Introduction
Irradiation-induced pulmonary fibrosis is a major
complication associated with total body irradiation for
hematopoietic stem cell transplantation, nuclear accidents, and
thoracic radiotherapy for lung cancer, breast cancer, thymoma, and
lymphoma (1,2). This complication develops ~6 months
to several years after radiation exposure in humans and 100–120
days post-irradiation in the C57BL/6J mouse model (2). Recent clinical data have
demonstrated that the incidence of irradiation-related pulmonary
injury among patients with cancer who received radiotherapy ranged
from 20.3% to 36.9% (3–6). The current clinical treatment for
pulmonary fibrosis primarily involves drugs such as steroids or
non-steroidal anti-inflammatory agents and immunosuppressive
agents. These drugs can decrease acute pneumonitis for 2–3 months
after irradiation, but they cannot effectively mitigate fibrosis
(2). In addition,
immunosuppressive agents can cause serious side-effects, including
death.
Oxidative stress begins at radiation exposure and is
sustained throughout the disease’s progression, presumably through
the radiation-induced activation of oxidant-generating enzymes,
mitochondrial leakage, and the activation of the respiratory burst
in the phagocytic cells that infiltrate damaged tissue (7). Therefore, antioxidant treatment
strategies are being developed to treat irradiation-induced
pulmonary fibrosis. Epigallocatechin-3-gallate (EGCG) is a natural
antioxidant derived from green tea that has attracted particular
attention and recognition for its potential applications in the
treatment of oxidative stress-related diseases including cancer,
cardiovascular diseases, and neurodegenerative diseases (8). EGCG is the primary component of tea
polyphenols, and it has shown a wide range of biological activities
and pharmacological effects in vitro and in vivo,
including antioxidant, anti-free radical, anti-mutagenic, and
antitumor effects (9). EGCG
inhibits chemical-induced lung fibrosis (10–13) and liver fibrosis (14). However, irradiation-induced
pulmonary fibrosis and acute chemical-induced lung injury are not
identical, particularly with respect to their pathogeneses. As yet,
no study has examined the efficacy or mechanism of action of EGCG
with regard to preventing or treating irradiation-induced pulmonary
fibrosis.
To counteract the oxidative stress induced by
reactive oxygen species (ROS), lung cells activate a wide variety
of endogenous antioxidant enzymes including catalase, superoxide
dismutases, and peroxiredoxins. The transcription of these
cytoprotective enzymes is regulated by the nuclear transcription
factor NF-E2-related factor 2 (Nrf2), which plays a central role in
the regulation of cellular redox status. Under normal homeostatic
conditions, Nrf2 transcription is repressed by its negative
regulator Kelch-like ECH-associated protein 1 (Keap1). However,
following exposure to ROS, Nrf2 dissociates from cytosolic Keap1
and translocates to the nucleus, where it binds to the antioxidant
response element (ARE) in the promoter regions of the genes that
encode antioxidant enzymes and induce their transcription (15). The antioxidant enzyme system
regulated by the Nrf2-ARE signaling pathway is primarily composed
of heme oxygenase-1 (HO-1), γ-glutamine cysteine synthetase
(γ-GCS), NAD(P)H:quinone oxidoreductase-1 (NQO-1) and superoxide
dismutase (SOD).
In the present study, we hypothesized that the
administration of EGCG would significantly inhibit
irradiation-induced pulmonary fibrosis. We first evaluated the
efficacy of EGCG to ameliorate irradiation-induced pulmonary
fibrosis and then examined whether EGCG treatment influenced Nrf-2,
HO-1 and NQO-1 levels in irradiated rats. To the best of our
knowledge, this study is the first to assess and highlight the
efficacy of EGCG in the treatment of irradiation-induced pulmonary
fibrosis.
Materials and methods
Animals
Male 6- to 8-week-old Sprague-Dawley (SD) rats
(Laboratory Animal Center, Academy of Military Medical Sciences,
Beijing, China) weighing 180–200 g were housed in an SPF-graded
animal care facility according to the guidelines of the National
Institutes of Health and Academy of Military Medical Sciences for
the Care and Use of Laboratory Animals. The Committee on the Ethics
of Animal Experiments of the Affiliated Hospital of Academy of
Military Medical Sciences approved the protocol. All interventions
were performed under sodium pentobarbital anesthesia, and all
efforts were made to minimize suffering. Rats were provided with
pathogen-free water and food for maintenance and caged in a
controlled SPF environment with a 12/12-h light/dark cycle. Rats
were observed daily up to 4 months post-irradiation, with
particular attention afforded to difficulties in breathing,
ruffling of the fur, hunched posture, and decreased breathing rate.
The natural death of the animals was recorded.
Irradiation and treatment
A 60Co irradiator [Reviss Services (UK),
Ltd., Buckinghamshire, UK] was used to generate gamma-ray
radiation. The rats were irradiated to 22 Gy at a dose rate of 290
cGy/min. The beam was restricted to the entire thorax. After
anesthetization with 3% sodium pentobarbital (45 mg/kg) and
irradiation as described above, the rats were intraperitoneally
injected with EGCG (25 mg/kg; Sigma, St. Louis, MO, USA) (n=40) or
dexamethasone (DEX; n=40; 5 mg/kg; Tianjing Pharmaceuticals Group
Corp., Tianjing, China) daily for 30 days. Irradiated rats
(radiation only; n=40) received radiation without treatment. The
control group (n=40) was composed of normally fed, age-matched
animals that were not irradiated. Similar doses of EGCG (25 mg/kg)
were used in previous studies (11–13).
Specimen processing and
histopathology
After measuring body weight, six rats from each
group were sacrificed at 15, 30, 60 or 120 days after initiation of
the experiment. The wet weight of the lungs was recorded for each
animal. The left lungs were frozen with dry ice powder and kept at
−70°C for later use. The right lungs were fixed with 4%
paraformaldehyde for histological and immunohistochemical analyses.
Blood samples were collected from the heart and allowed to clot for
1 h at room temperature. Serum samples were obtained by
centrifugation at 3,500 rpm for 5 min at 4°C and then stored at
−70°C. The right lungs were dehydrated in ethanol and embedded in
paraffin. Lung sections (5 μm) were stained with hematoxylin and
eosin (H&E), Masson’s trichrome (Masson) and Sirius red.
Lung index measurement
The ratio of the lung wet weight (mg) to body weight
(g) was used as the lung index.
Measurement of collagen content in the
lungs
Lung collagen content was determined using the
hydroxyproline (Hyp) assay according to the manufacturer’s protocol
(Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Approximately 100 mg of left lung tissue was hydrolyzed in 1 ml of
lysis buffer solution at 100°C for 20 min. The absorbance of
colored products was measured at 550 nm.
Malondialdehyde (MDA) content and SOD
activity measurements in serum
Serum samples were assayed for MDA content and SOD
activity using commercially available kits according to the
manufacturer’s instructions (Nanjing Jiancheng Bioengineering
Institute). MDA, an end product of ROS-induced peroxidation of cell
membrane lipids, is a reliable marker of oxidative damage (16). MDA content was determined by
measuring chromogen generation from the reaction of MDA with
2-thiobarbituric acid. SOD activity was measured by monitoring the
sample’s capacity to inhibit the reduction of ferricytochrome
c via xanthine/xanthine oxidase. Briefly, this method is
dependent on the inhibition of nitroblue tetrazolium (NBT)
reduction via the xanthine/xanthine oxidase system as a superoxide
generator (17).
Immunohistochemical analyses
Lung sections (5 μm) were deparaffinized, rehydrated
through a graded alcohol series, and exposed to a microwave-based
antigen retrieval with a citrate buffer (10 mM of sodium citrate,
pH 6.0 for 15 min). Endogenous peroxidases were quenched using 3%
H2O2 for 5 min. The sections were incubated
with surfactant protein-B (SPB; 1:200) or α smooth muscle actin
(α-SMA; 1:200) (both from Boster Biological Technology, Wuhan,
China) antibodies at 37°C for 2 h. The primary antibody was omitted
in the negative control samples. After washing with PBS, the
sections were incubated with poly-peroxidase-conjugated
anti-mouse/rabbit IgG for 30 min at 37°C using the Polymer-HRP
Detection System (Zymed Laboratories, South San Francisco, CA, USA)
according to the manufacturer’s instructions. The slides were
visualized with diaminobenzidine (DAB; Dako, Glostrup, Denmark),
counterstained with Mayer’s hematoxylin, dehydrated through
increasing concentrations of alcohol, cleared in xylene, and
mounted in neutral balsam (Sigma).
Serum cytokine levels
Serum levels of TGF-β1 were determined using the
commercially available TGF-β1 ELISA kit according to the
manufacturer’s instructions (Boster Biological Technology). The OD
value was determined at 450 nm using an ELISA reader and calculated
at the linear portion of the curve. Serum levels of IL-6, IL-10,
and TNF-α were measured using flow cytometric bead assays according
to the manufacturer’s instructions (BD™ CBA Flex Set; BD, Sparks,
MD, USA).
Western blot analysis
Frozen left lungs were pulverized and lysed in RIPA
buffer. Lysates were centrifuged at 12,000 rpm and 4°C for 10 min,
and the supernatants were collected for total protein analysis. A
BCA protein assay kit (Beyotime Institute of Biotechnology,
Jiangsu, China) was used to determine protein concentrations. Equal
amounts of protein were separated by SDS-PAGE, transferred to a
PVDF membrane (Millipore Corp., Billerica, MA, USA), and incubated
with 5% BSA at room temperature for 2.5 h to block non-specific
binding. The membranes were then incubated with the following
primary antibodies at room temperature for 3 h: Nrf-2 (1:200;
Sigma), HO-1 (1:200), NQO-1 (1:200) (both from Millipore) or
β-actin (1:2,000; Cell Signaling Technology, Inc., Danvers, MA,
USA). After washing with TBS-T, the membranes were incubated with
HRP-conjugated secondary antibodies. The protein bands were
visualized using enhanced chemiluminescence with a Super Signal
detection kit (Boster Biological Technology).
Morphometric analyses
Following the methodology described by Szapiel et
al (18), lung sections
stained with H&E or Masson’s trichrome were scored for
alveolitis and fibrosis, respectively. Briefly, the severity of
alveolitis and fibrosis was graded and scored on a scale of 0–3
(18): grade 0, normal lung;
grade 1, minimal lesion (lesion area <20%); grade 2, moderate
lesion (lesion area, 20–50%); or grade 3, severe lesion (lesion
area >50%). Ten fields per section at ×100 magnification were
randomly selected per rat, and two blinded pathologists carefully
and independently examined 60 fields per group using an Olympus
microscope (Olympus, Tokyo, Japan). The total score of each section
was calculated, and the mean score of each group was determined as
the total score of all sections divided by six. Lung sections
stained with Sirius red were observed and images were captured
using a polarizing microscope. For the immunohistochemical analyses
of SPB and α-SMA, staining density was determined using Image
Proplus software in one field with a prominent DAB reaction for
each section under ×200 magnification for a total of six fields per
group. Large airways and lung vessels were excluded from all
analyses.
Statistical analysis
Data were expressed as the means ± standard
deviations (SDs). Between-group differences were tested using a
two-way ANOVA followed by Tukey’s post-hoc test. Two-group
comparisons were performed using independent-samples Student’s
t-tests. P<0.05 was considered significant.
Results
EGCG reduces mortality and inhibits the
formation of fibrous nodules in pulmonary tissue
Irradiated rats treated with EGCG (17.5%, 7/40) had
a lower mortality rate compared with irradiated rats treated with
DEX (27.5%, 11/40) and those that received radiation only (25.0%,
10/40). All control animals survived to 4 months without death.
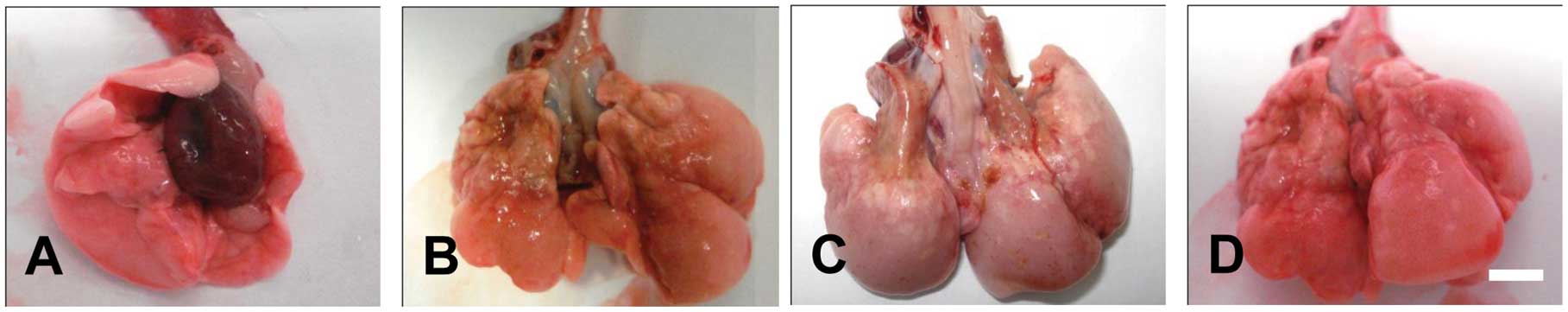
Congested edema and bleeding sites were
significantly attenuated in DEX-treated pulmonary tissues compared
with radiation-only animals at 15 and 30 days post-irradiation.
Pulmonary collapse and gray fibrous nodules were similar in
DEX-treated and radiation-only animals at 60 and 120 days
post-irradiation. Bleeding sites were seldom observed in
EGCG-treated pulmonary tissues at 15 days post-irradiation. Signs
of congested edema were also significantly attenuated in
EGCG-treated pulmonary tissues compared with DEX-treated and
radiation-only tissues at 15, 30 and 60 days post-irradiation. At
120 days post-irradiation, the lungs of EGCG-treated rats showed
signs of edema and uneven surfaces as well as scattered punctuate
bleeding points. However, neither pulmonary collapse nor gray
fibrous nodules were found in EGCG-treated animals at 120 days
post-irradiation (Fig. 1). These
results suggested that EGCG significantly ameliorates
irradiation-induced pulmonary fibrosis.
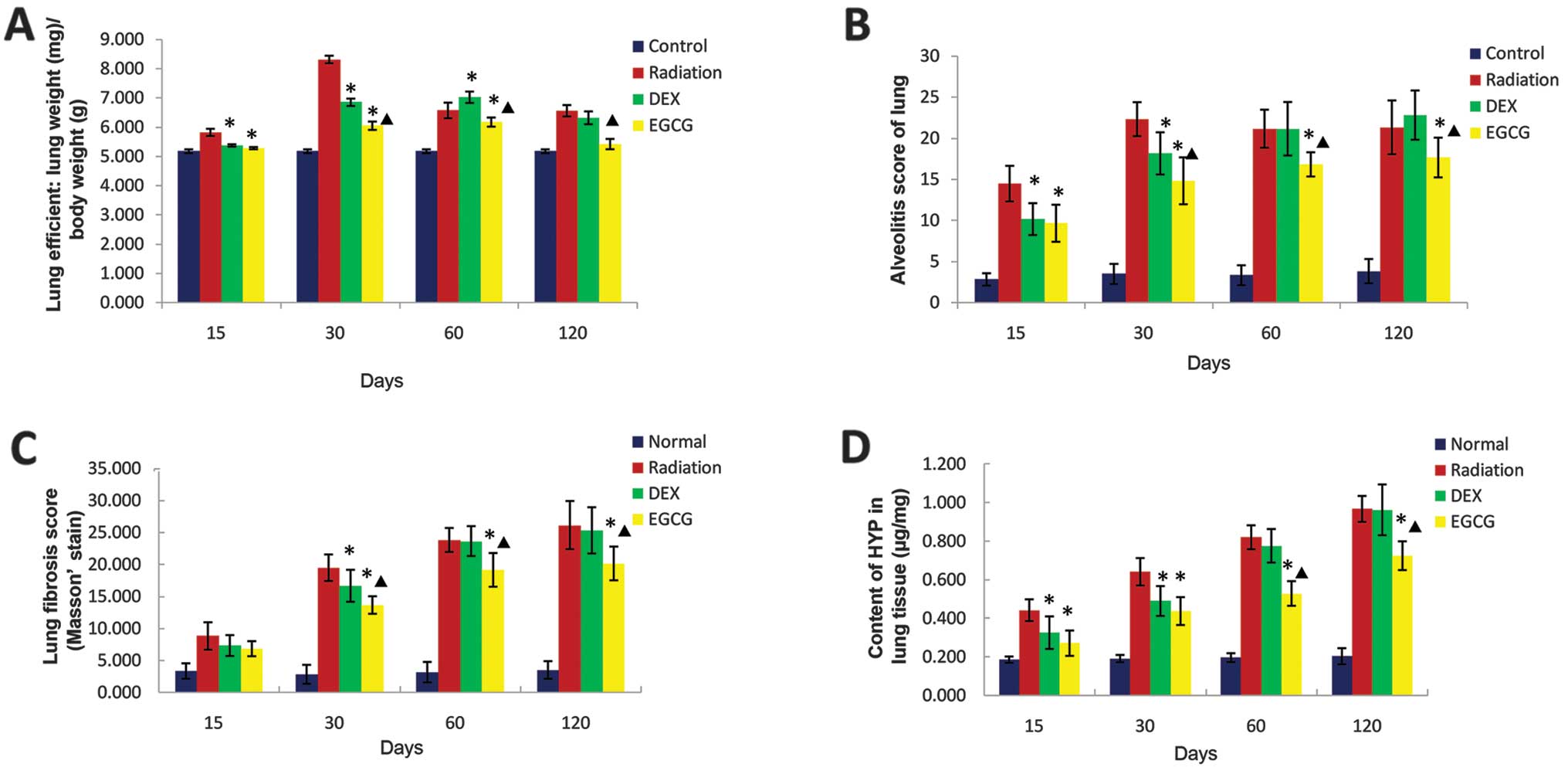
EGCG reduces the lung index score
We examined whether EGCG treatment influenced the
lung index, which refers to the ratio of lung wet weight to body
weight. The lung index was significantly lower (p<0.05) in
EGCG-treated animals relative to DEX-treated animals at 30 and 60
days post-irradiation but significantly higher (p<0.05) at 120
days post-irradiation. This result matches the morphometric
observations of lung appearance in EGCG-treated animals at 120 days
post-irradiation (Fig. 1). At
this time point, edema was still detectable in EGCG-treated animals
but was not detected in DEX-treated and untreated irradiated
animals. The lung index score was significantly lower (p<0.05)
among the DEX-treated animals relative to the radiation-only
animals at 15, 30 and 60 days post-irradiation but similar at 120
days post-irradiation (p>0.05; Fig. 2A). These results indicated that
EGCG significantly attenuated congested edema in pulmonary tissues
post-irradiation.
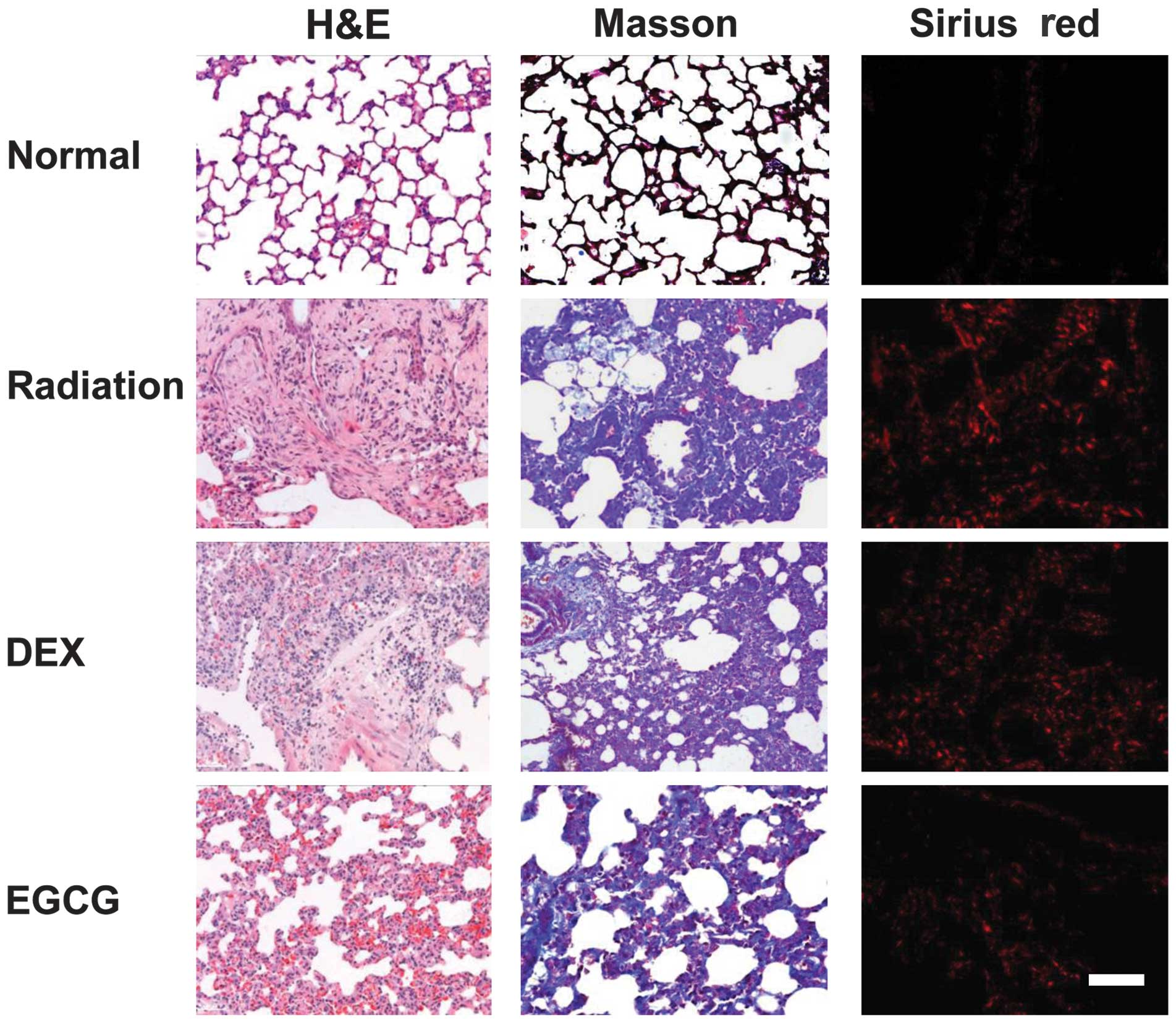
EGCG improves histological changes and
reduces collagen deposition in pulmonary tissues
We examined whether EGCG treatment improves the
histological changes that occur in pulmonary tissues
post-irradiation. We performed H&E, Masson’s trichrome and
Sirius red staining of the lung sections to observe histological
changes. A semi-quantitative analysis involving alveolitis and
fibrosis scores was performed on the H&E- and Masson-stained
sections following Szapiel’s method (18). Markedly thickened alveolar walls,
collapsed alveoli, foam-like cells in the alveolar space, diffuse
accumulations of inflammatory cells, extensive depositions of
collagen, and regional fibrotic foci were observed in the
H&E-stained irradiated pulmonary tissues at 120 days
post-irradiation. Treatment with EGCG, but not DEX, significantly
improved irradiation-induced pathological changes (Fig. 3, left column). Similarly, Masson’s
trichrome (Fig. 3, middle column)
and Sirius red staining (Fig. 3,
right column) of the lung sections revealed that the regional
fibrotic foci and collagen depositions were greatly reduced after
EGCG treatment.
The alveolitis and fibrosis scores of EGCG-treated
animals were significantly lower (p<0.05) than those of
DEX-treated animals at 30, 60 and 120 days post-irradiation. The
alveolitis score of the latter group was significantly lower
(p<0.05) than that of the radiation-only animals at 15 and 30
days post-irradiation. The fibrosis score of DEX-treated animals
was significantly lower (p<0.05) than that of radiation-only
animals at 30 days post-irradiation. The alveolitis and fibrosis
scores of DEX-treated animals were similar to those of
radiation-only animals but significantly higher (p<0.05) than
those of EGCG-treated animals at 60 and 120 days post-irradiation
(Fig. 2B and C).
We also examined the degree to which EGCG treatment
eliminated Hyp, the major constituent of collagen, from pulmonary
tissues. The amount of Hyp was significantly lower (p<0.05) in
EGCG-treated animals than DEX-treated animals at 60 and 120 days
post-irradiation. The Hyp content of the latter group was
significantly lower (p<0.05) than that of the radiation-only
animals at 15 and 30 days post-irradiation but similar to that of
these animals, and significantly higher (p<0.05) than that of
the EGCG-treated animals, at 60 and 120 days post-irradiation.
Together, these results showed marked anti-fibrotic effects of EGCG
in vivo (Fig. 2D).
EGCG modulates the serum redox state
We investigated whether EGCG treatment regulates the
redox balance post-irradiation. Serum MDA content and SOD activity
were measured to assess the oxidative and antioxidant statuses,
respectively. The serum MDA concentration was significantly lower
(p<0.05) in EGCG-treated animals than DEX-treated animals at 60
and 120 days post-irradiation. The MDA concentration in DEX-treated
animals was similar to that of the radiation-only animals at 15,
30, 60 and 120 days post-irradiation (Fig. 4A).
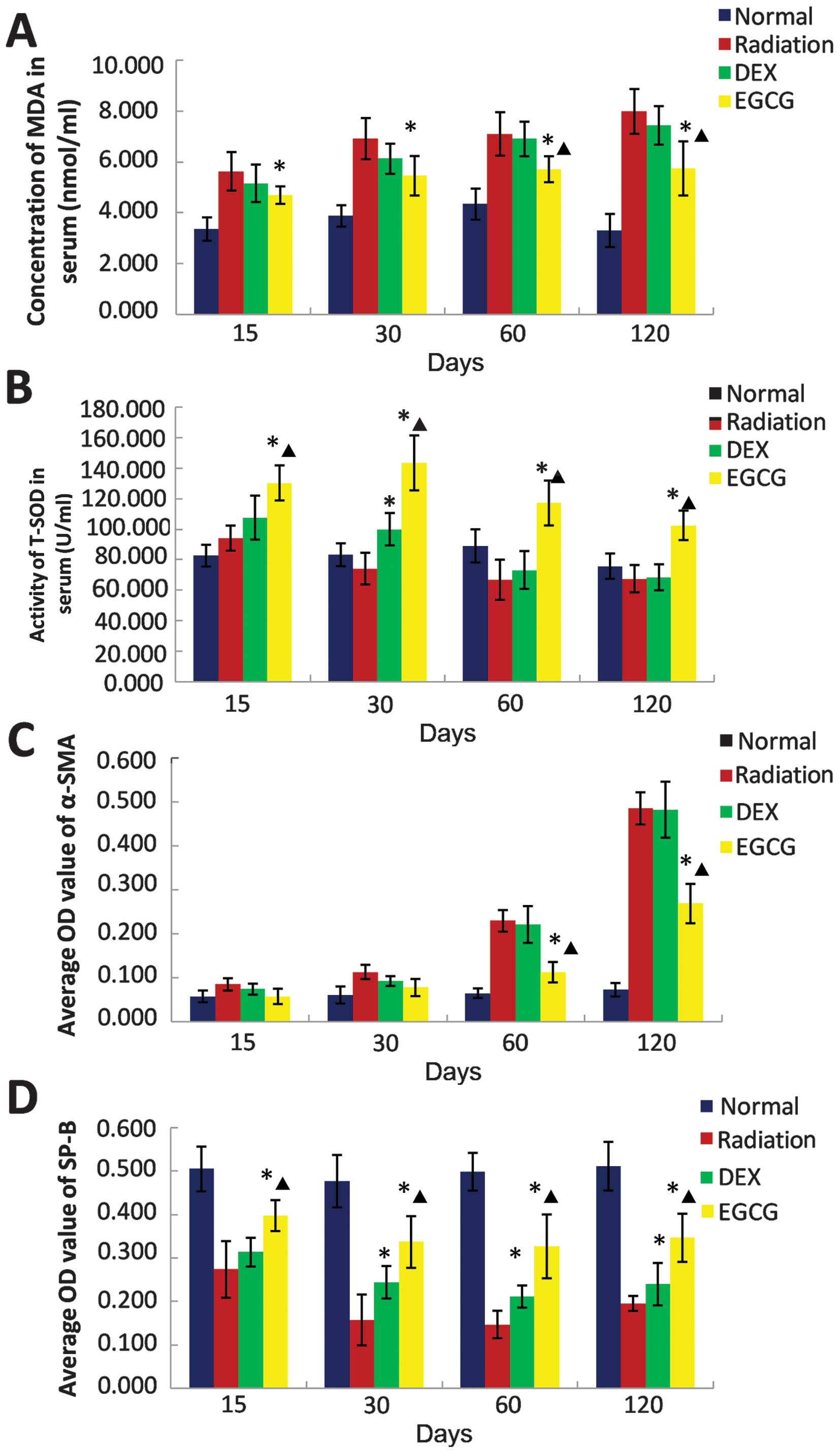 | Figure 4(A) The effect of
epigallocatechin-3-gallate (EGCG) on serum malondialdehyde (MDA)
concentrations, (B) superoxide dismutase (SOD) activity, (C) α
smooth muscle actin (α-SMA) levels and (D) surfactant protein-B
(SPB) levels at 15, 30, 60 and 120 days post-irradiation. (A) The
MDA concentration in serum was significantly lower (p<0.05)
among the EGCG-treated animals compared with that of the
dexamethasone (DEX)-treated animals at 60 and 120 days
post-irradiation. Conversely, the MDA concentrations of the
DEX-treated and untreated animals were comparable at 15, 30, 60 and
120 days post-irradiation. (B) Serum SOD activity was significantly
higher (p<0.05) among the EGCG-treated animals compared with the
DEX-treated animals at 15, 30, 60 and 120 days post-irradiation.
The SOD activity of the latter group was significantly higher
(p<0.05) than that of the radiation-only animals at 30 days
post-irradiation but similar at 15, 60 and 120 days
post-irradiation. (C) The OD value of α-SMA immunohistochemical
(IHC) was significantly lower (p<0.05) among EGCG-treated
animals than that of the DEX-treated animals at 60 and 120 days
post-irradiation. Conversely, the OD value of α-SMA IHC among the
DEX-treated animals was comparable to that of the untreated animals
at 15, 30, 60 and 120 days post-irradiation (p>0.05). (D) The OD
value of SPB IHC was significantly higher (p<0.05) among the
EGCG-treated animals relative to the DEX-treated animals at 15, 30,
60 and 120 days post-irradiation. The OD value of SPB IHC among the
DEX-treated animals was similar to that of the untreated animals at
15 days post-irradiation but significantly higher (p<0.05) at
30, 60 and 120 days post-irradiation. The bars in the graph are the
standard deviations (SDs). Asterisks show significance (p<0.05)
compared with the untreated radiation-only animals, and stars show
significance (p<0.05) compared with the DEX-treated animals. |
Serum SOD activity was significantly higher
(p<0.05) in EGCG-treated animals compared with DEX-treated
animals at 15, 30, 60 and 120 days post-irradiation. Levels of SOD
activity in the latter group were significantly higher (p<0.05)
than those of the radiation-only animals at 30 days
post-irradiation but similar at 15, 60 and 120 days
post-irradiation (Fig. 4B). These
results demonstrated that EGCG treatment modulates redox balance
in vivo.
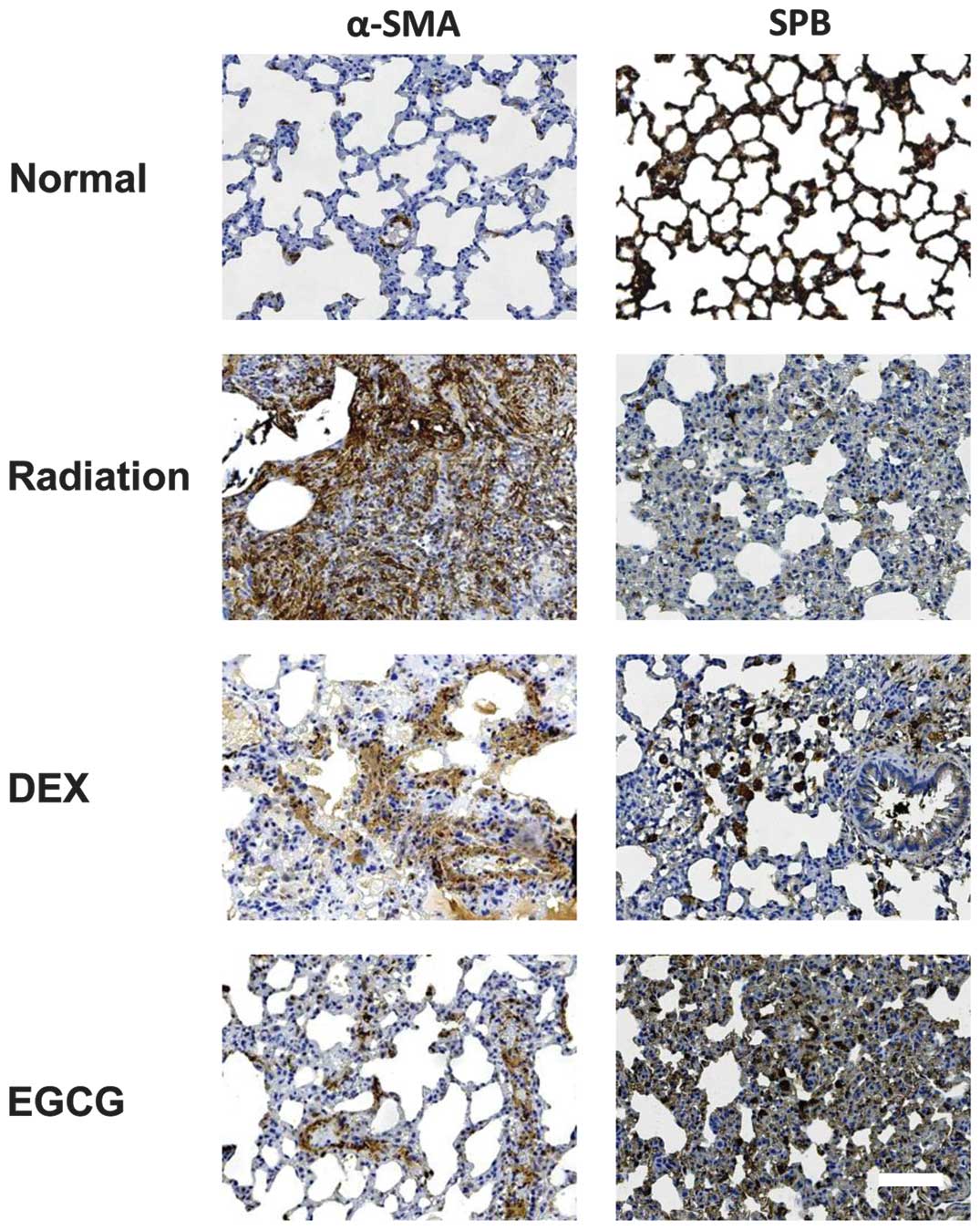
EGCG inhibits (myo)fibroblast
proliferation and protects alveolar epithelial type II (AE2) cells
from injury
The activation and proliferation of (myo)fibroblasts
are important contributors to pulmonary fibrosis. Injury to the AE2
cells directly and indirectly contributes to the effects of
irradiation-induced pulmonary injury. Therefore, we investigated
the effects of EGCG on (myo)fibroblasts and AE2 cells. The
expression of α-SMA, a (myo)fibroblast marker, and SPB, an AE2
marker, was investigated using immunohistochemistry on the lung
sections. Strong α-SMA expression was observed in the irradiated
pulmonary tissues at 120 days post-irradiation. Treatment with
EGCG, but not DEX, significantly reduced α-SMA expression (Fig. 5, left column). Conversely, weak
SPB expression was observed in the irradiated pulmonary tissues at
120 days post-irradiation. Treatment with EGCG, but not DEX,
significantly enhanced SPB expression (Fig. 5, right column).
A morphometric analysis of α-SMA and SPB
immunohistochemistry as described in Materials and methods was
performed. The OD value for α-SMA immunohistochemistry was
significantly lower (p<0.05) in the EGCG-treated animals
compared with the DEX-treated animals at 60 and 120 days
post-irradiation. The OD value for α-SMA immunohistochemistry in
the DEX-treated animals was similar to that of the radiation-only
animals at 15, 30, 60 and 120 days post-irradiation (p>0.05;
Fig. 4C). Conversely, the OD
value for SPB immunohistochemistry was significantly higher
(p<0.05) in the EGCG-treated animals compared with the
DEX-treated animals at 15, 30, 60 and 120 days post-irradiation.
The OD value for SPB immunohistochemistry in the latter group was
significantly higher (p<0.05) than that of the radiation-only
animals at 30, 60 and 120 days post-irradiation (Fig. 4D). These results confirmed that
EGCG inhibits (myo)fibroblast proliferation and protects AE2 cells
from injury post-irradiation.
EGCG regulates serum cytokine levels
We examined whether treatment with EGCG reverses the
abnormal expression of cytokines in serum following irradiation.
Therefore, we measured the serum levels of TGF-β1 using ELISA as
well as IL-6, IL-10, and TNF-α using the BD™ CBA Flex Set. The
serum level of TGF-β1 was significantly lower (p<0.05) in the
EGCG-treated animals compared with the DEX-treated animals at 30,
60 and 120 days post-irradiation, whereas the level observed in the
DEX-treated animals was similar to that of the radiation-only
animals at the same time points (Fig.
6A).
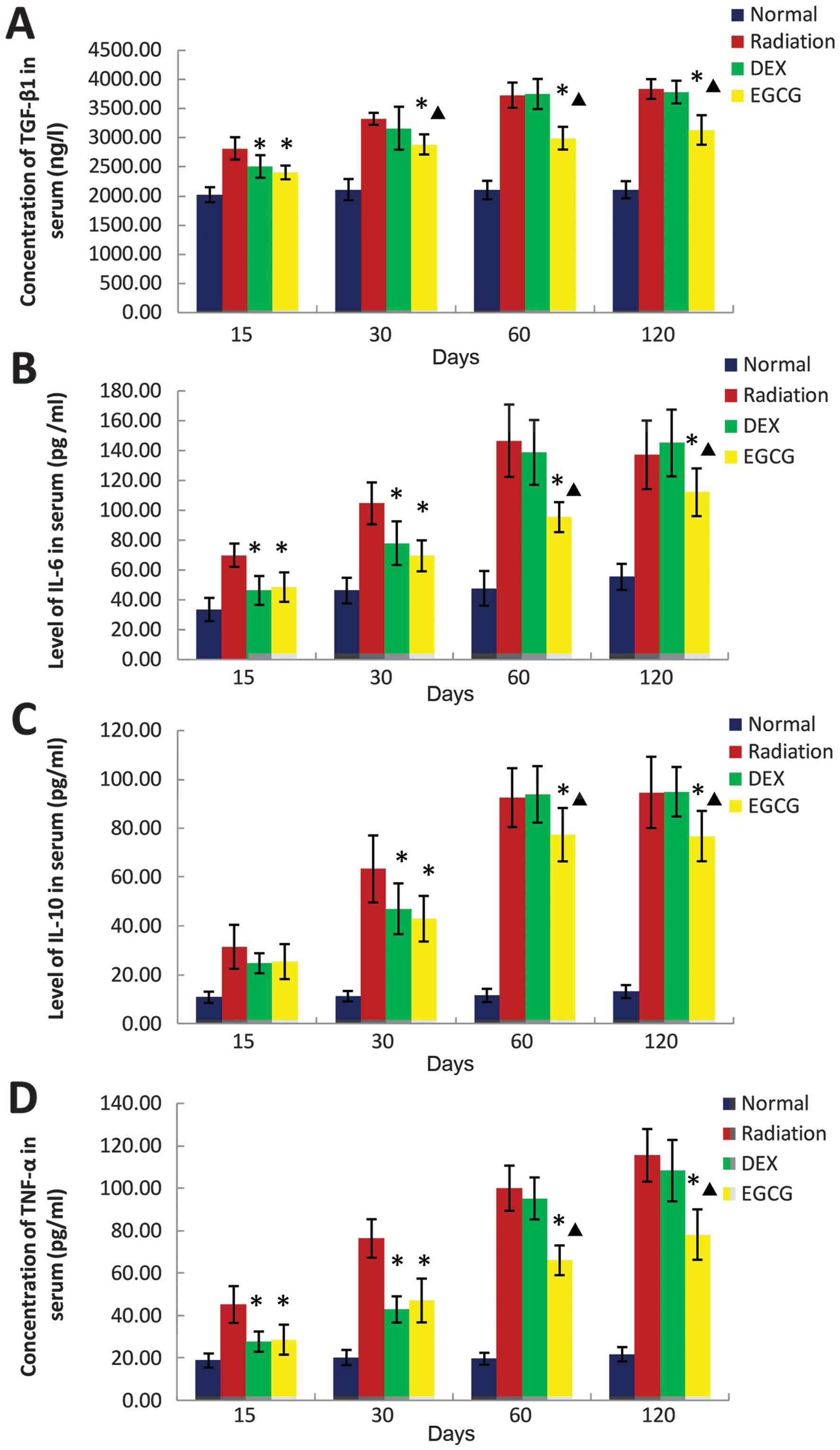 | Figure 6(A) The effect of
epigallocatechin-3-gallate (EGCG) on the serum levels of
transforming growth factor β1 (TGF-β1), (B) interleukin (IL)-6, (C)
IL-10, and (D) tumor necrosis factor α (TNF-α) at 15, 30, 60 and
120 days post-irradiation. (A) Serum TGF-β1 levels were
significantly lower (p<0.05) in the EGCG-treated animals
compared with those in the dexamethasone (DEX)-treated animals at
30, 60 and 120 days post-irradiation. The serum TGF-β1 levels in
the DEX-treated animals were similar to those of the untreated
animals at 30, 60 and 120 days post-irradiation. (B–D) The serum
levels of IL-6, IL-10, and TNF-α were significantly lower
(p<0.05) in the EGCG-treated animals compared with the
DEX-treated animals at 60 and 120 days post-irradiation. The serum
levels of IL-6 and TNF-α were significantly lower (p<0.05) in
the DEX-treated animals compared with those in the untreated
animals at 15 and 30 days post-irradiation. The serum levels of
IL-10 were significantly lower (p<0.05) in the DEX-treated
animals than those in the radiation-only animals at 30 days
post-irradiation. The serum levels of IL-6, IL-10, and TNF-α in the
DEX-treated animals were similar to those in the radiation-only
animals at 60 and 120 days post-irradiation. The bars in each graph
are the standard deviations (SDs). Asterisks show significance
(p<0.05) compared with the untreated animals and stars show
significance (p<0.05) compared with the DEX-treated animals. |
The serum levels of IL-6, IL-10 and TNF-α were
significantly lower (p<0.05) in the EGCG-treated animals than
the DEX-treated animals at 60 and 120 days post-irradiation. The
IL-6 and TNF-α levels were significantly lower (p<0.05) in the
DEX group compared with the radiation-only animals at 15 and 30
days post-irradiation. IL-10 was significantly reduced (p<0.05)
in the DEX-treated animals compared with the radiation-only animals
at 30 days post-irradiation. The levels of IL-6, IL-10 and TNF-α in
the DEX-treated animals were similar to those of the radiation-only
animals at 60 and 120 days post-irradiation (Fig. 6B–D).
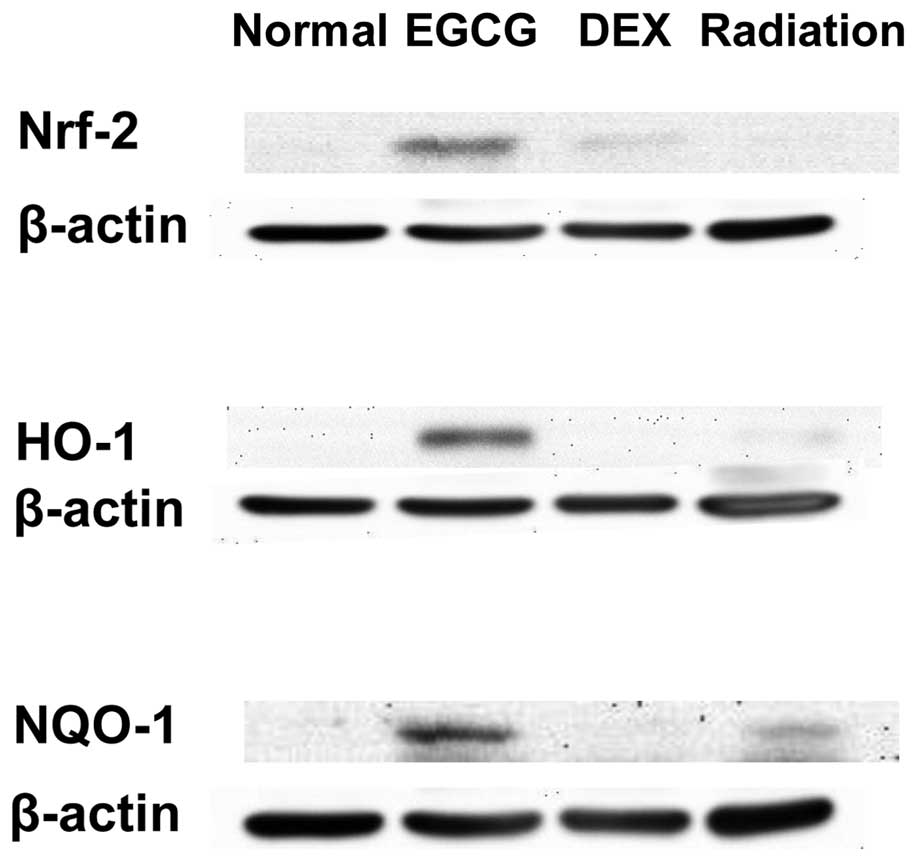
EGCG activates Nrf-2 and its downstream
antioxidant enzymes in pulmonary tissues
We also examined whether EGCG treatment activates
Nrf-2 signaling and its associated antioxidant enzymes. The protein
levels of Nrf-2, HO-1 and NQO-1 were assessed by western blot
analysis. This analysis revealed that EGCG administration strongly
activated Nrf-2, HO-1, and NQO-1 protein levels, whereas DEX
administration weakly activated Nrf-2 levels at 15 days post
irradiation (Fig. 7).
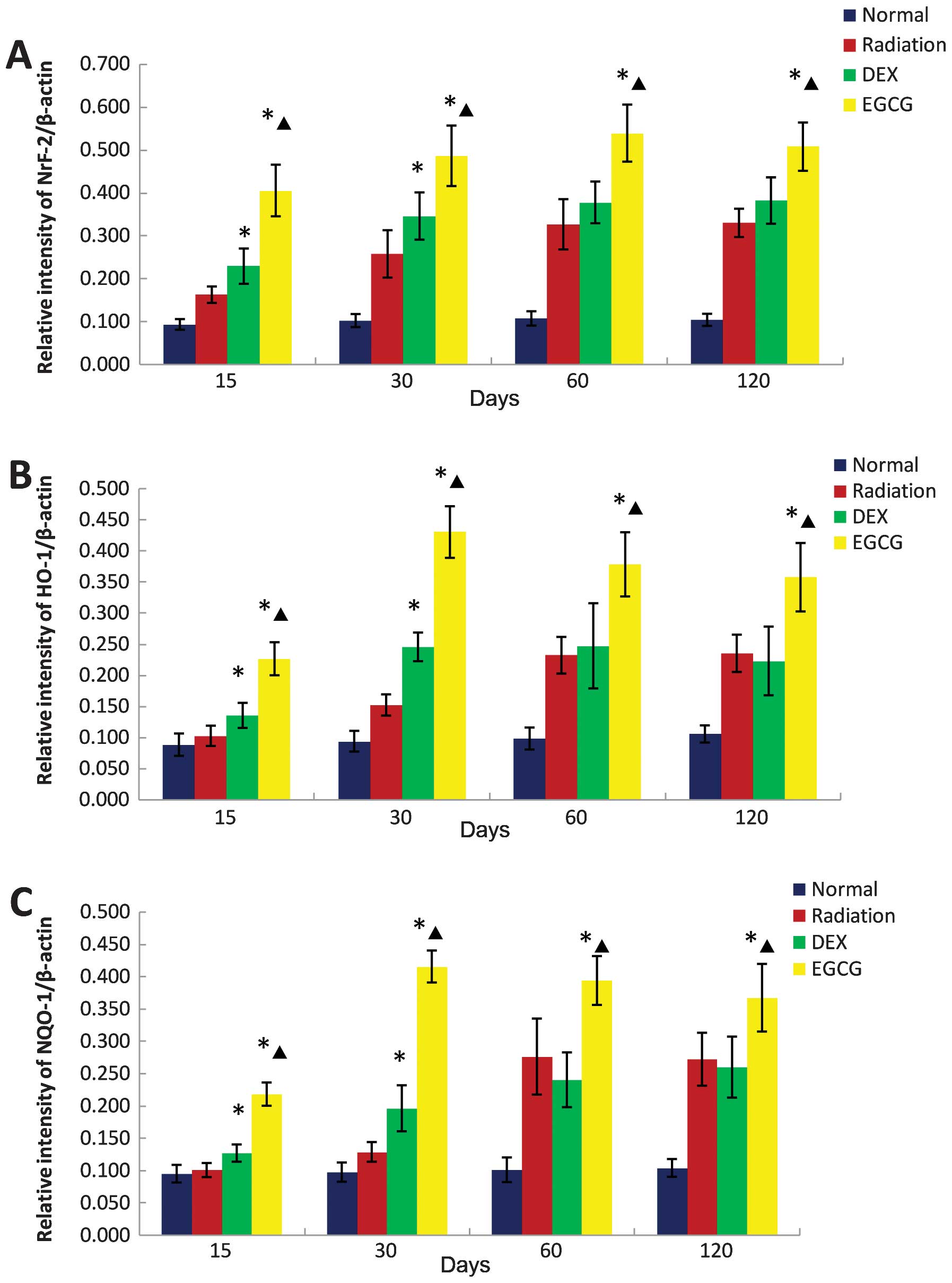
The protein levels of Nrf-2, HO-1, and NQO-1 were
significantly greater (p<0.05) in the pulmonary homogenates from
the EGCG-treated animals compared with those of the DEX-treated
animals at 15, 30, 60 and 120 days post-irradiation. The Nrf-2,
HO-1 and NQO-1 levels of the latter group were significantly higher
(p<0.05) than those of the radiation-only animals at 15 and 30
days post-irradiation but similar at 60 and 120 days
post-irradiation (Fig. 8).
Discussion
Results of the present study have shown that
irradiation-induced pulmonary fibrosis in rats is principally
ameliorated by EGCG administration. EGCG treatment reduced the
mortality rate and lung index score, alleviated lung histological
damage, reduced collagen deposition, modulated the redox state of
serum, inhibited (myo)fibroblast proliferation, protected AE2
cells, and regulated the serum levels of TGF-β1, IL-6, IL-10, and
TNF-α. We also showed that EGCG treatment activated Nrf-2 and its
downstream antioxidant enzymes HO-1 and NQO-1. The DEX treatment of
irradiation-induced pulmonary fibrosis did not produce similarly
ameliorative effects. Given these results, we demonstrated that
EGCG treatment significantly ameliorates irradiation-induced
pulmonary fibrosis. Our data reveal that EGCG has potential for
treatments of irradiation-induced pulmonary fibrosis.
Increasing evidence indicates that oxidative stress
and ROS contribute directly and indirectly to the formation of
irradiation-induced pulmonary fibrosis (19). The ROS-induced activation of
inflammatory cells (including macrophages, monocytes, and
neutrophils) can cause a positive feedback loop in which an
increased expression of a variety of intracellular oxidative
enzymes and large amounts of ROS and reactive nitrogen species
(RNS) are synthesized and released to remove necrotic tissue
(20,21). The dynamics of the
oxidant/antioxidant balance in the lung are destroyed, and tissue
damage persistently increases. Therefore, any therapeutic
intervention that defends against or alleviates oxidant insults may
be used to treat irradiation-induced pulmonary fibrosis. Due to its
potent antioxidant activity, a variety of animal models have shown
that the tea polyphenol EGCG is an effective scavenger of ROS and
free radicals with regard to tumors, cardiovascular diseases, and
neurological diseases both in vitro and in vivo
(15). The antioxidant activity
of EGCG (which likely involves the quenching of ROS, the
interception of free radicals, or both) is most likely mediated by
an H-atom transfer (HAT) reaction in which intramolecular hydrogen
bonding stabilizes the resultant phenoxy radical (22). Previous studies have suggested
that EGCG administration inhibits lipopolysaccharide (8) and bleomycin-induced pulmonary
fibrosis (11–13). Thus, we hypothesized that
scavenging free radicals with EGCG, a natural antioxidant extracted
from green tea, inhibits irradiation-induced pulmonary
fibrosis.
MDA levels and total SOD activity in serum are
important indicators of oxidative stress and the body’s capacity to
respond to induced oxidative stress. MDA levels most likely reflect
the degree of organic lipid peroxidation, which denotes the
severity of damage to cell membranes (23). Conversely, SOD plays a crucial
role in the organic oxidative/antioxidant balance. This enzyme can
neutralize free radical forms of oxygen, thereby protecting cells
from oxidative damage. Moreover, injections of SOD (24) and the SOD mimetic AEOL 10113
(25) have shown protective
effects in animal models of radiation-induced fibrosis. Our
investigation showed that the serum levels of MDA and inflammatory
cytokines decreased, and serum SOD activity increased in
DEX-treated animals compared with radiation-only animals at 15 and
30 days post-irradiation. However, these therapeutic benefits
ceased with treatment. EGCG-treated rats had greater SOD activity
than any other group of rats, including the non-irradiated normal
controls, at all time points (15–120 days post-irradiation). This
result suggests that EGCG reduced oxidative stress, at least in
part, by increasing the systemic production of antioxidant
proteins. Overall, our results have demonstrated that EGCG is
superior to DEX with regard to increasing the body’s capacity to
handle oxidative stress due to ROS/RNS, and these effects were
sustained long after treatment was discontinued.
The development of irradiation-induced pulmonary
fibrosis is also related to the expression of inflammatory
cytokines, which play an important role in creating a positive
feedback loop to reinforce the chemotaxis of macrophages and
neutrophils and sustaining oxidative stress by supporting the
enhanced production of ROS/RNS (26). TGF-β1 is a powerful cytokine that
can promote fibroblast proliferation and maturation, thereby
accelerating the development of pulmonary fibrosis (27). Wang et al demonstrated that
TGF-β1 levels were positively correlated with the incidence of
radiation-treatment-induced lung injury among patients with lung
cancer (28). TNF-α is a driving
factor within pro-inflammatory and immunoregulatory networks and is
likely involved in the development and progression of
radiation-induced pneumonitis (29). In addition, TNF-α stimulates the
proliferation of fibroblasts and the secretion of proinflammatory
cytokines, including IL-1 and IL-6, from neutrophils and
macrophages (30). IL-6 plays an
important role in the formation and proliferation of fibrous
connective tissue, potentially by increasing collagen aggregation,
inhibiting extracellular matrix (ECM) degradation, and stimulating
fibroblast proliferation. Previous studies have suggested that IL-6
leads to inflammation and fibrosis associated with hypersensitivity
pneumonitis in mice. These results suggest a close relationship in
IL-6 and pneumonitis and fibrotic development (31). IL-10 may inhibit monocytes,
macrophages, and Th1 cells as well as enhance B-cell immune
regulation function (32). In
addition, IL-10 is a T-cell-derived cytokine of the Th-2 family
that suppresses inflammation by inhibiting numerous
pro-inflammatory cytokines (33).
Findings of Barbarin et al have shown that silica-induced
pneumonia and pulmonary fibrosis in mice caused the overexpression
of IL-10, thereby contributing to the increased lung damage caused
by fibrosis (34).
To investigate the effects of EGCG treatment on
systemic inflammation, we measured the serum levels of key
inflammatory cytokines including TGF-β1, IL-6, IL-10, and TNF-α.
These cytokines were significantly reduced in the EGCG-treated
animals compared with the untreated and steroid-treated rats, and
this effect lasted for months after treatment ceased. The lower
alveolitis score of the EGCG-treated rats also suggested that EGCG
reduces the infiltration of inflammatory immune cells. Results of
this study are in agreement with those of previous studies that
have shown significant anti-inflammatory effects from the
administration of EGCG (10,35,36).
This study also investigated the protective effect
of EGCG on AE2 cells. AE2 and vascular endothelial cells are the
two major targets of radiation-induced lung injury from
inflammation and oxidative stress. Results of the SPB staining
analysis in the lung revealed that EGCG-treated rats showed a more
normalized distribution of AE2 cells in the parenchyma compared
with radiation-only and DEX-treated rats. AE2 cells were abundant
in alveolar walls of the lung tissues of the EGCG-treated rats,
although no evidence of dysplasia was found. These results suggest
that EGCG protected parenchymal and AE2 cells from free radical
damage. In addition, the myofibroblast proliferation in the lung
(as demonstrated by α-SMA staining) observed in the radiation-only
and DEX-treated groups was significantly reduced in rats treated
with EGCG at 60 and 120 days after irradiation, suggesting that
EGCG-inhibited pulmonary fibrosis partially inhibits myofibroblast
transformation and proliferation.
We also examined whether EGCG improves the ability
of the endogenous oxidative stress response system by activating
Nrf2 and its downstream antioxidant enzymes. Sriram et al
investigated the protective effects of EGCG in a bleomycin-induced
acute lung injury animal model and presented the first evidence
that EGCG protection against lung injury is associated with the
Nrf2-based activation of the oxidative stress response (12). Nrf2 plays a critical role in the
regulation of the major antioxidant enzymes HO-1 and NQO-1. Sahin
et al (37) reported that
EGCG significantly reduced the production of peroxides and the
subsequent peroxidation of lipids by enhancing the expression of
antioxidant enzymes (e.g., SOD, CAT, and GPx) to improve the
oxidative stress response. Those authors also showed that EGCG may
increase the downstream expression of other antioxidant enzymes by
activating Nrf2 and HO-1, thereby regulating oxidative stress. Our
western blot analysis results revealed that EGCG significantly
enhanced the expression levels of Nrf-2, HO-1, and NQO-1 in rat
lung tissues compared with radiation-only and DEX-treated rats,
thereby confirming the results of Sriram et al and Sahin
et al (12,37) as well as supporting our hypothesis
that EGCG relieves oxidative stress by activating Nrf2 and its
associated antioxidant enzymes.
Since glucocorticosteroids are commonly used to
treat irradiation-induced pulmonary fibrosis and other forms of
lung fibrosis in humans, DEX was selected as a baseline to compare
the efficacy of EGCG using various measures of lung inflammation,
the oxidative stress response, and fibrosis. A marginal
effectiveness was achieved with DEX therapy at 15 and 30 days
post-irradiation; however, these improvements ceased following
discontinuing steroid therapy (i.e., at 60 and 120 days
post-irradiation). The measures of lung inflammation, oxidative
stress, and fibrosis among the DEX-treated rats were similar to
those of the radiation-only group at 60 and 120 days
post-irradiation, suggesting a lack of persistent therapeutic
effects. Conversely, our results demonstrate that EGCG was superior
to glucocorticoids with regard to reducing inflammation, fibrosis,
and oxidative stress during the treatment period (which ended at 30
days post-irradiation). In addition, the therapeutic effects of
EGCG were sustained even after treatment ceased, unlike the steroid
treatment.
Collectively, results of the present study have
shown that EGCG treatment provides strong, persistent antioxidant,
anti-inflammatory, and anti-proliferative effects that protect
against irradiation-induced pulmonary fibrosis in rats. The
findings suggest that these effects are mediated by inhibiting
pro-inflammatory immune cells from infiltrating alveoli and lung
parenchyma, suppressing the expression of pro-inflammatory factors,
and inhibiting the synthesis and secretion of ROS/RNS-free radicals
that cause extensive oxidative damage to parenchymal cells. EGCG
also inhibited myofibroblast proliferation and AE2 cell dysplasia,
presumably by suppressing the secretion of TGF-β1. Of note, the
findings demonstrate that these effects reduced the rates of
morbidity and mortality compared with those among the rats in the
DEX group.
Acknowledgements
This study was mainly supported by grants from the
National Natural Science Foundation of China (NSFC contract nos.
81001220 and 81370077 to H.Y.), the Affiliated Hospital of the
Academy of Military Medical Sciences (to H.Y.), and the
12th Five-Year Project of PLA China (contract no.
CWS11J088 to H.Y.) and was partially supported by other grants from
the NSFC (NSFC no. 30972974 to W.J.Z. and NSFC contract no.
81202090 to K.Z.) and by grants from the Chongqing Science and
Technology Commission (contract no. 2013CSCT-JBKY-01703 to L.W.),
the Beijing Key Laboratory of Respiratory and Pulmonary Circulation
Disorders of the Capital Medical University (contract no.
RPCD201103 to H.Y.) and the Beijing Fengtai Local Government (to
H.Y.).
Abbreviations:
|
EGCG
|
epigallocatechin-3-gallate
|
|
DEX
|
dexamethasone
|
|
MDA
|
malondialdehyde
|
|
SOD
|
superoxide dismutase
|
|
TGF-β1
|
transforming growth factor β1
|
|
IL-6
|
interleukin-6
|
|
IL-10
|
interleukin-10
|
|
TNF-α
|
tumor necrosis factor α
|
|
SPB
|
surfactant protein-B
|
|
α-SMA
|
α smooth muscle actin
|
|
Nrf-2
|
nuclear transcription factor
NF-E2-related factor 2
|
|
HO-1
|
heme oxygenase-1 enzyme
|
|
NQO-1
|
NAD(P)H:quinone oxidoreductase-1
enzyme
|
|
AE2
|
alveolar epithelial type II
|
References
|
1
|
Almeida C, Nagarajan D, Tian J, et al: The
role of alveolar epithelium in radiation-induced lung injury. PLoS
One. 8:e536282013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Epperly MW, Guo H, Gretton JE and
Greenberger JS: Bone marrow origin of myofibroblasts in irradiation
pulmonary fibrosis. Am J Respir Cell Mol Biol. 29:213–224. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Matsuo Y, Shibuya K, Nakamura M, et al:
Dose-volume metrics associated with radiation pneumonitis after
stereotactic body radiation therapy for lung cancer. Int J Radiat
Oncol Biol Phys. 83:e545–e549. 2012.PubMed/NCBI
|
|
4
|
Minor GI, Yashar CM, Spanos WJ Jr, et al:
The relationship of radiation pneumonitis to treated lung volume in
breast conservation therapy. Breast J. 12:48–52. 2006.PubMed/NCBI
|
|
5
|
Rosenzweig KE, Zauderer MG, Laser B, et
al: Pleural intensity-modulated radiotherapy for malignant pleural
mesothelioma. Int J Radiat Oncol Biol Phys. 83:1278–1283. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu HW, Seftel MD, Rubinger M, et al:
Total body irradiation compared with BEAM: long-term outcomes of
peripheral blood autologous stem cell transplantation for
non-Hodgkin’s lymphoma. Int J Radiat Oncol Biol Phys. 78:513–520.
2010.PubMed/NCBI
|
|
7
|
Zhang Y, Zhang X, Rabbani ZN, Jackson IL
and Vujaskovic Z: Oxidative stress mediates radiation lung injury
by inducing apoptosis. Int J Radiat Oncol Biol Phys. 83:740–748.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mak JC: Potential role of green tea
catechins in various disease therapies: progress and promise. Clin
Exp Pharmacol Physiol. 39:265–273. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li CP, Yao J, Tao ZF, Li XM, Jiang Q and
Yan B: Epigallocatechin-gallate (EGCG) regulates autophagy in human
retinal pigment epithelial cells: a potential role for reducing UVB
light-induced retinal damage. Biochem Biophys Res Commun.
438:739–745. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Donà M, Dell’Aica I, Calabrese F, et al:
Neutrophil restraint by green tea: inhibition of inflammation,
associated angiogenesis, and pulmonary fibrosis. J Immunol.
170:4335–4341. 2003.PubMed/NCBI
|
|
11
|
Sriram N, Kalayarasan S and Sudhandiran G:
Epigallocatechin-3-gallate exhibits anti-fibrotic effect by
attenuating bleomycin-induced glycoconjugates, lysosomal hydrolases
and ultrastructural changes in rat model pulmonary fibrosis. Chem
Biol Interact. 180:271–280. 2009. View Article : Google Scholar
|
|
12
|
Sriram N, Kalayarasan S and Sudhandiran G:
Epigallocatechin-3-gallate augments antioxidant activities and
inhibits inflammation during bleomycin-induced experimental
pulmonary fibrosis through Nrf2-Keap1 signaling. Pulm Pharmacol
Ther. 22:221–236. 2009. View Article : Google Scholar
|
|
13
|
Sriram N, Kalayarasan S and Sudhandiran G:
Enhancement of antioxidant defense system by
epigallocatechin-3-gallate during bleomycin induced experimental
pulmonary fibrosis. Biol Pharm Bull. 31:1306–1311. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tipoe GL, Leung TM, Liong EC, Lau TY, Fung
ML and Nanji AA: Epigallocatechin-3-gallate (EGCG) reduces liver
inflammation, oxidative stress and fibrosis in carbon tetrachloride
(CCl4)-induced liver injury in mice. Toxicology. 273:45–52. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
de Vries HE, Witte M, Hondius D, et al:
Nrf2-induced antioxidant protection: a promising target to
counteract ROS-mediated damage in neurodegenerative disease? Free
Radic Biol Med. 45:1375–1383. 2008.PubMed/NCBI
|
|
16
|
Zhang L, Dong XW, Wang JN, et al:
PEP-1-CAT-transduced mesenchymal stem cells acquire an enhanced
viability and promote ischemia-induced angiogenesis. PLoS One.
7:e525372012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang YE, Fu SZ, Li XQ, et al: PEP-1-SOD1
protects brain from ischemic insult following asphyxial cardiac
arrest in rats. Resuscitation. 82:1081–1086. 2011. View Article : Google Scholar
|
|
18
|
Szapiel SV, Elson NA, Fulmer JD,
Hunninghake GW and Crystal RG: Bleomycin-induced interstitial
pulmonary disease in the nude, athymic mouse. Am Rev Respir Dis.
120:893–899. 1979.PubMed/NCBI
|
|
19
|
Williams JP, Johnston CJ and Finkelstein
JN: Treatment for radiation-induced pulmonary late effects: spoiled
for choice or looking in the wrong direction? Curr Drug Targets.
11:1386–1394. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rizvi M, Pathak D, Freedman JE and
Chakrabarti S: CD40-CD40 ligand interactions in oxidative stress,
inflammation and vascular disease. Trends Mol Med. 14:530–538.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mikkelsen RB and Wardman P: Biological
chemistry of reactive oxygen and nitrogen and radiation-induced
signal transduction mechanisms. Oncogene. 22:5734–5754. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hügel HM and Jackson N: Redox chemistry of
green tea polyphenols: therapeutic benefits in neurodegenerative
diseases. Mini Rev Med Chem. 12:380–387. 2012.PubMed/NCBI
|
|
23
|
Del Rio D, Stewart AJ and Pellegrini N: A
review of recent studies on malondialdehyde as toxic molecule and
biological marker of oxidative stress. Nutr Metab Cardiovasc Dis.
15:316–328. 2005.PubMed/NCBI
|
|
24
|
Lefaix JL, Delanian S, Leplat JJ, et al:
Successful treatment of radiation-induced fibrosis using Cu/Zn-SOD
and Mn-SOD: an experimental study. Int J Radiat Oncol Biol Phys.
35:305–312. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vujaskovic Z, Batinic-Haberle I, Rabbani
ZN, et al: A small molecular weight catalytic metalloporphyrin
antioxidant with superoxide dismutase (SOD) mimetic properties
protects lungs from radiation-induced injury. Free Radic Biol Med.
33:857–863. 2002. View Article : Google Scholar
|
|
26
|
Schaue D, Kachikwu EL and McBride WH:
Cytokines in radiobiological responses: a review. Radiat Res.
178:505–523. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Martin M, Lefaix J and Delanian S:
TGF-beta1 and radiation fibrosis: a master switch and a specific
therapeutic target? Int J Radiat Oncol Biol Phys. 47:277–290. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang J, Qiao XY, Lu FH, et al: TGF-beta1
in serum and induced sputum for predicting radiation pneumonitis in
patients with non-small cell lung cancer after radiotherapy. Chin J
Cancer. 29:325–329. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dong XR, Wang JN, Liu L, et al: Modulation
of radiation-induced tumour necrosis factor-α and transforming
growth factor β1 expression in the lung tissue by Shengqi Fuzheng
injection. Mol Med Rep. 3:621–627. 2010.
|
|
30
|
Wynn TA: Integrating mechanisms of
pulmonary fibrosis. J Exp Med. 208:1339–1350. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Denis M: Interleukin-6 in mouse
hypersensitivity pneumonitis: changes in lung free cells following
depletion of endogenous IL-6 or direct administration of IL-6. J
Leukoc Biol. 52:197–201. 1992.
|
|
32
|
Zdanov A: Structural features of the
interleukin-10 family of cytokines. Curr Pharm Des. 10:3873–3884.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Alhamad EH, Cal JG, Shakoor Z, Almogren A
and Alboukai AA: Cytokine gene polymorphisms and serum cytokine
levels in patients with idiopathic pulmonary fibrosis. BMC Med
Genet. 14:662013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Barbarin V, Xing Z, Delos M, Lison D and
Huaux F: Pulmonary overexpression of IL-10 augments lung fibrosis
and Th2 responses induced by silica particles. Am J Physiol Lung
Cell Mol Physiol. 288:L841–L848. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bae HB, Li M, Kim JP, et al: The effect of
epigallocatechin gallate on lipopolysaccharide-induced acute lung
injury in a murine model. Inflammation. 33:82–91. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sun Q, Zheng Y, Zhang X, et al: Novel
immunoregulatory properties of EGCG on reducing inflammation in
EAE. Front Biosci (Landmark Ed). 18:332–342. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sahin K, Tuzcu M, Gencoglu H, et al:
Epigallocatechin-3-gallate activates Nrf2/HO-1 signaling pathway in
cisplatin-induced nephrotoxicity in rats. Life Sci. 87:240–245.
2010. View Article : Google Scholar : PubMed/NCBI
|