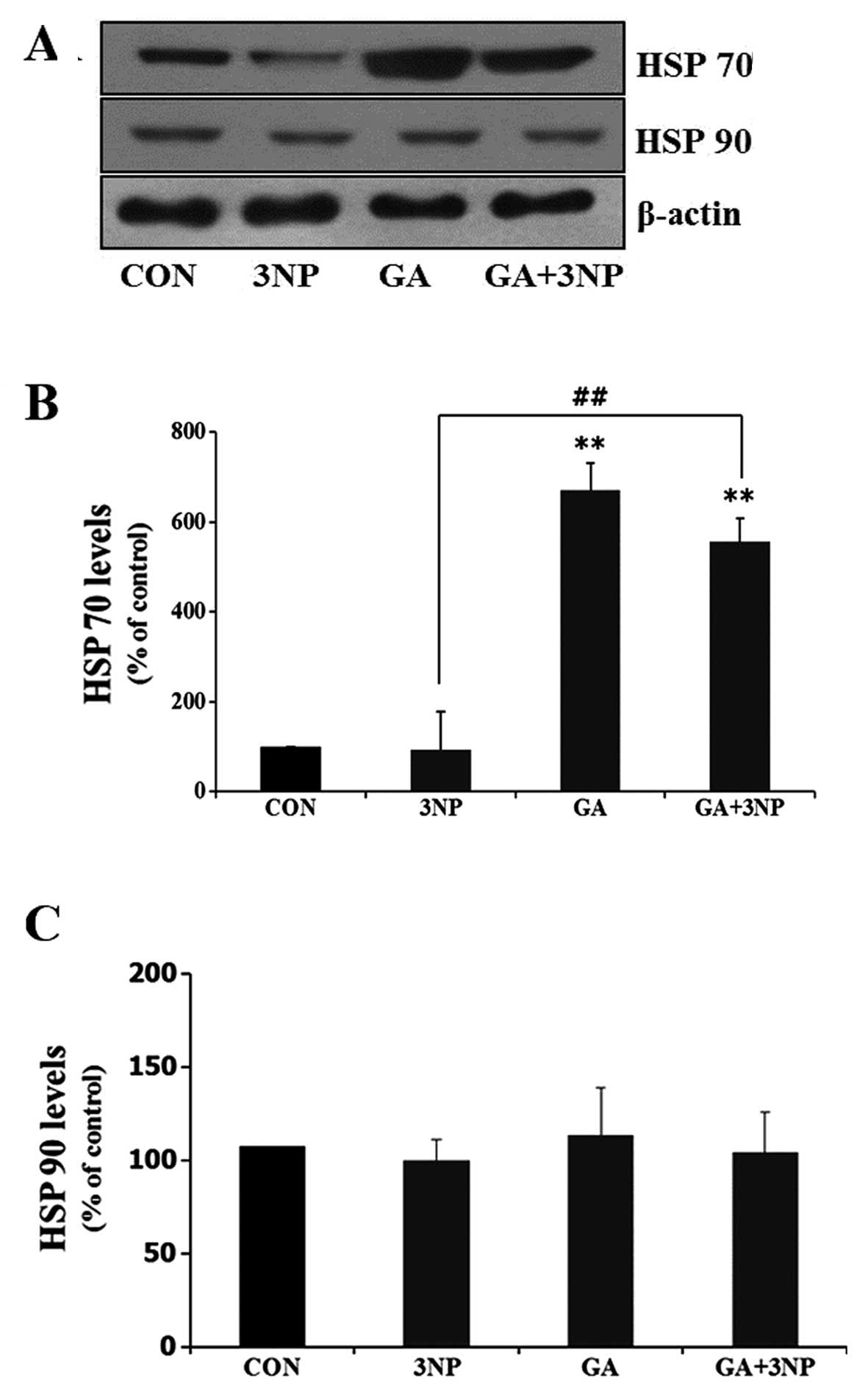
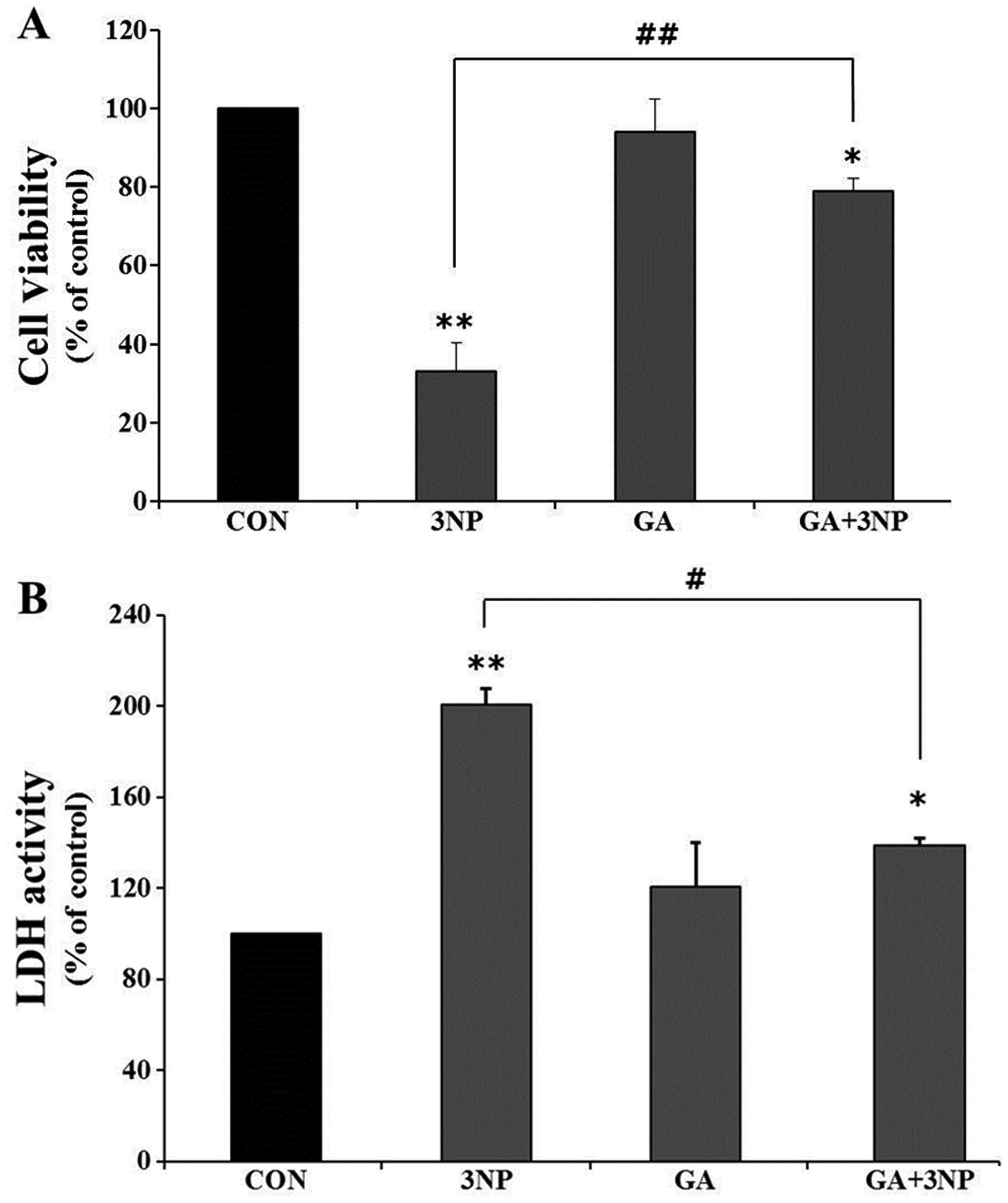
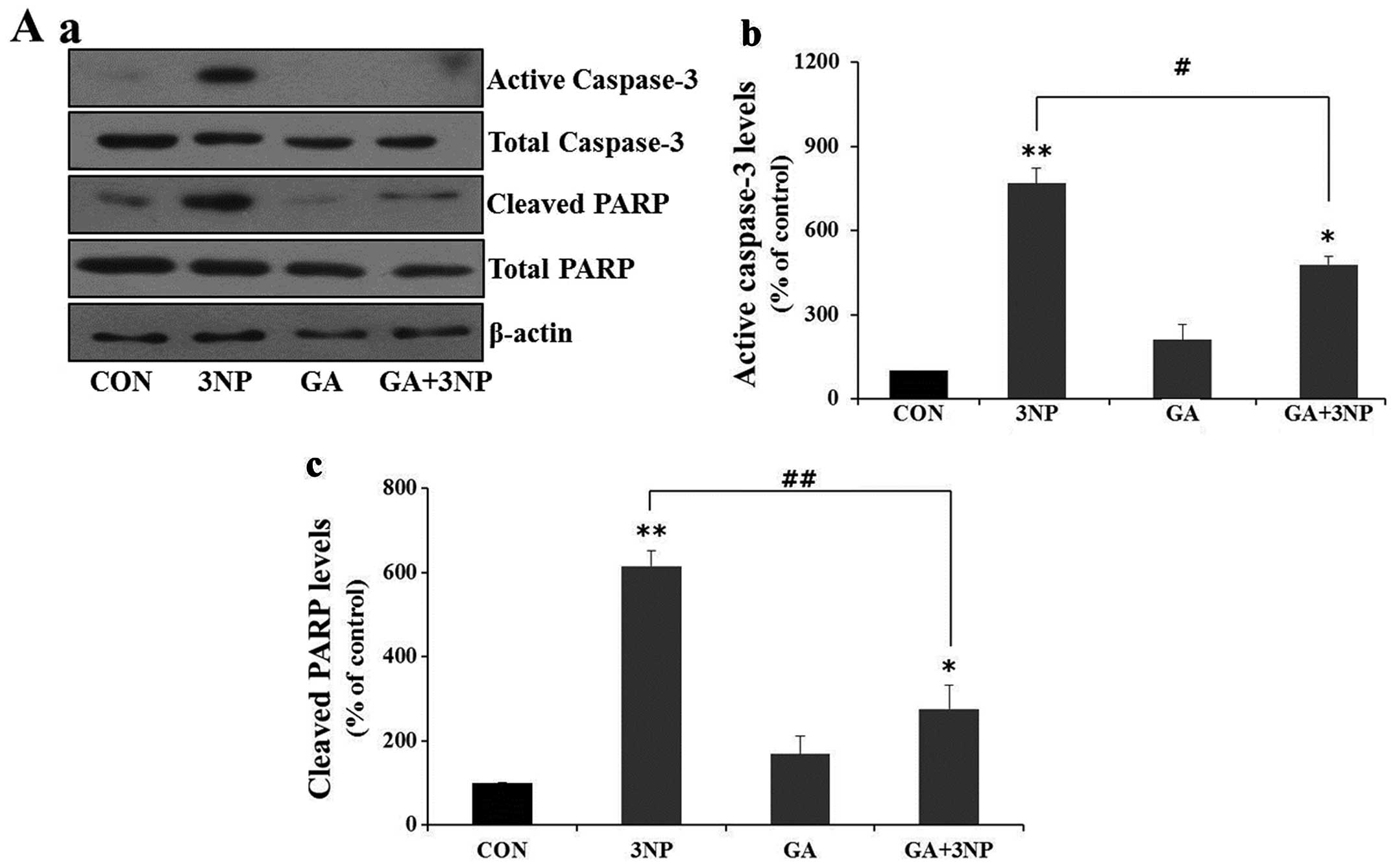
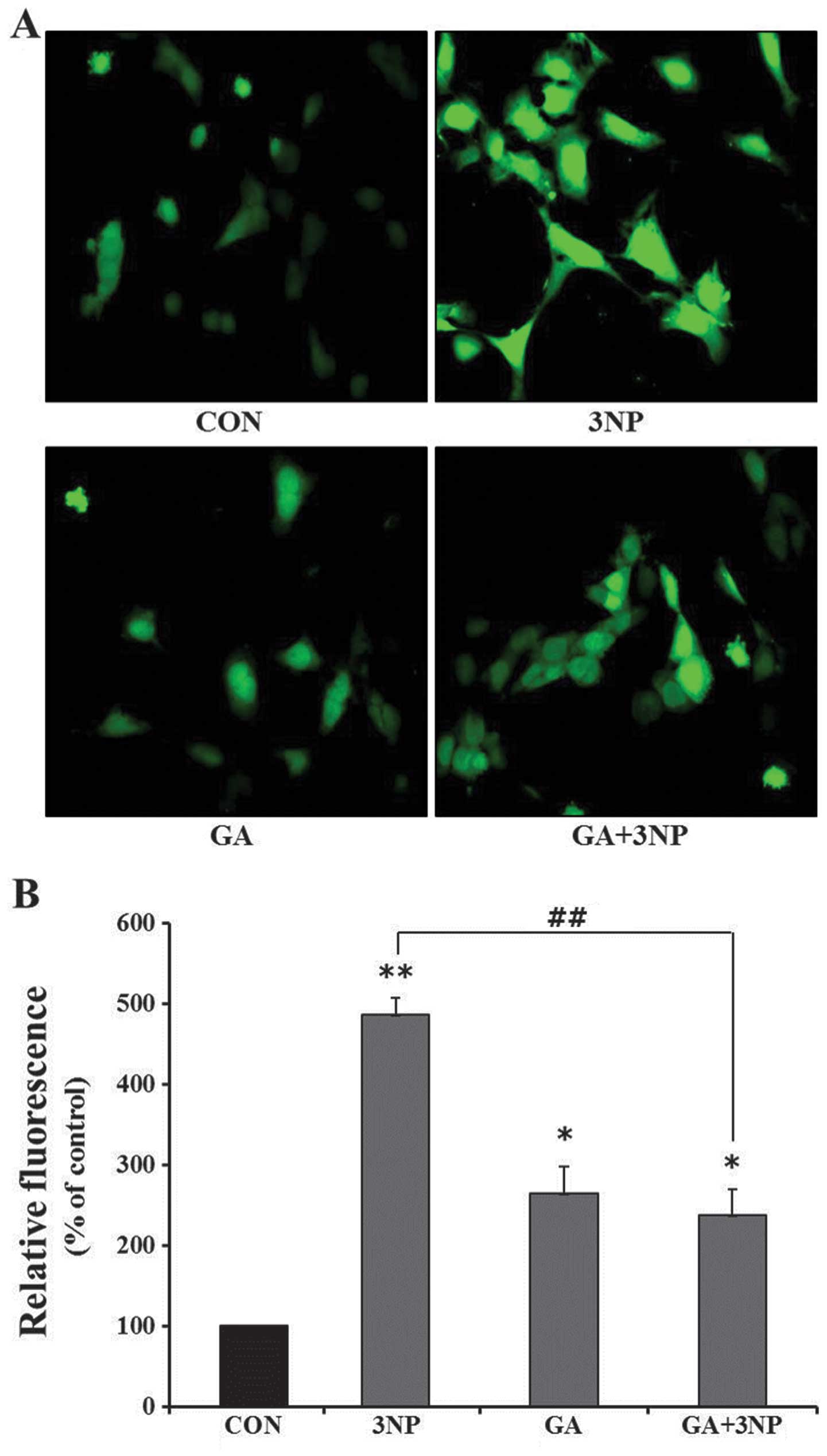
|
1
|
Andrew SE, Goldberg YP, Kremer B, et al:
The relationship between trinucleotide (CAG) repeat length and
clinical features of Huntington’s disease. Nat Genet. 4:398–403.
1993.
|
|
2
|
Vonsattel JP and DiFiglia M: Huntington
disease. J Neuropathol Exp Neurol. 57:369–384. 1998. View Article : Google Scholar
|
|
3
|
Beal MF: Mitochondria take center stage in
aging and neurodegeneration. Ann Neurol. 58:495–505. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Oliveira JM, Jekabsons MB, Chen S, et al:
Mitochondrial dysfunction in Huntington’s disease: the
bioenergetics of isolated and in situ mitochondria from transgenic
mice. J Neurochem. 101:241–249. 2007.
|
|
5
|
Garcia M, Vanhoutte P, Pages C, Besson MJ,
Brouillet E and Caboche J: The mitochondrial toxin 3-nitropropionic
acid induces striatal neurodegeneration via a c-Jun N-terminal
kinase/c-Jun module. J Neurosci. 22:2174–2184. 2002.PubMed/NCBI
|
|
6
|
Brouillet E, Condé F, Beal MF and Hantraye
P: Replicating Huntington’s disease phenotype in experimental
animals. Prog Neurobiol. 59:427–468. 1999.
|
|
7
|
Choi YJ, Om JY, Kim NH, et al: Heat shock
transcription factor-1 suppresses apoptotic cell death and ROS
generation in 3-nitropropionic acid-stimulated striatal cells. Mol
Cell Biochem. 375:59–67. 2013.PubMed/NCBI
|
|
8
|
Dedeoglu A, Ferrante RJ, Andreassen OA,
Dillmann WH and Beal MF: Mice overexpressing 70-kDa heat shock
protein show increased resistance to malonate and 3-nitropropionic
acid. Exp Neurol. 176:262–265. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takayama S, Reed JC and Homma S:
Heat-shock proteins as regulators of apoptosis. Oncogene.
22:9041–9047. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Beere HM, Wolf BB, Cain K, et al:
Heat-shock protein 70 inhibits apoptosis by preventing recruitment
of procaspase-9 to the Apaf-1 apoptosome. Nat Cell Biol. 2:469–475.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cummings CJ, Mancini MA, Antalffy B,
DeFranco DB, Orr HT and Zoghbi HY: Chaperone suppression of
aggregation and altered subcellular proteasome localization imply
protein misfolding in SCA1. Nat Genet. 19:148–154. 1998. View Article : Google Scholar
|
|
12
|
Kobayashi Y, Kume A, Li M, et al:
Chaperones Hsp70 and Hsp40 suppress aggregate formation and
apoptosis in cultured neuronal cells expressing truncated androgen
receptor protein with expanded polyglutamine tract. J Biol Chem.
275:8772–8778. 2000. View Article : Google Scholar
|
|
13
|
Wyttenbach A, Swartz J, Kita H, et al:
Polyglutamine expansions cause decreased CRE-mediated transcription
and early gene expression changes prior to cell death in an
inducible cell model of Huntington’s disease. Hum Mol Genet.
10:1829–1845. 2001.PubMed/NCBI
|
|
14
|
Mosser DD, Caron AW, Bourget L,
Denis-Larose C and Massie B: Role of the human heat shock protein
hsp70 in protection against stress-induced apoptosis. Mol Cell
Biol. 17:5317–5327. 1997.PubMed/NCBI
|
|
15
|
Jäättelä M, Wissing D, Kokholm K, Kallunki
T and Egeblad M: Hsp70 exerts its anti-apoptotic function
downstream of caspase-3-like proteases. EMBO J. 17:6124–6134.
1998.PubMed/NCBI
|
|
16
|
Mosser DD, Caron AW, Bourget L, et al: The
chaperone function of hsp70 is required for protection against
stress-induced apoptosis. Mol Cell Biol. 20:7146–7159. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ravagnan L, Gurbuxani S, Susin SA, et al:
Heat-shock protein 70 antagonizes apoptosis-inducing factor. Nat
Cell Biol. 3:839–843. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gurbuxani S, Schmitt E, Cande C, et al:
Heat shock protein 70 binding inhibits the nuclear import of
apoptosis-inducing factor. Oncogene. 22:6669–6678. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Koga F, Xu W, Karpova TS, McNally JG,
Baron R and Neckers L: Hsp90 inhibition transiently activates Src
kinase and promotes Src-dependent Akt and Erk activation. Proc Natl
Acad Sci USA. 103:11318–11322. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schulte TW, Akinaga S, Soga S, et al:
Antibiotic radicicol binds to the N-terminal domain of Hsp90 and
shares important biologic activities with geldanamycin. Cell Stress
Chaperones. 3:100–108. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zou J, Guo Y, Guettouche T, Smith DF and
Voellmy R: Repression of heat shock transcription factor HSF1
activation by HSP90 (HSP90 complex) that forms a stress-sensitive
complex with HSF1. Cell. 94:471–480. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bagatell R, Paine-Murrieta GD, Taylor CW,
et al: Induction of a heat shock factor 1-dependent stress response
alters the cytotoxic activity of hsp90-binding agents. Clin Cancer
Res. 6:3312–3318. 2000.PubMed/NCBI
|
|
23
|
Sittler A, Lurz R, Lueder G, et al:
Geldanamycin activates a heat shock response and inhibits
huntingtin aggregation in a cell culture model of Huntington’s
disease. Hum Mol Genet. 10:1307–1315. 2001.PubMed/NCBI
|
|
24
|
Bian X, McAllister-Lucas LM, Shao F, et
al: NF-kappa B activation mediates doxorubicin-induced cell death
in N-type neuroblastoma cells. J Biol Chem. 276:48921–48929. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bubici C, Papa S, Pham CG, Zazzeroni F and
Franzoso G: The NF-kappaB-mediated control of ROS and JNK
signaling. Histol Histopathol. 21:69–80. 2006.PubMed/NCBI
|
|
26
|
Dumont A, Hehner SP, Hofmann TG, Ueffing
M, Dröge W and Schmitz ML: Hydrogen peroxide-induced apoptosis is
CD95-independent, requires the release of mitochondria-derived
reactive oxygen species and the activation of NF-kappaB. Oncogene.
18:747–757. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Westerheide SD, Kawahara TL, Orton K and
Morimoto RI: Triptolide, an inhibitor of the human heat shock
response that enhances stress-induced cell death. J Biol Chem.
281:9616–9622. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wyttenbach A: Role of heat shock proteins
during polyglutamine neurodegeneration: mechanisms and hypothesis.
J Mol Neurosci. 23:69–96. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Muchowski PJ and Wacker JL: Modulation of
neurodegeneration by molecular chaperones. Nat Rev Neurosci.
6:11–22. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ciocca DR, Clark GM, Tandon AK, Fuqua SA,
Welch WJ and McGuire WL: Heat shock protein hsp70 in patients with
axillary lymph node-negative breast cancer: prognostic
implications. J Natl Cancer Inst. 85:570–574. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dive C and Hickman JA: Drug-target
interactions: only the first step in the commitment to a programmed
cell death? Br J Cancer. 64:192–196. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Seo JS, Park YM, Kim JI, et al: T cell
lymphoma in transgenic mice expressing the human Hsp70 gene.
Biochem Biophys Res Commun. 218:582–587. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wei YQ, Zhao X, Kariya Y, Teshigawara K
and Uchida A: Inhibition of proliferation and induction of
apoptosis by abrogation of heat-shock protein (HSP) 70 expression
in tumor cells. Cancer Immunol Immunother. 40:73–78. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Haldimann P, Muriset M, Vigh L and
Goloubinoff P: The novel hydroxylamine derivative NG-094 suppresses
polyglutamine protein toxicity in Caenorhabditis elegans. J
Biol Chem. 286:18784–18794
|
|
35
|
Neckers L: Hsp90 inhibitors as novel
cancer chemotherapeutic agents. Trends Mol Med. 8:S55–S61. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Panaretou B, Prodromou C, Roe SM, et al:
ATP binding and hydrolysis are essential to the function of the
Hsp90 molecular chaperone in vivo. EMBO J. 17:4829–4836. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Grenert JP, Johnson BD and Toft DO: The
importance of ATP binding and hydrolysis by hsp90 in formation and
function of protein heterocomplexes. J Biol Chem. 274:17525–17533.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schneider C, Sepp-Lorenzino L, Nimmesgern
E, et al: Pharmacologic shifting of a balance between protein
refolding and degradation mediated by Hsp90. Proc Natl Acad Sci
USA. 93:14536–14541. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Connell P, Ballinger CA, Jiang J, et al:
The co-chaperone CHIP regulates protein triage decisions mediated
by heat-shock proteins. Nat Cell Biol. 3:93–96. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xu W, Marcu M, Yuan X, Mimnaugh E,
Patterson C and Neckers L: Chaperone-dependent E3 ubiquitin ligase
CHIP mediates a degradative pathway for c-ErbB2/Neu. Proc Natl Acad
Sci USA. 99:12847–12852. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wacker JL, Huang SY, Steele AD, et al:
Loss of Hsp70 exacerbates pathogenesis but not levels of fibrillar
aggregates in a mouse model of Huntington’s disease. J Neurosci.
29:9104–9114. 2009.
|
|
42
|
Shen HY, He JC, Wang Y, Huang QY and Chen
JF: Geldanamycin induces heat shock protein 70 and protects against
MPTP-induced dopaminergic neurotoxicity in mice. J Biol Chem.
280:39962–39969. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Beal MF: Energetics in the pathogenesis of
neurodegenerative diseases. Trends Neurosci. 23:298–304. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gu M, Gash MT, Mann VM, Javoy-Agid F,
Cooper JM and Schapira AH: Mitochondrial defect in Huntington’s
disease caudate nucleus. Ann Neurol. 39:385–389. 1996.
|
|
45
|
Browne SE, Bowling AC, MacGarvey U, et al:
Oxidative damage and metabolic dysfunction in Huntington’s disease:
selective vulnerability of the basal ganglia. Ann Neurol.
41:646–653. 1997.
|
|
46
|
Sawa A, Wiegand GW, Cooper J, et al:
Increased apoptosis of Huntington disease lymphoblasts associated
with repeat length-dependent mitochondrial depolarization. Nat Med.
5:1194–1198. 1999. View
Article : Google Scholar
|
|
47
|
Beal MF, Brouillet E, Jenkins BG, et al:
Neurochemical and histologic characterization of striatal
excitotoxic lesions produced by the mitochondrial toxin
3-nitropropionic acid. J Neurosci. 13:4181–4192. 1993.
|
|
48
|
Hamilton BF and Gould DH: Nature and
distribution of brain lesions in rats intoxicated with
3-nitropropionic acid: a type of hypoxic (energy deficient) brain
damage. Acta Neuropathol. 72:286–297. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Brouillet E, Hantraye P, Ferrante RJ, et
al: Chronic mitochondrial energy impairment produces selective
striatal degeneration and abnormal choreiform movements in
primates. Proc Natl Acad Sci USA. 92:7105–7109. 1995. View Article : Google Scholar
|
|
50
|
Palfi S, Ferrante RJ, Brouillet E, et al:
Chronic 3-nitropropionic acid treatment in baboons replicates the
cognitive and motor deficits of Huntington’s disease. J Neurosci.
16:3019–3025. 1996.PubMed/NCBI
|
|
51
|
Gabai VL, Meriin AB, Yaglom JA, Volloch VZ
and Sherman MY: Role of Hsp70 in regulation of stress-kinase JNK:
implications in apoptosis and aging. FEBS Lett. 438:1–4. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Meriin AB, Yaglom JA, Gabai VL, et al:
Protein-damaging stresses activate c-Jun N-terminal kinase via
inhibition of its dephosphorylation: a novel pathway controlled by
HSP72. Mol Cell Biol. 19:2547–2555. 1999.PubMed/NCBI
|
|
53
|
Kyriakis JM and Avruch J: Protein kinase
cascades activated by stress and inflammatory cytokines. Bioessays.
18:567–577. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Park HS, Lee JS, Huh SH, Seo JS and Choi
EJ: Hsp72 functions as a natural inhibitory protein of c-Jun
N-terminal kinase. EMBO J. 20:446–456. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lee JS, Lee JJ and Seo JS: HSP70
deficiency results in activation of c-Jun N-terminal Kinase,
extracellular signal-regulated kinase, and caspase-3 in
hyperosmolarity-induced apoptosis. J Biol Chem. 280:6634–6641.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Li H, Liu L, Xing D and Chen WR:
Inhibition of the JNK/Bim pathway by Hsp70 prevents Bax activation
in UV-induced apoptosis. FEBS Lett. 584:4672–4678. 2010. View Article : Google Scholar : PubMed/NCBI
|