 101:427–433. 2002.
View Article : Google Scholar
|
|
3
|
Wright TC Jr, Massad LS, Dunton CJ, et al:
2006 consensus guidelines for the management of women with abnormal
cervical screening tests. J Low Genit Tract Dis. 11:201–222. 2007.
View Article : Google Scholar
|
|
4
|
Christopherson WM, Parker JE, Mendez WM
and Lundin FE Jr: Cervix cancer death rates and mass cytologic
screening. Cancer. 26:808–811. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Quinn M, Babb P, Jones J and Allen E:
Effect of screening on incidence of and mortality from cancer of
cervix in England: evaluation based on routinely collected
statistics. BMJ. 318:904–908. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pak SC, Martens M, Bekkers R, et al: Pap
smear screening history of women with squamous cell carcinoma and
adenocarcinoma of the cervix. Aust N Z J Obstet Gynaecol.
47:504–507. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mählck CG, Jonsson H and Lenner P: Pap
smear screening and changes in cervical cancer mortality in Sweden.
Int J Gynaecol Obstet. 44:267–272. 1994.
|
|
8
|
Liu S, Semenciw R, Probert A and Mao Y:
Cervical cancer in Canada: changing patterns in incidence and
mortality. Int J Gynecol Cancer. 11:24–31. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Etzioni R, Urban N, Ramsey S, et al: The
case for early detection. Nat Rev Cancer. 3:243–252. 2003.
View Article : Google Scholar
|
|
10
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Estimating the world cancer burden: Globocan 2000. Int J Cancer.
94:153–156. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cuzick J, Szarewski A, Cubie H, et al:
Management of women who test positive for high-risk types of human
papillomavirus: the HART study. Lancet. 362:1871–1876. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fahey MT, Irwig L and Macaskill P:
Meta-analysis of Pap test accuracy. Am J Epidemiol. 141:680–689.
1995.PubMed/NCBI
|
|
13
|
Koliopoulos G, Arbyn M, Martin-Hirsch P,
Kyrgiou M, Prendiville W and Paraskevaidis E: Diagnostic accuracy
of human papillomavirus testing in primary cervical screening: a
systematic review and meta-analysis of non-randomized studies.
Gynecol Oncol. 104:232–246. 2007. View Article : Google Scholar
|
|
14
|
Mayrand MH, Duarte-Franco E, Rodrigues I,
et al: Human papillomavirus DNA versus Papanicolaou screening tests
for cervical cancer. N Engl J Med. 357:1579–1588. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nanda K, McCrory DC, Myers ER, et al:
Accuracy of the Papanicolaou test in screening for and follow-up of
cervical cytologic abnormalities: a systematic review. Ann Intern
Med. 132:810–819. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guidos BJ and Selvaggi SM: Use of the Thin
Prep Pap Test in clinical practice. Diagn Cytopathol. 20:70–73.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zur Hausen H: Papillomaviruses causing
cancer: evasion from hostcell control in early events in
carcinogenesis. J Natl Cancer Inst. 92:690–698. 2000.PubMed/NCBI
|
|
18
|
Duensing S and Münger K: Mechanisms of
genomic instability in human cancer: insights from studies with
human papillomavirus oncoproteins. Int J Cancer. 109:157–162. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lazo PA: The molecular genetics of
cervical carcinoma. Br J Cancer. 80:2008–2018. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kurtycz D, Nuñez M, Arts T, Bauman C,
Harris C, Inhorn S and Meisner L: Use of fluorescent in-situ
hybridization to detect aneuploidy in cervical dysplasia. Diagn
Cytopathol. 15:46–51. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Heselmeyer K, Macville M, Schröck E,
Blegen H, Hellström AC, Shah K, Auer G and Ried T: Advanced-stage
cervical carcinomas are defined by a recurrent pattern of
chromosomal aberrations revealing high genetic instability and a
consistent gain of chromosome arm 3q. Genes Chromosomes Cancer.
19:233–240. 1997. View Article : Google Scholar
|
|
22
|
Thein A, Trková M, Fox M and Parrington J:
The application of comparative genomic hybridization to previously
karyotyped cervical cancer cell lines. Cancer Genet Cytogenet.
116:59–65. 2000. View Article : Google Scholar
|
|
23
|
Kirchhoff M, Rose H, Petersen BL, Maahr J,
Gerdes T, Lundsteen C, Bryndorf T, Kryger-Baggesen N, Christensen
L, Engelholm SA and Philip J: Comparative genomic hybridization
reveals a recurrent pattern of chromosomal aberrations in severe
dysplasia/carcinoma in situ of the cervix and in advanced-stage
cervical carcinoma. Genes Chromosomes Cancer. 24:144–150. 1999.
View Article : Google Scholar
|
|
24
|
Mullokandov MR, Kholodilov NG, Atkin NB,
Burk RD, Johnson AB and Klinger HP: Genomic alterations in cervical
carcinoma: losses of chromosome heterozygosity and human papilloma
virus tumor status. Cancer Res. 56:197–205. 1996.
|
|
25
|
Heselmeyer K, Schröck E, du Manoir S,
Blegen H, Shah K, Steinbeck R, Auer G and Ried T: Gain of
chromosome 3q defines the transition from severe dysplasia to
invasive carcinoma of the uterine cervix. Proc Natl Acad Sci USA.
93:479–484. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kersemaekers AM, van de Vijver MJ, Kenter
GG and Fleuren GJ: Genetic alterations during the progression of
squamous cell carcinomas of the uterine cervix. Genes Chromosomes
Cancer. 26:346–354. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dellas A, Torhorst J, Jiang F, Proffitt J,
Schultheiss E, Holzgreve W, Sauter G, Mihatsch MJ and Moch H:
Prognostic value of genomic alterations in invasive cervical
squamous cell carcinoma of clinical stage IB detected by
comparative genetic hybridization. Cancer Res. 59:3475–3479.
1999.
|
|
28
|
Hidalgo A, Schewe C, Petersen S, Salcedo
M, Gariglio P, Schlüns K, Dietel M and Petersen I: Human papilloma
virus status and chromosomal imbalances in primary cervical
carcinomas and tumor cell lines. Eur J Cancer. 36:542–548. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Todd R and Wong DT: Oncogenes. Anticancer
Res. 19:4729–4746. 1999.
|
|
30
|
Bishop JM: Molecular themes in
oncogenesis. Cell. 64:235–248. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Adhikary S and Eilers M: Transcriptional
regulation and transformation by Myc proteins. Nat Rev Mol Cell
Biol. 6:635–645. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Couturier J, Sastre-Garau X,
Schneider-Maunoury S, Labib A and Orth G: Integration of
papillomavirus DNA near myc genes in genital carcinomas and its
consequences for proto-oncogene expression. J Virol. 65:4534–4538.
1991.PubMed/NCBI
|
|
33
|
Sastre-Garau X, Favre M, Couturier J and
Orth G: Distinct patterns of alteration of myc genes associated
with integration of human papillomavirus type 16 or type 45 DNA in
two genital tumors. J Gen Virol. 81:1983–1993. 2000.PubMed/NCBI
|
|
34
|
Umayahara K, Hirai Y, Sugiyama Y, et al:
Genetic alterations during the progression of early cervical
neoplasm. Proc Amer Assoc Cancer Res. 46:57432005.
|
|
35
|
Heselmeyer-Haddad K, Sommerfeld K, White
NM, et al: Genomic amplification of the human telomerase gene
(TERC) in pap smears predicts the development of cervical cancer.
Am J Pathol. 166:1229–1238. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sokolova I, Algeciras-Schimnich A, Song M,
et al: Chromosomal biomarkers for detection of human papillomavirus
associated genomic instability in epithelial cells of cervical
cytology specimens. J Mol Diagn. 9:604–611. 2007. View Article : Google Scholar
|
|
37
|
Matthews CP, Shera KA and McDougall JK:
Genomic changes and HPV type in cervical carcinoma. Proc Soc Exp
Biol Med. 223:316–321. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Widschwendter A, Ivarsson L, Blassnig A,
et al: CDH1 and CDH13 methylation in serum is an independent
prognostic marker in cervical cancer patients. Int J Cancer.
109:163–166. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Apostolidou S, Hadwin R, Burnell M, et al:
DNA methylation analysis in liquid-based cytology for cervical
cancer screening. Int J Cancer. 125:2995–3002. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rosa MI, Medeiros LR, Bozzetti MC, et al:
Accuracy of telomerase in cervical lesions: a systematic review.
Int J Gynecol Cancer. 17:1205–1214. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li Y, Ye F, Lü WG, et al: Detection of
human telomerase RNA gene in cervical cancer and precancerous
lesions: comparison with cytological and human papillomavirus DNA
test findings. Int J Gynecol Cancer. 20:631–637. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Walboomers JM, Jacobs MV, Manos MM, et al:
Human papillomavirus is a necessary cause of invasive cervical
cancer worldwide. J Pathol. 189:12–19. 1999. View Article : Google Scholar : PubMed/NCBI
|