Introduction
The mammalian alveolar epithelium is composed of
type I alveolar epithelial cells (AECI) and type II alveolar
epithelial cells (AECII). AECI are broad and flat cells that cover
95% of the alveolar surface and comprise 40% of the alveolar
epithelium, which is important for gas exchange. AECII are cuboidal
cells characterized by the distinct morphological appearance of
lamellar bodies, which occupy only 5% of the alveolar surface and
comprise 60% of the alveolar epithelium. AECII synthesize, secrete
and recycle lung surfactant, which includes surfactant protein
(SP)A, SPB, SPC and SPD, reducing surface tension and preventing
the collapse of the alveolus (1).
AECII also provide pulmonary host defense by synthesizing and
secreting numerous cytokines and interleukins that affect
lymphocyte, macrophage, and neutrophil functions. Moreover, AECII
act as progenitor cells for AECI in the alveoli, and play a key
role in maintaining normal alveolar homeostasis and lung repair
(2). The inadequate, delayed, or
impaired re-epithelialization of the injured alveolus is known as a
key factor in the pathogenesis of several life-threatening
pulmonary diseases, such as chronic obstructive pulmonary disease
(COPD) (3) and acute respiratory
distress syndrome (ARDS) (4). A
variety of methods for isolating human AECII have been published
and some of their properties have been described (5–7);
however, it still remains unclear as to how to maintain the
proliferation and phenotype of the AECII. A number of difficulties
still exist in obtaining an adequate tissue supply to isolate human
AECII. Human embryonic stem cells (ESCs) are pluripotent stem cells
derived from embryos at the blastocyst stage. ESCs can proliferate
indefinitely while maintaining their capacity to differentiate into
a great variety of specialized cell types (8,9).
ESCs have been induced to differentiate into AECII in vitro
and in vivo (10,11). Furthermore, the transplantation of
human ESC-derived AECII has been shown to abrogate acute lung
injury in mice (12). Thus, AECII
derived from exogenous stem cells may provide novel treatment
options for COPD.
However, the acquirement of human ESCs implies the
destruction of human embryo, which results in obvious ethical
debates (13). Human amniotic
fluid stem cells (AFSCs) can be isolated from discarded
amniocentesis specimens or post-partum amniotic fluid, which is not
subject to teratocarcinoma formation and serious ethical issues
(14,15). AFSCs possess not only high
proliferation potential in vitro, but also a wide range of
differentiation potential into cell types from all 3 embryonic germ
layers, such as adipocytes, chondrocytes, osteocytes, hepatocytes,
neural cells and cardiomyocytes (16–18). Hence, AFSCs are considered new
pluripotent stem cells between ESCs and adult stem cells. Their
characteristics, including pluripotency, high proliferation rates,
multi-differentiation capabilities and lack of ethical issues
associated with the retrieval of these cells render them an
attractive source for cell therapy, drug discovery and toxicology
screening (14–18). Certain studies have demonstrated
that human AFSCs can integrate into the murine lung and
differentiate into pulmonary lineages following lung injury in
vivo (19). However, it
remains unknown as to whether amniotic fluid-derived mesenchymal
stem cells (AFMSCs) can be induced to differentiate into AECII
in vitro. Thus, in the present study, we investigated the
potential of AFMSC differentiation into AECII in vitro using
KnockOut™ serum replacement (KOSR), activin A and small airway
basal medium (SABM).
Materials and methods
Isolation and culture of AFMSCs
Fifteen independent samples of amniotic fluid (20 ml
each) were obtained from pregnant women at 16–20 weeks of pregnancy
who underwent amniocentesis for fetal karyotyping. The samples were
filtered through a 200 mesh filter and centrifuged for 5 min at
1,200 rpm. The cells derived from the amniotic fluid were
resuspended in low glucose Dulbecco’s modified Eagle’s medium
(DMEM; Gibco, Carlsbad, CA, USA) containing 5 mg/l basic fibroblast
growth factor (bFGF; Sigma, St. Louis, MO, USA) and 10% fetal
bovine serum (FBS; HyClone, Logan, UT, USA), and maintained in
6-well plates. When the cells were incubated for 3 days at 37°C in
a humidified atmosphere of 5% carbon dioxide and 95% air, the
supernatants were removed and the cell clones in spindle-shaped
growth were selected to other wells by cell scrapers. When the
cells reached 80–90% confluence, they were digested using 0.25%
trypsin (Sigma) and incubated in a 25 cm2 cell culture
flask (Corning Inc.-Life Sciences, Oneonta, NY, USA), labeled as P1
(the first generation). The surface antigens of the AFMSCs (P4)
were detected by flow cytometry with monoclonal antibodies as
follows: phycoerythrin (PE)-conjugated antibodies against CD29,
CD73, CD105 and CD166, and fluorescein isothiocyanate
(FITC)-conjugated antibodies against CD14, CD19, CD34, CD44, CD45
and CD90 (BD Biosciences, San Diego, CA, USA). The related isotype
control was used as a negative control. The results were analyzed
using a flow cytometer (Guava EasyCyte; Millipore, Billerica, MA,
USA). Octamer-binding transcription factor 4 (OCT4) mRNA
expression in the AFMSCs was detected by reverse
transcription-polymerase chain reaction (RT-PCR). The primers were
designed as follows (Invitrogen, Shanghai, China): OCT4
(GenBank: DQ486513.1), 5′-CGTG AAGCTGGAGAAGGAGAAGCTG-3′ (sense) and
5′-CAAGGG CCGCAGCTTACACATGTTC-3′ (antisense); and beta-actin
(ACTB), 5′-GGCACCACACCTTCTACAATGA-3′ (sense) and
5′-TCAGGAGGAGCAGCAATGATCTTG-3′ (antisense). The protein expression
of OCT4 was detected by western blot analysis. Human embryonic stem
cells (hES3; ES Cell International, Melbourne, Australia) were used
as positive controls and human lung fibroblasts (HFL1; ATCC,
Manassas, VA, USA) were used as negative controls. The protocol was
approved by the Ethics Committee of Zhejiang Provincial People’s
Hospital and each patient signed written informed consent.
Differentiation of AFMSCs in vitro
The AFMSCs (P4) were detached and dissociated into
single cells with a mixture of 0.25% trypsin (Sigma)/0.02%
EDTA-Na2 in phosphate-buffered saline (PBS). The AFMSCs
were seeded into 24-well low-adherence tissue culture plates
(Corning Inc.-Life Sciences) at the concentration of
2×107/l/well. They were then divided into 4 groups,
named groups I–IV. The AFMSCs were induced to differentiate in
vitro using KOSR (Invitrogen, Carlsbad, CA, USA), activin A
(PeproTech, Rocky Hill, NJ, USA) and SABM (Lonza, Basel,
Switzerland) by different methods. In group I, the AFMSCs were
cultured for 3 days suspended in DMEM (Gibco) containing 10% KOSR
firstly, and then transferred onto adherent agarose-coated plates.
The adherent cells were cultured for 4 days in DMEM containing 10%
KOSR with 100 mg/l activin A in a humidified atmosphere of 5%
carbon dioxide and 95% air at 37°C. The cells were then cultured
for 10 days in DMEM containing 15% KOSR. In group II, the AFMSCs
were cultured for 3 days suspended in DMEM containing 10% KOSR
firstly, and then adherently cultured for 4 days in DMEM containing
10% KOSR. The cells were then cultured for 10 days in DMEM
containing 15% KOSR. In group III, the AFMSCs were cultured for 3
days suspended in DMEM containing 10% KOSR with 100 mg/l activin A
firstly, and then cultured for 4 days adherently in DMEM containing
10% KOSR. The cells were then cultured for 10 days in DMEM
containing 15% KOSR. In group IV, the AFMSCs were cultured for 3
days suspended in DMEM containing 10% KOSR with 100 mg/l activin A
firstly, and then adherently cultured for 4 days in DMEM containing
10% KOSR with 100 mg/l activin A. The cells were then cultured for
10 days in DMEM containing 15% KOSR. Finally, all the cells were
cultured for 14 days in SABM.
Quantitative reverse transcription
PCR
Total RNA was extracted from the cells using TRIzol
reagent (Invitrogen, Carlsbad, CA, USA) according to the
instructions of the manufacturer and dissolved in nuclease-free
water. The final RNA concentrations were determined using a
spectrophotometer. Total RNA was reverse transcribed using MMLV
reverse transcriptase (Promega, Madison, WI, USA) at 42°C for 60
min and 70°C for 10 min. Real-time PCR analysis was carried out
using SYBR-Green Real-Time PCR Master Mix (Toyobo, Osaka, Japan)
and real-time PCR amplification equipment. The thermal profile
consisted of one cycle at 95°C for 15 min, followed by 45 cycles at
95°C for 15 sec and 60°C for 30 sec. The primers used were as
follows (Invitrogen, Shanghai, China): SPA forward,
5′-CCTATCATTTGCCAA GAACC-3′ and reverse, 5′-CAGGAAGATGGGTTTGGAT-3′;
SPC forward, 5′-CAACGGGAAAGGAAACGC-3′ and reverse,
5′-GCTGCTTTATTCTGTTGTGGC-3′; and ACTB forward,
5′-CTACAATGAGCTGCGTGTGGC-3′ and reverse, 5′-CAGG
TCCAGACGCAGGATGGC-3′. The mRNA expression of SPA and
SPC was determined by normalization of the threshold (Ct)
cycle of the target genes to that of the mRNA expression of
ACTB for each sample. The ΔCt value was determined using the
following equation: (ΔCt) = (Ct of the target genes) - (Ct of
ACTB). The ΔΔCt value was used to find the relative
expression of the target genes according to the following formula:
relative expression 0= 2−ΔΔCt. Data were analyzed using
the 2−ΔΔCt method. A549 lung epithelial cells (Shanghai
Institute of Biology, Chinese Academy of Sciences, Shanghai, China)
were used as the positive controls. Undifferentiated AFMSCs were
used as the negative controls.
Western blot analysis
The cells were lysed in ice-cold lysis buffer [50
mmol/l Tris (pH 7.5), 150 mmol/l NaCl, 1 mmol/l EDTA, 10 mmol/l
Na2P2O7, 100 mmol/l NaF and 1%
Triton X-100] and protease inhibitor cocktail (1 mmol/l
phenylmethylsulfonyl fluoride, 1 mg/l aprotinin and 1 mg/l
leupeptin). The protein concentration was measured using a protein
assay kit (Micro BCA; Pierce, Rockford, IL, USA). Equal amounts of
cell lysates containing 10 μg protein were incubated for 5 min in
boiling water. Proteins were separated by 8% sodium dodecyl sulfate
polyacrylamide gel electrophoresis and then electroblotted onto
Hybond-ECL nitrocellulose membranes. The membranes were blocked
with TBS solution containing 5% skim milk at room temperature for 1
h. The membranes were then incubated with primary antibodies for 1
h at room temperature on an orbital shaker. The membranes were then
washed 5 times, for 5 min each, in Tris-buffered saline with
Tween-20 (TBST) and incubated in diluted peroxidase-conjugated
immunopure goat anti-rabbit IgG (H+L) (Pierce) for 1 h at room
temperature on an orbital shaker. After the membranes were again
washed 5 times for 5 min each in TBST, the proteins were visualized
with an enhanced chemiluminescence solution (Amersham Pharmacia
Biotech, Buckinghamshire, UK). The images were developed on X-ray
film and the band densities were analyzed with a UVP-GDS8000 gel
analysis system. The same membranes were stripped and blotted with
anti-β-actin antibodies, which provided a loading control. The
primary antibodies were obtained from the following sources: OCT4
antibody, SPA antibody and SPC antibody from Abcam (Cambridge, MA,
USA), and β-actin (N-21) antibody from Santa Cruz Biotechnology,
Inc. (Santa Cruz, CA, USA). Protein markers were obtained from Cell
Signaling Technology (Danvers, MA, USA).
Immunofluorescence
The differentiated AFMSCs were fixed for 20 min at
4°C with 4% paraformaldehyde (Merck, Darmstadt, Germany). The cells
were blocked for 20 min at 37°C with 5% goat serum (diluted in TBS
and 0.25% Triton X-100; Sigma), followed by incubation for 2 h at
37°C with a polyclonal rabbit anti-human SPA and SPC antibody
(1:500; BD Biosciences). The cells were then rinsed twice for 5 min
with PBS, and further incubated for 1 h at 37°C with the secondary
antibody FITC-conjugated goat anti rabbit IgG (1:250; BD
Biosciences). Each antibody was diluted in PBS with 0.25% Triton
X-100. The cell nuclei were then counterstained with 1 g/l
4′,6-diamino-2-phenylindole (DAPI; Sigma) in PBS for 5 min and
covered with Immu-Mount (Thermo Electron Corp., Waltham, MA, USA).
Finally, all the slides were analyzed using an IX71 fluorescence
microscope (Olympus, Tokyo, Japan).
Electron microscopy
The cells were fixed for 1 h in 2.5% glutaraldehyde
and 0.1 mmol/l cacodylate buffer, pH 7.4. After a brief rinse in
0.2 mmol/l buffer, the cells were postfixed for 2 h in 1% osmium
tetroxide in 0.1 mmol/l cacodylate buffer, pH 7.4. The cells were
dehydrated in 90, 95 and 100% hydroxypropyl methacrylate and
embedded in Epon-Araldite. Ultrathin serial sections (100 nm) were
obtained by an ultramicrotome (RMC, Tucson, AZ, USA) equipped with
a diamond knife. The sections were stained with uranyl acetate and
lead citrate, and then observed under a transmission electron
microscope (Hitachi, Tokyo, Japan).
Statistical analysis
Data were analyzed for statistical significance by
one-way analysis of variance (ANOVA), paired-sample t-tests, and
multiple comparisons in ANOVA were analyzed using the
Student-Newman-Keuls test. Data are expressed as the means ±
standard deviation (SD). Values of P<0.05 were considered to
indicate statistically significant differences.
Results
Phenotypic characterization of
AFMSCs
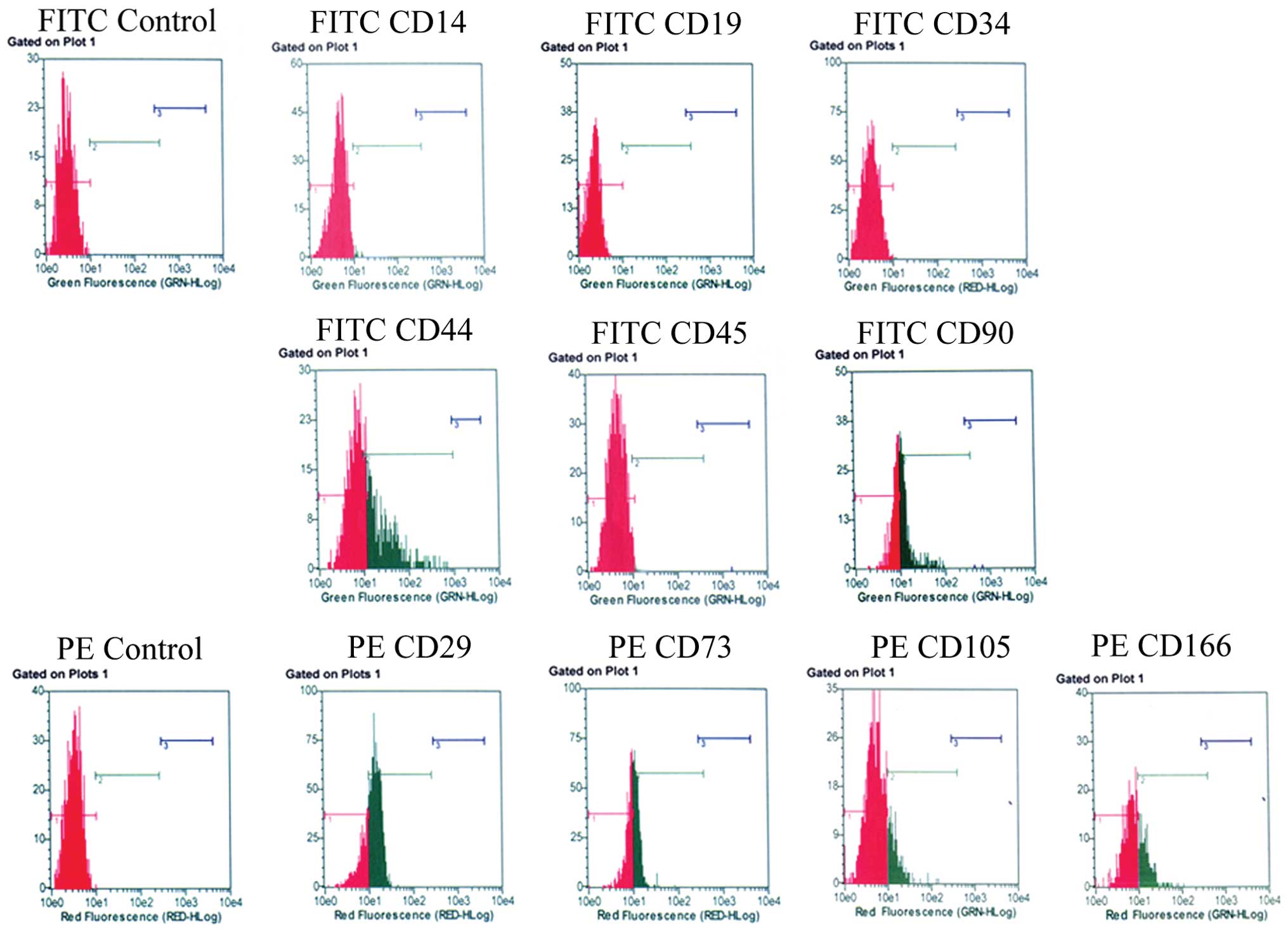
The surface antigenic characteristics of the AFMSCs
were analyzed using flow cytometry. The results revealed that the
expression of surface antigens, such as CD29 (73.7±6.6%), CD44
(49.7±4.7%), CD73 (53.0±3.6%), CD90 (54.4±4.35%), CD105 (22.5±2.7%)
and CD166 (22.7±3.6%) was positive; however, the expression of CD14
(0.25±0.02%), CD19 (0%), CD34 (0%) and CD45 (0%) was negative
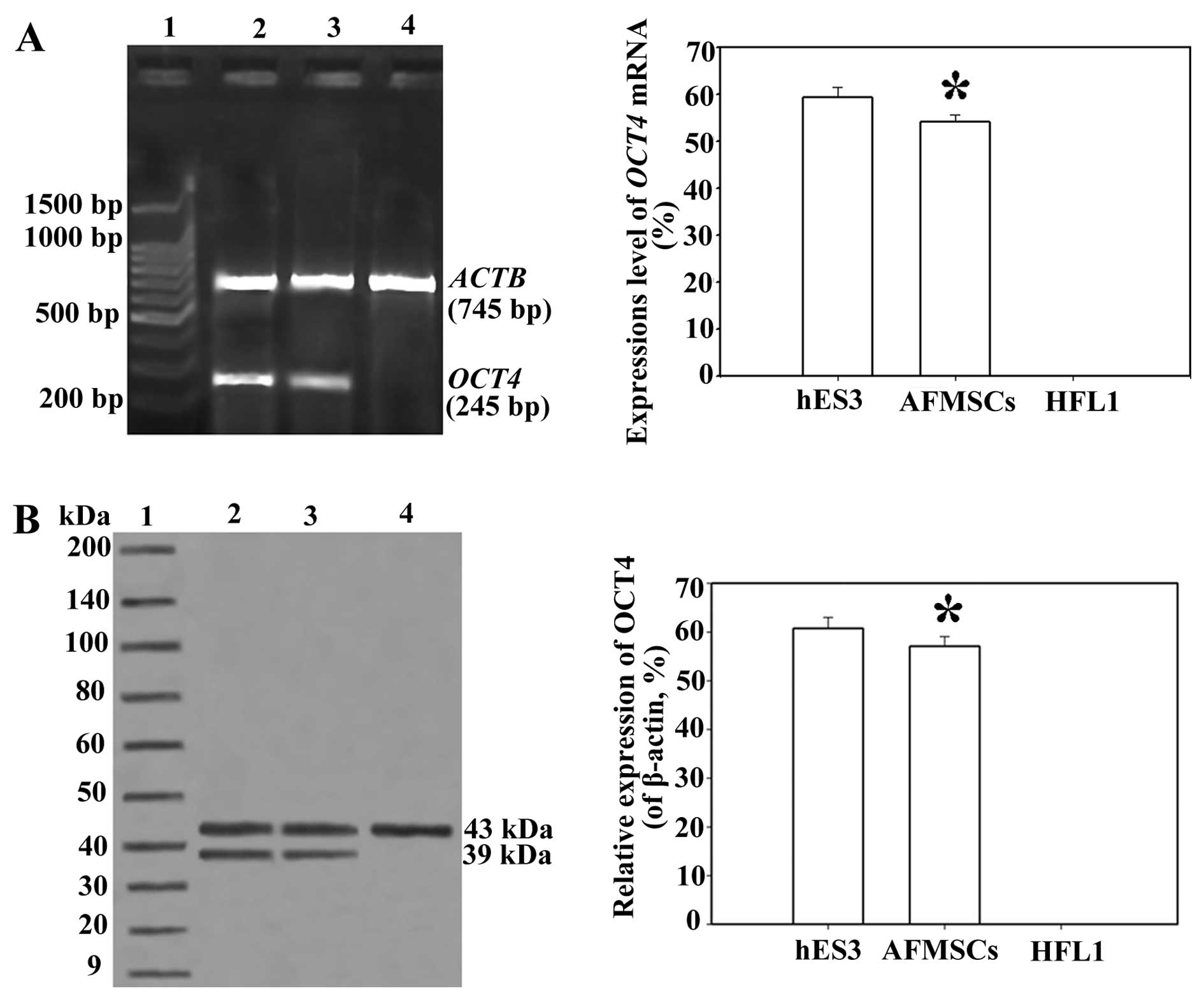
(Fig. 1). OCT4, at both the mRNA
and protein level was significantly expressed in the AFMSCs
(P<0.05) (Fig. 2).
mRNA expression of SPA and SPC in
differentiated AFMSCs
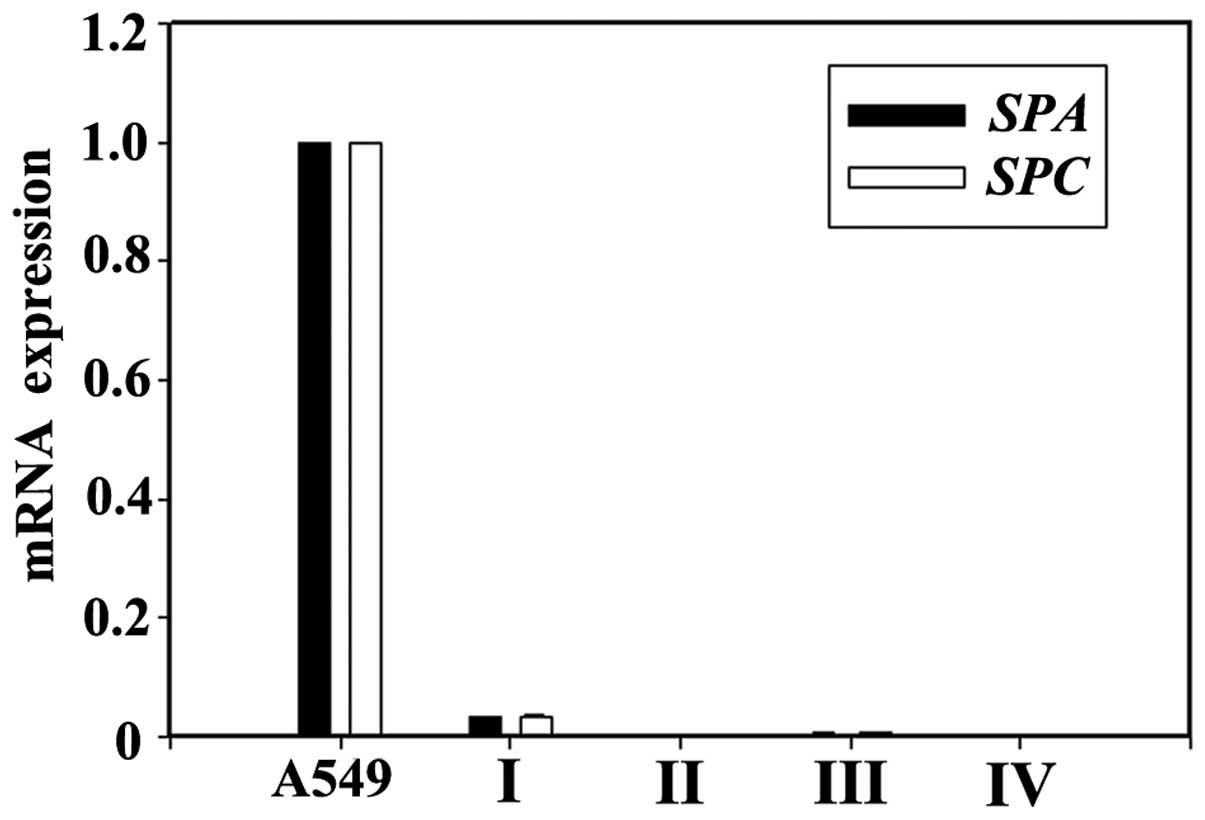
The mRNA expression of SPA and SPC in
the A549 lung epithelial cells (positive controls) was strongly
positive. The mRNA expression of SPA and SPC was not
detected in the undifferentiated AFMSCs. When the AFMSCs were
induced to differentiate using KOSR only, the mRNA expression of
SPA and SPC was negative (group II). When the AFMSCs
were induced to differentiate using a combination of KOSR, activin
A and SABM, the mRNA expression of SPA and SPC (group
III and IV) was still almost undetectable. The mRNA expression of
SPA and SPC in the cells in group I was significantly
induced, and was the highest amongst all the groups (P<0.05)
(Fig. 3).
Protein expression of SPA and SPC in
differentiated AFMSCs
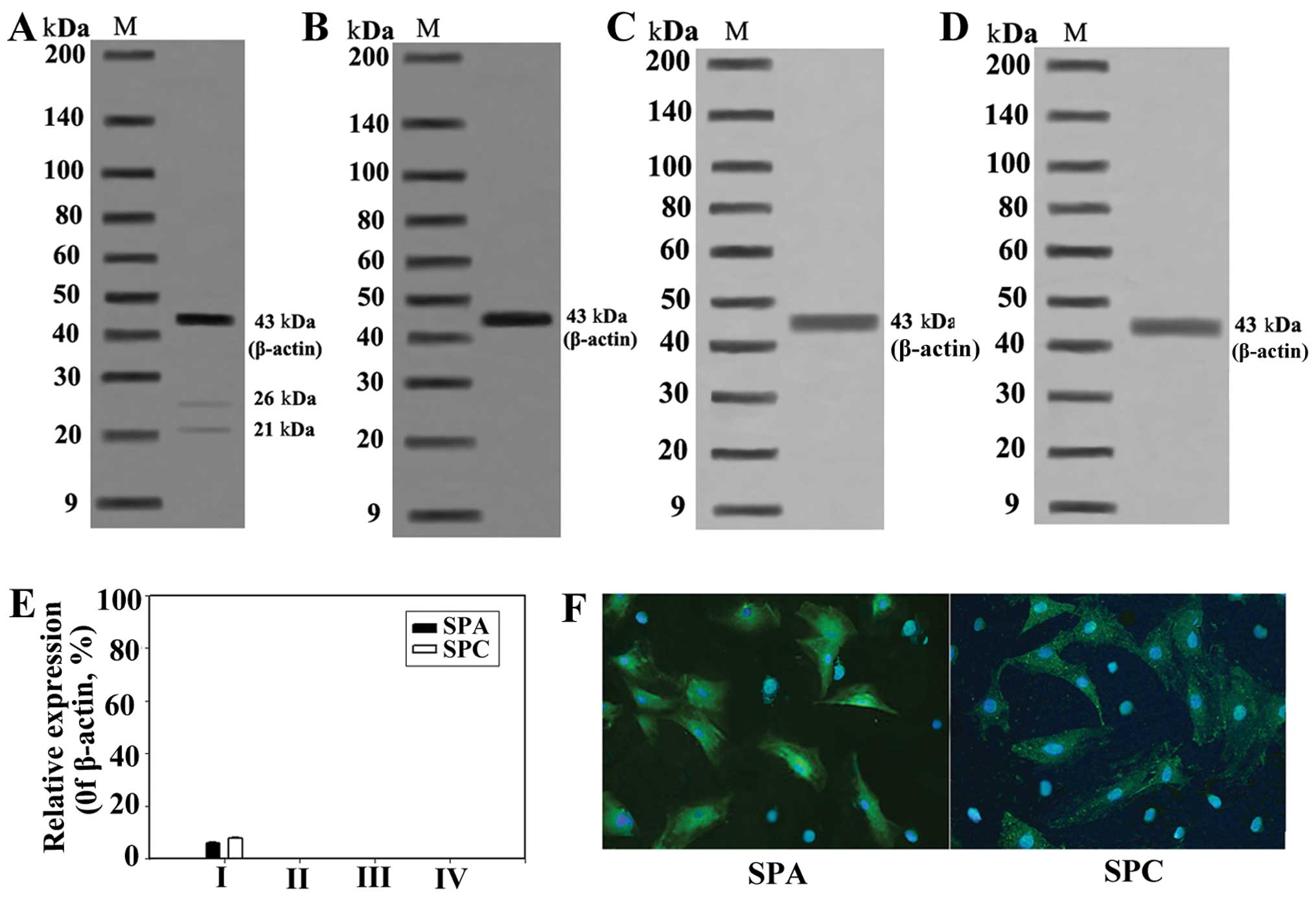
When the AFMSCs were induced to differentiate for 31
days, the protein expression of SPA and SPC was detected by western
blot analysis and immunofluorescence. The results revealed that the
expression of SPA and SPC in group I was significantly induced
(P<0.05), but was negative in the cells in the other groups
(Fig. 4), which indicates that
the AFMSCs can be induced to differentiate into AECII-like cells
under specific conditions.
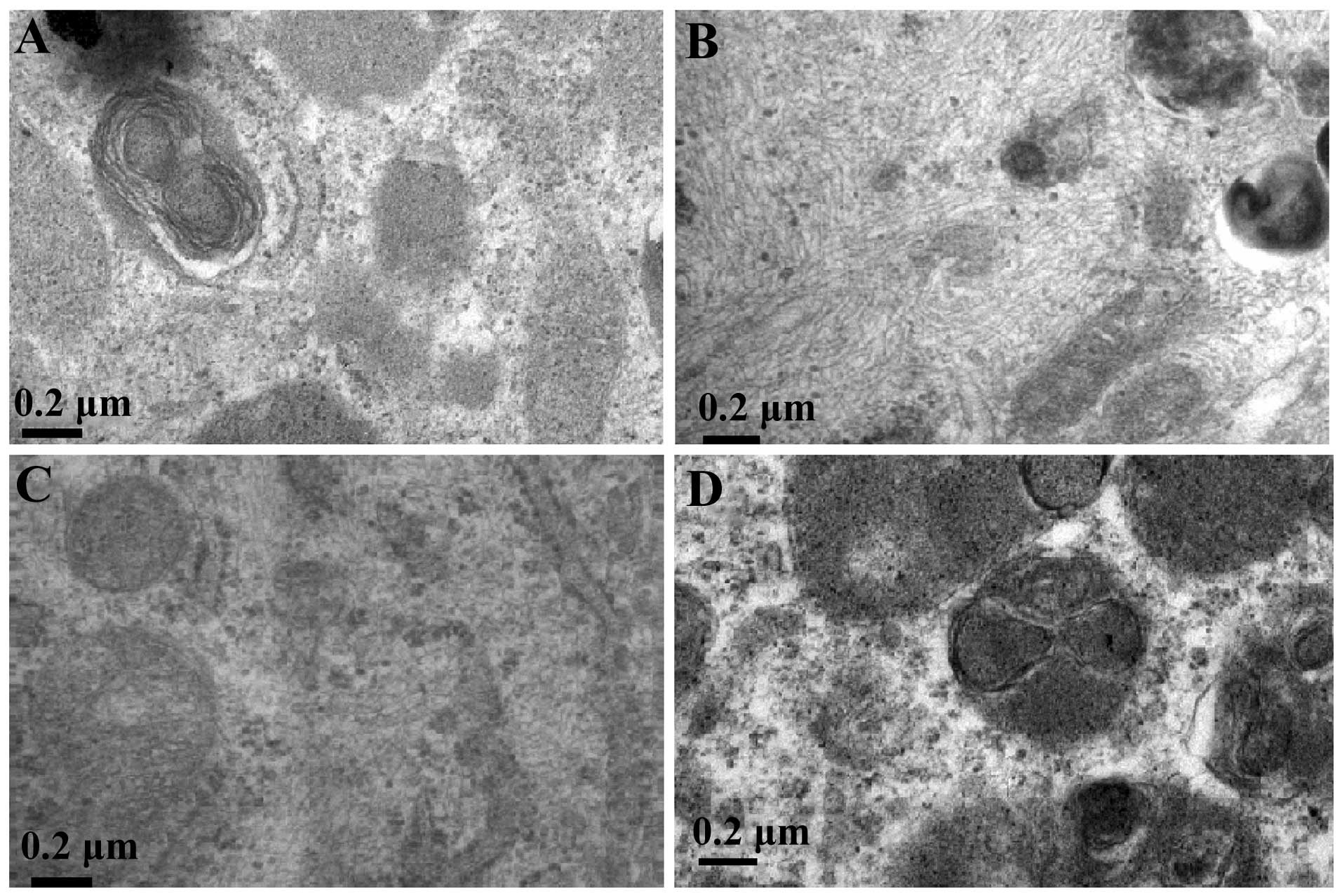
Ultrastructural evidence of AFMSCs
differentiating into AECII-like cells
When the AFMSCs were induced to differentiate for 31
days using KOSR, activin A and SABM, lamellar bodies that contained
pulmonary surfactant proteins and lipids were observed in the
differentiated AFMSCs in group I under a transmission electron
microscope (Fig. 5). However,
lamellar bodies were not observed in the cells in groups II–IV.
This indicates that AFMSCs can differentiate into AECII-like cells
by using the appropriate induction medium with KOSR, activin A and
SABM.
Discussion
AFMSCs are pluripotent stem cells, which can be
isolated from discarded amniocentesis specimens or post-partum
amniotic fluid (14,20). Previous studies have demonstrated
that AFMSCs express mesenchymal stem cell markers, such as CD90,
CD105, CD73 and CD166, but not do not express the hematopoietic
markers, CD45, CD34 and CD14 (20–22). In the present study, AFMSCs
significantly expressed surface antigens, such as CD29, CD44, CD73
and CD90, but did not express CD14, CD19, CD34 and CD45, which was
consistent with the study of Tsai et al (14). The potency of amniotic
fluid-derived stem cells in differentiating into cell types from
all 3 embryonic germ layers, including endothelial cells,
chondrocytes, adipocytes, hepatocytes, neuronal cells and myocytes
seems to be between ESCs and adult stem cells (16–18,23). The POU transcription factor, OCT4,
is a specific marker for embryonal carcinoma cells, embryonic germ
cells and embryonic stem cells (24). In the present study, OCT4 was
significantly expressed in the AFMSCs, which indicated that the
AFMSCs have pluripotent characteristics. Thus, their pluripotency,
lack of ethical issues associated with their acquirement and the
lack of teratoma formation when injected in vivo render them
attractive candidates for stem cell sources in the field of lung
regeneration (14–18).
As progenitors of AECI, AECII characterized by the
unique ability to synthesize and secrete SPC and the distinct
morphological appearance of lamellar bodies play important roles in
lung tissue repair (1). Severe
damage to or the loss of AECII may result in a considerable
vulnerability of the alveolus and the impairment of alveolar repair
(25–27). AECII derived from exogenous stem
cells are a promising source of cells which may be used
therapeutically to treat distal lung diseases, including COPD.
However, to date, there are still no reports on the differentiation
of AFMSCs into AECII in vitro (16,28). Small airway growth medium (SAGM)
composed of SABM and a commercial cocktail of growth factors is a
medium designed for the maintenance and growth of mature distal
airway epithelial cells. However, certain studies have shown that
SABM significantly improves distal lung epithelial differentiation
from mouse ESCs compared with SAGM (29). Thus, in the present study, SABM
was used to induce AFMSC differentiation in vitro in place
of SAGM. KOSR is a defined medium supplement designed to directly
replace FBS in ESC culture medium. Certain studies have shown that
definitive endoderm differentiation is improved (30) and human ESCs are induced to
differentiate into AECII by the replacement of FBS with KOSR
(11). In the present study,
AFMSCs failed to differentiate into AECII when induced with only
KOSR. Activin A belongs to the transforming growth factor-β (TGF-β)
family of growth and differentiation factors (31). Studies on amphibians and have mice
shown that high activity of activin signaling is necessary to
specify the endoderm germ layer (32,33). It has also been demonstrated that
activin A can increase the expression of early distal lung
epithelial markers, enhance endoderm formation and accelerate early
lung epithelial differentiation (29). In the present study, when the
AFMSCs were induced to differentiate for 31 days by KOSR, activin A
and SABM, the mRNA and protein expression of SPA and SPC was
strongly positive in the differentiated AFMSCs in group I, and
lamellar bodies were also observed in the differentiated AFMSCs.
However, negative results were observed for the other cell groups,
which suggests that the differentiation of AFMSCs is precisely
controlled by both time and space. In conclusion, AFMSCs can be
induced to differentiate into AECII-like cells in vitro
using a combination of KOSR, activin A and SABM under the
appropriate conditions, which indicates that AFMSCs have the
potential for use in lung regenerative therapy. However, the exact
mechanisms of AFMSC differentiation into AECII reamin unknown, and
thus, further studies are required in the near future.
Acknowledgements
This study was supported by a grant from the
National Natural Science Foundation of China (no. 81000016).
References
|
1
|
Whitsett JA, Wert SE and Weaver TE:
Alveolar surfactant homeostasis and the pathogenesis of pulmonary
disease. Annu Rev Med. 61:105–119. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hoffman AM and Ingenito EP: Alveolar
epithelial stem and progenitor cells: emerging evidence for their
role in lung regeneration. Curr Med Chem. 19:6003–6008. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tsuji T, Aoshiba K and Nagai A: Alveolar
cell senescence exacerbates pulmonary inflammation in patients with
chronic obstructive pulmonary disease. Respiration. 80:59–70. 2010.
View Article : Google Scholar
|
|
4
|
Miyake Y, Kaise H, Isono K, Koseki H,
Kohno K and Tanaka M: Protective role of macrophages in
noninflammatory lung injury caused by selective ablation of
alveolar epithelial type II Cells. J Immunol. 178:5001–5009. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Corti M, Brody AR and Harrison JH:
Isolation and primary culture of murine alveolar type II cells. Am
J Respir Cell Mol Biol. 14:309–315. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ehrhardt C, Kim KJ and Lehr CM: Isolation
and culture of human alveolar epithelial cells. Methods Mol Med.
107:207–216. 2005.PubMed/NCBI
|
|
7
|
Fujino N, Kubo H, Suzuki T, et al:
Isolation of alveolar epithelial type II progenitor cells from
adult human lungs. Lab Invest. 91:363–378. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gaur M, Ritner C, Sievers R, Pedersen A,
Prasad M, Bernstein HS and Yeqhiazarians Y: Timed inhibition of
p38MAPK directs accelerated differentiation of human embryonic stem
cells into cardiomyocytes. Cytotherapy. 12:807–817. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Muguruma K and Sasai Y: In vitro
recapitulation of neural development using embryonic stem cells:
from neurogenesis to histogenesis. Dev Growth Differ. 54:349–357.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Samadikuchaksaraei A and Bishop AE:
Derivation and characterization of alveolar epithelial cells from
murine embryonic stem cells in vitro. Methods Mol Biol.
330:233–248. 2006.PubMed/NCBI
|
|
11
|
Wang D, Haviland DL, Burns AR, Zsiqmond E
and Wetsel RA: A pure population of lung alveolar epithelial type
II cells derived from human embryonic stem cells. Proc Natl Acad
Sci USA. 104:4449–4454. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang D, Morales JE, Calame DG, Alcorn JL
and Wetsel RA: Transplantation of human embryonic stem cell-derived
alveolar epithelial type II cells abrogates acute lung injury in
mice. Mol Ther. 18:625–634. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sugarman J: Human stem cell ethics: beyond
the embryo. Cell Stem Cell. 2:529–533. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tsai MS, Lee JL, Chang YJ and Hwang SM:
Isolation of human multipotent mesenchymal stem cells from
second-trimester amniotic fluid using a novel two-stage culture
protocol. Hum Reprod. 19:1450–1456. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sessarego N, Parodi A, Podestà M, et al:
Multipotent mesenchymal stromal cells from amniotic fluid: solid
perspectives for clinical application. Haematologica. 93:339–346.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Joo S, Ko IK, Atala A, Yoo JJ and Lee SJ:
Amniotic fluid-derived stem cells in regenerative medicine
research. Arch Pharm Res. 35:271–280. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hartmann K, Raabe O, Wenisch S and Arnhold
S: Amniotic fluid derived stem cells give rise to neuron-like cells
without a further differentiation potential into retina-like cells.
Am J Stem Cells. 2:108–118. 2013.PubMed/NCBI
|
|
18
|
Sun H, Feng K, Hu J, Soker S, Atala A and
Ma PX: Osteogenic differentiation of human amniotic fluid-derived
stem cells induced by bone morphogenetic protein-7 and enhanced by
nanofibrous scaffolds. Biomaterials. 31:1133–1139. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Carraro G, Perin L, Sedrakyan S, et al:
Human amniotic fluid stem cells can integrate and differentiate
into epithelial lung lineages. Stem Cells. 26:2902–2911. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Perin L, Sedrakyan S, Da Sacco S and De
Filippo R: Characterization of human amniotic fluid stem cells and
their pluripotential capability. Methods Cell Biol. 86:85–99. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
De Coppi P, Bartsch G Jr, Siddiqui MM, et
al: Isolation of amniotic stem cell lines with potential for
therapy. Nat Biotechnol. 25:100–106. 2007.PubMed/NCBI
|
|
22
|
Roubelakis MG, Trohatou O and Anagnou NP:
Amniotic fluid and amniotic membrane stem cells: marker discovery.
Stem Cells Int. 2012:1078362012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Benavides OM, Petsche JJ, Moise KJ Jr,
Johnson A and Jacot JG: Evaluation of endothelial cells
differentiated from amniotic fluid-derived stem cells. Tissue Eng
Part A. 18:1123–1131. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tondeur S, Assou S, Nadal L, Hamamah S and
De Vos J: Biology and potential of human embryonic stem cells. Ann
Biol Clin (Paris). 66:241–247. 2008.(In French).
|
|
25
|
Sisson TH, Mendez M, Choi K, et al:
Targeted injury of type II alveolar epithelial cells induces
pulmonary fibrosis. Am J Respir Crit Care Med. 181:254–263. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kuwano K: Epithelial cell apoptosis and
lung remodeling. Cell Mol Immunol. 4:419–429. 2007.
|
|
27
|
Zhao CZ, Fang XC, Wang D, Tang FD and Wang
XD: Involvement of type II pneumocytes in the pathogenesis of
chronic obstructive pulmonary disease. Respir Med. 104:1391–1395.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Antonucci I, Pantalone A, Tete S, Salini
V, Borlongan CV, Hess D and Stuppia L: Amniotic fluid stem cells: a
promising therapeutic resource for cell-based regenerative therapy.
Curr Pharm Des. 18:1846–1863. 2012.PubMed/NCBI
|
|
29
|
Rippon HJ, Polak JM, Qin M and Bishop AE:
Derivation of distal lung epithelial progenitors from murine
embryonic stem cells using a novel three-step differentiation
protocol. Stem Cells. 24:1389–1398. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kubo A, Shinozaki K, Shannon JM, et al:
Development of definitive endoderm from embryonic stem cells in
culture. Development. 131:1651–1662. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Massagué J and Gomis RR: The logic of
TGFbeta signaling. FEBS Lett. 580:2811–2820. 2006.PubMed/NCBI
|
|
32
|
Cao Y, Siegel D and Knöchel W: Xenopus POU
factors of subclass V inhibit activin/nodal signaling during
gastrulation. Mech Dev. 123:614–625. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hansson M, Olesen DR, Peterslund JM, et
al: A late requirement for Wnt and FGF signaling during
activin-induced formation of foregut endoderm from mouse embryonic
stem cells. Dev Biol. 330:286–304. 2009. View Article : Google Scholar : PubMed/NCBI
|