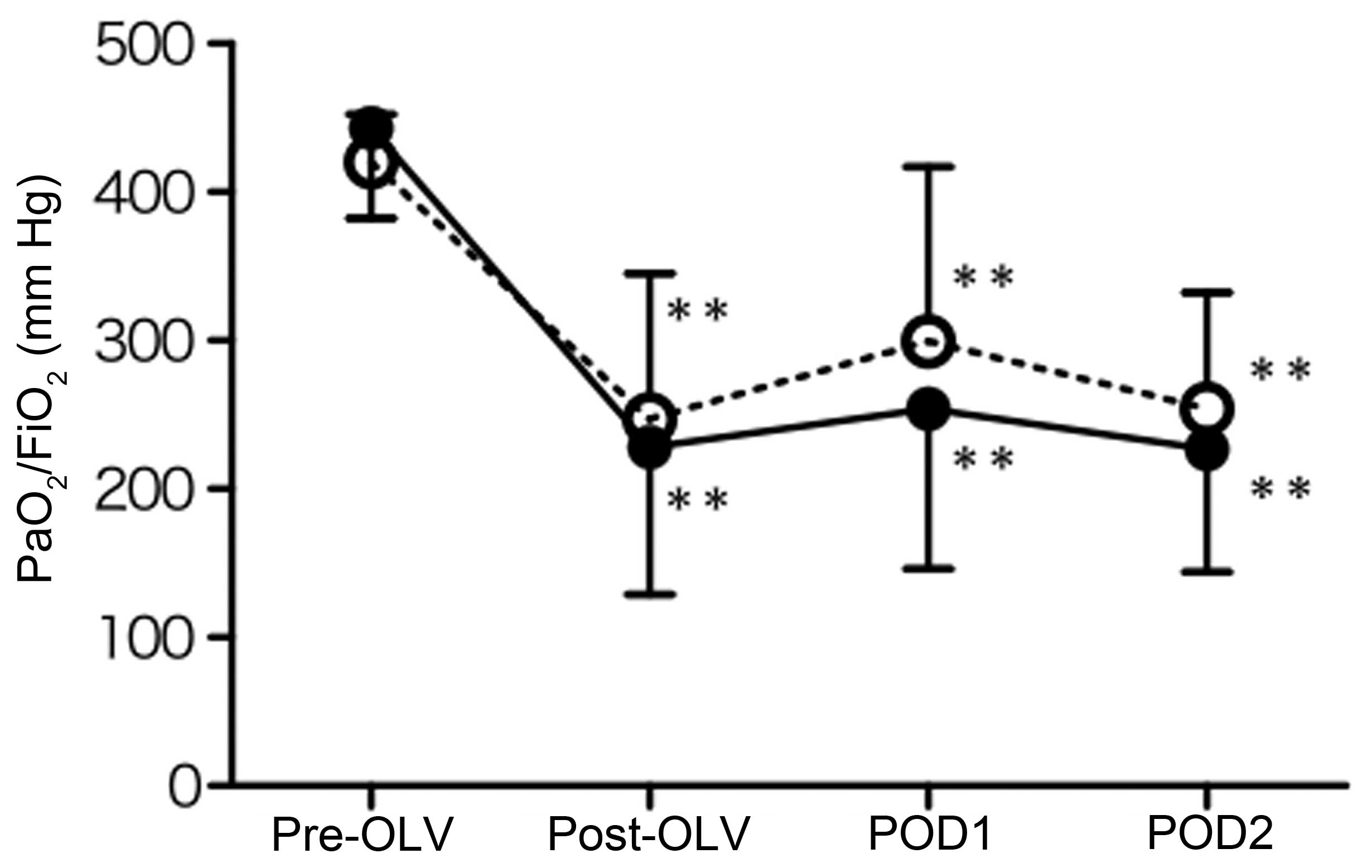
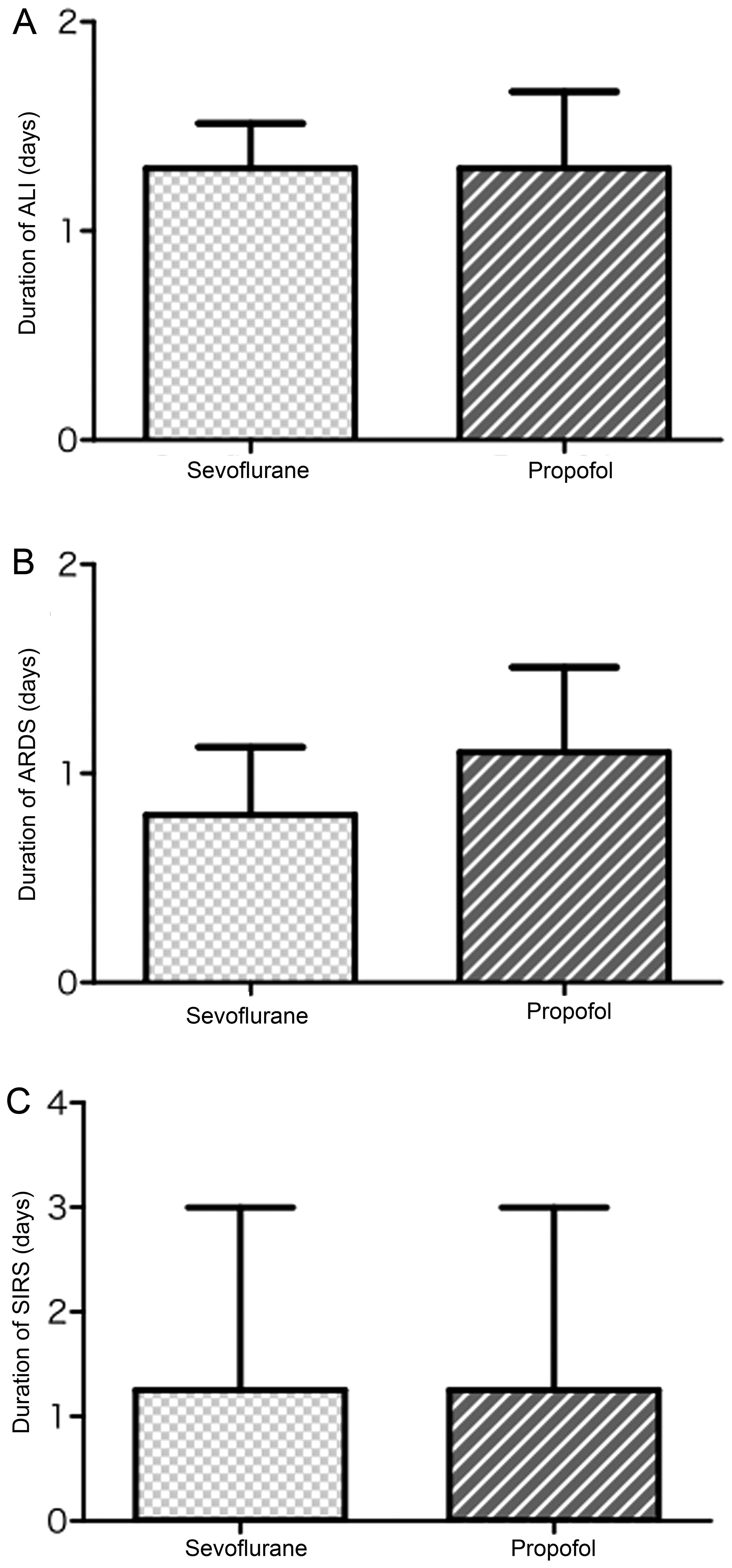
|
1
|
Yamaguchi K, Sugasawa Y, Takeuchi K, et
al: Effects of sivelestat on bronchial inflammatory responses after
esophagectomy. Int J Mol Med. 28:187–192. 2011.PubMed/NCBI
|
|
2
|
Sato N, Endo S, Kimura Y, et al: Influence
of a human protease inhibitor on surgical stress induced
immunosuppression. Dig Surg. 19:300–305. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sugasawa Y, Yamaguchi K, Kumakura S, et
al: The effect of one-lung ventilation upon pulmonary inflammatory
responses during lung resection. J Anesth. 25:170–177. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sugasawa Y, Yamaguchi K, Kumakura S, et
al: Effects of sevoflurane and propofol on pulmonary inflammatory
responses during lung resection. J Anesth. 26:62–69. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
De Conno E, Steurer MP, Wittlinger M, et
al: Anesthetic-induced improvement of the inflammatory response to
one-lung ventilation. Anesthesiology. 110:1316–1326.
2009.PubMed/NCBI
|
|
6
|
Schilling T, Kozian A, Kretzschmar M, et
al: Effects of propofol and desflurane anaesthesia on the alveolar
inflammatory response to one-lung ventilation. Br J Anaesth.
99:368–375. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schilling T, Kozian A, Senturk M, et al:
Effects of volatile and intravenous anesthesia on the alveolar and
systemic inflammatory response in thoracic surgical patients.
Anesthesiology. 115:65–74. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bernard GR, Artigas A, Brigham KL, et al:
The American-European Consensus Conference on ARDS. Definitions,
mechanisms, relevant outcomes, and clinical trial coordination. Am
J Respir Crit Care Med. 149:818–824. 1994. View Article : Google Scholar
|
|
9
|
American College of Chest
Physicians/Society of Critical Care Medicine Consensus Conference:
definitions for sepsis and organ failure and guidelines for the use
of innovative therapies in sepsis. Crit Care Med. 20:864–874. 1992.
View Article : Google Scholar
|
|
10
|
Nakayama H, Kitayama J, Muto T and Nagawa
H: Characterization of intracellular cytokine profile of
CD4+ T cells in peripheral blood and tumor-draining
lymph nodes of patients with gastrointestinal cancer. Jpn J Clin
Oncol. 30:301–305. 2000.PubMed/NCBI
|
|
11
|
Simon RH and Paine R III: Participation of
pulmonary alveolar epithelial cells in lung inflammation. J Lab
Clin Med. 126:108–118. 1995.PubMed/NCBI
|
|
12
|
Takizawa H: Airway epithelial cells as
regulators of airway inflammation (Review). Int J Mol Med.
1:367–378. 1998.PubMed/NCBI
|
|
13
|
Vozzelli MA, Mason SN, Whorton MH and
Auten RL Jr: Antimacrophage chemokine treatment prevents neutrophil
and macrophage influx in hyperoxia-exposed newborn rat lung. Am J
Physiol Lung Cell Mol Physiol. 286:L488–L493. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Beck-Schimmer B, Schwendener R, Pasch T,
Reyes L, Booy C and Schimmer RC: Alveolar macrophages regulate
neutrophil recruitment in endotoxin-induced lung injury. Respir
Res. 6:612005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Asadullah K, Sterry W and Volk HD:
Interleukin-10 therapy-review of a new approach. Pharmacol Rev.
55:241–269. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kumakura S, Yamaguchi K, Sugasawa Y, et
al: Effects of nitrous oxide on the production of cytokines and
chemokines by the airway epithelium during anesthesia with
sevoflurane and propofol. Mol Med Rep. 8:1643–1648. 2013.PubMed/NCBI
|
|
17
|
Kotani N, Hashimoto H, Sessler DI, et al:
Intraoperative modulation of alveolar macrophage function during
isoflurane and propofol anesthesia. Anesthesiology. 89:1125–1132.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sato N, Koeda K, Kimura Y, et al: Cytokine
profile of serum and bronchoalveolar lavage fluids following
thoracic esophageal cancer surgery. Eur Surg Res. 33:279–284. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Abe T, Oka M, Tangoku A, et al:
Interleukin-6 production in lung tissue after transthoracic
esophagectomy. J Am Coll Surg. 192:322–329. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Galley HF, Dubbels AM and Webster NR: The
effect of midazolam and propofol on interleukin-8 from human
polymorphonuclear leukocytes. Anesth Analg. 86:1289–1293.
1998.PubMed/NCBI
|
|
21
|
O’Donnell NG, McSharry CP, Wilkinson PC
and Asbury AJ: Comparison of the inhibitory effect of propofol,
thiopentone and midazolam on neutrophil polarization in vitro in
the presence or absence of human serum albumin. Br J Anaesth.
69:70–74. 1992.
|
|
22
|
Ma L, Wu X, Chen W and Fujino Y: Propofol
has anti-inflammatory effects on alveolar type II epithelial cells.
Acta Anaesthesiol Scand. 54:362–369. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Murphy PG, Ogilvy AJ and Whiteley SM: The
effect of propofol on the neutrophil respiratory burst. Eur J
Anaesthesiol. 13:471–473. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mathy-Hartert M, Mouithys-Mickalad A,
Kohnen S, Deby-Dupont G, Lamy M and Hans P: Effects of propofol on
endothelial cells subjected to a peroxynitrite donor (SIN-1).
Anaesthesia. 55:1066–1071. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ledowski T, Paech MJ, Patel B and Schug
SA: Bronchial mucus transport velocity in patients receiving
propofol and remifentanil versus sevoflurane and remifentanil
anesthesia. Anesth Analg. 102:1427–1430. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Takala RS, Soukka HR, Salo MS, et al:
Pulmonary inflammatory mediators after sevoflurane and thiopentone
anaesthesia in pigs. Acta Anaesthesiol Scand. 48:40–45. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kotani N, Takahashi S, Sessler DI, et al:
Volatile anesthetics augment expression of proinflammatory
cytokines in rat alveolar macrophages during mechanical
ventilation. Anesthesiology. 91:187–197. 1999. View Article : Google Scholar
|
|
28
|
Koksal GM, Sayilgan C, Gungor G, et al:
Effects of sevoflurane and desflurane on cytokine response during
tympanoplasty surgery. Acta Anaesthesiol Scand. 49:835–839. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kalimeris K, Christodoulaki K, Karakitsos
P, et al: Influence of propofol and volatile anaesthetics on the
inflammatory response in the ventilated lung. Acta Anaesthesiol
Scand. 55:740–748. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wei H, Liang G, Yang H, et al: The common
inhalational anesthetic isoflurane induces apoptosis via activation
of inositol 1,4,5-trisphosphate receptors. Anesthesiology.
108:251–260. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Moore KW, de Waal Malefyt R, Coffman RL
and O’Garra A: Interleukin-10 and the interleukin-10 receptor. Annu
Rev Immunol. 19:683–765. 2001. View Article : Google Scholar
|
|
32
|
Gilliland HE, Armstrong MA, Carabine U and
McMurray TJ: The choice of anesthetic maintenance technique
influences the antiinflammatory cytokine response to abdominal
surgery. Anesth Analg. 85:1394–1398. 1997.PubMed/NCBI
|
|
33
|
Dimopoulou I, Armaganidis A, Douka E, et
al: Tumour necrosis factor-alpha (TNFα) and interleukin-10 are
crucial mediators in post-operative systemic inflammatory response
and determine the occurrence of complications after major abdominal
surgery. Cytokine. 37:55–61. 2007.
|
|
34
|
Hudson LD, Milberg JA, Anardi D and
Maunder RJ: Clinical risks for development of the acute respiratory
distress syndrome. Am J Respir Crit Care Med. 151:293–301. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Helmy SA, Wahby MA and El-Nawaway M: The
effect of anaesthesia and surgery on plasma cytokine production.
Anaesthesia. 54:733–738. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Michelet P, D’Journo XB, Roch A, et al:
Protective ventilation influences systemic inflammation after
esophagectomy: a randomized controlled study. Anesthesiology.
105:911–919. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
D’Journo XB, Michelet P, Marin V, et al:
An early inflammatory response to oesophagectomy predicts the
occurrence of pulmonary complications. Eur J Cardiothorac Surg.
37:1144–1151. 2010.PubMed/NCBI
|