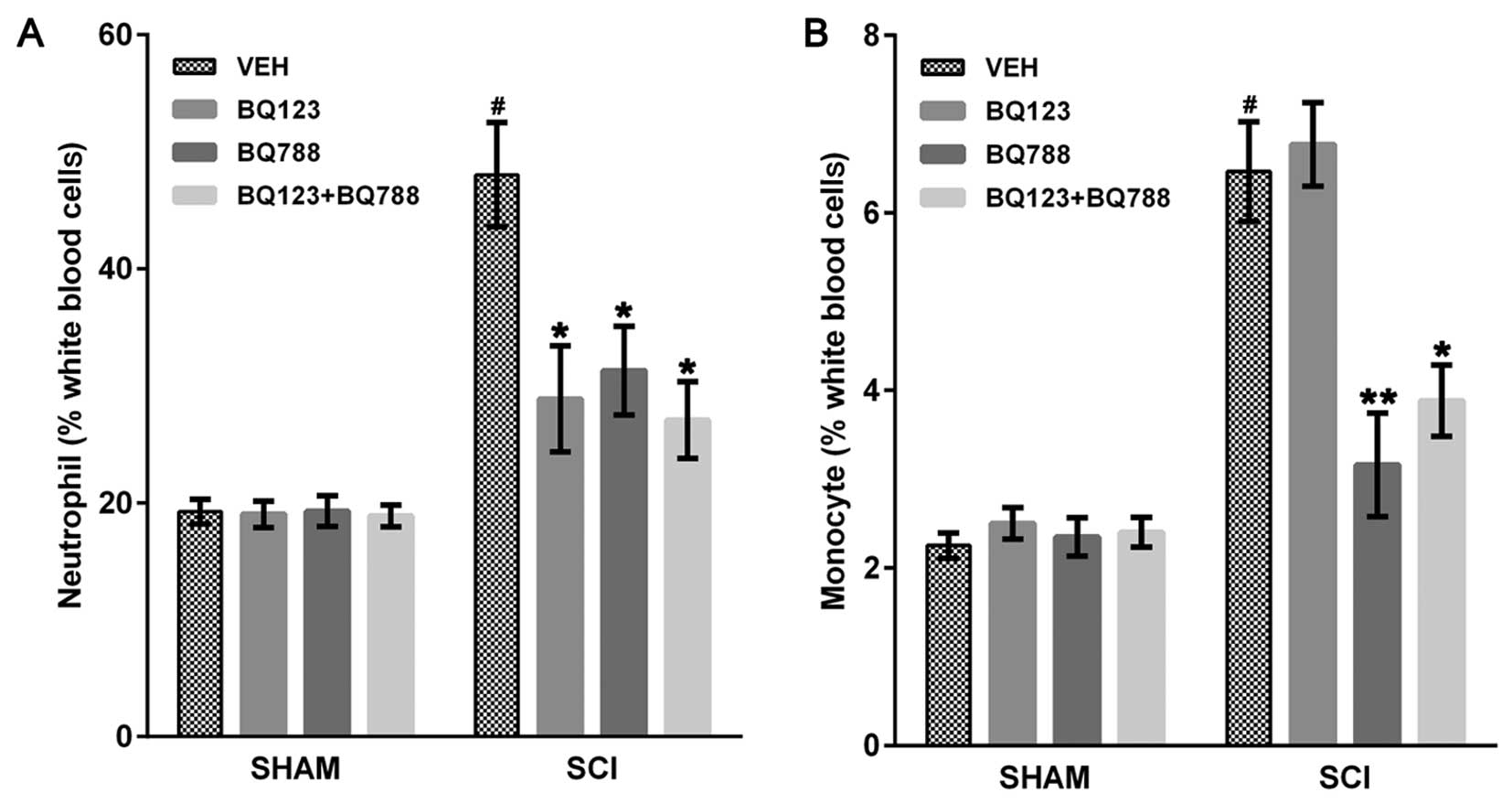
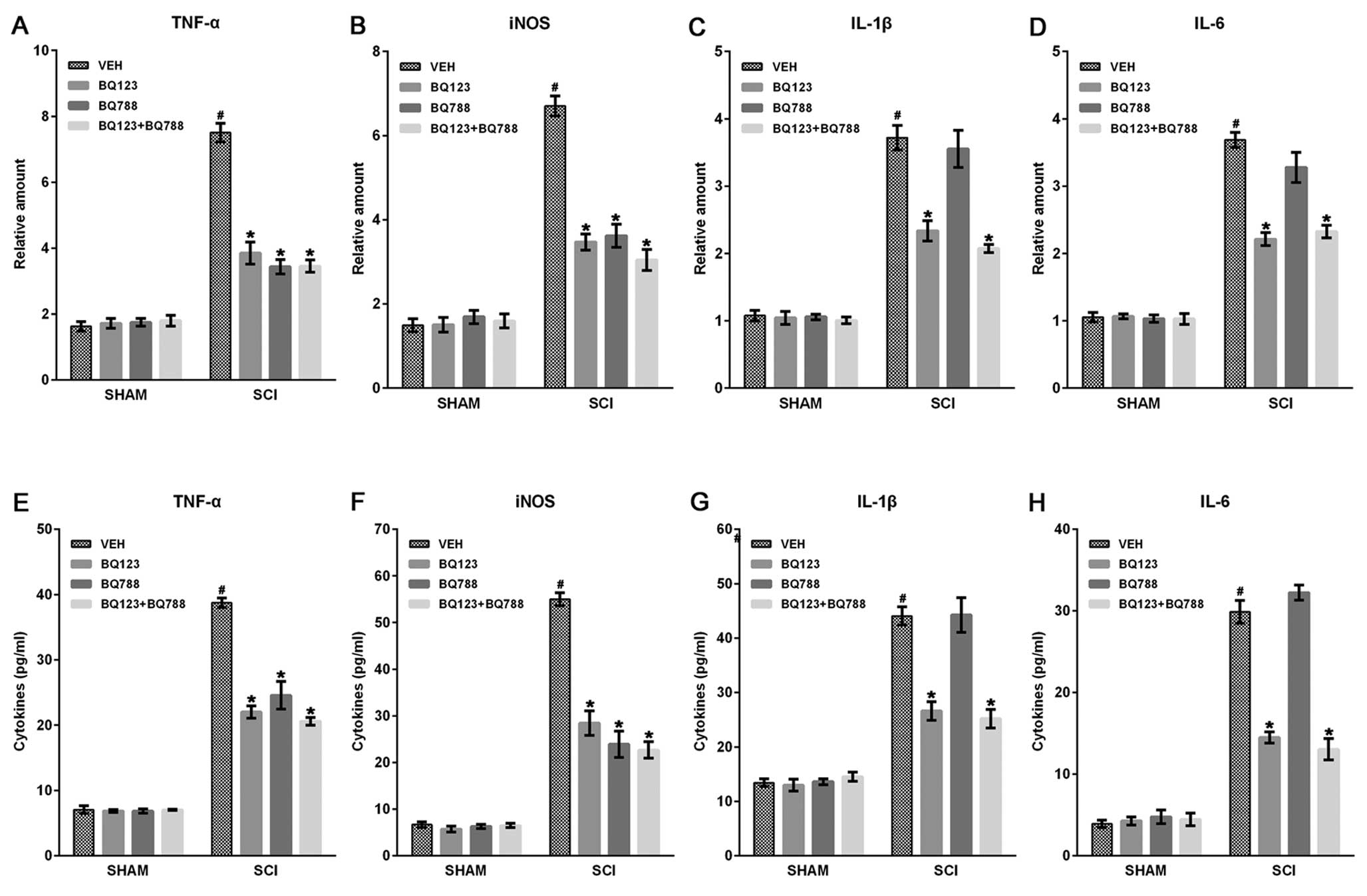
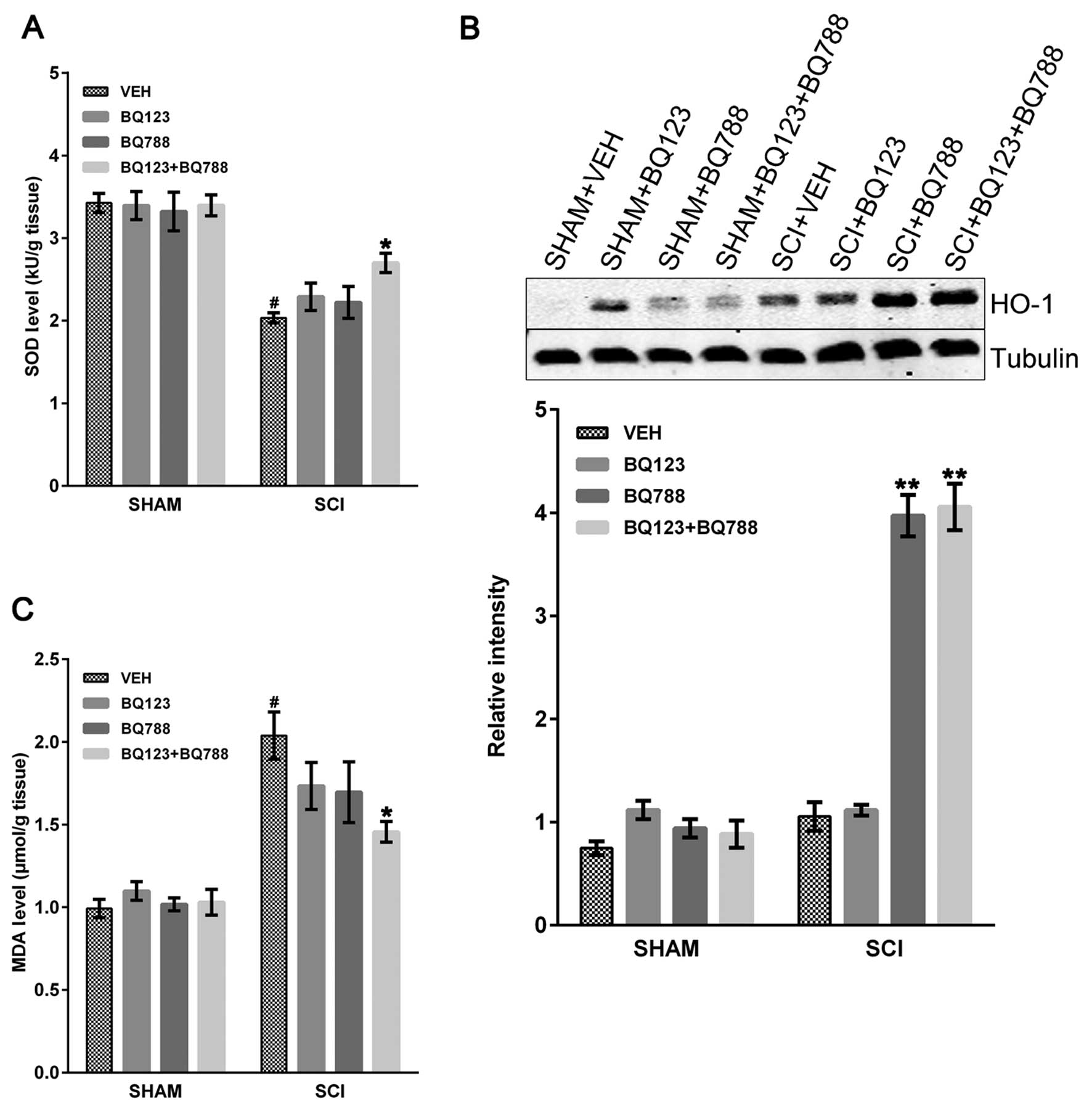
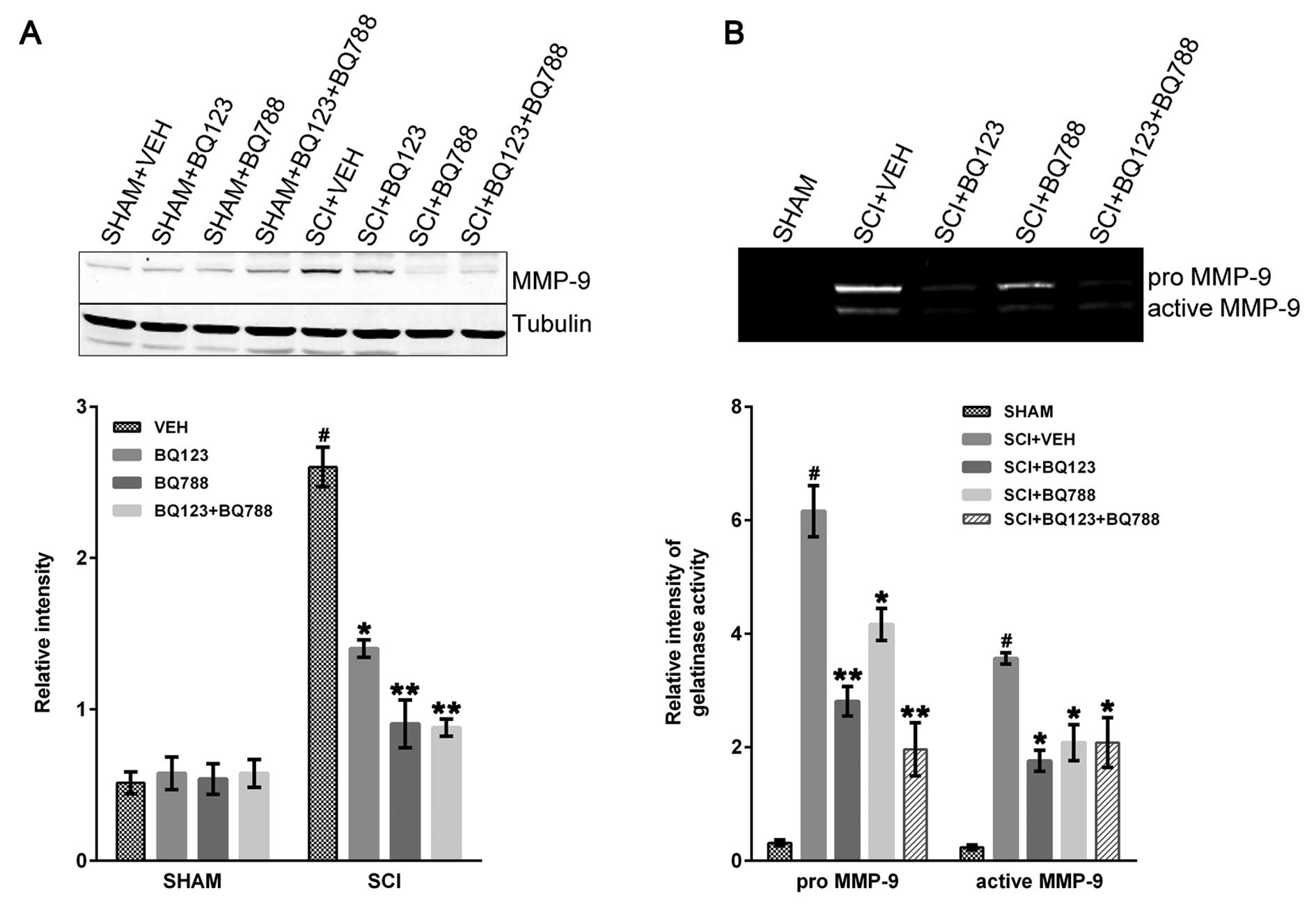
|
1
|
Hawkins B and Davis T: The blood-brain
barrier/neurovascular unit in health and disease. Pharmacol Rev.
57:173–185. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Abbott N, Rönnbäck L and Hansson E:
Astrocyte-endothelial interactions at the blood-brain barrier. Nat
Rev Neurosci. 7:41–53. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bao F, Chen Y, Schneider K and Weaver L:
An integrin inhibiting molecule decreases oxidative damage and
improves neurological function after spinal cord injury. Exp
Neurol. 214:160–167. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Goldbaum O and Richter-Landsberg C: Stress
proteins in oligodendrocytes: differential effects of heat shock
and oxidative stress. J Neurochem. 78:1233–1242. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lin Q, Weis S, Yang G, et al: Heme
oxygenase-1 protein localizes to the nucleus and activates
transcription factors important in oxidative stress. J Biol Chem.
282:20621–20633. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lindsey M, Wedin K, Brown M, et al:
Matrix-dependent mechanism of neutrophil-mediated release and
activation of matrix metalloproteinase 9 in myocardial
ischernia/reperfusion. Circulation. 103:2181–2187. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu D, Li L and Augustus L: Prostaglandin
release by spinal cord injury mediates production of hydroxyl
radical, malondialdehyde and cell death: a site of the
neuroprotective action of methylprednisolone. J Neurochem.
77:1036–1047. 2001. View Article : Google Scholar
|
|
8
|
Liu Y, Tachibana T, Dai Y, et al: Heme
oxygenase-1 expression after spinal cord injury: the induction in
activated neutrophils. J Neurotrauma. 19:479–490. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nguyen H, O’Barr T and Anderson A:
Polymorphonuclear leukocytes promote neurotoxicity through release
of matrix metalloproteinases, reactive oxygen species, and
TNF-alpha. J Neurochem. 102:900–912. 2007. View Article : Google Scholar
|
|
10
|
Dang A, Tay B, Kim H, Nauth A,
Alfonso-Jaume M and Lovett D: Inhibition of MMP2/MMP9 after spinal
cord trauma reduces apoptosis. Spine (Phila Pa 1976). 33:E576–E579.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dehouck M, Vigne P, Torpier G, Breittmayer
J, Cecchelli R and Frelin C: Endothelin-1 as a mediator of
endothelial cell-pericyte interactions in bovine brain capillaries.
J Cereb Blood Flow Metab. 17:464–469. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hama H, Kasuya Y, Sakurai T, et al: Role
of endothelin-1 in astrocyte responses after acute brain damage. J
Neurosci Res. 47:590–602. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Siren A, Knerlich F, Schilling L,
Kamrowski-Kruck H, Hahn A and Ehrenreich H: Differential glial and
vascular expression of endothelins and their receptors in rat brain
after neurotrauma. Neurochem Res. 25:957–969. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Martin D, Schoenen J, Delrée P, et al:
Experimental acute traumatic injury of the adult rat spinal cord by
a subdural inflatable balloon: methodology, behavioral analysis,
and histopathology. J Neurosci Res. 32:539–550. 1992. View Article : Google Scholar
|
|
15
|
Lampl Y, Fleminger G, Gilad R, Galron R,
Sarova-Pinhas I and Sokolovsky M: Endothelin in cerebrospinal fluid
and plasma of patients in the early stage of ischemic stroke.
Stroke. 28:1951–1955. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Reijerkerk A, Lakeman K, Drexhage J, et
al: Brain endothelial barrier passage by monocytes is controlled by
the endothelin system. J Neurochem. 121:730–737. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Leonard M, Briyal S and Gulati A:
Endothelin B receptor agonist, IRL-1620, reduces neurological
damage following permanent middle cerebral artery occlusion in
rats. Brain Res. 1420:48–58. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chou A, Chen T, Winardi W, et al:
Functional neuroprotective effect of CGS 26303, a dual ECE
inhibitor, on ischemic-reperfusion spinal cord injury in rats. Exp
Biol Med (Maywood). 232:214–218. 2007.PubMed/NCBI
|
|
19
|
Barnes K and Turner A: The endothelin
system and endothelin-converting enzyme in the brain: molecular and
cellular studies. Neurochem Res. 22:1033–1040. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kallakuri S, Kreipke C, Schafer P, Schafer
S and Rafols J: Brain cellular localization of endothelin receptors
A and B in a rodent model of diffuse traumatic brain injury.
Neuroscience. 168:820–830. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
McKenzie A, Hall J, Aihara N, Fukuda K and
Noble L: Immunolocalization of endothelin in the traumatized spinal
cord: relationship to blood-spinal cord barrier breakdown. J
Neurotrauma. 12:257–268. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
MacCumber M, Ross C and Snyder S:
Endothelin in brain: receptors, mitogenesis, and biosynthesis in
glial cells. Proc Natl Acad Sci USA. 87:2359–2363. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Peters C, Rogers S, Pomonis J, et al:
Endothelin receptor expression in the normal and injured spinal
cord: potential involvement in injury-induced ischemia and gliosis.
Exp Neurol. 180:1–13. 2003. View Article : Google Scholar
|
|
24
|
Huggins J, Pelton J and Miller R: The
structure and specificity of endothelin receptors: their importance
in physiology and medicine. Pharmacol Ther. 59:55–123. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Koyama Y, Takemura M, Fujiki K, Ishikawa
N, Shigenaga Y and Baba A: BQ788, an endothelin ET(B) receptor
antagonist, attenuates stab wound injury-induced reactive
astrocytes in rat brain. Glia. 26:268–271. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Noble L, Donovan F, Igarashi T, Goussev S
and Werb Z: Matrix metalloproteinases limit functional recovery
after spinal cord injury by modulation of early vascular events. J
Neurosci. 22:7526–7535. 2002.PubMed/NCBI
|
|
27
|
Fu L, Guo Z and Longhurst J: Endogenous
endothelin stimulates cardiac sympathetic afferents during
ischaemia. J Physiol. 588:2473–2486. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Douglas SA, Vickery-Clark LM, Louden C and
Ohlstein EH: Selective ETA receptor antagonism with BQ-123 is
insufficient to inhibit angioplasty induced neointima formation in
the rat. Cardiovasc Res. 29:641–646. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Okada M and Nishikibe M: BQ-788, a
selective endothelin ET(B) receptor antagonist. Cardiovasc Drug
Rev. 20:53–66. 2002. View Article : Google Scholar
|
|
30
|
Lee J, Kim H, Choi H, Oh T and Yune T:
Fluoxetine inhibits matrix metalloprotease activation and prevents
disruption of blood-spinal cord barrier after spinal cord injury.
Brain. 135:2375–2389. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Basso D, Fisher L, Anderson A, Jakeman L,
McTigue D and Popovich P: Basso mouse scale for locomotion detects
differences in recovery after spinal cord injury in five common
mouse strains. J Neurotrauma. 23:635–659. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee S, Rosen S, Weinstein P, van Rooijen N
and Noble-Haeusslein L: Prevention of both neutrophil and monocyte
recruitment promotes recovery after spinal cord injury. J
Neurotrauma. 28:1893–1907. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu W, Chi L, Xu R, et al: Increased
production of reactive oxygen species contributes to motor neuron
death in a compression mouse model of spinal cord injury. Spinal
Cord. 43:204–213. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cuadrado A and Rojo A: Heme oxygenase-1 as
a therapeutic target in neurodegenerative diseases and brain
infections. Curr Pharm Des. 14:429–442. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Salzman S, Acosta R, Beck G, Madden J,
Boxer B and Ohlstein E: Spinal endothelin content is elevated after
moderate local trauma in the rat to levels associated with
locomotor dysfunction after intrathecal injection. J Neurotrauma.
13:93–101. 1996. View Article : Google Scholar
|
|
36
|
Nagasaka J, Tsuji M, Takeda H and
Matsumiya T: Role of endothelin receptor subtypes in the behavioral
effects of the intracerebroventricular administration of
endothelin-1 in conscious rats. Pharmacol Biochem Behav.
64:171–176. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ishikawa K, Fukami T, Nagase T, et al:
Cyclic pentapeptide endothelin antagonists with high ETA
selectivity. Potency- and solubility-enhancing modifications. J Med
Chem. 35:2139–2142. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Khimji A and Rockey D: Endothelin -
biology and disease. Cell Signal. 22:1615–1625. 2010. View Article : Google Scholar
|
|
39
|
Beck K, Nguyen H, Galvan M, Salazar D,
Woodruff T and Anderson A: Quantitative analysis of cellular
inflammation after traumatic spinal cord injury: evidence for a
multiphasic inflammatory response in the acute to chronic
environment. Brain. 133:433–447. 2010. View Article : Google Scholar
|
|
40
|
Letellier E, Kumar S, Sancho-Martinez I,
et al: CD95-ligand on peripheral myeloid cells activates Syk kinase
to trigger their recruitment to the inflammatory site. Immunity.
32:240–252. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Stirling D and Yong V: Dynamics of the
inflammatory response after murine spinal cord injury revealed by
flow cytometry. J Neurosci Res. 86:1944–1958. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ahnstedt H, Stenman E, Cao L, Henriksson M
and Edvinsson L: Cytokines and growth factors modify the
upregulation of contractile endothelin ET(A) and ET(B) receptors in
rat cerebral arteries after organ culture. Acta Physiol (Oxf).
205:266–278. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zarpelon A, Pinto L, Cunha T, et al:
Endothelin-1 induces neutrophil recruitment in adaptive
inflammation via TNFα and CXCL1/CXCR2 in mice. Canadian Can J
Physiol Pharmacol. 90:187–199. 2012.PubMed/NCBI
|
|
44
|
Tonari M, Kurimoto T, Horie T, Sugiyama T,
Ikeda T and Oku H: Blocking endothelin-B receptors rescues retinal
ganglion cells from optic nerve injury through suppression of
neuroinflammation. Invest Ophthalmol Vis Sci. 53:3490–3500. 2012.
View Article : Google Scholar
|
|
45
|
Wang H, Hsieh H and Yang C: Nitric oxide
production by endothelin-1 enhances astrocytic migration via the
tyrosine nitration of matrix metalloproteinase-9. J Cell Physiol.
226:2244–2256. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gallelli L, Pelaia G, D’Agostino B, et al:
Endothelin-1 induces proliferation of human lung fibroblasts and
IL-11 secretion through an ET(A) receptor-dependent activation of
MAP kinases. J Cell Biochem. 96:858–868. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Solini A, Santini E, Madec S, Cuccato S
and Ferrannini E: Effects of endothelin-1 on fibroblasts from type
2 diabetic patients: possible role in wound healing and tissue
repair. Growth Factors. 25:392–399. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yin X, Yin Y, Cao F, et al: Tanshinone IIA
attenuates the inflammatory response and apoptosis after traumatic
injury of the spinal cord in adult rats. PLoS One. 7:e383812012.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kanno H, Ozawa H, Dohi Y, Sekiguchi A,
Igarashi K and Itoi E: Genetic ablation of transcription repressor
Bach1 reduces neural tissue damage and improves locomotor function
after spinal cord injury in mice. J Neurotrauma. 26:31–39. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Goven D, Boutten A, Lecon-Malas V,
Boczkowski J and Bonay M: Prolonged cigarette smoke exposure
decreases heme oxygenase-1 and alters Nrf2 and Bach1 expression in
human macrophages: roles of the MAP kinases ERK(1/2) and JNK. FEBS
Lett. 583:3508–3518. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sallum CO, Wilson JL, Rupasinghe C, et al:
Enhancing and limiting endothelin-1 signaling with a
cell-penetrating peptide mimicking the third intracellular loop of
the ETB receptor. Chem Biol Drug Des. 80:374–381. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Romanic AM 1, White RF, Arleth AJ,
Ohlstein EH and Barone FC: Matrix metalloproteinase expression
increases after cerebral focal ischemia in rats: inhibition of
matrix metalloproteinase-9 reduces infarct size. Stroke.
29:1020–1030. 1998. View Article : Google Scholar
|