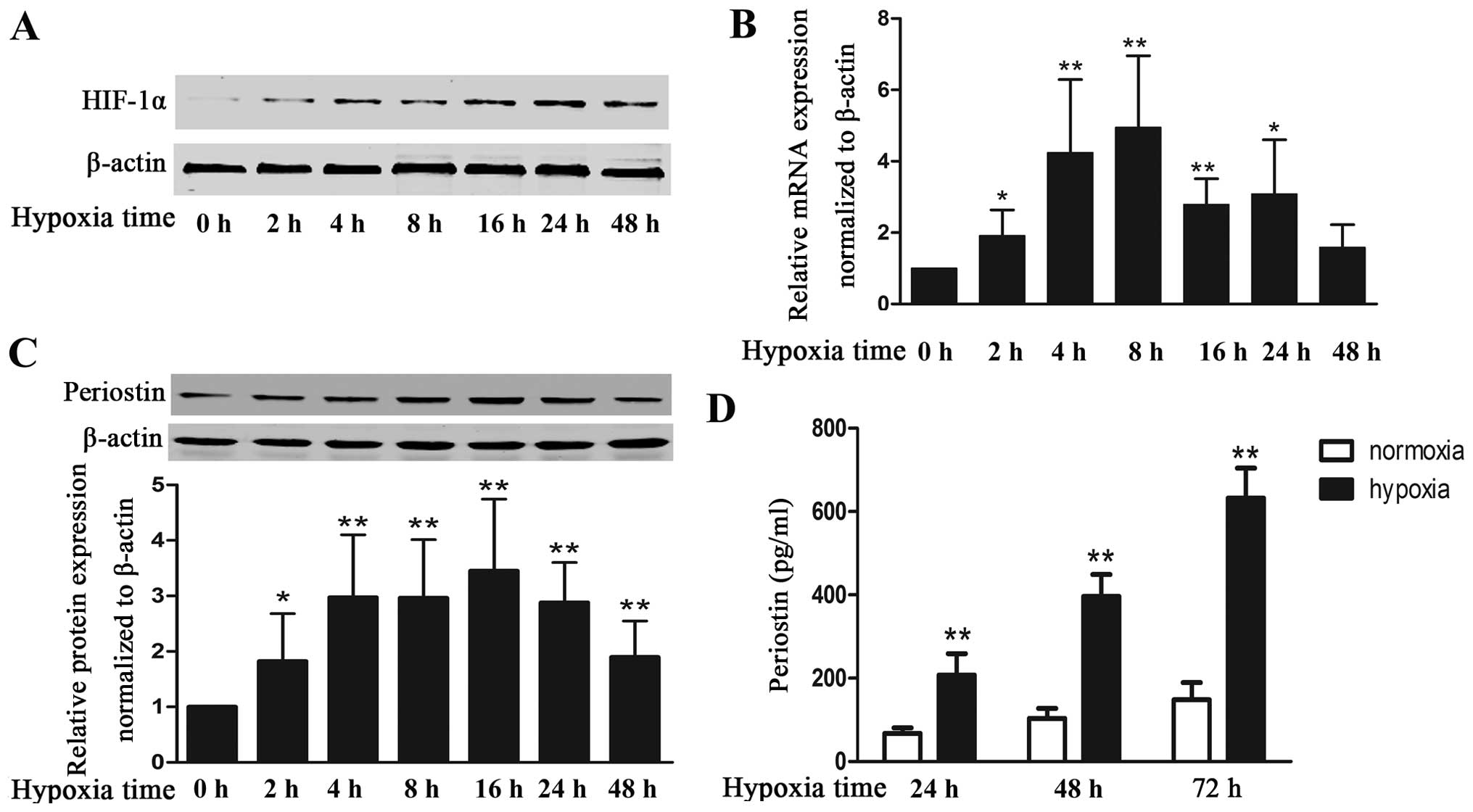
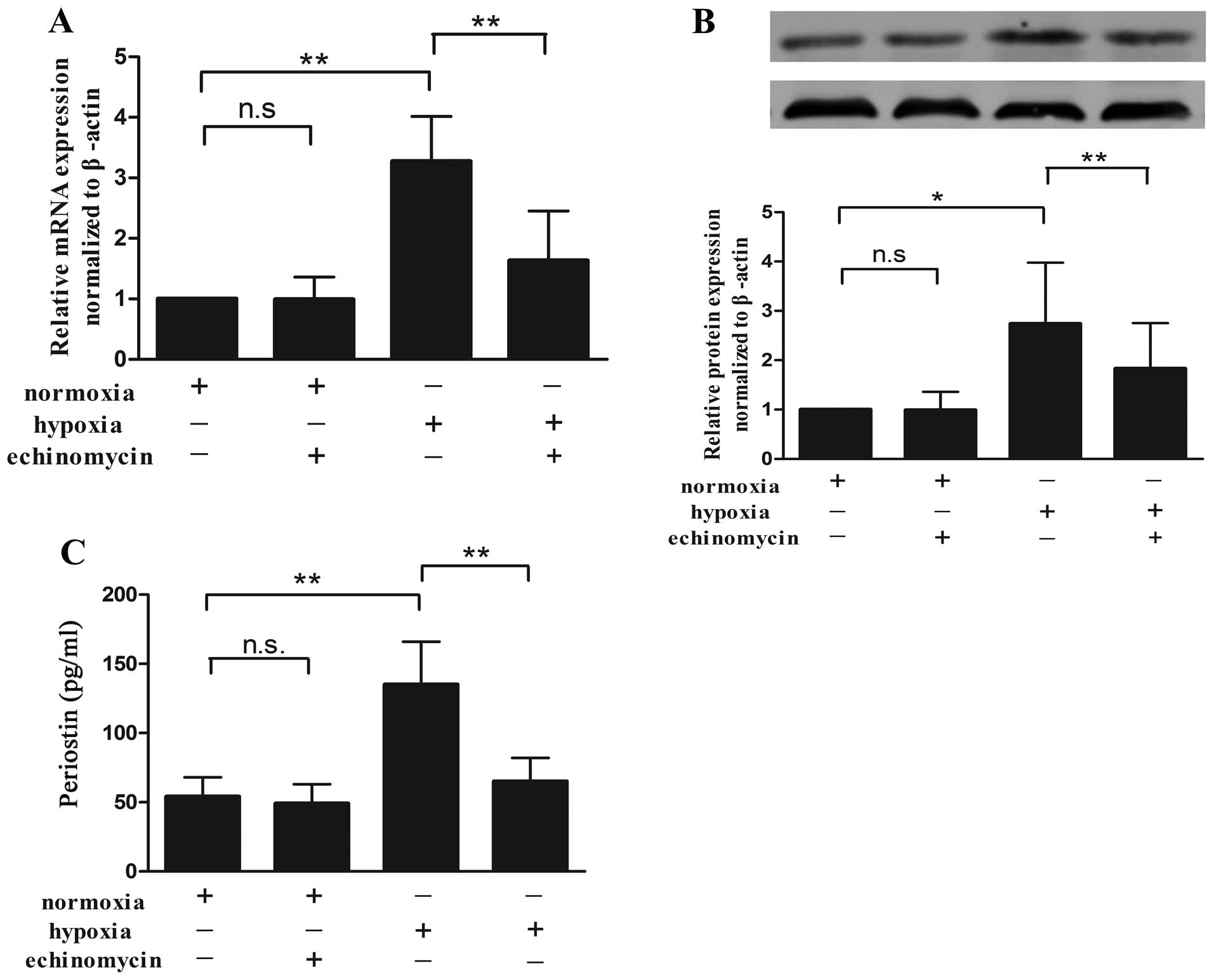
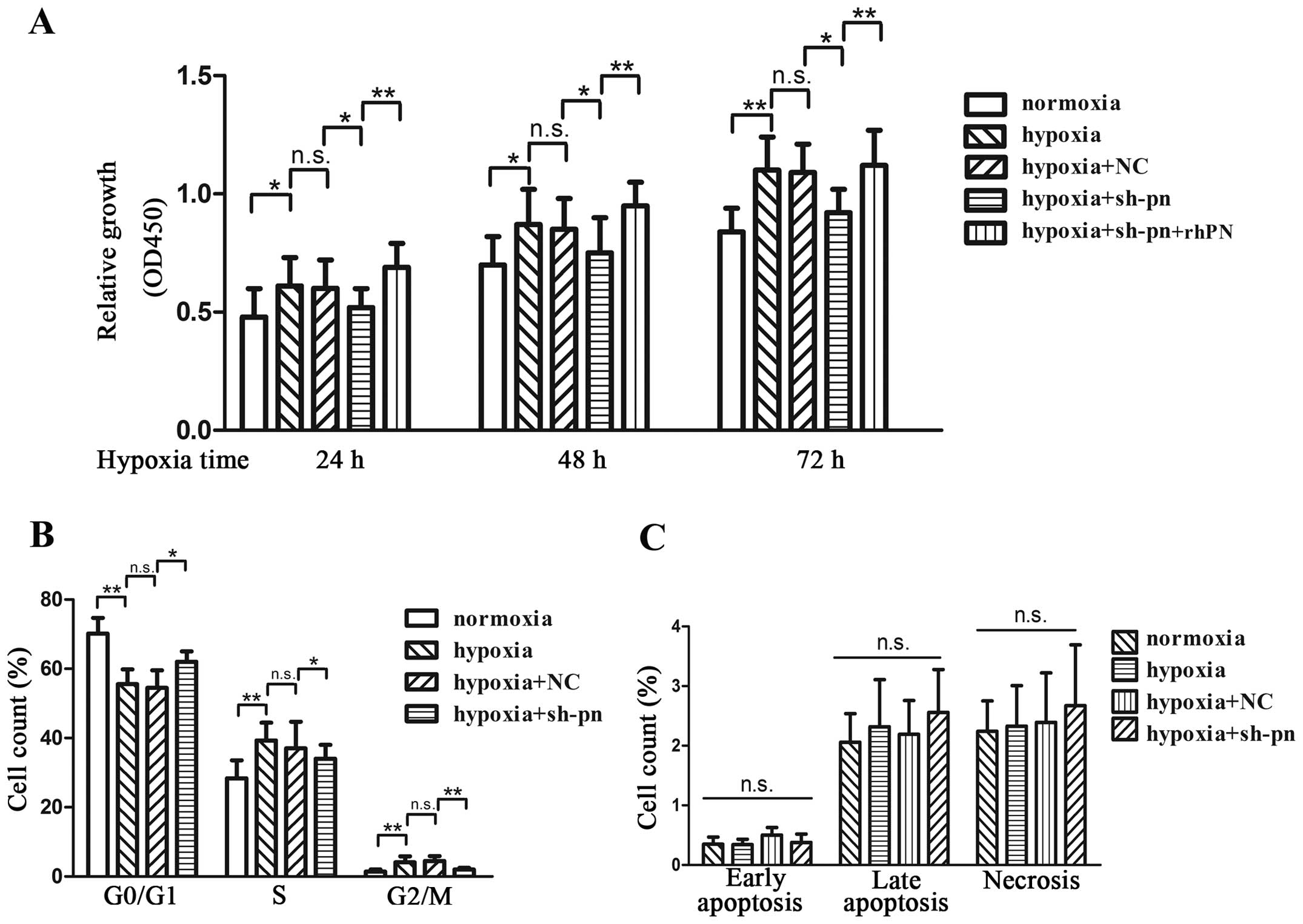
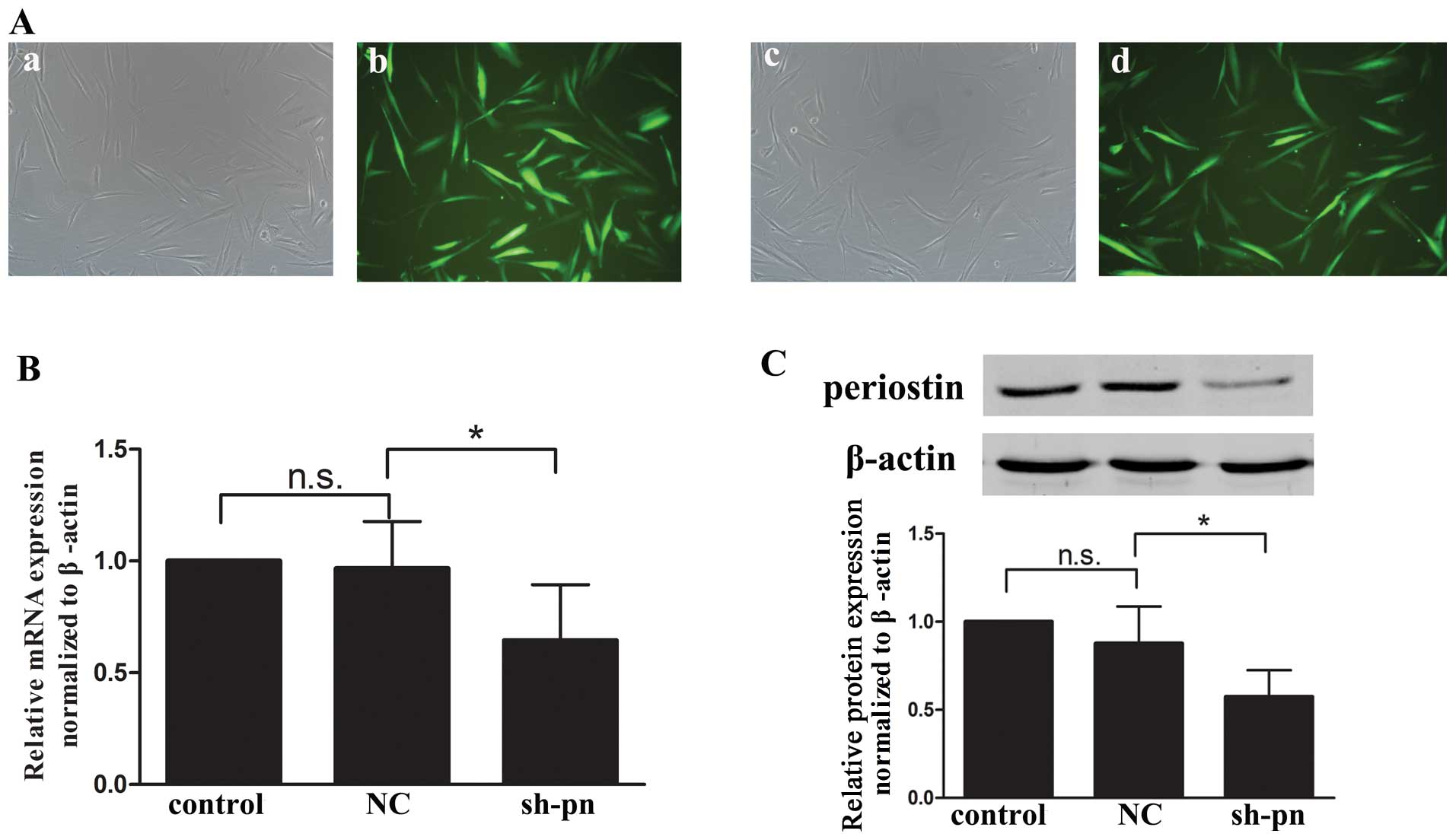
|
1
|
Niessen FB, Spauwen PH, Schalkwijk J and
Kon M: On the nature of hypertrophic scars and keloids: a review.
Plast Reconstr Surg. 104:1435–1458. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bran GM, Goessler UR, Hormann K, Riedel F
and Sadick H: Keloids: Current concepts of pathogenesis (Review).
Int J Mol Med. 24:283–293. 2009.PubMed/NCBI
|
|
3
|
Love PB and Kundu RV: Keloids: an update
on medical and surgical treatments. J Drugs Dermatol. 12:403–409.
2013.PubMed/NCBI
|
|
4
|
Zhang Q, Wu Y, Ann DK, et al: Mechanisms
of hypoxic regulation of plasminogen activator inhibitor-1 gene
expression in keloid fibroblasts. J Invest Dermatol. 121:1005–1012.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ueda K, Yasuda Y, Furuya E and Oba S:
Inadequate blood supply persists in keloids. Scand J Plast Reconstr
Surg Hand Surg. 38:267–271. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Steinbrech DS, Mehrara BJ, Chau D, et al:
Hypoxia upregulates VEGF production in keloid fibroblasts. Ann
Plast Surg. 42:514–520. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang Q, Wu Y, Chau CH, Ann DK, Bertolami
CN and Le AD: Crosstalk of hypoxia-mediated signaling pathways in
upregulating plasminogen activator inhibitor-1 expression in keloid
fibroblasts. J Cell Physiol. 199:89–97. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ahluwalia A and Tarnawski AS: Critical
role of hypoxia sensor - HIF-1alpha in VEGF gene activation.
Implications for angiogenesis and tissue injury healing. Curr Med
Chem. 19:90–97. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ben-Yosef Y, Lahat N, Shapiro S, Bitterman
H and Miller A: Regulation of endothelial matrix
metalloproteinase-2 by hypoxia/reoxygenation. Circ Res. 90:784–791.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Horiuchi K, Amizuka N, Takeshita S, et al:
Identification and characterization of a novel protein, periostin,
with restricted expression to periosteum and periodontal ligament
and increased expression by transforming growth factor beta. J Bone
Miner Res. 14:1239–1249. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou HM, Wang J, Elliott C, Wen W,
Hamilton DW and Conway SJ: Spatiotemporal expression of periostin
during skin development and incisional wound healing: lessons for
human fibrotic scar formation. J Cell Commun Signal. 4:99–107.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kyutoku M, Taniyama Y, Katsuragi N, et al:
Role of periostin in cancer progression and metastasis: Inhibition
of breast cancer progression and metastasis by anti-periostin
antibody in a murine model. Int J Mol Med. 28:181–186.
2011.PubMed/NCBI
|
|
13
|
Wang Q, Nie FF, Zhao X and Qin ZL: The
expression of periostin in hyperplasic scars and the relations to
TGF-beta1 and its receptors. Zhonghua Zheng Xing Wai Ke Za Zhi.
23:229–232. 2007.(In Chinese).
|
|
14
|
Liu C, Song ZH and Qin ZL: Construction of
periostin shRNA vectors and their effects on the expression of
periostin in fibroblasts. Beijing Da Xue Xue Bao. 42:503–508.
2010.PubMed/NCBI
|
|
15
|
Kong D, Park EJ, Stephen AG, et al:
Echinomycin, a small-molecule inhibitor of hypoxia-inducible
factor-1 DNA-binding activity. Cancer Res. 65:9047–9055. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Alster TS and Tanzi EL: Hypertrophic scars
and keloids: etiology and management. Am J Clin Dermatol.
4:235–243. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Song J, Xu H, Lu Q, et al: Madecassoside
suppresses migration of fibroblasts from keloids: involvement of
p38 kinase and PI3K signaling pathways. Burns. 38:677–684. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Syed F, Sherris D, Paus R, Varmeh S,
Pandolfi PP and Bayat A: Keloid disease can be inhibited by
antagonizing excessive mTOR signaling with a novel dual TORC1/2
inhibitor. Am J Pathol. 181:1642–1658. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gillan L, Matei D, Fishman DA, Gerbin CS,
Karlan BY and Chang DD: Periostin secreted by epithelial ovarian
carcinoma is a ligand for alpha(V)beta(3) and alpha(V)beta(5)
integrins and promotes cell motility. Cancer Res. 62:5358–5364.
2002.PubMed/NCBI
|
|
20
|
Chung KC, Disa JJ, Gosain AK, Kinney BM
and Rubin JP: Plastic Surgery. 1st edition. Elsevier; New York:
2009
|
|
21
|
Lokmic Z, Musyoka J, Hewitson TD and Darby
IA: Hypoxia and hypoxia signaling in tissue repair and fibrosis.
Int Rev Cell Mol Biol. 296:139–185. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kischer CW: The microvessels in
hypertrophic scars, keloids and related lesions: a review. J
Submicrosc Cytol Pathol. 24:281–296. 1992.PubMed/NCBI
|
|
23
|
Kischer CW, Thies AC and Chvapil M:
Perivascular myofibroblasts and microvascular occlusion in
hypertrophic scars and keloids. Hum Pathol. 13:819–824. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Watanabe T, Yasue A, Fujihara S and Tanaka
E: PERIOSTIN regulates MMP-2 expression via the αvβ3 integrin/ERK
pathway in human periodontal ligament cells. Arch Oral Biol.
57:52–59. 2012.PubMed/NCBI
|
|
25
|
Li P, Oparil S, Feng W and Chen YF:
Hypoxia-responsive growth factors upregulate periostin and
osteopontin expression via distinct signaling pathways in rat
pulmonary arterial smooth muscle cells. J Appl Physiol.
1985.97:1550–1558; discussion 1549, 2004.
|
|
26
|
Deschene K, Céleste C, Boerboom D and
Theoret CL: Hypoxia regulates the expression of extracellular
matrix associated proteins in equine dermal fibroblasts via HIF1. J
Dermatol Sci. 65:12–18. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vincent AS, Phan TT, Mukhopadhyay A, Lim
HY, Halliwell B and Wong KP: Human skin keloid fibroblasts display
bioenergetics of cancer cells. J Invest Dermatol. 128:702–709.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mucaj V, Shay JE and Simon MC: Effects of
hypoxia and HIFs on cancer metabolism. Int J Hematol. 95:464–470.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen J, Zeng B, Yao H and Xu J: The effect
of TLR4/7 on the TGF-β-induced Smad signal transduction pathway in
human keloid. Burns. 39:465–472. 2013.PubMed/NCBI
|
|
30
|
Bran GM, Goessler UR, Schardt C, Hormann
K, Riedel F and Sadick H: Effect of the abrogation of TGF-β1 by
antisense oligonucleotides on the expression of TGF-β-isoforms and
their receptors I and II in isolated fibroblasts from keloid scars.
Int J Mol Med. 25:915–921. 2010.
|
|
31
|
Wu CS, Wu PH, Fang AH and Lan CC: FK506
inhibits the enhancing effects of transforming growth factor
(TGF)-β1 on collagen expression and TGF-β/Smad signalling in keloid
fibroblasts: implication for new therapeutic approach. Br J
Dermatol. 167:532–541. 2012.
|
|
32
|
Bettinger DA, Yager DR, Diegelmann RF and
Cohen IK: The effect of TGF-beta on keloid fibroblast proliferation
and collagen synthesis. Plast Reconstr Surg. 98:827–833. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lindsley A, Li W, Wang J, Maeda N, Rogers
R and Conway SJ: Comparison of the four mouse fasciclin-containing
genes expression patterns during valvuloseptal morphogenesis. Gene
Expr Patterns. 5:593–600. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Naik PK, Bozyk PD, Bentley JK, et al:
Periostin promotes fibrosis and predicts progression in patients
with idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol
Physiol. 303:L1046–L1056. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Huang L, Wong YP, Cai YJ, Lung I, Leung CS
and Burd A: Low-dose 5-fluorouracil induces cell cycle G2 arrest
and apoptosis in keloid fibroblasts. Br J Dermatol. 163:1181–1185.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ouyang G, Liu M, Ruan K, Song G, Mao Y and
Bao S: Upregulated expression of periostin by hypoxia in
non-small-cell lung cancer cells promotes cell survival via the
Akt/PKB pathway. Cancer Lett. 281:213–219. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Greijer AE and van der Wall E: The role of
hypoxia inducible factor 1 (HIF-1) in hypoxia induced apoptosis. J
Clin Pathol. 57:1009–1014. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bao S, Ouyang G, Bai X, et al: Periostin
potently promotes metastatic growth of colon cancer by augmenting
cell survival via the Akt/PKB pathway. Cancer Cell. 5:329–339.
2004. View Article : Google Scholar
|
|
39
|
Barrientos S, Stojadinovic O, Golinko MS,
Brem H and Tomic-Canic M: Growth factors and cytokines in wound
healing. Wound Repair Regen. 16:585–601. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hou JJ, Nie FF, Li BL, et al: The
expressions of periostin and the related factors during healing
process of full-thickness cutaneous wound in rat. Zhongguo Wei
Zhong Bing Ji Jiu Yi Xue. 24:334–337. 2012.(In Chinese).
|
|
41
|
Elliott CG, Wang J, Guo X, et al:
Periostin modulates myofibroblast differentiation during
full-thickness cutaneous wound repair. J Cell Sci. 125:121–132.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shimazaki M, Nakamura K, Kii I, et al:
Periostin is essential for cardiac healing after acute myocardial
infarction. J Exp Med. 205:295–303. 2008. View Article : Google Scholar : PubMed/NCBI
|