|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar
|
|
2
|
Irvin WJ Jr and Carey LA: What is
triple-negative breast cancer? Eur J Cancer. 44:2799–2805. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bauer KR, Brown M, Cress RD, Parise CA and
Caggiano V: Descriptive analysis of estrogen receptor
(ER)-negative, progesterone receptor (PR)-negative, and
HER2-negative invasive breast cancer, the so-called triple-negative
phenotype: a population-based study from the California cancer
Registry. Cancer. 109:1721–1728. 2007. View Article : Google Scholar
|
|
4
|
Lin NU, Claus E, Sohl J, Razzak AR,
Arnaout A and Winer EP: Sites of distant relapse and clinical
outcomes in patients with metastatic triple-negative breast cancer:
high incidence of central nervous system metastases. Cancer.
113:2638–2645. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Haffty BG, Yang Q, Reiss M, et al:
Locoregional relapse and distant metastasis in conservatively
managed triple negative early-stage breast cancer. J Clin Oncol.
24:5652–5657. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dent R, Trudeau M, Pritchard KI, et al:
Triple-negative breast cancer: clinical features and patterns of
recurrence. Clin Cancer Res. 13:4429–4434. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kassam F, Enright K, Dent R, et al:
Survival outcomes for patients with metastatic triple-negative
breast cancer: implications for clinical practice and trial design.
Clin Breast Cancer. 9:29–33. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
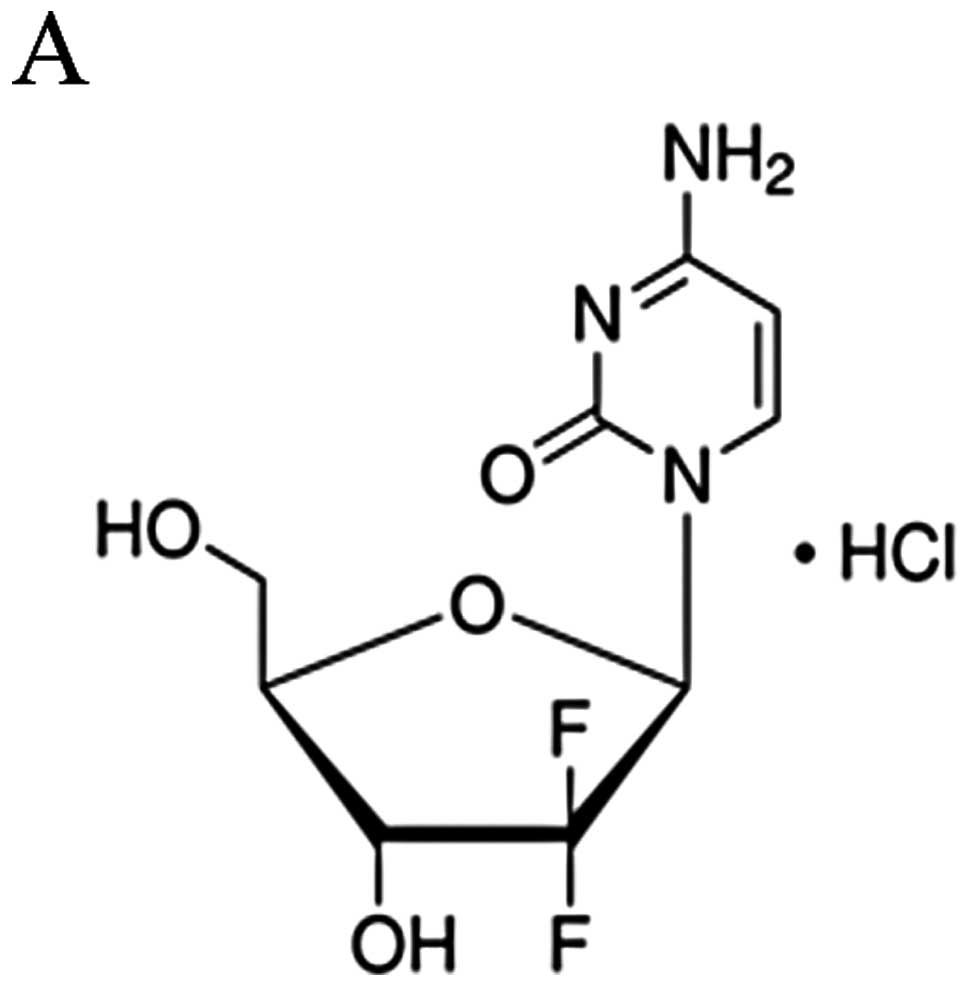
Huang P, Chubb S, Hertel LW, Grindey GB
and Plunkett W: Action of 2′,2′-difluorodeoxycytidine on DNA
synthesis. Cancer Res. 51:6110–6117. 1991.
|
|
9
|
Silvestris N, D’Aprile M, Andreola G,
Locopo N, Marini L, Crucitta E, De Lena M and Lorusso V: Rationale
for the use of gemcitabine in breast cancer (Review). Int J Oncol.
24:389–398. 2004.PubMed/NCBI
|
|
10
|
Passardi A, Massa I, Zoli W, et al: Phase
II study of gemcitabine, doxorubicin and paclitaxel (GAT) as
first-line chemotherapy for metastatic breast cancer: a
translational research experience. BMC Cancer. 6:762006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
O’Shaughnessy JA, Pluenneke R, Sternberg
J, Khandelwal P, Ilegbodu D and Asmar L: Phase II trial of weekly
docetaxel/gemcitabine as first-line chemotherapy in patients with
locally recurrent or metastatic breast cancer. Clin Breast Cancer.
6:505–510. 2006.
|
|
12
|
Tomao S, Romiti A, Tomao F, et al: A phase
II trial of a biweekly combination of paclitaxel and gemcitabine in
metastatic breast cancer. BMC Cancer. 6:1372006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hernández-Vargas H, Rodríguez-Pinilla SM,
Julián-Tendero M, et al: Gene expression profiling of breast cancer
cells in response to gemcitabine: NF-kappaB pathway activation as a
potential mechanism of resistance. Breast Cancer Res Treat.
102:157–172. 2007.PubMed/NCBI
|
|
14
|
Mizushima N and Komatsu M: Autophagy:
renovation of cells and tissues. Cell. 147:728–741. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rubinsztein DC, Codogno P and Levine B:
Autophagy modulation as a potential therapeutic target for diverse
diseases. Nat Rev Drug Discov. 11:709–730. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kroemer G and Levine B: Autophagic cell
death: the story of a misnomer. Nat Rev Mol Cell Biol. 9:1004–1010.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Baehrecke EH: Autophagy: dual roles in
life and death? Nat Rev Mol Cell Biol. 6:505–510. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Levine B and Yuan J: Autophagy in cell
death: an innocent convict? J Clin Invest. 115:2679–2688. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kroemer G and Jäättelä M: Lysosomes and
autophagy in cell death control. Nat Rev Cancer. 5:886–897. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gozuacik D and Kimchi A: Autophagy and
cell death. Curr Top Dev Biol. 78:217–245. 2007. View Article : Google Scholar
|
|
21
|
Maiuri MC, Zalckvar E, Kimchi A and
Kroemer G: Self-eating and self-killing: crosstalk between
autophagy and apoptosis. Nat Rev Mol Cell Biol. 8:741–752. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Donohue E, Thomas A, Maurer N, et al: The
autophagy inhibitor verteporfin moderately enhances the antitumor
activity of gemcitabine in a pancreatic ductal adenocarcinoma
model. J Cancer. 4:585–596. 2013. View
Article : Google Scholar
|
|
23
|
Pardo R, Lo Ré A, Archange C, et al:
Gemcitabine induces the VMP1-mediated autophagy pathway to promote
apoptotic death in human pancreatic cancer cells. Pancreatology.
10:19–26. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mukubou H, Tsujimura T, Sasaki R and Ku Y:
The role of autophagy in the treatment of pancreatic cancer with
gemcitabine and ionizing radiation. Int J Oncol. 37:821–828.
2010.PubMed/NCBI
|
|
25
|
Papademetrio DL, Cavaliere V, Simunovich
T, et al: Interplay between autophagy and apoptosis in pancreatic
tumors in response to gemcitabine. Target Oncol. Apr 16–2013.(Epub
ahead of print).
|
|
26
|
Liang XH, Jackson S, Seaman M, et al:
Induction of autophagy and inhibition of tumorigenesis by Beclin 1.
Nature. 402:672–676. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jain MV, Paczulla AM, Klonisch T, et al:
Interconnections between apoptotic, autophagic and necrotic
pathways: implications for cancer therapy development. J Cell Mol
Med. 17:12–29. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kondo Y, Kanzawa T, Sawaya R and Kondo S:
The role of autophagy in cancer development and response to
therapy. Nat Rev Cancer. 5:726–734. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li J, Hou N, Faried A, Tsutsumi S and
Kuwano H: Inhibition of autophagy augments 5-fluorouracil
chemotherapy in human colon cancer in vitro and in vivo model. Eur
J Cancer. 46:1900–1909. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu D, Yang Y, Liu Q and Wang J:
Inhibition of autophagy by 3-MA potentiates cisplatin-induced
apoptosis in esophageal squamous cell carcinoma cells. Med Oncol.
28:105–111. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sun WL, Chen J, Wang YP and Zheng H:
Autophagy protects breast cancer cells from epirubicin-induced
apoptosis and facilitates epirubicin-resistance development.
Autophagy. 7:1035–1044. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Carew JS, Medina EC, Esquivel JA II, et
al: Autophagy inhibition enhances vorinostat-induced apoptosis via
ubiquitinated protein accumulation. J Cell Mol Med. 14:2448–2459.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Klionsky DJ, Abdalla FC, Abeliovich H, et
al: Guidelines for the use and interpretation of assays for
monitoring autophagy. Autophagy. 8:445–544. 2012. View Article : Google Scholar
|