Introduction
Myocardial infarction (MI) is one of the most
important health challenges worldwide (1,2).
Although the mortality rate associated with MI has decreased due to
early thrombolysis, percutaneous coronary intervention or coronary
artery bypass grafting, patients who survive inevitably suffer from
consequent cardiac remodeling and heart failure (HF) (3–8).
Thus, it is urgent to explore effective therapeutic approaches in
order to prevent cardiac remodeling induced by MI.
As a highly valued traditional Chinese medicine,
musk is adopted extensively by clinicians for the treatment of
cardio-cerebrovascular diseases, such as angina, vascular headaches
and stroke (9–17). Musk is believed to have
antioxidant, anti-inflammatory, vasodilator and angiogenic effects
(18,19). As the most important monomer of
musk, muscone has been found in in vitro studies to have
multiple cardioprotective effects on injury caused by myocardial
ischemia/reperfusion (20).
However, it remains unclear as to whether muscone is also effective
in persistent myocardial ischemia in vivo.
It has been confirmed that myocardial fibrosis,
inflammation, apoptosis and endothelial dysfunction are typical
characteristics of cardiac remodeling following MI (4). Pro-inflammatory mediators, such as
transforming growth factor (TGF)-β1, tumor necrosis factor (TNF)-α
and interleukin (IL)-1β amplify the inflammatory response and play
pathogenic roles in myocardial fibrosis and remodeling (21,22). The decreased expression of Bax
protein and the increased expression of Bcl-2 inhibit apoptosis,
both contributing to reserve cardiac function. The activation of
the phosphatidylinositol 3-kinase (PI3K) signaling pathway,
including protein kinase B (Akt) and endothelial nitric oxide
synthase (eNOS) phosphorylation promote the synthesis of nitric
oxide (NO), which is considered to be involved in the protection of
endothelial function (16,23–25).
In light of the established pharmacological effects
of muscone, we hypothesized that the administration of muscone may
ameliorate cardiac remodeling and improve cardiac function
following MI. In the present study, we used a mouse model of
persistent myocardial ischemia by permanent ligation of the left
anterior descending (LAD) coronary artery (26) in order to investigate the
cardioprotective effects of muscone on MI in vivo, as well
as the potential mechanisms involved.
Materials and methods
Mouse model of myocardial infarction
All animal care and experimental procedures were in
accordance with the Ethics Review Committee of Nanjing Medical
University (Nanjing, China) for the Use and Application of
Laboratory Animals (Permit No. NJMU-ERLAUA-2012-11-01-01). C57BL/6J
mice (Laboratory Animal Center of Nanjing Medical University, male,
aged 6–8 weeks, weighing 18–24 g) were anesthetized with sodium
pentobarbital (50 mg/kg, intraperitoneally) followed by tracheal
intubation with artificial ventilation. Following anesthesia, the
mice were fixed on a heating pad to maintain normothermia. A
thoracotomy was then performed to expose the heart. The respiratory
conditions were monitored and an electrocardiogram was performed.
MI was induced by the permanent ligation of the LAD coronary artery
with an 8-0 polypropylene suture passing 2–3 mm from the inferior
margin of left auricle. MI was confirmed by myocardial blanching
and ST segment elevation of the electrocardiogram. The
sham-operated group was subjected to a similar procedure without
ligating the LAD coronary artery. The thorax was closed layer by
layer, and penicillin was injected intramuscularly. Following
recovery of spontaneous breathing, the mice were extubated and
placed on an electric blanket for recovery.
Muscone treatment protocol
Muscone was purchased from the National Institute
for the Control of Pharmaceutical and Biological Products (Beijing,
China; purity, >98%; concentration, 1 g/ml). The mice that
survived were randomly treated with muscone (2 mg/kg/day,
intragastric administration, n=27), as previously described
(17,27) or the vehicle (normal saline;
intragastric administration, n=27) for 3 weeks. The sham-operated
mice also received the vehicle (normal saline; n=9). The mice were
observed every 12 h, weighed each week, and sacrificed by
suffocation with carbon dioxide on the 21st day. The survival rate
was measured by Kaplan-Meier survival curve analysis.
Measurement of time to exhaustion during
swimming
The mice were placed in a pool (temperature, 31±1°C;
depth, 20 cm; surface area, 0.25 m2) for swimming. The
time to exhaustion during swimming was defined as a consecutive
sinking of >3 times. The time to exhaustion during swimming for
each mouse was measured and recorded.
Echocardiographic measurement
Cardiac structure and function on the 1st, 10st and
21st day following treatment were evaluated by using a
high-frequency ultrasound system Vevo 2100 (VisualSonics, Inc.,
Toronto, ON, Canada) with a 30-MHz central frequency scan head.
After the mice were anesthetized, two-dimensional echocardiographic
measurements were obtained. The left ventricular end-systolic
diameter (LVESd), left ventricular end-diastolic diameter (LVEDd),
as well as the left ventricular ejection fraction (LVEF) and left
ventricular fractional shortening (LVFS) were recorded.
Measurement of myocardial fibrosis
The hearts were harvested and weighed, washed in
phosphate-buffered saline, fixed in 4% paraformaldehyde overnight
and embedded in paraffin. Each paraffin-embedded heart was cut into
sections (4 μm thick) through the infarct area and stained with
Masson’s trichrome. Each section was imaged under a microscope
(Nikon, Tokyo, Japan). Fibrosis was calculated by computerized
planimetry using ImageJ software, version 1.44 (NIH, Bethesda, MD,
USA).
Measurement of apoptotic cells by TUNEL
staining
On the 21st day after treatment, the distribution of
apoptotic cells was detected using the In Situ Cell Death Detection
kit (Biunique, Nanjing, China). All the slices were stained with
DAPI (1 μg/ml; Sigma, St. Louis, MO, USA) for the assessment of
nuclear morphology. The FITC-labeled TUNEL-positive cells were
imaged under a fluorescence microscope at a magnification of ×400
(Nikon) and 3 horizons were randomly selected in the marginal zone
of infarction from each slice. The FITC-labeled TUNEL-positive
cells were counted using Image-Pro Plus software (Media
Cybernetics, Rockville, MD, USA) and the apoptotic index was
calculated as follows: apoptotic cell number/1,000 cells ×100%.
Western blot analysis
Total protein was obtained from left ventricular
myocardial tissues by sonication, centrifugation and heat
denaturation. The protein lysates were electrophoresed and
separated on 6–12% SDS-PAGE and transferred onto nitrocellulose
membranes (Bio-Rad, Hercules, CA, USA). The membranes were blocked
with 5% skim milk at room temperature for 1 h, and then incubated
overnight at 4°C with primary antibodies, including rabbit anti-Akt
(1:1,000; Cell Signaling Technology, Inc., Danvers, MA, USA),
rabbit anti-phospho Akt (1:1,000; Cell Signaling Technology, Inc.),
rabbit anti-eNOS (1:1,000; Sigma), rabbit anti-phospho-eNOS (1:200;
Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), rabbit
anti-NF-κB (1:800; Cell Signaling Technology, Inc.), rabbit
anti-Bcl-2 (1:800; BioWorld, Inc., Visalia, CA, USA), rabbit
anti-Bax (1:800; BioWorld, Inc.), and rabbit anti-GAPDH (1:1,000;
Cell Signaling Technology, Inc.). The membranes were then incubated
with HRP-conjugated secondary antibodies (1:500; Santa Cruz
Biotechnology, Inc.) at room temperature for 1 h. The SuperSignal
ECL kit (Thermo Fisher Scientific, Rockville, MD, USA) was used to
detect the antigen-antibody complexes in a western blotting
detection system (Bio-Rad). The results were expressed as density
values normalized to GAPDH.
ELISA measurement of TGF-β1, TNF-α and
IL-1β
The levels of TGF-β1, TNF-α and IL-1β (Bio-Swamp,
Shanghai, China) in the left ventricular myocardium were measured
by ELISA. Myocardial samples (20 mg) were homogenized in 200 μl of
1× phosphate-buffered saline (pH 7.4), then stored overnight at
−20°C. The homogenates were determined by 2 freeze-thaw cycles to
break the cell membranes followed by centrifugation at 5000 × g for
10 min. The samples were then tested immediately following the
procedure recommended by the manufacturer.
Statistical analysis
The GraphPad Prism 5 Demo and SPSS 13.0 software
were used for statistical analyses and graphing. The Student’s
t-test or one-way ANOVA were used to evaluate the mean difference
(MD) ± standard error of the mean (SEM) and 95% confidence interval
(CI) of the values of the parameters between the groups.
Kaplan-Meier survival analysis was performed to evaluate the
overall survival of the mice following the induction of MI by the
log-rank test, and a two-sided value of P<0.05 was considered to
indicate a statistically significant difference.
Results
Treatment with muscone improves the
survival rate and time to exhaustion during swimming
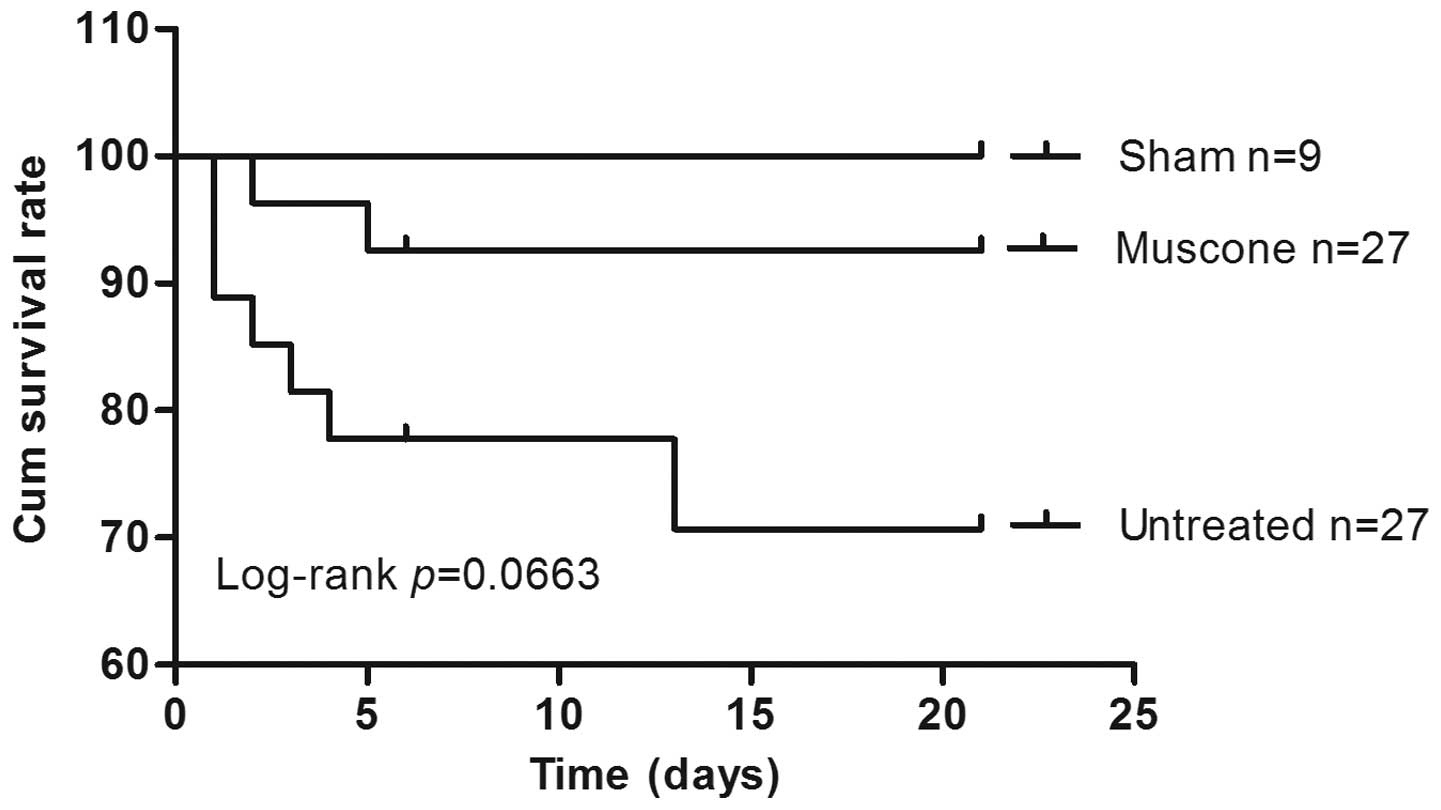
Compared to the untreated mice, the muscone-treated
mice showed an improved survival rate [93 vs. 74%; hazard ration
(HR) 0.29; 95% CI 0.07745–1.087; P=0.066] (Fig. 1). A post-mortem examination
indicated that the main cause of death was HF. The time to
exhaustion during swimming was reduced in the mice with MI compared
to the sham-operated mice (18.70±0.5867 min vs. 28.21±0.4911 min;
MD −9.515±0.9072; P<0.0001), which was improved following
treatment with muscone compared with the untreated mice with MI
(20.71±0.7342 min vs. 18.70±0.5867 min; MD 2.014±0.9756; 95% CI
0.004332–4.024; P=0.0495).
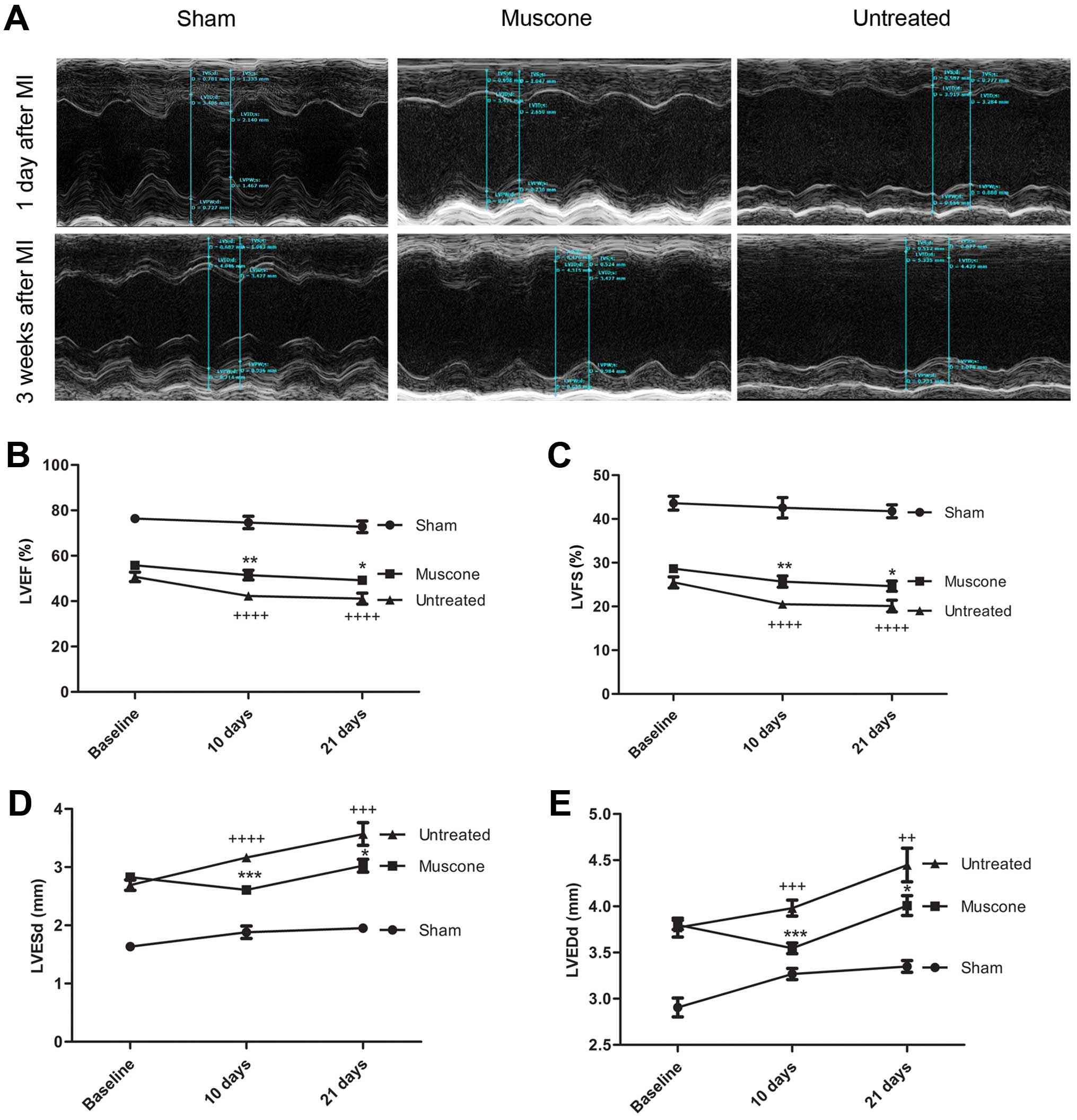
Treatment with muscone improves cardiac
structure and function
No significant difference in cardiac function was
observed between the muscone-treated and untreated group at
baseline. On the 21st day following the induction of MI, LVESd and
LVEDd were higher, while LVFS and LVEF were lower in the mice with
MI compared to the sham-operated group (P<0.05). Following
treatment for 3 weeks, LVESd (3.025±0.1089 vs. 3.570±0.1965 mm; MD
−0.5451±0.2246; 95% CI −1.011 to −0.07918; P=0.0239) and LVEDd
(4.008±0.1073 vs. 4.447±0.1822 mm; MD −0.4398±0.2114; 95% CI
−0.8782 to −0.001284; P=0.0494) were significantly lower compared
to the untreated group, while LVEF (49.23±1.906 vs. 41.12±2.441%;
MD 8.112±3.097; 95% CI 1.688–14.54; P=0.0157) and LVFS (24.65±1.159
vs. 20.10±1.347%; MD 4.551±1.777; 95% CI 0.8654–8.237; P=0.0178)
were significantly higher in the muscone-treated group compared
with the untreated group (Fig.
2A–E). In addition, the heart weight/body weight ratio was also
higher in the mice with MI compared to the sham-operated group
(P<0.01), which was reduced following treatment with muscone
compared with the untreated mice (0.6120±0.01866 vs.
0.6939±0.02428; MD −0.08195±0.03062; 95% CI −0.1455 to −0.01844;
P=0.0138).
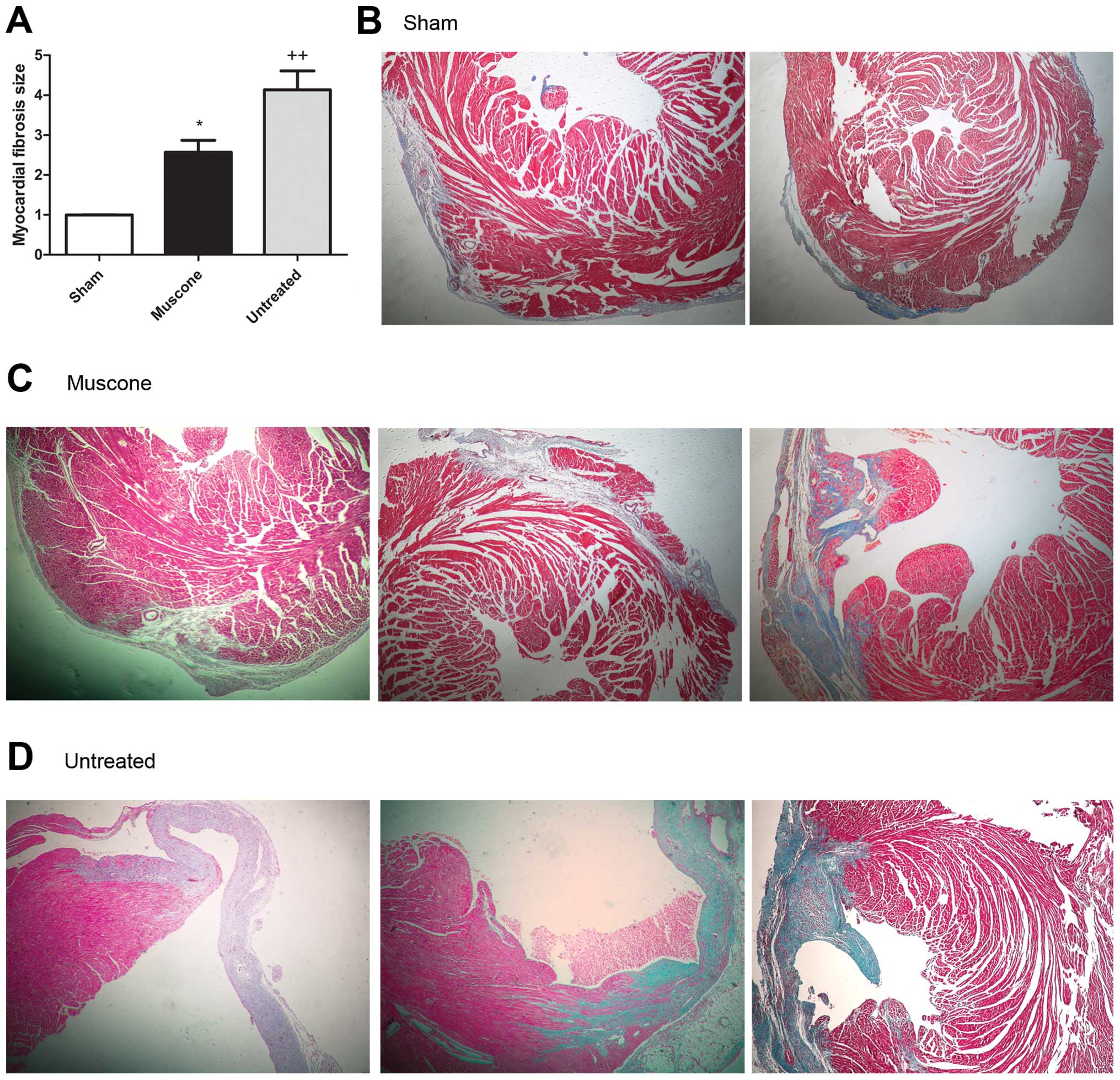
Treatment with muscone reduces myocardial
fibrosis
Masson’s trichrome staining was used to detect
myocardial fibrosis. The results revealed that the fibrotic area
was significantly increased in the mice with MI compared to the
sham-operated group (P<0.05); treatment with muscone
significantly decreased myocardial fibrosis and collagen deposition
compared to the untreated group (2.567±0.3032 vs. 4.136±0.4741; MD
−1.568±0.5628; 95% CI −2.822 to −0.3144; P=0.0192) (Fig. 3).
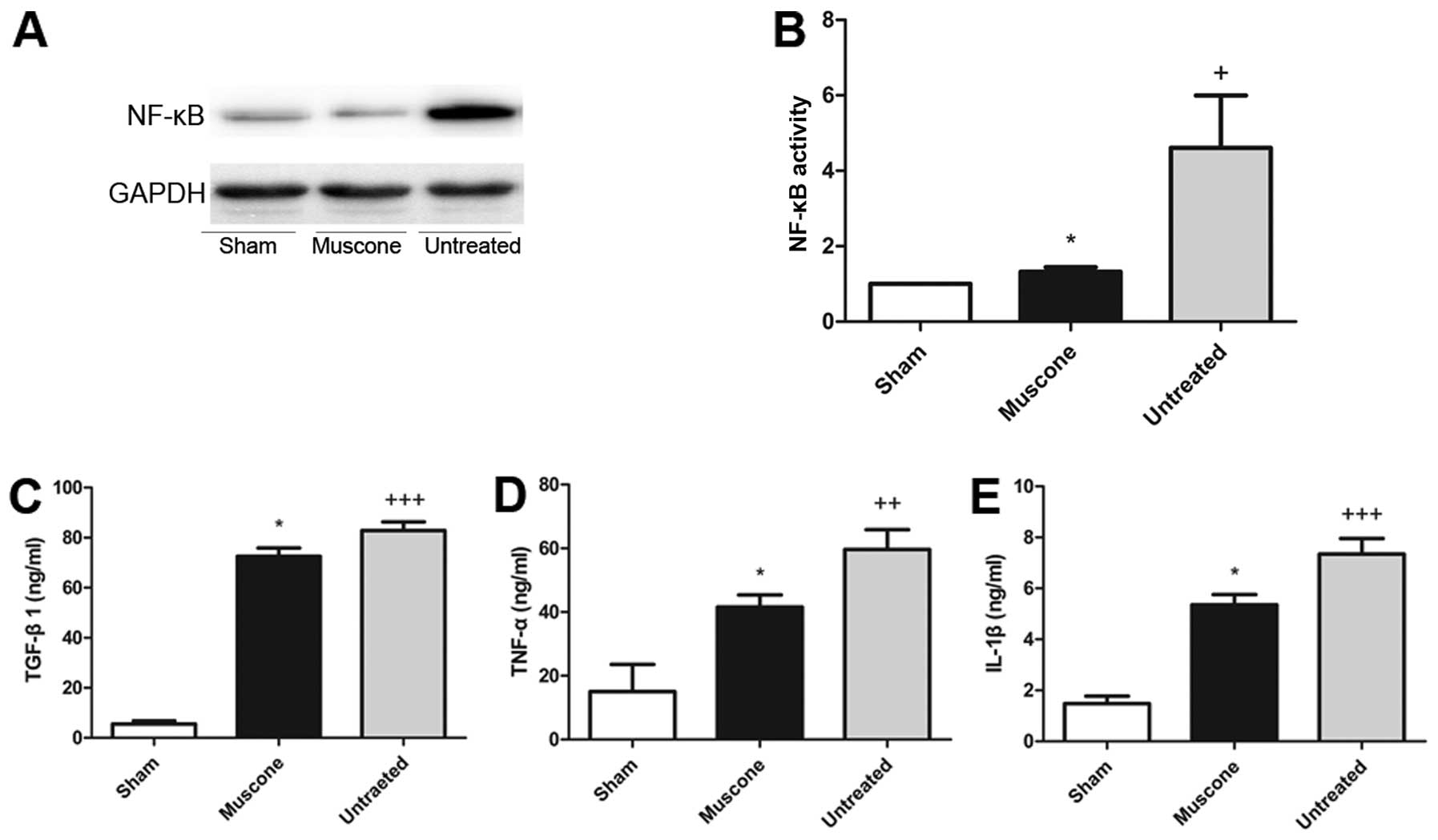
Treatment with muscone suppresses the
expression of inflammatory cytokines
Compared to the sham-operated mice, the expression
levels of TGF-β1, TNF-α, IL-1β and NF-κB were higher in the
untreated group (P<0.05), indicating that MI upregulated the
levels of pro-inflammatory cytokines. The muscone-treated group
showed significantly lower expression levels of TGF-β1 (72.56±3.302
vs. 109.0±12.74; MD −36.41±12.25; 95% CI −63.36 to −9.452;
P=0.0127), TNF-α (41.56±3.802 vs. 59.66±6.164l MD −18.09±7.011; 95%
CI −33.53 to −2.665; P=0.0255), IL-1β (5.354±0.3977 vs.
7.345±0.6096; MD −1.990±0.7075; 95% CI −3.548 to −0.4330; P=0.0169)
and NF-κB (1.321±0.1247 vs. 4.817±1.185; MD −3.496±1.192; 95% CI
−6.804 to −0.1870; P=0.0427) compared to the untreated group
(Fig. 4).
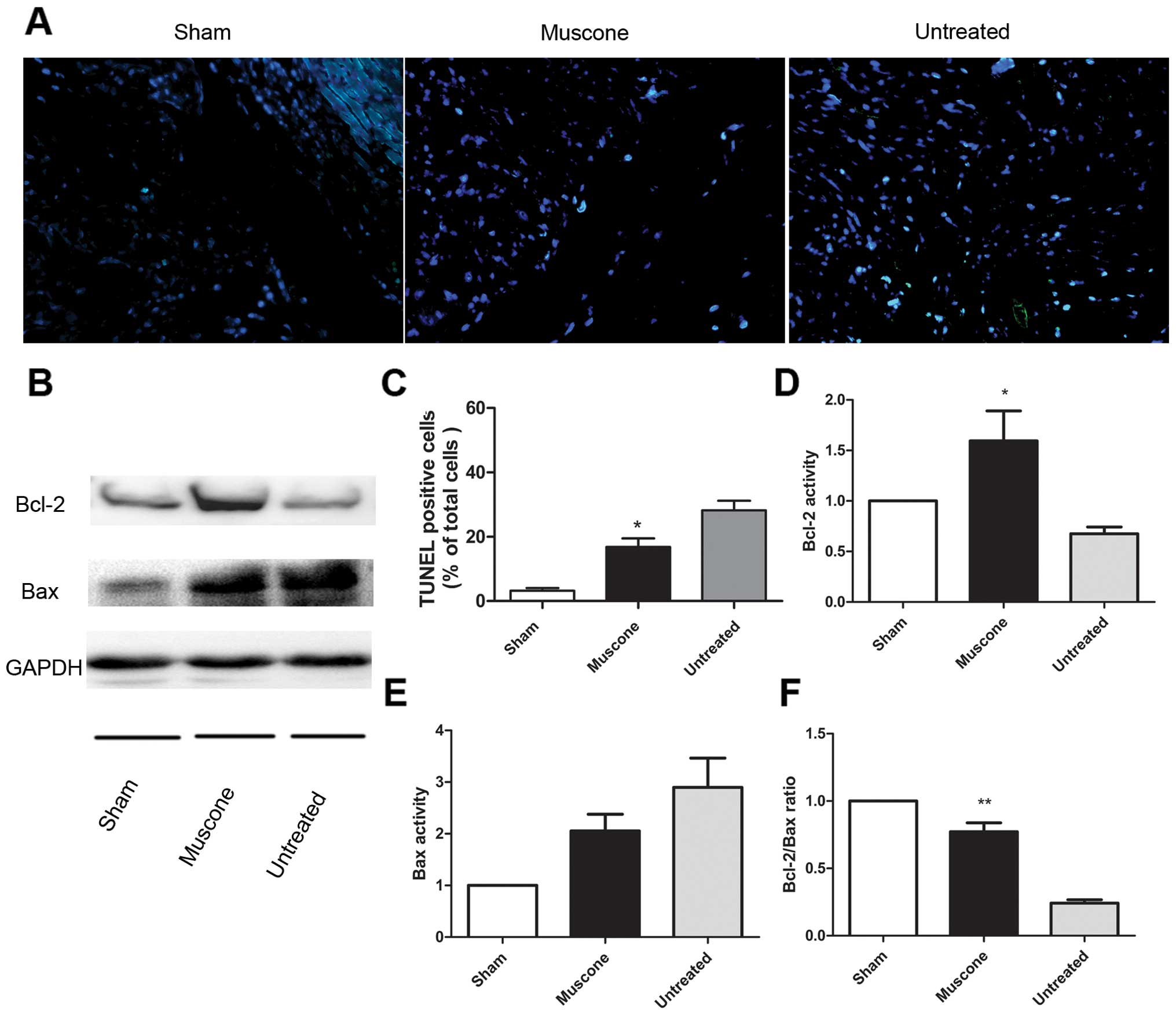
Treatment with muscone decreases
myocardial apoptosis
Compared to the sham-operated group, the untreated
group showed a significantly higher apoptotic index in the marginal
zone of the nfarcted myocardium (P<0.05). The apoptotic index
was significantly lower in the muscone treated group compared to
the untreated group (16.80±2.672 vs. 28.20±2.973; MD −11.40±3.997;
95% CI −20.62 to −2.182; P=0.0214) (Fig. 5A and B). The expression level of
Bcl-2 (1.594±0.2966 vs. 0.6756±0.06635; MD 0.9180±0.3039; 95% CI
0.07424–1.762; P=0.0392) and the Bcl-2/Bax ratio (0.7715±0.06642
vs. 0.2418±0.02536; MD 0.5297±0.07110; 95% CI 0.3323–0.7271;
P=0.0017) were significantly increased in the treated group
compared to the untreated group (Fig.
5C–F).
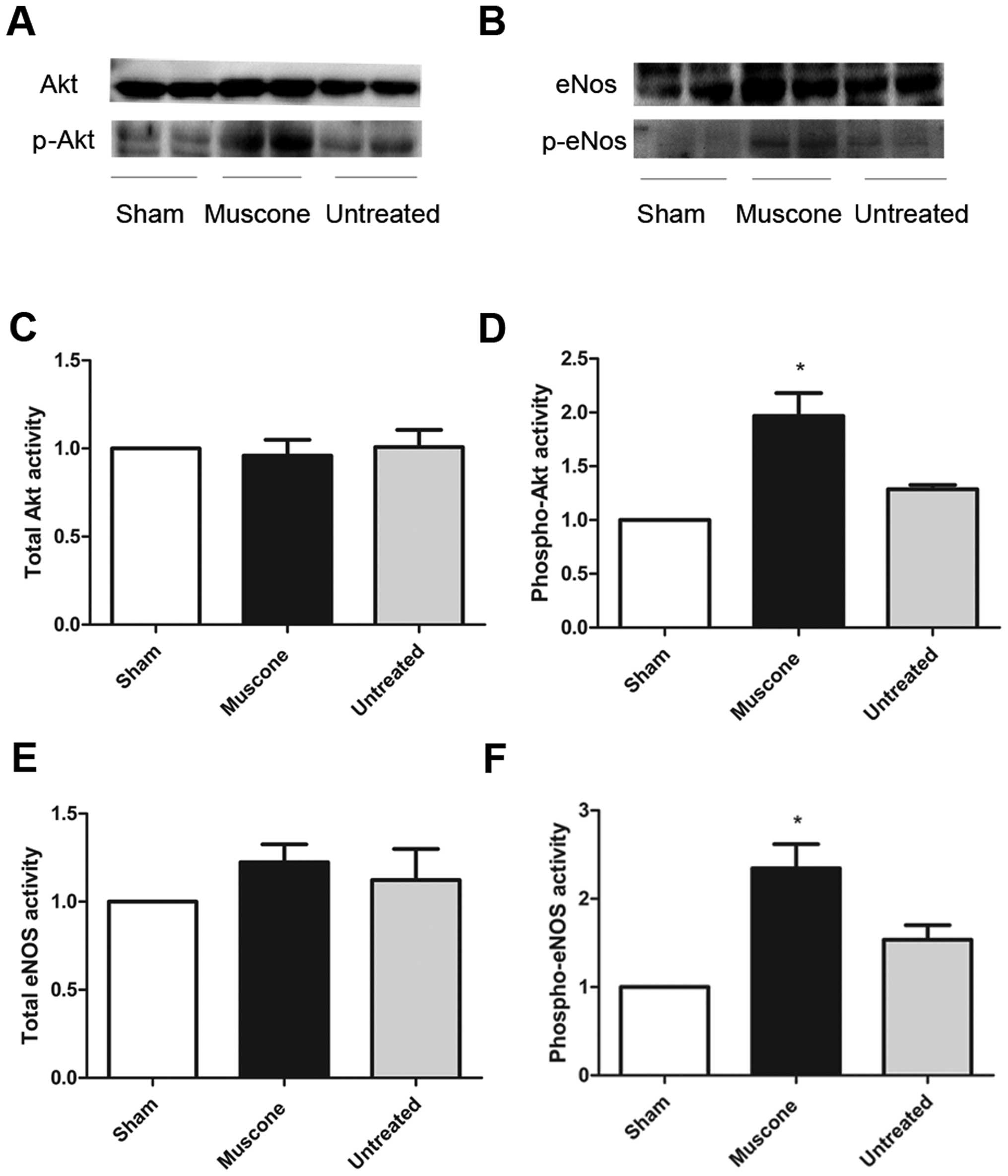
Treatment with muscone activates Akt-eNOS
signaling
The expression of total Akt and eNOS in the ischemic
myocardium was similar among the 3 groups. However, the expression
levels of phosphorylated Akt and phosphorylated eNOS were
significantly lower in the mice with MI compared with the
sham-operated group (P<0.05); treatment with muscone increased
the phosphorylation of Akt (1.968±0.2110 vs. 1.286±0.04034; MD
0.6825±0.2149; 95% CI 0.08610–1.279; P=0.0336) and phosphorylated
eNOS (2.347±0.2729 vs. 1.537±0.1645; MD 0.8095±0.3186; 95% CI
0.02995–1.589; P=0.044) compared to the untreated group (Fig. 6).
Discussion
In this study, we investigated the cardioprotective
effects of muscone in mice following the induction of MI. The key
findings were as follows: i) muscone improved the survival rate and
exercise tolerance, as well as the cardiac structure and function;
ii) muscone attenuated myocardial fibrosis and inflammation
following MIl iii) muscone exerted anti-apoptotic effects by
increasing Bcl-2 and decreasing Bax expression; iv) muscone
increased the phosphorylation of Akt-eNOS. Our results demonstrated
that muscone is effective in preventing the progression of cardiac
remodeling following MI.
Inflammatory cytokines, including TGF-β1, TNF-α and
IL-1β, play crucial roles in myocardial fibrosis and the
pathological progression of left ventricular remodeling following
MI (28–30). NF-κB is a key transcription
factor, which induces the expression of a number of target genes,
including pro-inflammatory cytokines, endothelial adhesion
molecules and oxidative-stress related enzymes (31). Activated NF-κB increases the
expression of TGF-β1, TNF-α and IL-1β, which subsequently activates
collagen deposition and myocardial fibrosis that lead to myocardial
remodeling and HF (32,33). Morishita et al (34) reported that the inhibition of
NF-κB binding decreased cardiac damage following MI. In the present
study, we also observed the increased expression of TGF-β1, TNF-α,
IL-1β and NF-κB following the induction of MI, and treatment with
muscone significantly decreased the expression of these
inflammatory markers, which indicated that the cardioprotective
effects of muscone may be attributed to its anti-inflammation
effects.
Apoptosis plays an important role in MI and HF.
Previous studies have demonstrated that ischemia-induced apoptosis
and necrosis contribute to autophagic cardiomyocyte death and
cardiomyocyte loss in myocardial ischemic injury (35,36). Apoptosis is characterized by the
increased expression of pro-apoptotic Bax family proteins and the
decreased expression of anti-apoptotic Bcl-2 family proteins
(7). Grimm et al (38) proved that Bcl-2 also inhibited the
activity of NF-κB. It has also been demonstrated that the
amplification of apoptosis is sufficient to increase fibrosis,
indicating a critical role for cardiomyocyte apoptosis in the
progression of myocardial fibrosis (39). Inflammatory cytokines, such as
TNF-α and IL-1β also induce apoptosis and contribute to the process
of myocardial remodeling (21,36). Consistently, we demonstrated that
the apoptotic level in the marginal zone of the infarcted
myocardium was decreased by treatment with muscone. Furthermore,
our results also revealed the upregulated expression of Bcl-2 and
the downregulated expression of Bax in the muscone-treated group.
Thus, we hypothesized that treatment with muscone reduced cardiac
remodeling due to its anti-apoptotic effects.
The balance of eNOS activity is the hallmark of
vascular endothelial function, such as endothelium-dependent
relaxation, the integrity of the vascular endothelium and
angiogenesis (40,41). The activation of eNOS promotes the
synthesis of NO, which then protects the ischemic heart by
regulating vascular remodeling and angiogenesis (42,43). It has been shown that the
activation of the PI3K-Akt-eNOS pathway alleviates cardiac
ischemia-reperfusion injury (44,45), while eNOS deficiency causes
myocardial apoptosis and HF (46). In our study, we observed that the
administration of muscone significantly induced the phosphorylation
of Akt and eNOS, indicating that treatment with muscone may exert
protective effects on the ischemic myocardium by activating the
PI3K-Akt-eNOS pathway.
There were some limitations of the present study.
Firstly, although treatment with muscone led to a marked
improvement in left ventricular morphology and function, our study
investigated only some of its possible mechanisms of action.
Further studies are required to reveal additional pathways.
Secondly, the observation period was relatively short. Thirdly,
whether muscone can function in conjunction with other medications
to achieve better therapeutic effects needs to be further
explored.
In conclusion, in the present study, we identified
that the administration of muscone has notable benefits, preventing
cardiac remodeling following MI. The potential mechanisms may be
associated with the anti-fibrotic, anti-inflammatory and
anti-apoptotic effects of muscone on the ischemic myocardium. Given
our increased understanding of muscone and cardiovascular diseases,
muscone is highly valued as a novel therapeutic application in
myocardial ischemic injury following MI.
Acknowledgements
We would like to thank the Jiangsu People’s Hospital
for supplying the reagents free of charge for this study and for
funding.
References
|
1
|
Lin DL, Chang HC and Huang SH:
Characterization of allegedly musk-containing medicinal products in
Taiwan. J Forensic Sci. 49:1187–1193. 2004.PubMed/NCBI
|
|
2
|
Go AS, Mozaffarian D, Roger VL, Benjamin
EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco
S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ,
Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH,
Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK,
Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner
PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D and
Turner MB: Heart disease and stroke statistics--2013 update: a
report from the American Heart Association. Circulation.
127:e6–e245. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hung J, Teng TH, Finn J, Knuiman M, Briffa
T, Stewart S, Sanfilippo FM, Ridout S and Hobbs M: Trends from 1996
to 2007 in incidence and mortality outcomes of heart failure after
acute myocardial infarction: a population-based study of 20,812
patients with first acute myocardial infarction in Western
Australia. J Am Heart Assoc. 2:e0001722013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sun Y, Zhang JQ, Zhang J and Lamparter S:
Cardiac remodeling by fibrous tissue after infarction in rats. J
Lab Clin Med. 135:316–323. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
See F, Kompa A, Martin J, Lewis DA and
Krum H: Fibrosis as a therapeutic target post-myocardial
infarction. Curr Pharm Des. 11:477–487. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tucci PJ: Pathophysiological
characteristics of the post-myocardial infarction heart failure
model in rats. Arq Bras Cardiol. 96:420–424. 2011.PubMed/NCBI
|
|
7
|
Rohde LE, Ducharme A, Arroyo LH, Aikawa M,
Sukhova GH, Lopez-Anaya A, McClure KF, Mitchell PG, Libby P and Lee
RT: Matrix metalloproteinase inhibition attenuates early left
ventricular enlargement after experimental myocardial infarction in
mice. Circulation. 99:3063–3070. 1999. View Article : Google Scholar
|
|
8
|
Lindsey ML, Mann DL, Entman ML and Spinale
FG: Extracellular matrix remodeling following myocardial injury.
Ann Med. 35:316–326. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yan SK, Zhang WD, Liu RH and Zhan YC:
Chemical fingerprinting of Shexiang Baoxin Pill and simultaneous
determination of its major constituents by HPLC with evaporative
light scattering detection and electrospray mass spectrometric
detection. Chem Pharm Bull (Tokyo). 54:1058–1062. 2006. View Article : Google Scholar
|
|
10
|
Shen W, Fan WH and Shi HM: Effects of
shexiang baoxin pill on angiogenesis in atherosclerosis plaque and
ischemic myocardium. Zhongguo Zhong Xi Yi Jie He Za Zhi.
30:1284–1287. 2010.(In Chinese).
|
|
11
|
Fan X, Shi M, Wang Y, Liang Q and Luo G:
Transcriptional profiling analysis of HMP-treated rats with
experimentally induced myocardial infarction. J Ethnopharmacol.
137:199–204. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu DJ, Hong HS and Jiang Q: Effect of
shexiang baoxin pill in alleviating myocardial fibrosis in
spontaneous hypertensive rats. Zhongguo Zhong Xi Yi Jie He Za Zhi.
25:350–353. 2005.(In Chinese).
|
|
13
|
Cai YM, He Y, Qiu T, Zou J, Sun DP, Peng
QH, Jia RX and Zhao HR: Research on frequency of application with
modern Chinese herbal medicine. Chin J Integr Med. 17:64–70. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang LJ, Luo XP and Wang Y: Evaluation on
tolerability and safety of long-term administration with shexiang
baoxin pill in patients with coronary heart disease of stable
angina pectoris. Zhongguo Zhong Xi Yi Jie He Za Zhi. 28:399–401.
2008.(In Chinese).
|
|
15
|
Wang S, Zheng Z, Weng Y, Yu Y, Zhang D,
Fan W, Dai R and Hu Z: Angiogenesis and anti-angiogenesis activity
of Chinese medicinal herbal extracts. Life Sci. 74:2467–2478. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xiang L, Jiang P, Zhan C, Chen Z, Liu X,
Huang X, Wang S, Hu Y, Zhang W and Liu R: The serum metabolomic
study of intervention effects of the traditional Chinese medicine
Shexiang Baoxin Pill and a multi-component medicine polypill in the
treatment of myocardial infarction in rats. Mol Biosyst.
8:2434–2442. 2012. View Article : Google Scholar
|
|
17
|
Sun R, Zhang ZP, Huang W, Lv LP and Ren
HY: Protective effects of muskone on rats with complete cerebral
ischemia. Trad Chin Drug Res Clin Pharmacol. 20:197–200. 2009.(In
Chinese).
|
|
18
|
Wei G, Chen DF, Lai XP, Liu DH, Deng RD,
Zhou JH, Zhang SX, Li YW, Li H and Zhang QD: Muscone exerts
neuroprotection in an experimental model of stroke via inhibition
of the fas pathway. Nat Prod Commun. 7:1069–1074. 2012.PubMed/NCBI
|
|
19
|
Tanaka E, Funae Y, Imaoka S and Misawa S:
Characterization of liver microsomal cytochrome P450 from rats
treated with muscone (3-methylcyclopentadecanone). Biochem
Pharmacol. 41:472–473. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu Q, Li H, Wu Y, Shen W, Zeng L, Cheng H
and He L: Protective effects of muscone on ischemia-reperfusion
injury in cardiac myocytes. J Ethnopharmacol. 138:34–39. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hori M and Nishida K: Oxidative stress and
left ventricular remodelling after myocardial infarction.
Cardiovasc Res. 81:457–464. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vilahur G, Juan-Babot O, Pena E, Onate B,
Casani L and Badimon L: Molecular and cellular mechanisms involved
in cardiac remodeling after acute myocardial infarction. J Mol Cell
Cardiol. 50:522–533. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang C, Talukder MA, Varadharaj S,
Velayutham M and Zweier JL: Early ischaemic preconditioning
requires Akt- and PKA-mediated activation of eNOS via serine1176
phosphorylation. Cardiovasc Res. 97:33–43. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen LL, Zhu TB, Yin H, Huang J, Wang LS,
Cao KJ and Yang ZJ: Inhibition of MAPK signaling by eNOS gene
transfer improves ventricular remodeling after myocardial
infarction through reduction of inflammation. Mol Biol Rep.
37:3067–3072. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu JX, Liang C and Ren YS: Effects of
shexiang baoxin pill on function and nitric oxide secretion of
endothelial progenitor cells. Zhongguo Zhong Xi Yi Jie He Za Zhi.
29:511–513. 2009.(In Chinese).
|
|
26
|
Salto-Tellez M, Yung Lim S, El-Oakley RM,
Tang TP, Za AL and Lim SK: Myocardial infarction in the C57BL/6J
mouse: a quantifiable and highly reproducible experimental model.
Cardiovasc Pathol. 13:91–97. 2004. View Article : Google Scholar
|
|
27
|
Liang H and Luo BY: The study of muscone
on attenuating excitotoxicity during acute cerebral ischemia. Zhong
Yao Yao Li Yu Lin Chuang. 21:12–13. 2005.(In Chinese).
|
|
28
|
Vivar R, Humeres C, Ayala P, Olmedo I,
Catalan M, Garcia L, Lavandero S and Diaz-Araya G: TGF-beta1
prevents simulated ischemia/reperfusion-induced cardiac fibroblast
apoptosis by activation of both canonical and non-canonical
signaling pathways. Biochim Biophys Acta. 1832.754–762.
2013.PubMed/NCBI
|
|
29
|
Gu Q, Yang XP, Bonde P, DiPaula A,
Fox-Talbot K and Becker LC: Inhibition of TNF-alpha reduces
myocardial injury and proinflammatory pathways following
ischemia-reperfusion in the dog. J Cardiovasc Pharmacol.
48:320–328. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Siwik DA, Chang DL and Colucci WS:
Interleukin-1beta and tumor necrosis factor-alpha decrease collagen
synthesis and increase matrix metalloproteinase activity in cardiac
fibroblasts in vitro. Circ Res. 86:1259–1265. 2000. View Article : Google Scholar
|
|
31
|
Stephenson D, Yin T, Smalstig EB, Hsu MA,
Panetta J, Little S and Clemens J: Transcription factor nuclear
factor-kappa B is activated in neurons after focal cerebral
ischemia. J Cereb Blood Flow Metab. 20:592–603. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ogawa K, Chen F, Kuang C and Chen Y:
Suppression of matrix metalloproteinase-9 transcription by
transforming growth factor-beta is mediated by a nuclear
factor-kappaB site. Biochem J. 381:413–422. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hirotani S, Otsu K, Nishida K, Higuchi Y,
Morita T, Nakayama H, Yamaguchi O, Mano T, Matsumura Y, Ueno H,
Tada M and Hori M: Involvement of nuclear factor-kappaB and
apoptosis signal-regulating kinase 1 in G-protein-coupled receptor
agonist-induced cardiomyocyte hypertrophy. Circulation.
105:509–515. 2002. View Article : Google Scholar
|
|
34
|
Morishita R, Sugimoto T, Aoki M, Kida I,
Tomita N, Moriguchi A, Maeda K, Sawa Y, Kaneda Y, Higaki J and
Ogihara T: In vivo transfection of cis element ‘decoy’ against
nuclear factor-kappaB binding site prevents myocardial infarction.
Nat Med. 3:894–899. 1997.
|
|
35
|
Elsasser A, Vogt AM, Nef H, Kostin S,
Mollmann H, Skwara W, Bode C, Hamm C and Schaper J: Human
hibernating myocardium is jeopardized by apoptotic and autophagic
cell death. J Am Coll Cardiol. 43:2191–2199. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Crow MT, Mani K, Nam YJ and Kitsis RN: The
mitochondrial death pathway and cardiac myocyte apoptosis. Circ
Res. 95:957–970. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kluck RM, Bossy-Wetzel E, Green DR and
Newmeyer DD: The release of cytochrome c from mitochondria: a
primary site for Bcl-2 regulation of apoptosis. Science.
275:1132–1136. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Grimm S, Bauer MK, Baeuerle PA and
Schulze-Osthoff K: Bcl-2 down-regulates the activity of
transcription factor NF-kappaB induced upon apoptosis. J Cell Biol.
134:13–23. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Syed FM, Hahn HS, Odley A, Guo Y, Vallejo
JG, Lynch RA, Mann DL, Bolli R and Dorn GW: Proapoptotic effects of
caspase-1/interleukin-converting enzyme dominate in myocardial
ischemia. Circ Res. 96:1103–1109. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sharma S, Singh M and Sharma PL: Mechanism
of hyperhomocysteinemia-induced vascular endothelium dysfunction -
possible dysregulation of phosphatidylinositol-3-kinase and its
downstream phosphoinositide dependent kinase and protein kinase B.
Eur J Pharmacol. 721:365–372. 2013. View Article : Google Scholar
|
|
41
|
Yasuda S, Kobayashi H, Iwasa M, Kawamura
I, Sumi S, Narentuoya B, Yamaki T, Ushikoshi H, Nishigaki K,
Nagashima K, Takemura G, Fujiwara T, Fujiwara H and Minatoguchi S:
Antidiabetic drug pioglitazone protects the heart via activation of
PPAR-gamma receptors, PI3-kinase, Akt, and eNOS pathway in a rabbit
model of myocardial infarction. Am J Physiol Heart Circ Physiol.
296:H1558–H1565. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fulton D, Gratton JP, McCabe TJ, Fontana
J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A and Sessa WC:
Regulation of endothelium-derived nitric oxide production by the
protein kinase Akt. Nature. 399:597–601. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bell RM and Yellon DM: Bradykinin limits
infarction when administered as an adjunct to reperfusion in mouse
heart: the role of PI3K, Akt and eNOS. J Mol Cell Cardiol.
35:185–193. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tsutsumi YM, Tsutsumi R, Mawatari K,
Nakaya Y, Kinoshita M, Tanaka K and Oshita S: Compound K, a
metabolite of ginsenosides, induces cardiac protection mediated
nitric oxide via Akt/PI3K pathway. Life Sci. 88:725–729. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Balakumar P, Kathuria S, Taneja G, Kalra S
and Mahadevan N: Is targeting eNOS a key mechanistic insight of
cardiovascular defensive potentials of statins? J Mol Cell Cardiol.
52:83–92. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhao X, Lu X and Feng Q: Deficiency in
endothelial nitric oxide synthase impairs myocardial angiogenesis.
American journal of physiology Am J Physiol Heart Circ Physiol.
283:H2371–H2378. 2002.PubMed/NCBI
|