Introduction
Tumor necrosis factor-induced protein 1 (Tnfaip1),
which was also termed B12 and previously identified as a tumor
necrosis factor-α (TNF-α)-inducible protein (1), is highly conserved in a wide range
of species including human (1),
mouse (1), rat (2), and Caenorhabditis elegans
(C. elegans) (3). In
mammals, Tnfaip1 has at least two paralogs, KCTD10 and KCTD13,
which share >60% amino acid sequence identities, and a conserved
BTB/POZ domain at their N-terminus. Besides sequence similarity,
all of the paralogs show some common functional features. They may
play roles in the cytokinesis signaling pathway and DNA synthesis
by interacting with proliferating cell nuclear antigen (PCNA) and
small subunit (p50) of polymerase δ (2,4,5).
Tnfaip1 is also involved in the process of Alzheimer’s disease (AD)
development (3), the innate
immunity against hepatitis B virus (HBV) (6), the apoptosis of cancer cells
(7), the phosphorylation
procedure (8) and type 2 diabetic
nephropathy (9). Tnfaip1 is
differentially expressed in various tissues such as liver, kidney,
heart, placenta and brain of developing mouse embryo (1) and has a lower expression in cancer
cells (7). However, the detailed
expression of Tnfaip1 and its exact function in these physiological
processes has yet to be thoroughly investigated. The detailed
expression in nerve system has also not been reported, particularly
in hippocampus, which is one of the brain regions affected early in
AD, although it was previously reported that Tnfaip1 was involved
in response to β-amyloid peptide accumulation in a transgenic C.
elegans AD model (3).
The sex hormone estrogen has multiple functions,
including the differentiation and function of the reproductive
tract, memory storage and bone growth (10,11). Usually, estrogen exhibits its
function by binding to the classic estrogen receptors, ERα and ERβ,
which are nuclear transcription factors that activate or repress
gene expression (12).
Hippocampal neurons express ERα and ERβ mRNA in tissue sections as
well as in slice cultures and dispersion cultures (13,14). Results of previous studies
strongly suggest that hippocampal neurons are able to synthesize
estrogens de novo (14,15), and hippocampus-derived endogenous
estrogen is essential for hippocampal synaptic plasticity (16). Peripheral estrogen has also been
found to exert a protective effect in hippocampal neurons (17). For more than two decades, it is
known that estrogens influence synaptic plasticity in the
hippocampus. Gould et al (18) showed that ovariectomy (OVX) of
female rats resulted in a decrease of dendritic spine density on
CA1 pyramidal neurons in the hippocampus. Accordingly, systemic
estradiol treatment of these ovariectomized animals caused an
increase in the number of dendritic spines in this particular
hippocampal region. Consistently, spine synapse density varied in
response to fluctuating estrogen levels during the estrous cycle in
female rats (19).
In this study, in order to study the function of
Tnfaip1 in physiological processes, such as AD disease, we focused
on the Tnfaip1 expression in mouse hippocampus. Results of the
present study showed that Tnfaip1 expression is associated with age
and gender, possibly regulated by estrogen in hippocampus. This
result is supported by in vitro and in vivo studies.
Through promoter analysis, a potential binding site for ERβ was
identified in the Tnfaip1 promoter region and Tnfaip1 expression
was upregulated by ERβ. The identification of a novel regulatory
factor and mechanism for Tnfaip1 in hippocampus indicates the
involvement of Tnfaip1 in hippocampus-related disease, such as
AD.
Materials and methods
Mice and estrogen manipulations
C57Bl/6J (B6) mice were housed in a 12:12 h
light:dark cycle. Sham, OVX and 17β-estradiol pellet implantation
were performed as previously described (20,21). All the OVX (n=12) and Sham (n=6)
mice were 2 months old. The OVX mice had access to
phytoestrogen-free food, receiving either a placebo pellet (OVX +
placebo; n=6) or a 17β-estradiol pellet (OVX + E2; n=6). The
pellets contained 0.18 mg of 17β-estradiol or placebo in a matrix
that is designed to release contents (17β-estradiol or placebo)
over a 60-day period (Innovative Research of America, Sarasota, FL,
USA). Sham operations were performed (Sham; n=6) on an additional
group of mice. Treatment with placebo or 17β-estradiol lasted for 4
weeks. Unless otherwise indicated, the reported results were
obtained from homozygous mutants. The animal procedures were
approved by the Institutional Animal Care and Use Committee of
Hunan Agriculture University (approval no. 09-01).
Primary hippocampal neuronal culture
Hippocampal neuronal cells were prepared from mouse
embryos using the methods described previously (22). Briefly, female C57Bl/6J (B6) mice
with 18.5 days of gestation were anesthetized with isoflurane, and
embryonic mouse pups were surgically removed and decapitated.
Hippocampuses were harvested under sterile conditions. The
hippocampal tissue was enzymatically dissociated in 0.125% trypsin
II (Sigma-Aldrich, St. Louis, MO, USA). Isolated neural cells were
placed in poly-D-lysine-coated 35-mm plastic culture dishes
containing 3 ml of neurobasal medium to a culture surface cell
density of 5×105/ml (400–500/mm2). The
cultures were maintained in neurobasal medium supplemented with B27
(2%, v/v; Invitrogen, Carlsbad, CA, USA), glutamine (0.5 mM) and
penicillin/streptomycin (100 units) for at least 7–10 days prior to
being used for experiments. To test the potential function of ERs,
hippocampal neuronal cultures were treated with 1 μM ER antagonist
ICI182,780 (Sigma-Aldrich), or 1 μM selective ERα antagonist
methyl-piperidino-pyrazole (MPP; Sigma-Aldrich), or 5 μM selective
ERβ antagonist
4-[2-phenyl-5,7-bis(trifluoromethyl)pyrazolo[1,5-a]pyrimidin-3-yl]
phenol (PHTPP; Sigma-Aldrich), with the presence of 10 nM
17β-estradiol (E2; Sigma-Aldrich). The antagonists and E2 were
dissolved in dimethylsulfoxide (DMSO). Vehicle-treated controls
received the same volume of DMSO. Medium was changed with fresh
medium every 4 days.
Quantitative real-time-polymerase chain
reaction (qRT-PCR)
Total RNA was prepared using TRIzol (Invitrogen),
and an equal amount of RNA from each tissue (50–100 ng) was reverse
transcribed using the SuperScript III first-strand synthesis system
(Invitrogen). SYBR-Green PCR Master Mix for qRT-PCR was purchased
from ABI (Applied Biosystems, Foster City, CA, USA). qRT-PCR was
performed according to the manufacturer’s instructions, with minor
modifications based on cDNA samples and primers. The primers used
in this study were: Tnfaip1 forward,
5′-gcgtgctcttcatcaaggat-3′ and reverse, 5′-ttccaccttggtctgcttct-3′;
β-actin forward, 5′-cggttccgatgccctgaggctctt-3′ and reverse,
5′-cgtcacacttcatgatggaattga-3′; and 40 cycles of amplification was
achieved in a 96-well plate consisting of SYBR-Green Master Mix,
primers and cDNA. The standard mode of an ABI 7500 Fast Sequence
detection system was used. β-actin was amplified in parallel
as an endogenous control to standardize the amount of sample and
for the quantification of the relative gene expression within and
between samples. Reactions were performed in triplicate.
Fluorescent immunostaining
Frozen tissue sections (10 mm) were treated with
blocking solution containing 5% goat serum and 0.2% Triton X-100 at
room temperature for 30 min. Primary antibodies were added at a
dilution of 1:200, and incubated at 4°C overnight. After three
washes in 1X phosphate-buffered saline (PBS), Alexa-568 (Molecular
Probes, Carlsbad, CA, USA) conjugated secondary antibodies were
added at a dilution of 1:600, together with DAPI (1:10,000), and
incubated at room temperature for 2 h in the dark. Non-immune sera
(instead of primary antibodies) were used as negative controls in
the preliminary experiment. Slides were mounted in Fluoromount-G
(Southern Biotech, Birmingham, AL, USA) and viewed using a Carl
Zeiss Microscope Objective (Microscope Axio Observer.A1; Carl Zeiss
Microscopy, LLC, San Diego, CA, USA).
Western blot analysis
To study the effect of estrogen on the expression of
Tnfiap1 in vitro and in vivo, western blot assay was
performed as previously described (8). The proteins were extracted with RIPA
buffer and boiled together with loading buffer. After separation
with SDS/PAGE, the proteins were transferred to a polyvinylidene
difluoride membrane (Millipore), blocked in 5% non-fat milk for 1
h, and probed overnight with the rabbit polyclonal anti-Tnfaip1
(Abcam) in the presence of 5% non-fat milk. Immunoblots were washed
3 times (5–10 min each) in PBS containing 0.1% Tween-20 and
incubated with the goat anti-rat-HRP (Amersham Biosciences)
(1:10,000) for 1 h. Blots were washed 3 times (10 min each) in PBS
containing 0.1% Tween-20, developed in ECL reagent (Millipore) for
2–5 min, and exposed to Blue XB-1 film (Kodak).
Promoter analysis
The promoter region of mouse Tnfaip1 (GenBank
accession no. NM_009395.4) was predicted using the Promoter
Inspector program (www.genomatix.de) and ProScan
(www.bimas.cit.nih.gov). Transcription factor
binding sites were predicted by online software JASPAR (http://jaspar.cgb.ki.se/). The CpG were also predicted
by online software (http://www.bio-soft.net/sms/cpg_island.html). The
promoter region (−2166/+61) was amplified from mouse genomic DNA
using primer pairs with KpnI site at the forward primers and
XhoI site at the reverse primers. A series of fragments
including (−1072/+61), (−734/+61) and (−273/+61) were amplified
from the promoter region. The primers used were: forward primers
with KpnI site, for −2166/+61,
5′-ggggtacccagacacgtgaacctgaagga-3′; −1072/+61,
5′-ggggtacctgataaccgctagcagtggat-3′; −734/+61,
5′-ggggtaccgatgggtaagatagatatggt-3′; −273/+61,
5′-ggggtaccgtcccaagtcctccacg-3′; the common reverse
primer with XhoI site,
5′-ccgctcgagagcagtgggtcgaaaacttgac-3′. The letters in
bold italic refer to the recognition site of the restriction
enzyme. These amplified fragments were inserted into the
promoterless-luciferase gene upstream of plasmid pTAL-luc,
separately denoted p(−2166/+61)-Luc, p(−1072/+61)-Luc,
p(−734/+61)-Luc and p(−273/+61)-Luc. Site-directed mutagenesis for
the ERβ binding site construct was performed by overlapping
extension PCR as previously described (2) and termed p(−Δ734/+61)-Luc.
Luciferase assay
The primary hippocampal cells were plated in 6-well
tissue culture dishes at 9×104 cells/well and used for
transient transfection at day 7 in vitro (DIV). Cells were
cotransfected with 1.5 μg of luciferase reporter plasmid and 0.5 μg
of β-gaglactosidase expression vector pCMVβ as an internal control.
At 36 h after transfection, the cells were lysed to measure the
luciferase activity using the Luciferase Assay System (Promega,
Madison, WI, USA).
To study the regulation of ERβ on Tnfaip1 using the
luciferase assay, the coding sequences of mouse ERβ were amplified
from mouse hippocampal cDNA and cloned into the eukaryotic
expression vector pCMV-Myc. The construct was designated as
pCMV-Myc-ERβ. The luciferase reporter plasmid including the
site-directed mutagenesis for the ERβ binding site and pCMV-Myc-ERβ
were cotransfected.
ChIP
To detect the association of ERβ with Tnfaip1,
chromatin immunoprecipitation (ChIP) was performed using
overexpressed ERβ protein. The primary hippocampal cells were
cultured and transfected with an ERβ-expressing pCMV-Myc vector as
previously described (23). ChIP
was conducted following the recommended protocols (Active Motif).
Specifically, enzymatic shearing was conducted for 10 min and IP
was performed with the ERβ rabbit polyclonal IgG (Sigma-Aldrich) at
a concentration of 3 μg/100 μl or the pre-immune mouse IgG as the
negative control. Potential ERβ binding sites were predicted using
the JASPAR TF Search program. PCR primers were designed flanking
putative ERβ binding sites (forward, 5′-tcatgtggaaacagaaggctg-3′
and reverse primer, 5′-agttcttcctgtgtctacgcc-3′) and ChIP pull-down
products were amplified for 30 cycles with a 60°C annealing
temperature and imaged by agarose gel electrophoresis.
Results
Expression of Tnfaip1 in nerve
tissues
To study the function of Tnfaip1 in the nerve
system, we compared the expression of Tnfaip1 in nerve system using
quantitative PCR (qPCR). The 1.5 M mouse tissues including cerebral
cortex, hippocampus, cerebellar, hypothalamus, and nerve in spine
were dissected and qPCR was performed as described in Materials and
methods. Positive signals were detected in all the tissues. In
these tissues, the a significant difference in Tnfaip1 expression
in hippocampus and cerebellar with a 45-fold difference was
identified (Fig. 1A). This result
suggested that Tnfaip1 may play a critical role in the nerve
system, particularly in the hippocampus.
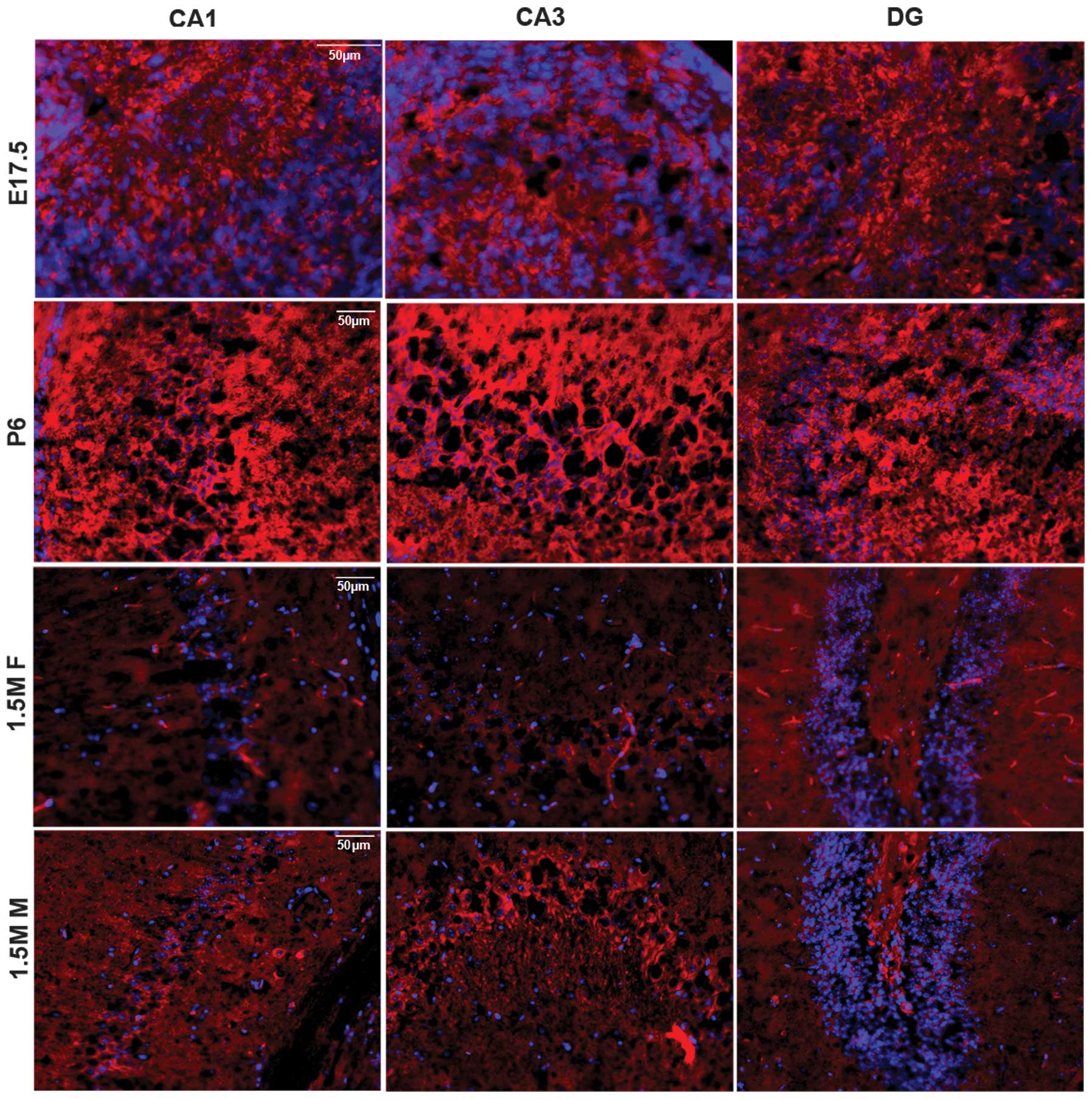
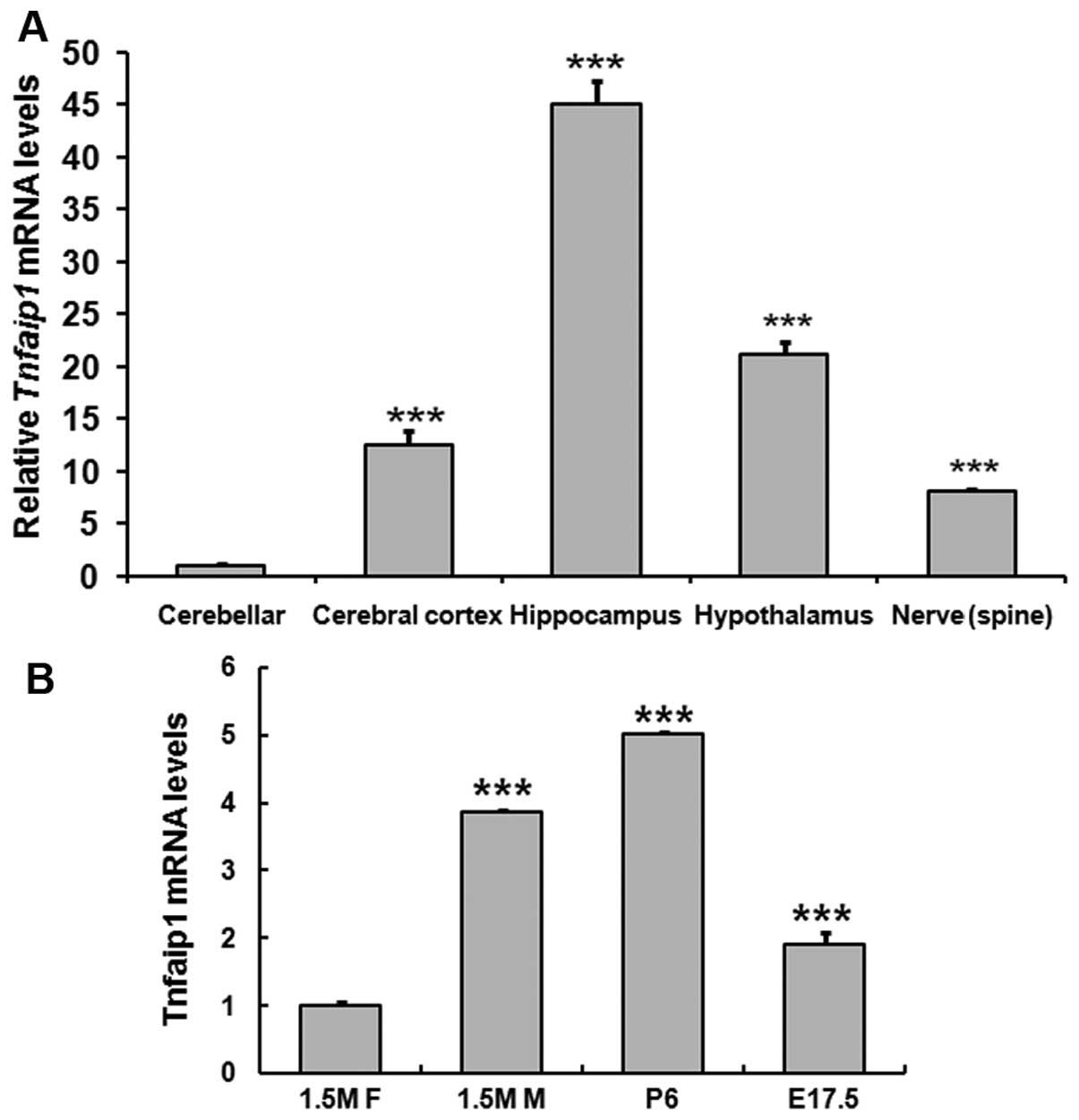 | Figure 1Tumor necrosis factor-induced protein
1 (Tnfaip1) mRNA levels in the mouse nerve system. Tissues were
dissected and quantitative polymerase chain reaction (qRT-PCR) was
performed. (A) Tnfaip1 mRNA levels in the nerve system including
cerebellar, cerebral cortex, hippocampus, hypothalamus and
peripheral nerve, respectively. ***P<0.001 when
cerebellar was compared with the cerebral cortex, hippocampus,
hypothalamus and peripheral nerve. (B) Tnfaip1 mRNA levels in
hippocampus of different age and gender. E17.5, embryonic 17.5 day;
P6, postnatal 6 day; 1.5 M F, 1.5-month-old female; 1.5 M M,
1.5-month-old male. ***P<0.001 when 1.5 M F was
compared with 1.5 M M, P6 or E17.5. |
Age- and gender-related difference in the
expression of Tnfaip1 in hippocampus
Previously it was reported that Tnfaip11 was
involved in response to β-amyloid peptide accumulation in a
transgenic C. elegans AD model (3). However, little is known with regard
to the exact role of Tnfaip1 in these diseases. Evidence that the
parahippocampal cortex (including the entorhinal cortex) undergoes
pathological changes during the early stages of the disease is
theoretically significant (24).
To investigate the role of Tnfaip1 in these diseases, three check
points were selected: embryonic (E17.5), postnatal (P6), and adult
(1.5 M), with a focus on the expression of Tnfaip1 in hippocampus.
The hippocampal tissues were dissected from mice of different age
and gender. The results of qPCR showed that Tnfaip1 expression was
low in E17.5, high in P6, and lowest in 1.5 M. Of note, at the 1.5
M stage, male mice had a stronger expression than the female mice
(Fig. 1B).
To map Tnfaip1 in hippocampus, the brains including
the hippocampus were dissected, the slides were cut, and
fluorescent immunostaining was performed using anti-Tnfaip1
antibodies as primary antibodies. Tnfaip1 showed strong staining in
the hippocampus of P6 mouse, but faint staining in the 1.5 M female
hippocampus. The 1.5 M male exhibited stronger immunoreactivity
than the female counterpart (Fig.
2). The results were consistent with qPCR results, showing age-
and gender-related differences in the expression of Tnfaip1. The
CA3 region can be divided into CA3a,b,c subareas (25). Fig.
2 shows data of only CA3a subareas, with the data of CA3b and c
not being shown. Among the slides including all the ages and both
genders, the pyramidal cells in CA3 regions including the CA3a,b,c
subareas in the adult mouse have the strongest staining, higher
than in the subregions of CA1 and DG (Fig. 2). These data suggested that
Tnfaip1 plays an important role in short-term memory and pattern
separation of the geometry of environment through the CA3
region.
Effect of peripheral estrogen on the
expression of Tnfaip1 in hippocampus
Findings of previous studies suggest that the
systemic estradiol treatment of ovariectomized animals caused an
increase in the number of dendritic spines in this particular
hippocampal region (18) and
spine synapse density varied in response to fluctuating estrogen
levels during the estrous cycle in female rats (19). To study the effect of systemic
estrogen on the expression of Tnfaip1 in hippocampus, the OVX mice
were implanted with placebo pellet or 17β-estradiol pellet for 4
weeks. The entire brain tissue was dissected for fluorescent
immunostaining and the hippocampus was dissected for western blot
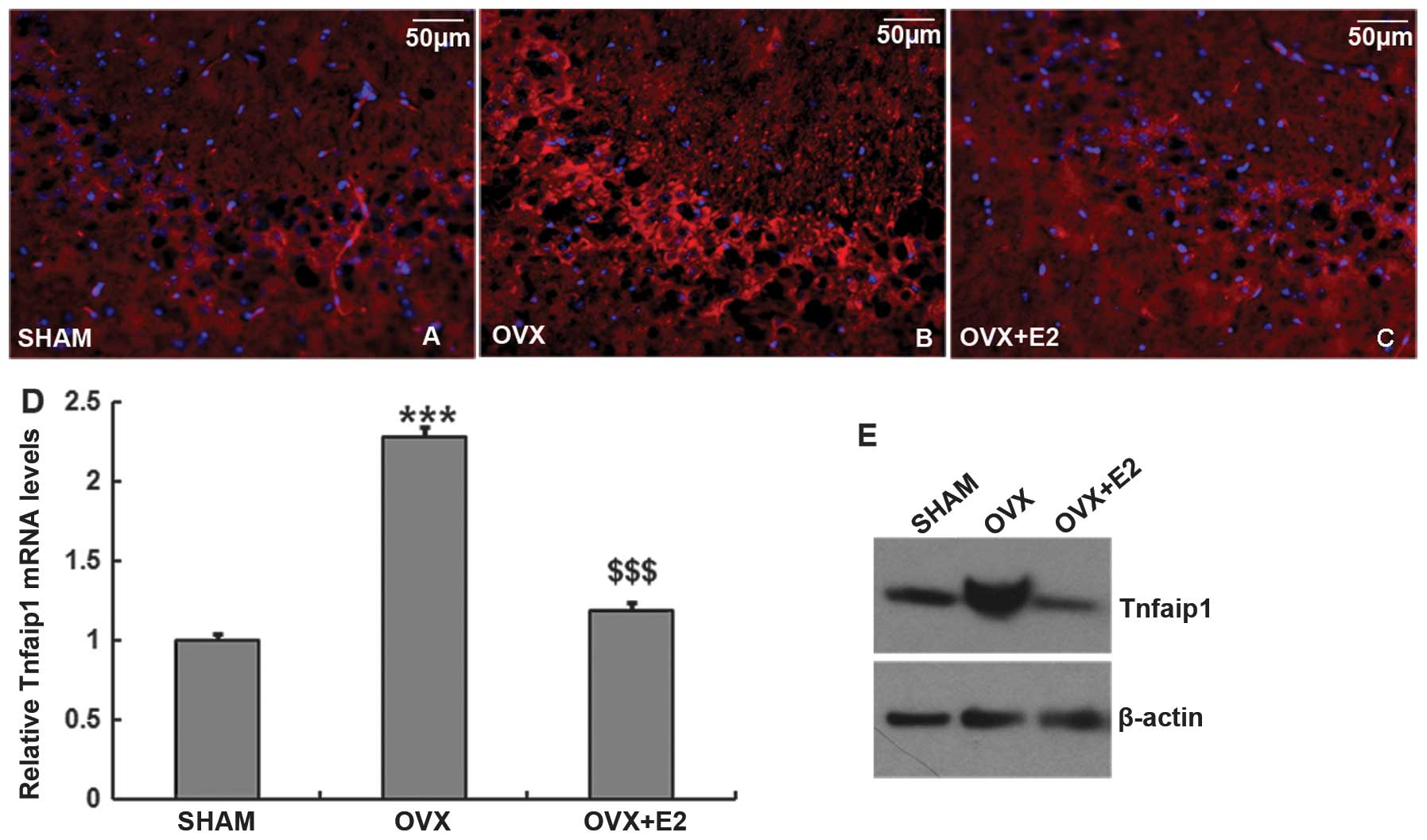
or real-time analysis. The Sham mice were used as the control. The
results with fluorescent immunostaining showed that Tnfaip1 had
stronger staining in the hippocampal CA3 subregion of OVX + placebo
mice than the OVX + E2 or Sham mice (Fig. 3A–C). The staining was not
different in the CA1 or DG subregions (data not shown).
Furthermore, the mRNA level (Fig.
3D) and protein level (Fig.
3E) of Tnfaip1 in hippocampus was higher in the OVX + placebo
mice than in the OVX + E2 (P<0.001) or in Sham mice
(P<0.001). These results suggested that systemic estrogen
inhibits Tnfaip1 expression in hippocampus.
Effect of estrogen on Tnfaip1 expression
in in vitro hippocampal neuronal cells
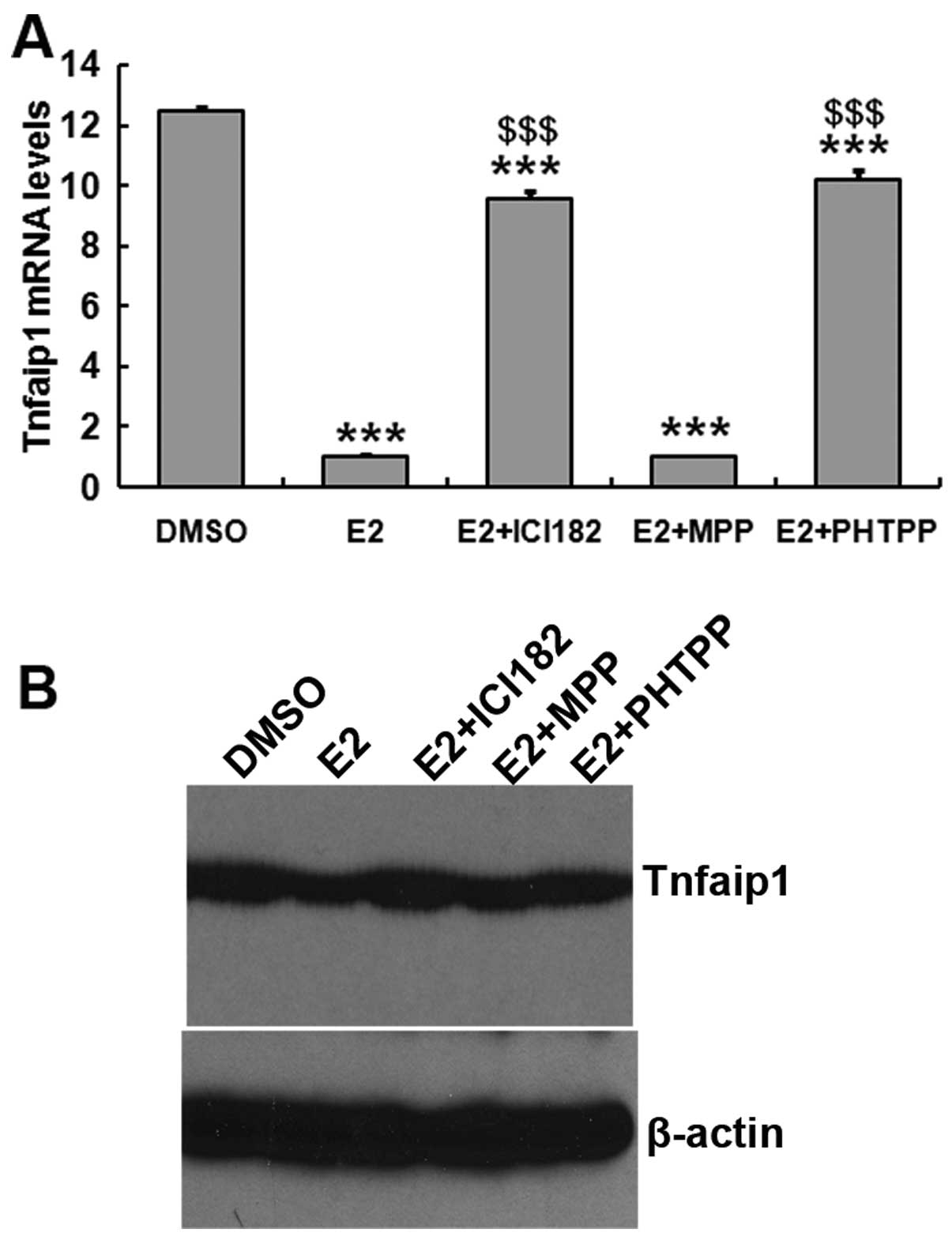
To examine the effect of estrogen on Tnfaip1
expression, hippocampal neuronal cells were prepared from mouse
embryos and cultured in the presence or absence of E2. Tnfaip1
expression was lower in the cells treated with estrogen compared to
the control (Fig. 4).
The classic estrogen receptors, known as ERα and
ERβ, are nuclear transcription factors that activate or repress
gene expression (12). To
determine whether ERα and ERβ were involved in the inhibition
progress, the cells were treated with DMSO (control) or estrogen
receptor antagonists (ICI182,780, MPP or PHTPP) in the presence of
E2. Inhibition of Tnfaip1 expression by E2 was reversed by ER
non-selective antagonist ICI182,780 and ERβ-selective antagonist
PHTPP, but not by ERα-selective antagonist MPP. These data
suggested that estrogen downregulates Tnfaip1 expression in in
vitro hippocampal cells, which was consistent with the in
vivo results. Additionally, this function of estrogen may be
mediated by ERβ.
ERβ upregulates Tnfaip1 expression by
binding to the promoter of Tnfaip1
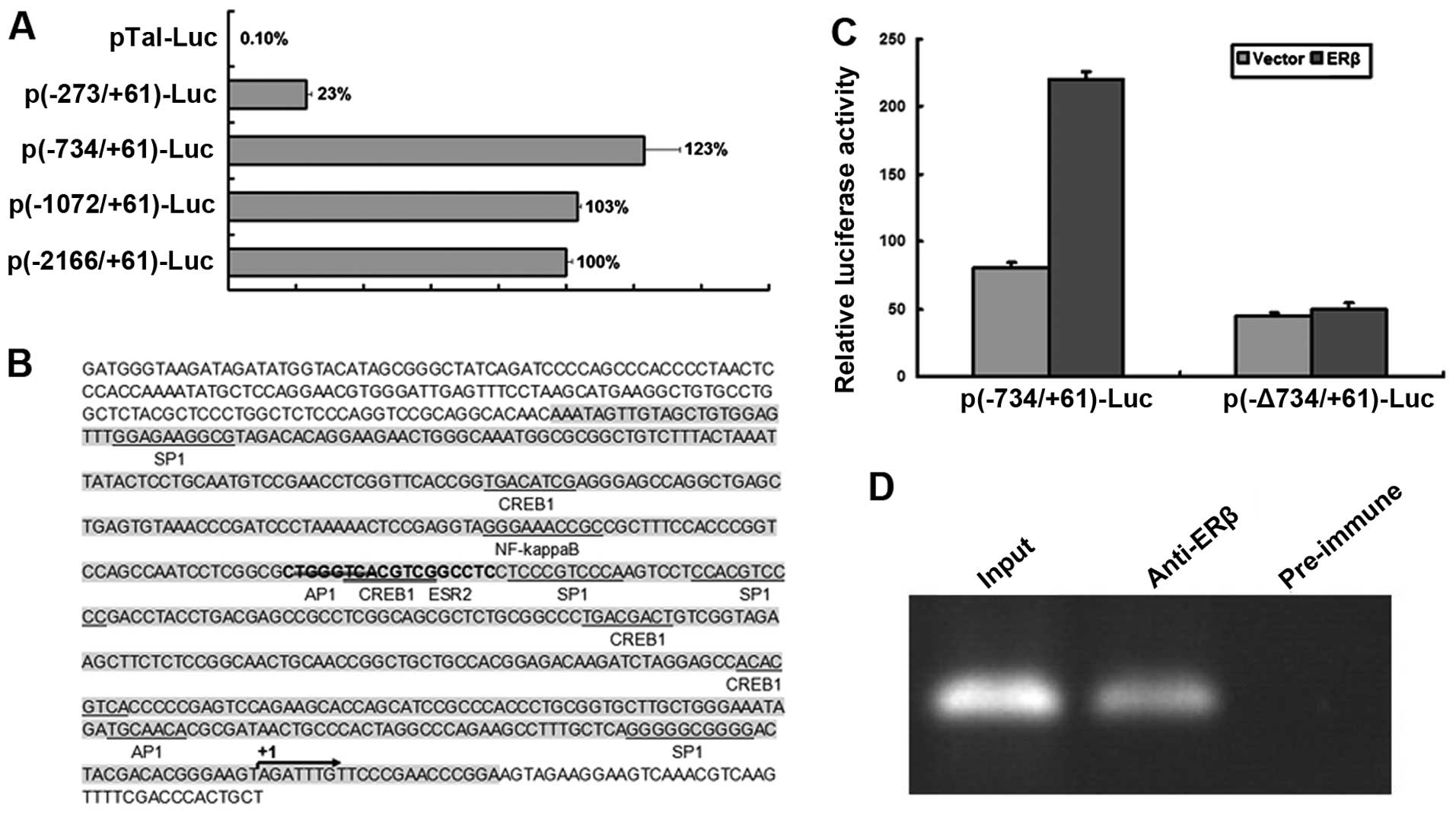
To examine the regulation of ERβ on Tnfaip1
expression, a promoter analysis was performed. The promoter region
of mouse Tnfaip1 was predicted to be a 2166-bp genomic region. A
series of 5′-deletion promoter regions upstream of the first exon
of the Tnfaip1 gene were amplified and inserted into the
promoterless luciferase reporter construct. Insertion of longer
regions (−2166/+61, −1072/+61 and −734/+61) significantly enhanced
the promoter activity compared to the promoterless luciferase
control (Fig. 5A). The deletion
−734/+61 had the strongest enhancement, followed by the −1072/+61
deletion. Both were stronger than the longest insertion
(−2166/+61). However, the deletion −237/+61 showed a significant
decrease in promoter activity. These results showed that the
upstream region −2166/+61 of the mouse Tnfaip1 gene contained a
functional promoter and the main regulatory element resided in the
range of −734 to +61.
To further determine the potential estrogen response
element (ERE), the region −734 to +61 was scanned using the JASPAR
and CpG-prediction software. No classical TATA box or CCAAT box was
found in the promoter region. Instead, a CpG island was detected
from −607 to +21, whose sequence was shaded in Fig. 5B. A potential ERβ (known as ERS2
in the software) binding site was identified in this region.
However, the ERα binding site was not found (Fig. 5B). To study the regulation of ERβ
on Tnfaip1 expression, the p(−734/+61)-Luc or the mutant
p(−Δ734/+61)-Luc was co-transfected with pCMV-Myc-ERβ into cultured
cells. The luciferase activity analysis revealed that the
overexpression of ERβ enhanced the Tnfaip1 promoter activity,
compared to the empty or mutated vector (Fig. 5C). To confirm the involvement of
ERβ in the Tnfaip1 promoter regulation, we performed ChIP assay
using the hippocampal primary cells. (Fig. 5D) The cross-linked mouse Tnfaip1
promoter-ERβ complexes immunoprecipitated with anti-ERβ antibody
were detected by PCR amplification with primers covering the
potential binding sites of mouse Tnfaip1 promoter. No band was
detected with a non-specific antibody as a negative control. These
results demonstrated that ERβ can directly bind to the Tnfaip1
promoter, providing novel evidence for Tnfaip1 transcriptional
regulation.
Discussion
Tnfaip1 was initially identified as a tumor necrosis
factor α-induced protein (1).
Although TNF-α treatment was reported to protect hippocampal
neurons against Aβ toxicity (26), no study has shown Tnfaip1 to be
associated with Aβ toxicity since its identification. Until 10
years ago, the homolog gene of Tnfiap1 was upregulated in response
to Aβ treatment, using the C. elegans AD model. The
hippocampus is a complex structure, most commonly known for its
role in learning and memory. The hippocampus consists of CA1, CA3,
and the dentate gyrus regions, and comprises distinct neuronal cell
types including pyramidal neurons, interneurons, and granule cells.
The hippocampus has been the target for extensive studies regarding
spatial and non-spatial memory, long-term potentiation, adult
neurogenesis, neurodegeneration and neurodegenerative diseases,
such as Alzheimer’s. In this study, we focused on the expression of
Tnfaip1 in the mouse hippocampus, which is one of the brain regions
affected early in the AD process. The present study showed high
mRNA levels in hippocampus (Fig.
1A), and strong staining in the CA3 subregions in mice of all
ages and both genders (Fig. 2).
The data suggest that Tnfaip1 potentially plays an important role
in the hippocampus. However, the exact function remains to be
investigated in Tnfaip1 knock-out or transgenic mice.
Despite being markedly different in anatomy, the
subregions CA3, CA1 and dentate gyrus (DG) in hippocampus have
distinct functions (27,28). In particular, the extensive
excitatory recurrent connections of region CA3 have been suggested
to play an important role in the encoding and retrieval of new
spatial information within short-term memory (29,30) and spatial information requiring
multiple trials (31,32). The CA3 region also supports
sequential processing of information in cooperation with CA1
(32) and processing of the
geometry of the environment in cooperation with DG (33,34). In this study, our data showed that
the expression of Tnfaip1 was stronger in the CA3 region of the
adult hippocampus (Fig. 2). The
high expression of Tnfaip1 suggests its important role in the
above-mentioned functions. A recent study has highlighted
functional deficits in the DG/CA3 hippocampal subfields in animal
models of AD pathology and in patients in the early stages of
dementia (35). Additionally, its
findings showed that activity in the DG/CA3 networks was
compromised in transgenic mice expressing an APP (amyloid precursor
protein) mutation (35). To the
best of our knowledge, the present study provides new evidence for
the association between Tnfaip1 and AD although it was previously
reported that the homolog gene of Tnfaip1 was upregulated in the
superior frontal gyrus and cerebellum but not significantly
increased in the hippocampus of a transgenic C. elegans AD
model (3). This conflict may have
resulted from the difference in study species, analytical methods
and models.
In this study, the age- and gender-related
expression of Tnfaip1 in hippocampus was detected using qPCR
(Fig. 1B) and fluorescent
immunostaining (Fig. 2). The
result is consistent with the study of Wolf et al (1), who investigated the developmental
expression in heart and liver. Our data provide complementary
evidence withe regard to the developmental expression of Tnfaip1,
particularly in the hippocampus. Based on the result of the age-
and gender-related expression of Tnfaip1, we hypothesized that
Tnfaip1 is regulated by estrogen. Previous findings strongly
suggest that hippocampal neurons are able to synthesize estrogens
de novo (14,15), and hippocampus-derived endogenous
estrogen is essential for hippocampal synaptic plasticity (16). However, a number of other studies
have suggested that peripheral estrogen exerts a protective effect
in hippocampal neurons. Woolley et al (19) were the first to show cyclic
fluctuations in dendritic spine density as estradiol and
progesterone levels vary across the estrous cycle in adult female
rats. At the same time, they also reported that OVX resulted in a
decrease in dendritic spine density in the hippocampus in female
rat, which could be prevented by systemic estradiol treatment.
Several subsequent experiments confirmed the connection between
ovarian estrogens and spine synapse density in rodents (36) and in non-human primates (37). Another study found that synaptic
plasticity highly depends on endogenous estrogen and cannot be
influenced by exogenous estrogen (16). A study performed by Leranth et
al (38) may help to explain
this discrepancy. Their studies showed an indirect action of
estrogen on hippocampal spinogenesis, i.e., the systemic
application of estradiol results in an increase of spine density
indirectly via the subcortical regions. Similar results strongly
suggest that systemic estrogen effects on hippocampus may be
mediated by various subcortical regions, such as medial
septum/diagonal band of Broca (MSDB), the supramammillary area
(SUM) and the median raphe (MR) (39–41). The MSDB may play a key role in
this process since it is not only sensitive to local estrogen
stimulation but its septohippocampal neurons receive stimulatory
signals from the SUM and MR (42,43). To examine the regulation of
systemic estrogen on Tnfaip1 expression in hippocampus, OVX and
Sham mice were used. Compared to the control, Sham mice, a high
expression was detected in the hippocampus of OVX mice using
fluorescent immunostaining, qPCR and western blot analysis
(Fig. 3). The enhanced expression
was reversed by complementary estrogen (Fig. 3). These data provide novel
evidence that systemic estrogen downregulates Tnfaip1 expression in
hippocampus.
The classic estrogen receptors, known as ERα and
ERβ, are nuclear transcription factors that activate or repress
gene expression (12).
Hippocampal neurons in tissue sections as well as in slice cultures
and dispersion cultures express ERα and ERβ mRNA (13,14). To determine whether ERα and ERβ
were involved in the inhibition process, the primary hippocampal
cells were cultured and treated with estrogen receptor antagonists.
In cultured hippocampal neuronal cells, estrogen inhibited Tnfaip1
expression compared to the control (Fig. 4). The decrease of Tnfaip1
expression was reversed by both the ER non-selective antagonist and
ERβ-selective antagonist, but not by the ERα-selective antagonist
(Fig. 4). These data suggest that
estrogen actually downregulated Tnfaip1 expression in vitro,
consistent with the in vivo results (Fig. 3). These results also suggest that
the regulation of estrogen on Tnfaip1 expression may be through ERβ
mediation. Previous studies have described the acute,
membrane-initiated signaling actions of E2 in the brain through
multiple signaling pathways (44), such as G-protein-activated
pathways (45), the
mitogen-activated protein kinase (MAPK) pathway (46,47) and the phosphatidylinositol
3-kinase (PI3K)/Akt pathway (48–50). At least some of these rapid
actions of E2 cannot be attributed to classical nuclear-initiated
steroid signaling of ERα or ERβ. There are several potential
candidates for novel membrane ERs including ER-X and two
G-protein-coupled receptors, GPR30 and Gq-mER (51,52). However, the mechanism whereby
membrane ERs activate the neuroprotective cascade is largely
unknown.
Mounting evidence suggests that ERα and ERβ can
regulate transcription of some of these ‘estrogen-responsive’ genes
by interacting with other DNA-bound transcription factors, such as
specificity protein-1 (SP-1) and activator protein-1 (AP-1), rather
than binding directly to DNA (53,54). When scanning the promoter region
of the mouse Tnfaip1 gene, we found the potential binding sites of
ERβ and other transcription factors (Fig. 5B), but no binding sites of ERα.
This may explain why the effect of estrogen on Tnfaip1 expression
occurs through the ERβ but not the ERα pathway. ERβ upregulates
Tnfaip1 expression (Fig. 5C) and
is able to physically bind to the Tnfaip1 promoter (Fig. 5D). In the study of Prange-Kiel
et al (14), treating the
primary hippocampal neurons with additional estradiol resulted in
the upregulation of ERα and downregulation of ERβ, as
immunohistochemistry and subsequent semiquantitative image analysis
revealed. Expression of ERs is known to be regulated by estrogen in
both reproductive system (55,56) and brain regions (57). The possible mechanism can be
deduced based on these studies and our study for the effect of
estrogen on Tnfaip1 expression. Estrogen upregulates ERα and
subsequently downregulates ERβ. Although ERβ is able to upregulate
Tnfaip1 expression, its function is inhibited by estrogen. To the
best of our knowledge, our data provide a novel regulatory
mechanism Tnfaip1 expression in hippocampus. This study also
elucidated the potential function of Tnfaip1 in hippocampus-related
disease, such as AD, which may be affected by the estrogen
level.
Acknowledgements
This study was supported by the foundation from the
National Natural Science Foundation of China (81173177), the
Science and Technology Planning Project of Hunan (2009FJ3012), the
Educational Commission of Hunan (09C1062 and 10C0018), the
Environmental Scientific Research Foundation of Hunan (2009364),
China.
References
|
1
|
Wolf FW, Marks RM, Sarma V, et al:
Characterization of a novel tumor necrosis factor-alpha-induced
endothelial primary response gene. J Biol Chem. 267:1317–1326.
1992.PubMed/NCBI
|
|
2
|
Zhou J, Hu X, Xiong X, et al: Cloning of
two rat PDIP1 related genes and their interactions with
proliferating cell nuclear antigen. J Exp Zool A Comp Exp Biol.
303:227–240. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Link CD, Taft A, Kapulkin V, et al: Gene
expression analysis in a transgenic Caenorhabditis elegans
Alzheimer’s disease model. Neurobiol Aging. 24:397–413. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhou J, Ren K, Liu X, Xiong X, Hu X and
Zhang J: A novel PDIP1-related protein, KCTD10, that interacts with
proliferating cell nuclear antigen and DNA polymerase delta.
Biochim Biophys Acta. 1729:200–203. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Smith TC, Fang Z and Luna EJ: Novel
interactors and a role for supervillin in early cytokinesis.
Cytoskeleton (Hoboken). 67:346–364. 2010.PubMed/NCBI
|
|
6
|
Liu XW, Lu FG, Zhang GS, et al: Proteomics
to display tissue repair opposing injury response to LPS-induced
liver injury. World J Gastroenterol. 10:2701–2705. 2004.PubMed/NCBI
|
|
7
|
Yang LP, Zhou AD, Li H, et al: Expression
profile in the cell lines of human TNFAIP1 gene. Yi Chuan.
28:918–922. 2006.(In Chinese).
|
|
8
|
Yang L, Liu N, Hu X, et al: CK2
phosphorylates TNFAIP1 to affect its subcellular localization and
interaction with PCNA. Mol Biol Rep. 37:2967–2973. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gupta J, Gaikwad AB and Tikoo K: Hepatic
expression profiling shows involvement of PKC epsilon, DGK eta,
Tnfaip, and Rho kinase in type 2 diabetic nephropathy rats. J Cell
Biochem. 111:944–954. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kuiper GG, Shughrue PJ, Merchenthaler I
and Gustafsson JA: The estrogen receptor beta subtype: a novel
mediator of estrogen action in neuroendocrine systems. Front
Neuroendocrinol. 19:253–286. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hultcrantz M, Simonoska R and Stenberg AE:
Estrogen and hearing: a summary of recent investigations. Acta
Otolaryngol. 126:10–14. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nilsson S, Mäkelä S, Treuter E, et al:
Mechanisms of estrogen action. Physiol Rev. 81:1535–1565. 2001.
|
|
13
|
Wehrenberg U, Prange-Kiel J and Rune GM:
Steroidogenic factor-1 expression in marmoset and rat hippocampus:
co-localization with StAR and aromatase. J Neurochem. 76:1879–1886.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Prange-Kiel J, Wehrenberg U, Jarry H and
Rune GM: Para/autocrine regulation of estrogen receptors in
hippocampal neurons. Hippocampus. 13:226–234. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kretz O, Fester L, Wehrenberg U, et al:
Hippocampal synapses depend on hippocampal estrogen synthesis. J
Neurosci. 24:5913–5921. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Prange-Kiel J and Rune GM: Direct and
indirect effects of estrogen on rat hippocampus. Neuroscience.
138:765–772. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Green PS, Yang SH, Nilsson KR, Kumar AS,
Covey DF and Simpkins JW: The nonfeminizing enantiomer of
17beta-estradiol exerts protective effects in neuronal cultures and
a rat model of cerebral ischemia. Endocrinology. 142:400–406.
2001.PubMed/NCBI
|
|
18
|
Gould E, Woolley CS, Frankfurt M and
McEwen BS: Gonadal steroids regulate dendritic spine density in
hippocampal pyramidal cells in adulthood. J Neurosci. 10:1286–1291.
1990.PubMed/NCBI
|
|
19
|
Woolley CS, Gould E, Frankfurt M and
McEwen BS: Naturally occurring fluctuation in dendritic spine
density on adult hippocampal pyramidal neurons. J Neurosci.
10:4035–4039. 1990.PubMed/NCBI
|
|
20
|
Moran AL, Warren GL and Lowe DA: Removal
of ovarian hormones from mature mice detrimentally affects muscle
contractile function and myosin structural distribution. J Appl
Physiol. 100:548–559. 2006. View Article : Google Scholar
|
|
21
|
Moran AL, Nelson SA, Landisch RM, Warren
GL and Lowe DA: Estradiol replacement reverses ovariectomy-induced
muscle contractile and myosin dysfunction in mature female mice. J
Appl Physiol. 102:1387–1393. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Flavin MP, Coughlin K and Ho LT: Soluble
macrophage factors trigger apoptosis in cultured hippocampal
neurons. Neuroscience. 80:437–448. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Viesselmann C, Ballweg J, Lumbard D and
Dent EW: Nucleofection and primary culture of embryonic mouse
hippocampal and cortical neurons. J Vis Exp. pii: 2373 View Article : Google Scholar
|
|
24
|
Hyman BT, Van Hoesen GW, Kromer LJ and
Damasio AR: Perforant pathway changes and the memory impairment of
Alzheimer’s disease. Ann Neurol. 20:472–481. 1986.
|
|
25
|
Li XG, Somogyi P, Ylinen A and Buzsaki G:
The hippocampal CA3 network: an in vivo intracellular labeling
study. J Comp Neurol. 339:181–208. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Barger SW, Hörster D, Furukawa K, Goodman
Y, Krieglstein J and Mattson MP: Tumor necrosis factors alpha and
beta protect neurons against amyloid beta-peptide toxicity:
evidence for involvement of a kappa B-binding factor and
attenuation of peroxide and Ca2+ accumulation. Proc Natl
Acad Sci USA. 92:9328–9332. 1995. View Article : Google Scholar
|
|
27
|
Amaral DG and Witter MP: The
three-dimensional organization of the hippocampal formation: a
review of anatomical data. Neuroscience. 31:571–591. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hasselmo ME: The role of hippocampal
regions CA3 and CA1 in matching entorhinal input with retrieval of
associations between objects and context: theoretical comment on
Lee et al (2005). Behav Neurosci. 119:342–345. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee I and Kesner RP: Differential
contribution of NMDA receptors in hippocampal subregions to spatial
working memory. Nat Neurosci. 5:162–168. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kesner RP, Lee I and Gilbert P: A
behavioral assessment of hippocampal function based on a
subregional analysis. Rev Neurosci. 15:333–351. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hunsaker MR, Allan KD and Kesner RP: Role
of dCA3 efferents via the fimbria in the acquisition of a delay
nonmatch to place task. Hippocampus. 17:494–502. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee I, Jerman TS and Kesner RP: Disruption
of delayed memory for a sequence of spatial locations following
CA1- or CA3-lesions of the dorsal hippocampus. Neurobiol Learn Mem.
84:138–147. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tanila H: Hippocampal place cells can
develop distinct representations of two visually identical
environments. Hippocampus. 9:235–246. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Leutgeb JK, Leutgeb S, Moser MB and Moser
EI: Pattern separation in the dentate gyrus and CA3 of the
hippocampus. Science. 315:961–966. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Palmer A and Good M: Hippocampal synaptic
activity, pattern separation and episodic-like memory: implications
for mouse models of Alzheimer’s disease pathology. Biochem Soc
Trans. 39:902–909. 2011.PubMed/NCBI
|
|
36
|
McEwen B: Estrogen actions throughout the
brain. Recent Prog Horm Res. 57:357–384. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Leranth C, Shanabrough M and Redmond DE
Jr: Gonadal hormones are responsible for maintaining the integrity
of spine synapses in the CA1 hippocampal subfield of female
nonhuman primates. J Comp Neurol. 447:34–42. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Leranth C, Shanabrough M and Horvath TL:
Hormonal regulation of hippocampal spine synapse density involves
subcortical mediation. Neuroscience. 101:349–356. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Prange-Kiel J, Rune GM and Leranth C:
Median raphe mediates estrogenic effects to the hippocampus in
female rats. Eur J Neurosci. 19:309–317. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Leranth C and Shanabrough M:
Supramammillary area mediates subcortical estrogenic action on
hippocampal synaptic plasticity. Exp Neurol. 167:445–450. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lâm TT and Leranth C: Role of the medial
septum diagonal band of Broca cholinergic neurons in
oestrogen-induced spine synapse formation on hippocampal CA1
pyramidal cells of female rats. Eur J Neurosci. 17:1997–2005.
2003.
|
|
42
|
Leranth C, Shanabrough M and Horvath TL:
Estrogen receptor-alpha in the raphe serotonergic and
supramammillary area calretinin-containing neurons of the female
rat. Exp Brain Res. 128:417–420. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Leranth C and Vertes RP: Median raphe
serotonergic innervation of medial septum/diagonal band of broca
(MSDB) parvalbumin-containing neurons: possible involvement of the
MSDB in the desynchronization of the hippocampal EEG. J Comp
Neurol. 410:586–598. 1999. View Article : Google Scholar
|
|
44
|
Bryant DN, Sheldahl LC, Marriott LK,
Shapiro RA and Dorsa DM: Multiple pathways transmit neuroprotective
effects of gonadal steroids. Endocrine. 29:199–207. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kelly MJ and Rønnekleiv OK:
Membrane-initiated estrogen signaling in hypothalamic neurons. Mol
Cell Endocrinol. 290:14–23. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Singer CA, Figueroa-Masot XA, Batchelor RH
and Dorsa DM: The mitogen-activated protein kinase pathway mediates
estrogen neuroprotection after glutamate toxicity in primary
cortical neurons. J Neurosci. 19:2455–2463. 1999.
|
|
47
|
Mize AL, Shapiro RA and Dorsa DM: Estrogen
receptor-mediated neuroprotection from oxidative stress requires
activation of the mitogen-activated protein kinase pathway.
Endocrinology. 144:306–312. 2003. View Article : Google Scholar
|
|
48
|
Honda K, Sawada H, Kihara T, et al:
Phosphatidylinositol 3-kinase mediates neuroprotection by estrogen
in cultured cortical neurons. J Neurosci Res. 60:321–327. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Harms C, Lautenschlager M, Bergk A, et al:
Differential mechanisms of neuroprotection by 17 beta-estradiol in
apoptotic versus necrotic neurodegeneration. J Neurosci.
21:2600–2609. 2001.PubMed/NCBI
|
|
50
|
Cimarosti H, Zamin LL, Frozza R, et al:
Estradiol protects against oxygen and glucose deprivation in rat
hippocampal organotypic cultures and activates Akt and inactivates
GSK-3beta. Neurochem Res. 30:191–199. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Funakoshi T, Yanai A, Shinoda K, Kawano MM
and Mizukami Y: G protein-coupled receptor 30 is an estrogen
receptor in the plasma membrane. Biochem Biophys Res Commun.
346:904–910. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Toran-Allerand CD: Estrogen and the brain:
beyond ER-alpha, ER-beta, and 17beta-estradiol. Ann NY Acad Sci.
1052:136–144. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Gruber CJ, Gruber DM, Gruber IM, Wieser F
and Huber JC: Anatomy of the estrogen response element. Trends
Endocrinol Metab. 15:73–78. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Paech K, Webb P, Kuiper GG, et al:
Differential ligand activation of estrogen receptors ERalpha and
ERbeta at AP1 sites. Science. 277:1508–1510. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Xiao CW and Goff AK: Hormonal regulation
of oestrogen and progesterone receptors in cultured bovine
endometrial cells. J Reprod Fertil. 115:101–109. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Murata T, Narita K, Honda K and Higuchi T:
Changes of receptor mRNAs for oxytocin and estrogen during the
estrous cycle in rat uterus. J Vet Med Sci. 65:707–712. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Tena-Sempere M, Navarro VM, Mayen A,
Bellido C and Sanchez-Criado JE: Regulation of estrogen receptor
(ER) isoform messenger RNA expression by different ER ligands in
female rat pituitary. Biol Reprod. 70:671–678. 2004. View Article : Google Scholar : PubMed/NCBI
|