Introduction
Hepatitis C virus (HCV) is a major cause of chronic
liver disease. An estimated 170 million individuals worldwide are
infected with HCV, including 1.5 million individuals in Japan
(1,2). The main goal of treatment for
chronic HCV is to prevent the progression to cirrhosis and the
development of hepatocellular carcinoma by eradicating the virus.
Interferon (IFN)-based therapy was first used in 1992 (3), and the treatment regimens have
improved since then. In Japan, a combination of pegylated
interferon (PEG-IFN) and ribavirin (RBV) has been used since 2004
(4). However, almost half of the
patients treated with this combination do not achieve a sustained
viral response (SVR), despite long-term therapy (5).
Several factors have been reported to influence the
efficacy of PEG-IFN/RBV therapy. Host factors associated with
unfavorable outcomes, include advanced age, the female gender and
advanced fibrosis. Additionally, the IL28B single nucleotide
polymorphism (SNP) has been reported to be a strong genomic
predictor of the efficacy of PEG-IFN/RBV therapy, such that the
IL28B SNP is routinely examined before beginning any treatment
regimen (6). As regards viral
factors, a mutation at amino acid 70 of the core region of HCV
(core 70) is an important predictor of the therapeutic efficacy
(7). The non-structural (NS)5A
protein combines with the core protein in the formation of the
viral particle. It has been reported that several regions in domain
II of NS5A are associated with the therapeutic efficacy of IFN
monotherapy and PEG-IFN/RBV combination therapy. For example, in
1996, it was reported that a large number of mutations in the IFN
sensitivity-determining region (ISDR) (amino acids, 2209-2248) were
associated with the SVR to IFN monotherapy in Japanese patients
with genotype 1b HCV (8). In
2008, it was reported that mutations in the IFN-RBV
resistance-determining region (IRRDR) (amino acids, 2334-2379) were
also associated with the SVR to combined PEG-IFN/RBV therapy
(9).
Telaprevir (TPV), an NS3/4A protease inhibitor, is
the first commercially available direct-acting antiviral (DAA) that
directly inhibits viral replication. Although TPV/PEG-IFN/RBV
combination therapy has achieved a viral eradication rate of up to
70–80% (10), 20–30% of patients
did not achieve SVR during treatment due to side-effects (e.g.,
skin rash and renal dysfunction), loss of compliance and the
development of antiviral resistance.
Thus, taking the above data into consideration, we
conducted a collaborative study in Kobe, Japan to identify which
factors, including NS5A mutations, are associated with the SVR in
patients with genotype 1b HCV and a high viral load who were
treated with TPV/PEG-IFN/RBV.
Materials and methods
Patients and sample collection
Serum samples were collected from patients with
genotype 1b chronic HCV infection and a high viral load. Overall,
48 patients (31 males/17 females; mean ± standard deviation age,
57.7±8.3 years) were enrolled in this study. The patients were
treated with TPV/PEG-IFN/RBV combination therapy for 12 weeks
followed by PEG-IFN/RBV for 12 weeks. TPV (Mitsubishi Tanabe Pharma
Corp., Osaka, Japan) was orally administered at doses of 750 or 500
mg 3 times daily (every 8 h). PEG-IFN (Pegintron®;
Schering-Plough, Innishannon, County Cork, Ireland) was
subcutaneously injected once weekly (1.5 μg/kg). RBV was orally
administered at 400–800 mg daily.
The serum HCV-RNA status was assessed at 4 weeks, at
the end of treatment, and at 6 months after the end of treatment.
Serum samples were collected and stored at −80°C until virological
examination. The rapid virological response (RVR) was defined as
undetectable HCV-RNA at 4 weeks. SVR12 was defined as persistent
undetectable serum HCV-RNA and normal serum alanine
aminotransferase levels at 12 weeks after the end of treatment.
This study was conducted between November 2011 and
June 2013 at Kobe University Hospital and at 3 affiliated hospitals
in Hyogo prefecture. The study was approved by the Ethics Committee
of Kobe University Hospital. Written informed consent was obtained
from each patient prior to enrollment in the study.
NS3 and NS5A sequence analysis
HCV-RNA was extracted from 140 μl of serum using a
commercial kit according to the manufacturer’s instructions (QIAamp
viral RNA kit; Qiagen, Tokyo, Japan). The NS3 and NS5A regions of
the HCV genome were amplified and sequenced by nested RT-PCR using
primer sets, as previously described (9,11).
The amino acid sequences were deduced and aligned using Genetyx Win
software version 7.0 (Genetyx Corp., Tokyo, Japan).
Statistical analysis
All statistical analyses were performed using SPSS
software version 16 (SPSS Inc., Chicago, IL, USA). P-values of
<0.05 were considered to indicate statistically significant
differences.
Results
Baseline characteristics and on-treatment
responses
Among the 48 patients, 22 were treatment-naïve and
26 were receiving IFN-based therapy. Of these latter 26 patients,
17 experienced relapse on prior therapy and 9 were non-responders.
As regards the IL28B SNP (rs8099917), 32 patients had the major
allele (TT) and 16 patients had the minor allele (TG/GG). Overall,
37/48 patients (77%) achieved SVR12 following triple therapy. The
mean age of the patients who achieved SVR12 was less than that of
those who did not achieve SVR12, although the difference was not
significant. The SVR12 rate was significantly lower in
non-responders to previous therapy (44%) than in relapsed patients
(82%) and treatment-naïve patients (86%) (P<0.05). The SVR12
rate was significantly higher in patients with the IL28B major
allele than in patients with the minor allele (88 vs. 56%;
P<0.05).
Effect of mutations in the core protein
or NS5A region on therapeutic outcomes
We then sought to identify factors associated with
the SVR12 by intent-to-treat analysis. The frequency of mutations
amino acid 70 in the core region of HCV and the number of mutations
in the ISDR did not differ significantly between patients who
achieved SVR12 and those who did not achieve SVR12 (Table I). The amino acid alignment in
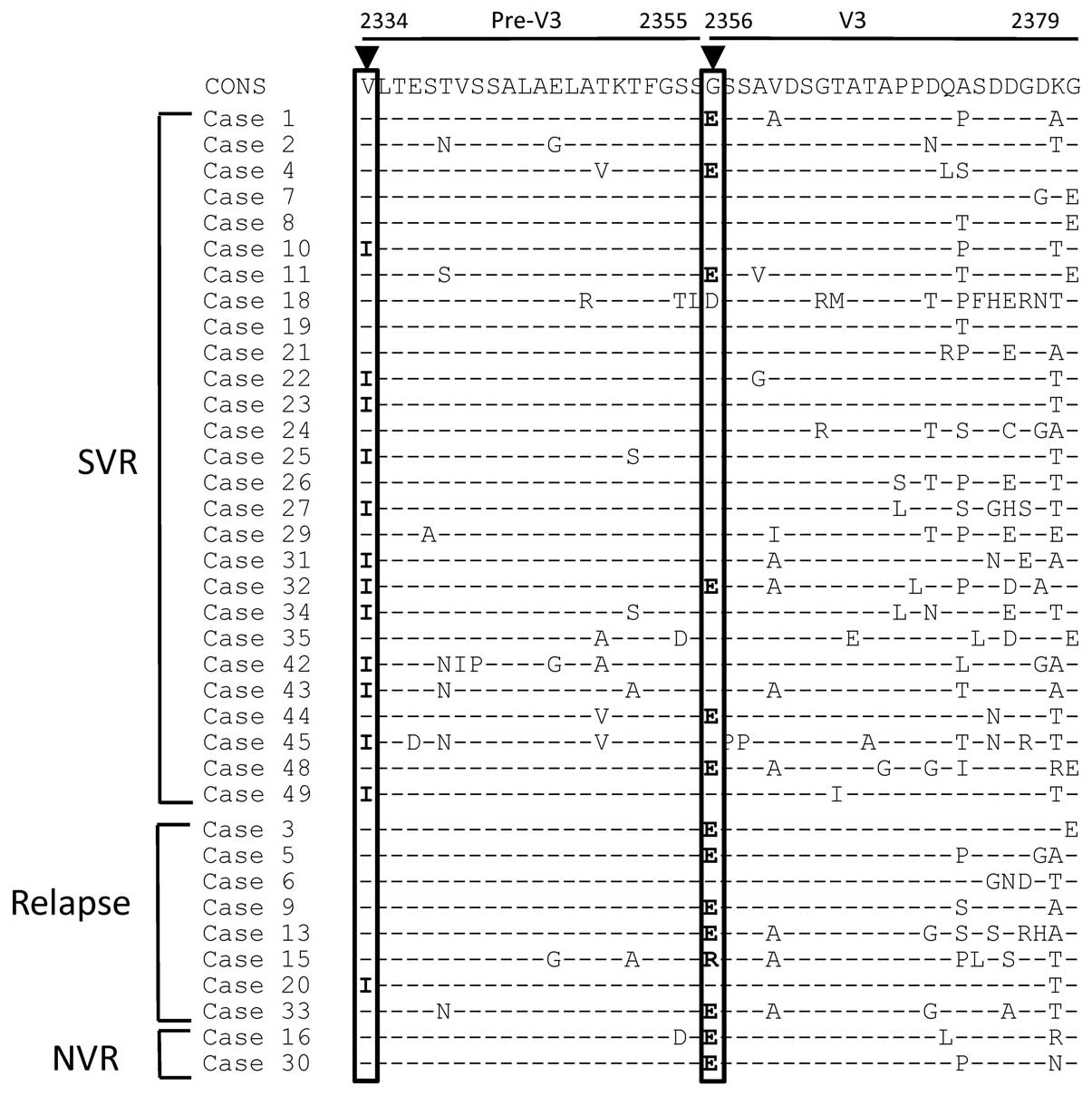
IRRDR is shown in Fig. 1. The
mean number of mutations in the IRRDR did not differ significantly
between patients who achieved SVR12 and those who did not achieve
SVR12 (5.1±2.9 vs. 4.4±2.2). However, the frequencies of 2 amino
acid mutations differed significantly the 2 groups of patients. The
V2334I mutation was significantly more frequent in patients who
achieved SVR12 than in those who did not achieve SVR12 (44 vs. 10%;
P<0.05), and the frequency of the G2356E mutation was lower in
patients who achieved SVR12 than in those who did not achieve SVR12
(22 vs. 70%; P<0.05).
 | Table IComparison between patients who
achieved SVR and those who did not (no SVR). |
Table I
Comparison between patients who
achieved SVR and those who did not (no SVR).
| Factor | SVR | No SVR | P-value |
|---|
| Number of
patients | 37 (77%) | 11 (23%) | |
| Age | 56.0±9.2 | 59.5±12.4 | NS |
| Gender (M/F) | 24/13 | 7/4 | NS |
| Previous therapy |
| Naïve | 19 (86%)a | 3 (14%) | |
| Relapse | 14 (82%)a | 3 (18%) | |
| NVR | 4 (44%) | 5 (56%) | |
| IL28B SNP |
| TT | 28 (88%) | 4 (12%) | <0.05 |
| TG/GG | 9 (56%) | 7 (44%) | |
| Core 70 wild | 17/28 | 5/11 | |
| ISDR ≥1 | 5/16 (31%) | 4/9 (44%) | NS |
| IRRDR ≥4 | 19/27 (70%) | 6/10 (60%) | NS |
| V2360A and/or
K2378T | 17/27 (63%) | 5/10 (50%) | NS |
| V2334I | 12/27 (44%) | 1/10 (10%) | 0.039 |
| G2356E | 6/27 (22%) | 7/10 (70%) | 0.016 |
Mutations in the NS3 region before and
after treatment
We then examined whether mutations in the NS3 region
were associated with antiviral resistance, using sera obtained
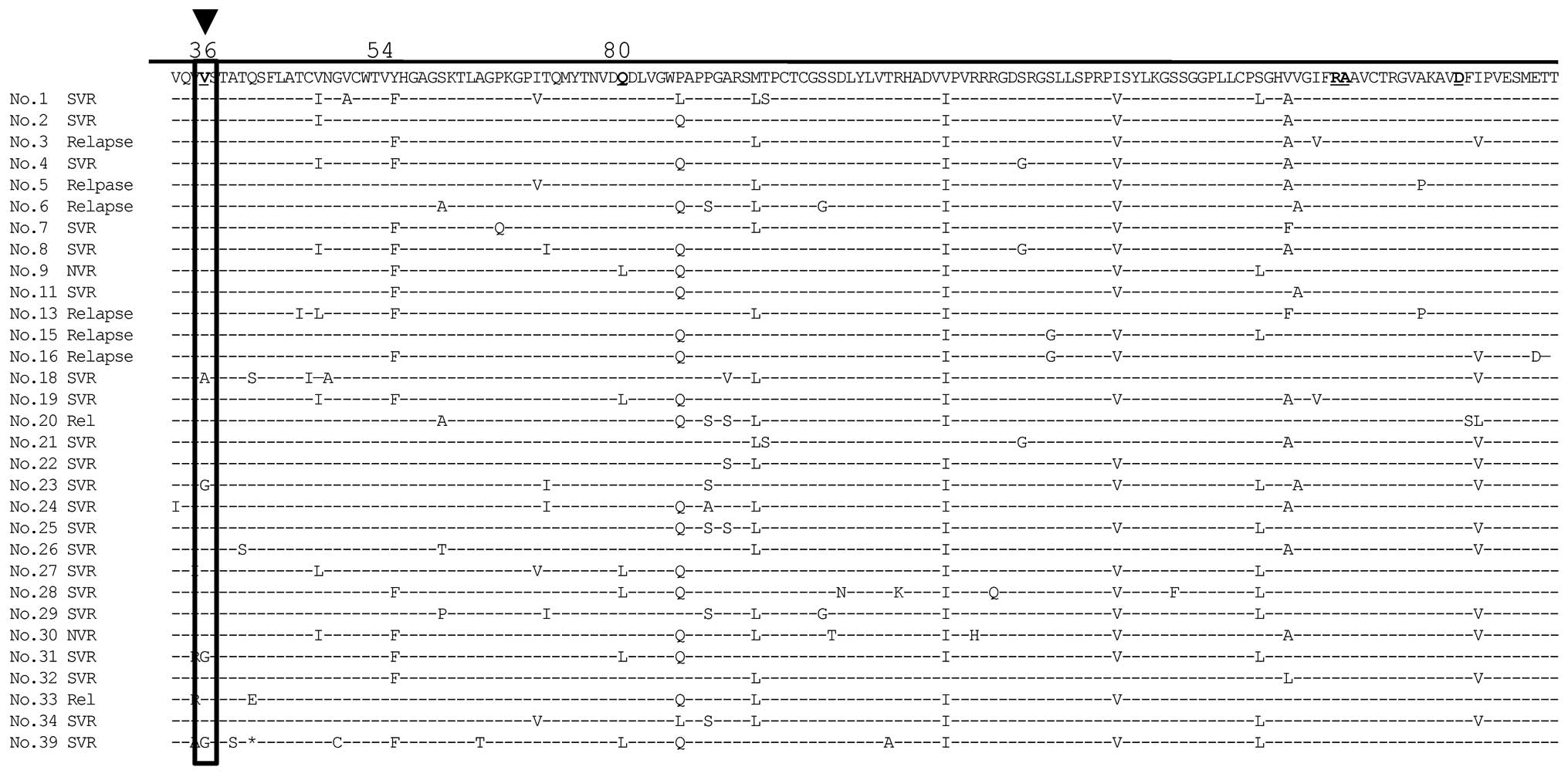
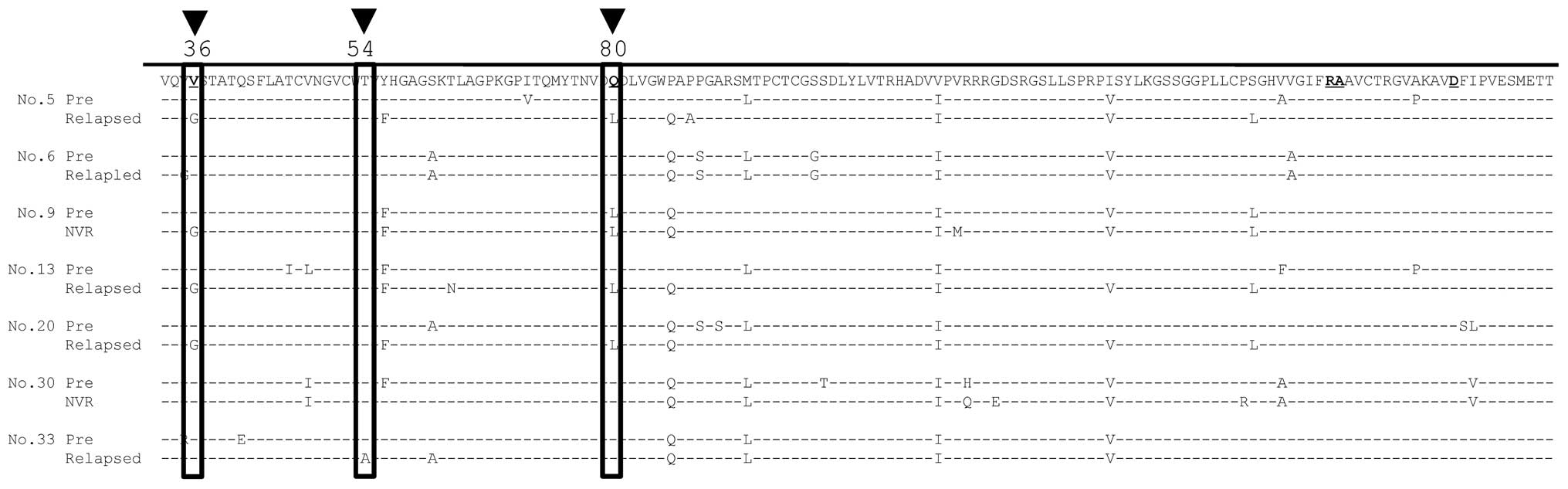
before and after therapy. Three patients who achieved SVR12 had the
Val36 mutation in the NS3 region, which is thought to confer
resistance to TVR (Fig. 2). In
addition, 5/10 patients who did not achieve SVR12 had mutations
conferring resistance to TVR, including 4 patients with Val36 and 1
with Thr54 (Fig. 3).
Discussion
In recent years, many DAAs have been developed and
thus clinical trials are examining their efficacy for the treatment
of chronic HCV infection (12,28). TPV was approved in Japan in
November 2011, and the triple therapy (TVR/PEG-IFN/RBV) has become
a standard regimen for patients with genotype 1b chronic HCV
infection with a high viral load. In the US, TVR was approved by
the Food and Drug Administration in May 2011 for use in combination
with PEG-IFN/RBV for the treatment of genotype 1b chronic HCV
infection (13). The SVR rate in
patients with genotype 1 HCV treated with triple therapy was 61–69%
in the PROVE1 and PROVE2 studies published in 2009 (10,14). Since then, several clinical
studies have confirmed the effectiveness of TVR-based therapy and
it has now become a standard component of therapeutic regimens
worldwide (15,16). In our study, the SVR12 rate was
77%, which is as high as that in earlier studies. However, 31% of
patients classified as non-responders to prior therapy achieved SVR
during treatment with a TVR-based regimen in the REALIZE trial
published in 2012 (17). We
obtained similar results in our study, as the SVR12 was
significantly lower in non-responders (44%) than in relapsed
patients (82%) and treatment-naïve patients (86%) (P<0.05).
The NS3 protein is approximately 67 kDa and has
serine proteinase activity. The NS3 protein forms a complex with
NS4 and serves to process NS4 and NS5 proteins. NS3/4A protease
inhibitors exhibit strong antiviral effects as the NS3/4A protease
activity is necessary for the lifecycle of HCV. Although TVR is
very effective, it is frequently associated with side-effects,
including skin rash and renal dysfunction, which lead to treatment
discontinuation. Therefore, it is essential to increase compliance
to improve the therapeutic efficacy of TPV. In this study, 7/48
patients discontinued treatment due to side-effects and 4 of these
patients experienced disease relapse. Accordingly, it will be
important to investigate how to maintain compliance, particulalry
among older patients.
Although TVR-based therapy is highly effective,
approximatley 30% of patients do not achieve SVR, despite triple
therapy. In trials of boceprevir-based therapy, it was found that
RVR and the IL28B SNP were predictive of SVR (18). Similarly, Chayama et al
reported that RVR, the IL28B SNP, and the response to prior therapy
were predictors of SVR during triple therapy (19). In terms of viral factors, it has
been reported that substitutions of amino acid 70 in the core
region of HCV genotype 1b were significant predictors of SVR
(20). In our study, IL28B and
the response to prior therapy were significant predictors of
SVR.
Mutations in several amino acids in the NS5A protein
have been reported and appear to play an important role in the
response to IFN. HCV NS5A is a zinc-containing phosphoprotein that
regulates HCV RNA replication and particle production. A previous
study using bioinformatics-assisted modeling suggested that there
were 3 principal domains (21),
with domain I (amino acids, 1973-2185) located in the N-terminal
region, and domain II (amino acids, 2222-2314) and domain III
(amino acids, 2328-2419) in the C-terminal region. Another study
revealed that domain III was important for HCV particle formation
(22). We have also previously
reported that IRRDR in domain III is strongly associated with SVR
(23). Although the number of
ISDRs and IRRDRs did not affect the therapeutic efficacy of triple
therapy in this study, 2 novel mutations were potentially
associated with SVR. V2334 is located in the putative nuclear
localization signal sequence (PPRKKRTVV; amino acids, 2326-2334)
within the C-terminal region of NS5A (24). This mutation may affect the
sensitivity to antiviral therapy by changing the transition of HCV
during intracellular localization. Another study suggested that a
specific C-terminal region (amino acids, 2350-2419) is involved in
basal phosphorylation (25).
G2356 is located in this region and may affect cellular signaling
mechanisms by altering NS5A phosphorylation.
TPV has a covalent linear structure and is a
first-generation NS3/4A inhibitor. Several mutations, including
V36A/M/L, T54A/S, R155K/M/S/T, A156S and A156T/Y, have been
reported to confer resistance to TPV (26). In this study, we also examined
mutations associated with resistance to antiviral drugs. Using
direct sequencing analysis, we found that 3 patients had the Val36
mutation in NS3 before therapy, which may confer resistance to TVR.
However, all of these patients achieved SVR12, which suggests that
antiviral therapy should not be contraindicated in patients with
mutations conferring low levels of resistance, such as Val36 and
Thr54. Simeprevir, which should soon be available for the treatment
of genotype 1 chronic HCV infection (27), is a non-covalent macrocyclic
inhibitor and is classified as a second-generation inhibitor with a
different resistant profile to first-generation inhibitors. While
the Q80 and D168 mutations are specific to non-covalent
peptidomimetic inhibitors, Arg155 and A156 confer cross-resistance
to all proteinase inhibitors (28). Although the Val36 and Thr54
mutations were detected in relapsed patients and in non-responders
of the present study, we found none of the cross-resistant
mutations. These patients may benefit from second-generation DAAs,
but it is important to determine the presence of mutations that may
confer resistance to these drugs.
Although TVR-based IFN therapy is effective, this
treatment regimen is often limited by the side-effects of IFN. It
is necessary that future therapies should be associated with
greater SVR, greater compliance, shorter treatment duration, less
viral resistance and better safety profiles than existing drugs.
Previous studies demonstrated that dual oral therapy with
daclatasvir, a NS5A inhibitor, and asnaprevir, an NS3 protease
inhibitor, was well tolerated and the SVR was high (29,30). Based on these results, we predict
that a combination of two or more DAAs could achieve complete viral
clearance in all patients with chronic HCV infection.
In conclusion, the IL28B SNP is strongly associated
with SVR in patients receiving TVR/PEG-IFN/RBV triple therapy.
Mutations in V2334 and G2356 are potential viral factors associated
with the therapeutic efficacy of this regimen. Mutations in NS3
were found in approximately half of patients who did not achieve
SVR and may confer resistance to second-generation proteinase
inhibitors.
Acknowledgements
This study was supported by a Grant-in-Aid from the
Japan Initiative for Global Research Network on Infectious Disease
(J-GRID) supported by The Ministry of Education, Culture, Sports,
Science and Technology, Japan and a SATREPS Grant from Japan
Science and Technology Agency and Japan International Cooperation
Agency.
References
|
1
|
Seeff LB: Natural history of chronic
hepatitis C. Hepatology. 36:S35–S46. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lavanchy D: The global burden of hepatitis
C. Liver Int. 29:74–81. 2009. View Article : Google Scholar
|
|
3
|
Hagiwara H, Hayashi N, Mita E, et al:
Detection of hepatitis C virus RNA in serum of patients with
chronic hepatitis C treated with interferon-alpha. Hepatology.
15:37–41. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
National Institutes of Health. National
Institutes of Health Consensus Development Conference Statement:
Management of hepatitis C. Hepatology. 36(Suppl 1): S3–S20. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fried MW, Shiffman ML, Reddy KR, et al:
Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus
infection. N Engl J Med. 347:975–982. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tanaka Y, Nishida N, Sugiyama M, et al:
Genome-wide association of IL28B with response to pegylated
interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat
Genet. 41:1105–1109. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Akuta N, Suzuki F, Kawamura Y, et al:
Predictive factors of early and sustained responses to
peginterferon plus ribavirin combination therapy in Japanese
patients infected with hepatitis C virus genotype 1b: amino acid
substitutions in the core region and lowdensity lipoprotein
cholesterol levels. J Hepatol. 46:403–410. 2007.
|
|
8
|
Enomoto N, Sakuma I, Asahina Y, et al:
Mutations in the nonstructural protein 5A gene and response to
interferon in patients with chronic hepatitis C virus 1b infection.
N Engl J Med. 334:77–81. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
El-Shamy A, Nagano-Fujii M, Sasase N,
Imoto S, Kim SR and Hotta H: Sequence variation in hepatitis C
virus nonstructural protein 5A predicts clinical outcome of
pegylated interferon/ribavirin combination therapy. Hepatology.
48:38–47. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
McHutchison JG, Everson GT, Gordon SC,
Jacobson IM, Sulkowski M, Kauffman R, McNair L, Alam J and Muir AJ;
PROVE1 Study Team. Telaprevir with peginterferon and ribavirin for
chronic HCV genotype 1 infection. N Engl J Med. 360:1827–1838.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sanjo M, Saito T, Ishii R, et al:
Secondary structure of the amino-terminal region of HCV NS3 and
virological response to pegylated interferon plus ribavirin therapy
for chronic hepatitis C. J Med Virol. 82:1364–1370. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Welsch C, Jesudian A, Zeuzem S and
Jacobson I: New direct-acting antiviral agents for the treatment of
hepatitis C virus infection and prospectives. Gut. 61(Suppl 1):
i36–i46. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jesudian AB, Gambarin-Gelwan M and
Jacobson IM: Advances in the treatment of hepatitis C virus
infection. Gastroenterol Hepatol. 8:91–101. 2012.
|
|
14
|
Hézode C, Forestier N, Dusheiko G, et al;
PROVE2 Study Team. Telaprevir and peginterferon with or without
ribavirin for chronic HCV infection. N Engl J Med. 360:1839–1850.
2009.
|
|
15
|
Yee HS, Chang MF, Pocha C, et al: Update
on the management and treatment of hepatitis C virus infection:
recommendations from the department of veterans affairs hepatitis C
resource center program and the national hepatitis C program
office. Am J Gastroenterol. 107:669–689. 2012.
|
|
16
|
Chayama K, Hayes CN, Ohishi W and Kawakami
Y: Treatment of chronic hepatitis C virus infection in Japan:
update on therapy and guidelines. J Gastroenterol. 48:1–12. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Forestier N and Zeuzem S: Triple therapy
with telaprevir: results in hepatitis C virus-genotype 1 infected
relapsers and non-responders. Liver Int. 32:44–50. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Poordad F, Bronowicki JP, Gordon SC, et
al; SPRINT-2 and RESPOND-2 Investigators. Factors that predict
response of patients with hepatitis C virus infection to
boceprevir. Gastroenterology. 143:608–618. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chayama K, Hayes CN, Abe H, et al: IL28B
but not ITPA polymorphism is predictive of response to pegylated
interferon, ribavirin, and telaprevir triple therapy in patients
with genotype 1 hepatitis C. J Infect Dis. 204:84–93. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Akuta N, Suzuki F, Seko Y, et al:
Determinants of response to triple therapy of telaprevir,
peginterferon, and ribavirin in previous non-responders infected
with HCV genotype 1. J Med Virol. 84:1097–1105. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tellinghuisen TL, Marcotrigiano J,
Gorbalenya AE and Rice CM: The NS5A protein of hepatitis C virus is
a zinc metalloprotein. J Biol Chem. 279:48576–48587. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Moradpour D, Penin F and Rice CM:
Replication of hepatitis C virus. Nat Rev Microbiol. 5:453–463.
2007. View Article : Google Scholar
|
|
23
|
Yano Y, Seo Y, Miki A, et al: Mutations in
non-structural 5A and rapid viral response to pegylated
interferon-α-2b plus ribavirin therapy are associated with
therapeutic efficacy in patients with genotype 1b chronic hepatitis
C. Int J Mol Med. 30:1048–1052. 2012.PubMed/NCBI
|
|
24
|
Ide Y, Zhang L, Chen M, et al:
Characterization of the nuclear localization signal and subcellular
distribution of hepatitis C virus nonstructural protein NS5A. Gene.
182:203–211. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tanji Y, Kaneko T, Satoh S and Shimotohno
K: Phosphorylation of hepatitis C virus-encoded nonstructural
protein NS5A. J Virol. 69:3980–3986. 1995.
|
|
26
|
Vermehren J and Sarrazin C: The role of
resistance in HCV treatment. Best Pract Res Clin Gastroenterol.
26:487–503. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hayashi N, Seto C, Kato M, Komada Y and
Goto S: Once-daily simeprevir (TMC435) with peginterferon/ribavirin
for treatment naïve hepatitis C genotype 1-infected patients in
Japan: the DRAGON study. J Gastroetnterol. 49:138–147.
2014.PubMed/NCBI
|
|
28
|
Sarrazin C, Hezode C, Zeuzem S and
Pawlotski JM: Antiviral strategies in hepatitis C virus infection.
J Hepatol. 56(Suppl 1): S88–S100. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chayama K, Takahashi S, Toyoda J, et al:
Dual therapy with the nonstructural protein 5A inhibitor,
daclatasvir, and the nonstructural protein 3 protease inhibitor,
asunaprevir, in hepatitis C virus genotype 1b-infected null
responders. Hepatology. 55:742–748. 2012. View Article : Google Scholar
|
|
30
|
Suzuki Y, Ikeda K, Suzuki F, et al: Dual
oral therapy with daclatasvir and asunaprevir for patients with HCV
genotype 1b infection and limited treatment options. J Hepatol.
58:655–662. 2013. View Article : Google Scholar : PubMed/NCBI
|