Introduction
Astrocytes are major components of the adult
neurogenic niche and play a crucial role in regulating neural stem
cell proliferation and differentiation (1–4).
Following acute brain injury, such as cerebral ischemia,
intracerebral hemorrhage (ICH) or traumatic brain injury,
astrocytes become activated and proliferate in the context of brain
damage (5–7). Reactive astrocytes secrete numerous
pro-inflammatory cytokines which play pathophysiological roles in
the subsequent damage.
High-mobility group box 1 (HMGB1) protein is one of
the most important pro-inflammatory cytokines secreted by reactive
astrocytes (8,9), and mediates a variety of
inflammatory responses following insults that include sepsis,
pancreatitis and pneumonia (10–12). HMGB1 is a highly conserved
non-histone DNA-binding protein that stabilizes nucleosome
formation and regulates gene expression by facilitating
transcription (13,14). Previous studies have confirmed
that following brain injury, HMGB1 acts as an important
pro-inflammatory cytokine in mediating inflammation, neuronal
apoptosis and tissue damage (15–18). However, emerging data suggest that
HMGB1 also appears to have beneficial effects during the recovery
stage of brain injury. HMGB1 promotes neurovascular remodeling by
recruiting endothelial progenitor cells (EPCs) and increasing EPC
proliferation (7,19,20). Roles for HMGB1 in brain
development have also been reported, as HMGB1 is associated with
neurogenesis, neural progenitor cells survival and proliferation,
neurite outgrowth and neuronal differentiation (21–23).
Following brain injury, endogenous neural
stem/progenitor cells (NS/PCs) spontaneously proliferate in
proximity to the damaged area (24–26). However, the precise mechanisms
driving the NS/PC spontaneous proliferation in response to damage
remain unknown. Considering that HMGB1 can induce NS/PC
proliferation, and is secreted by reactive astrocytes following
brain injury, it is highly possible that HMGB1 released by
astrocytes may potentiate NS/PC proliferation following brain
injury.
In the present study, we examined the hypothesis
that HMGB1 released by astrocytes promotes NS/PC proliferation, and
investigated the possible intracellular signaling pathways within
the NS/PCs involved in this process. Astrocytes were stimulated
with low levels of interleukin (IL)-1β to mimic a reactive
phenotype. Subsequently, we used astrocyte-conditioned medium (ACM)
with or without HMGB1 through the use of RNA interference (RNAi) to
evaluate the effects of astrocyte-derived HMGB1 on NS/PC
proliferation. We then explored the potential underlying mechanisms
in vitro, through the blockade of the receptor for advanced
glycation endproducts (RAGE) with an anti-RAGE antibody and by
inhibiting the c-Jun N-terminal protein kinase (JNK) signaling
pathway with the potent JNK inhibitor, SP600125. The present study
provides an experimental basis to explain the potential mechanisms
through which NS/PCs spontaneously proliferate following brain
injury.
Materials and methods
Animals
The Ethics Committee of Chongqing Medical
University, Chongqing, China approved all protocols for the animal
experiments. All procedures were carried out in accordance with the
National Institutes of Health (NIH) Guide for the Care and Use of
Laboratory Animals (NIH, Bethesda, MD, USA). All animals were
provided by the Experimental Animal Center of Chongqing Medical
University. Neonatal Sprague-Dawley (SD) rats (1–2 days old) were
used for primary astrocyte culture and primary NS/PC culture. All
efforts were made to minimize the number of animals used, as well
as their suffering.
Study design
In the experiments using ACM, the astrocytes were
assigned to 4 groups, and cultured for 24 h in serum-free NS/PC
medium with or without IL-1β (0.1 ng/ml), as previously described
by Hayakawa et al (7)
(Table I). Four types of
conditioned medium were collected and used undiluted in NS/PC
proliferation assays: normal ACM (nACM), stimulated ACM (sACM),
HMGB1 shRNA interference sACM (HMGB1 shRNA sACM) and control shRNA
interference sACM (control shRNA sACM).
 | Table IStudy design. |
Table I
Study design.
| Experiment | Group | Number | Explanation |
|---|
|
Astrocyte-conditioned media (ACM)
synthesis of HMGB1 | nACM | 9 | Astrocytes were
cultured in NS/PC medium for 24 h in the absence of IL-1β
stimulation |
| sACM | 9 | Astrocytes were
cultured for 24 h in NS/PC medium containing IL-1β (0.1 ng/ml;
Prospec, East Brunswick, NJ, USA) |
| HMGB1 shRNA
sACM | 9 | Astrocytes
expressing HMGB1 shRNA were cultured for 24 h in NS/PC medium
containing 0.1 ng/ml IL-1β |
| Control shRNA
sACM | 9 | Astrocytes
expressing control shRNA were cultured for 24 h in NS/PCs medium
containing 0.1 ng/ml IL-1β |
| NS/PC
proliferation | Vehicle | 9a | NS/PCs were
cultured in serum-free NS/PC medium |
| Control | 9a | NS/PCs were
cultured in nACM containing IL-1β |
| sACM | 9a | NS/PCs were
cultured in sACM |
| HMGB1 shRNA
sACM | 9a | NS/PCs were
cultured in sACM from astrocytes expressing HMGB1 shRNA |
| Control shRNA
sACM | 9a | NS/PCs were
cultured in sACM from astrocytes expressing control shRNA |
| HMGB1 | 9a | NS/PCs were
cultured in serum-free NS/PC medium containing 7 ng/ml recombinant
human HMGB1 (Prospec) |
| RAGE pathway
experiment | IgG | 9 | NS/PCs were
cultured for 96 h in NS/PC culture medium containing 7 ng/ml HMGB1
and 20 μg/ml control IgG (Beyotime Institute of Biotechnology,
Wuhan, China) |
| Anti-RAGE | 9 | NS/PCs were
cultured for 96 h in NS/PC culture medium containing 7 ng/ml HMGB1
and 20 μg/ml anti-RAGE antibody (Santa Cruz Biotechnology, Santa
Cruz, CA, USA) |
| JNK pathway
analysis | Vehicle | 9 | NS/PCs were
cultured for 96 h in serum-free NS/PC medium |
| 0 μM | 9 | NS/PCs were
cultured for 96 h in NS/PC culture medium containing 7 ng/ml
HMGB1 |
| 1 μM | 9 | NS/PCs were
cultured for 96 h in NS/PC culture medium containing 7 ng/ml HMGB1
and 1 μM SP600125 |
| 10 μM | 9 | NS/PCs were
cultured for 96 h in NS/PC culture medium containing 7 ng/ml HMGB1
and 10 μM SP600125 |
In the NS/PC proliferation experiments, the NS/PCs
were assigned to 6 groups, and cultured in NS/PC culture medium
(vehicle), nACM with IL-1β (0.1 ng/ml) (control), sACM, HMGB1 shRNA
sACM, control shRNA sACM and HMGB1 culture medium (Table I). To eliminate interference by
IL-1β and cytokines that were secreted by normal astrocytes, we
used nACM containing IL-1β (0.1 ng/ml) as a control culture
(control group). To further confirm that HMGB1 promotes NS/PC
proliferation, we added 7 ng/ml recombinant human HMGB1
(approximate to the HMGB1 level in sACM) to serum-free NS/PC medium
as an additional control (HMGB1 group). Each group was further
divided into 4 subgroups for time course analysis and cultured for
24, 48, 72 and 96 h.
In the analysis of the dual RAGE-JNK signaling
pathway, we selected HMGB1 culture medium (NS/PC culture medium
containing 7 ng/ml recombinant human HMGB1). In the RAGE pathway
experiments, the NS/PCs were cultured in HMGB1 culture medium in
the presence of IgG (20 μg/ml) or anti-RAGE antibody (20 μg/ml) for
96 h (Table I). In the JNK
signaling pathway experiments, the NS/PCs were cultured in HMGB1
culture medium in the presence of increasing concentrations of the
potent JNK inhibitor, SP600125 (0, 1 and 10 μM), and serum-free
NS/PC culture medium for 96 h (Table
I).
Primary astrocyte culture
Primary astrocytes were prepared from the cortices
of 1–2-day-old neonatal SD rats as previously described (7), with some modifications. The cells
were grown in high-glucose Dulbecco’s modified Eagle’s medium
(DMEM; Invitrogen, Beijing, China), containing 10% fetal bovine
serum (FBS; Gibco, Life Technologies Ltd., Mount Waverley,
Victoria, Australia) and 25 μg/ml penicillin/streptomycin in 95%
air/5% CO2. To remove non-astrocytic cells, which were
mainly microglia, the flasks were shaken overnight at 200 rpm. The
cells were then trypsinized and seeded into T50 flasks at a density
of 6x105 cells/cm2. The cells were cultured
for 16–18 days before preparing the ACM and were only used for
study when the purity of the astrocytes reached a level of 95%
[verified with Glial fibrillary acidic protein (GFAP)
staining].
Primary NS/PC culture
NS/PCs were prepared from the cortices of
1–2-day-old neonatal SD rats as previously described (27), with minor modifications. Single
cells were plated in uncoated T50 flasks at a density of
1x105 cells/cm2 with NS/PC culture medium
composed of DMEM/F12 (Invitrogen) supplemented with 2% B27
supplement (Invitrogen), 20 ng/ml epidermal growth factor (EGF;
Peprotech, Rocky Hill, NJ, USA) and 20 ng/ml basic fibroblast
growth factor (bFGF; Peprotech). The cultures were maintained for 7
days in a humidified incubator at 37°C and 5% CO2, with
half-volume changes of fresh medium every 2–3 days. Neurospheres
that formed during the period were dissociated into single cells
with 0.125% trypsin (HyClone, Logan, UT, USA), and the cells were
regenerated into neurospheres using NS/PC culture medium. These
procedures were repeated 2–3 times before the cells were treated
and single-cell suspensions from neurospheres were utilized to
examine cell proliferation.
Lentivirus infection of astrocytes
Lentiviral vectors expressing HMGB1 shRNA or control
shRNA were obtained from Shanghai Genechem Co. Ltd, Shanghai,
China. The sequences of HMGB1 shRNA were as follows:
5′-GATCCCGAAGCA CCCGGATGCTTCTTTCAAGAGAAGAAGCATCCGGG
TGCTTTTTTGGAAA-3′ (15). Control
shRNA consisted of a scrambled sequence that fails to target any
known cellular mRNA.
The shRNA-carrying lentiviral vector was used to
infect the astrocytes at a multiplicity of infection (MOI) of 10,
30 and 50. The lentiviral vectors were prepared according to the
transduction protocol for cell cultures provided by Shanghai
Genechem. Five days after infection, the cells were collected and
the percentage of GFP-positive cells was quantified by fluorescence
microscopy. The optimum MOI was found to be 30, which resulted in a
transduction efficiency of 95%. Western blot analysis was then
performed to determine the efficiency of HMGB1 knockdown.
Enzyme-linked immunosorbent assay
(ELISA)
The concentrations of HMGB1 in the nACM, sACM, HMGB1
shRNA sACM and control shRNA sACM were analyzed with a commercially
available ELISA kit according to the manufacturer’s instructions
(Cat. no. CSB-E08224r/96T; Cusabio Biotech Co., Ltd., Wuhan,
China).
CCK-8 proliferation assays for
NS/PCs
The CCK-8 (Cat. no. C0038; Beyotime Institute of
Biotechnology, Shanghai, China) proliferation assay was used to
determine the rate of NS/PC proliferation. Single NS/PCs were
seeded into 96-well plates (10,000 cells/well in 100 μl medium)
with corresponding medium. CCK-8 solution (10 μl) was added to the
cell culture medium and following incubation for 2 h at 37°C, the
absorbance of the culture medium was determined at a wavelength of
450 nm with a reference wavelength of 630 nm by using a multiskan
spectrum scanning spectrophotometer (Thermo Labsystems, Vantaa,
Finland). Using these procedures, a good linear correlation was
obtained between the absorbance and viable cell number. Each
experiment was performed in triplicate and the results were
collected as the average of at least 3 independent experiments.
NS/PC cell cycle analysis
The effects of the different culture media on cell
cycle distribution were measured by flow cytometry, as previously
described (28). The NS/PCs were
dissociated into single-cell suspensions, and fixed in 70% ice-cold
ethanol overnight at 4°C. The fixed cells were stained with
propidium iodide (50 μg/ml; BD Biosciences Franklin Lakes, NJ,
USA), and 50 μg/ml RNAse A (BD Biosciences) at 37°C for 30 min in
the dark, and subsequently analyzed using a flow cytometer
(FACSVantage SE; BD Biosciences). The changes in cell cycle
distribution were determined by calculating the proliferation index
(PI). The following formula was used: PI = (S + G2/M)/(G0/G1 + S +
G2/M), as previously described (28).
Double-immunofluoresence labeling
The cultured astrocytes and neurospheres were washed
and fixed with 4% paraformaldehyde at 4°C for 30 min. The
astrocytes were incubated overnight at 4°C with the following
primary antibodies: mouse anti-GFAP (1:300; Cell Signaling
Technology, Danvers, MA, USA) and rabbit anti-HMGB1 antibodies
(1:100; Abcam, Cambridge, MA, USA). Neurospheres were incubated
overnight at 4°C with the following primary antibodies: rabbit
anti-nestin (1:50; Proteintech, Wuhan, China) and mouse anti-Sox-2
antibodies (1:100; Cell Signaling Technology). The cells were then
incubated with secondary antibodies, including goat anti-mouse
Alexa Fluor 647 (1:200; Beyotime Institute of Biotechnology), goat
anti-rabbit TRITC (1:100) and goat anti-mouse FITC (1:100) (all
from Beijing Ding Guo Changsheng Biotech Co., Ltd., Beijing, China)
for 1 h at 37°C in the dark. Finally, the cells were examined by
laser-scanning confocal microscopy on an Olympus IX70 inverted
microscope (Olympus, Tokyo, Japan) equipped with a FluoView FVX
confocal scan head (Leica Microsystems GmbH, Wetzlar, Germany).
Western blot analysis
Total protein derived from the astrocytes and NS/PCs
was harvested with RIPA lysis buffer containing a protease and
phosphatase inhibitor cocktail (KeyGen Biotech Co., Ltd., Nanjing,
China). The protein concentrations were measured using the Bradford
method (Beyotime Institute of Biotechnology). The protein samples
(50 μg) were fractionated by 10% SDS-polyacrylamide gel
electrophoresis, electroblotted onto a polyvinylidene difluoride
(PVDF; Millipore, Billerica, MA, USA) membrane and immunoblotted
with primary antibodies, including: rabbit anti-HMGB1 (1:1,000),
rabbit anti-phosphorylated JNK (anti-p-JNK, 1:1,000; Cell Signaling
Technology), rabbit anti-JNK (1:1,000; Cell Signaling Technology)
and mouse anti-β-actin anbitody (1:1000, Beijing Ding Guo
Changsheng Biotech) as an internal control. A Bio-Rad apparatus
(Bio-Rad Laboratories, Richmond, CA, USA) and Quantity One software
version 4.6.2 (Bio-Rad Laboratories) were used to scan the
immunoblots for semi-quantitative analysis.
Statistical analysis
The statistical software program SPSS 18.0 for
Windows (SPSS Inc., Chicago, IL,USA) was used to conduct
statistical analysis. All data are presented as the means ±
standard deviation (SD). Statistical analysis was performed by
one-way ANOVA, and when α values were at P<0.05, the Bonferroni
test was used for homogeneity of variance, and the Tamhane test was
used for heterogeneity of variance. Values of P<0.05 were
considered to indicate statistically significant differences.
Results
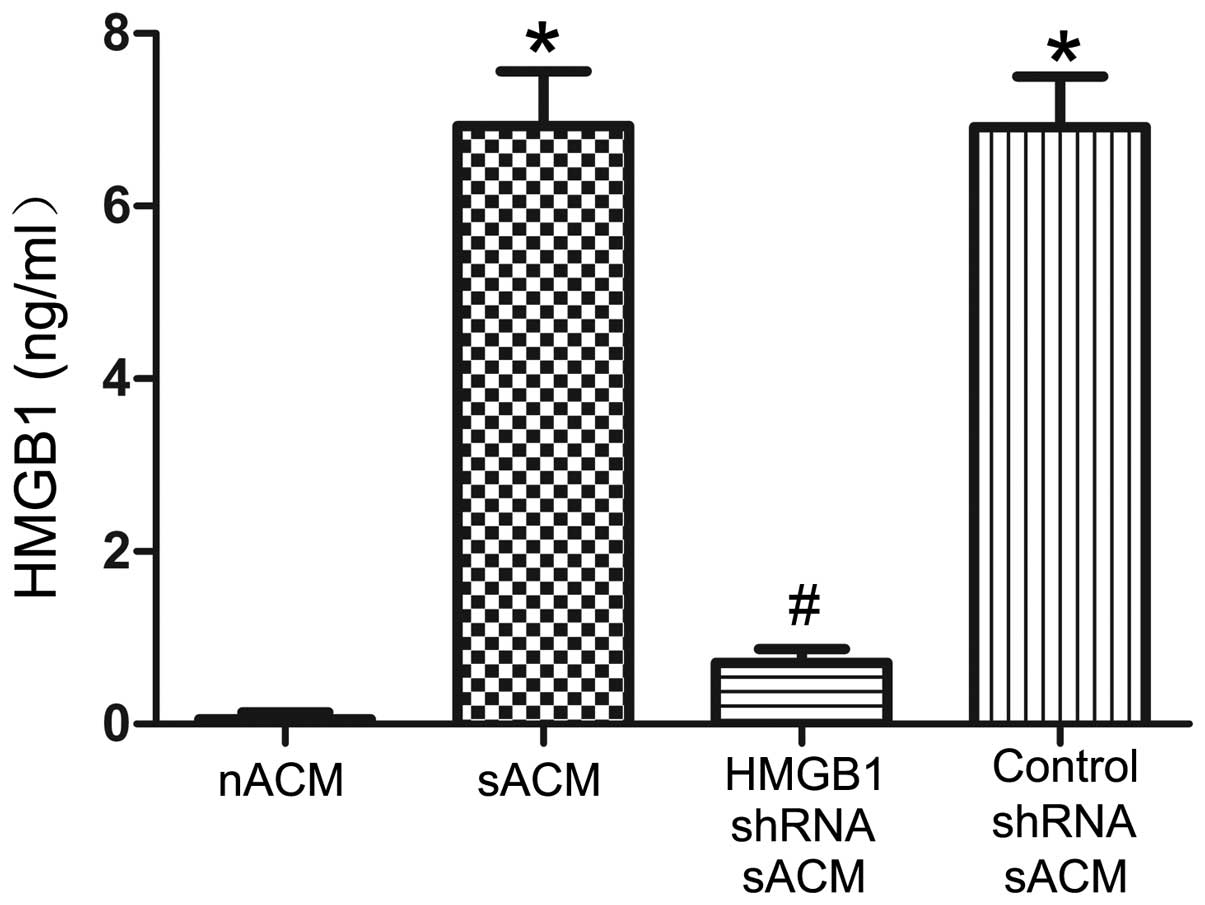
IL-1β-stimulated astrocytes can release
HMGB1, and RNAi successfully suppresses the release of HMGB1
To determine the effects of IL-1β and RNAi on HMGB1
secretion, we examined the HMGB1 concentrations in the ACM. HMGB1
protein expression was barely detectable in the nACM (0.060±0.078
ng/ml). After 24 h of stimulation with IL-1β, sACM showed a clear
accumulation of soluble HMGB1 (6.929±0.630 ng/ml). Compared with
that in sACM, the HMGB1 concentration in the HMGB1 shRNA sACM
(0.707±0.164 ng/ml) was markedly decreased by RNAi (Fig. 1). Additionally, there was almost
no effect on the HMGB1 concentration in the control shRNA sACM
(6.917±0.586 ng/ml), compared with that in the sACM (Fig. 1).
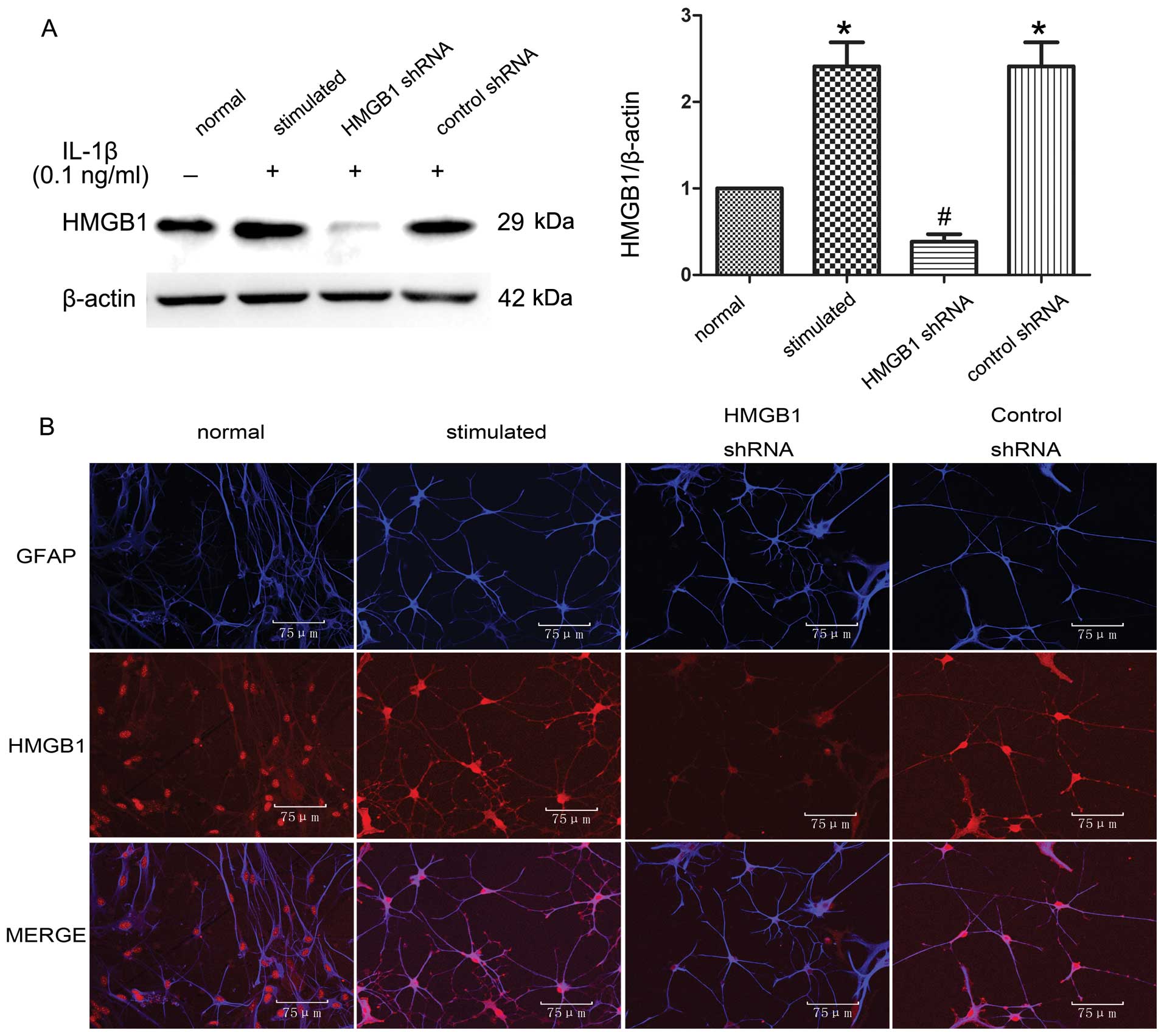
IL-1β upregulates HMGB1 protein
expression in astrocytes, and RNAi successfully suppresses the
expression of HMGB1
To determine the effects of IL-1β and RNAi on
intracellular HMGB1 expression, we examined the HMGB1 protein
levels in cell lysates obtained from astrocytes and
double-immunofluorescence-stained astrocytes. Western blots of
astrocyte lysates (Fig. 2A) and
the double-immunofluorescence staining of astrocytes
(HMGB1/astrocytes red/blue; Fig.
2B) confirmed that endogenous HMGB1 protein was indeed
upregulated in the IL-1β-stimulated astrocytes compared to the
normal astrocytes. HMGB1 shRNA effectively suppressed HMGB1 protein
expression in the IL-1β-stimulated astrocytes. Control shRNA had no
effect on HMGB1 expression in the IL-1β-stimulated astrocytes,
compared to that of the IL-1β-stimulated astrocytes.
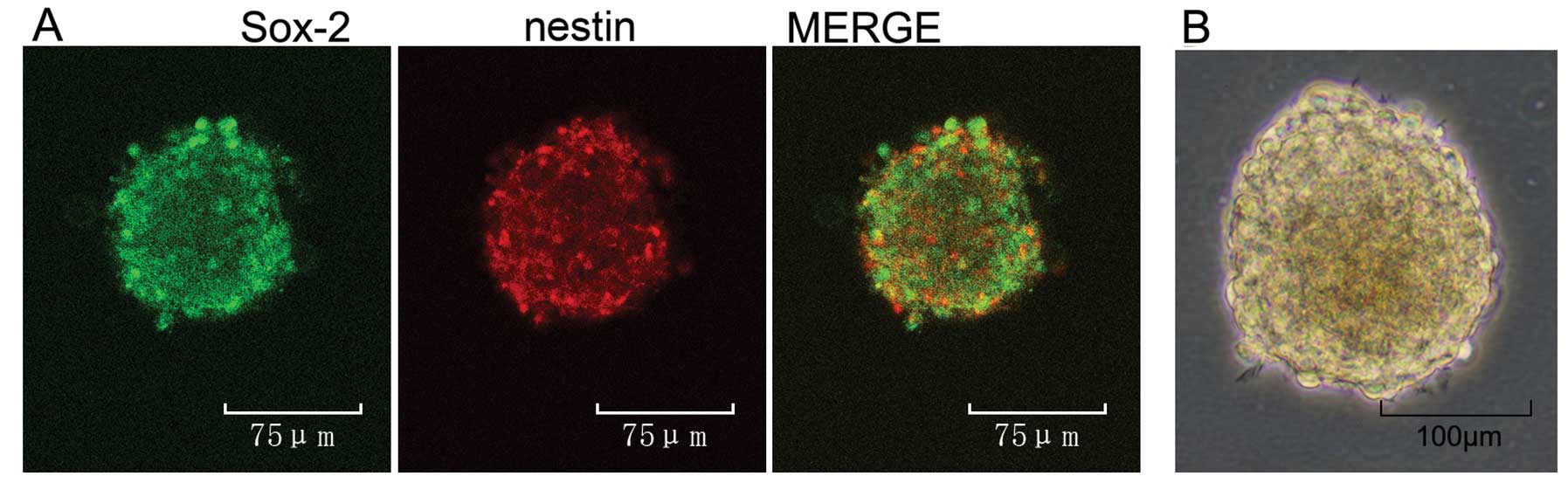
Neurospheres cultured in vitro were
identified with the representative markers, nestin and SRY-re lated
HMG-box gene 2 (Sox-2)
To characterize the cell population, we performed
double-labelling experiments with markers of undifferentiated
neural stem/progenitor cells, including the intermediate
neurofilament protein, nestin, and Sox-2, a transcription factor
expressed in undifferentiated stem/progenitor cell nuclei (29) (Fig.
3A). Infant rat cortex-derived neurospheres grown in the
presence of EGF and bFGF are mainly composed of nestin (red) and
Sox-2 (green)-positive cells. The results of double-immunolabelling
experiments and the light microscopy of neurospheres (Fig. 3B) suggested that they are composed
of undifferentiated NS/PCs.
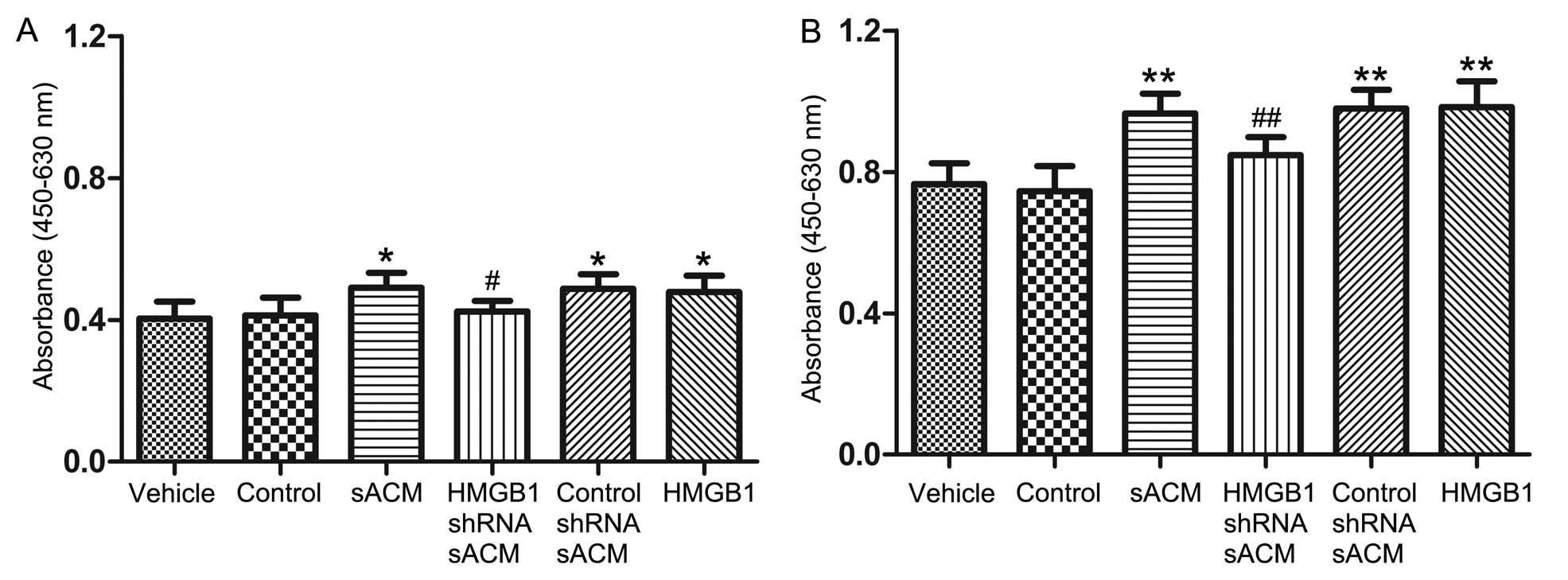
HMGB1 released by astrocytes promotes
NS/PC proliferation in vitro
To determine whether HMGB1 released from astrocytes
affects NS/PC proliferation, we performed an in vitro CCK-8
assay. The absorbance of CCK-8 in the NS/PC proliferation
experiments is shown in Table
II. The results indicatedd that there were no differences in
the proliferation rates among each of the groups at 24 or 48 h
(P>0.05). However, as shown in Fig. 4, after 72 and 96 h of exposure,
the NS/PC proliferation rates in the sACM group, control shRNA sACM
group and HMGB1 group increased significantly compared to the
vehicle control group. Compared to the sACM group, the NS/PC
proliferation rates in the HMGB1 shRNA sACM group exhibited a
statistically significant decrease at 72 and 96 h, while the NS/PC
proliferation rates in the control group showed no statistical
differences at 72 or 96 h compared to the vehicle group
(P>0.05).
 | Table IIEffects of astrocyte-conditioned
medium on the CCK-8 absorbance of NS/PCs (n=9, values indicate the
means ± SD). |
Table II
Effects of astrocyte-conditioned
medium on the CCK-8 absorbance of NS/PCs (n=9, values indicate the
means ± SD).
| Time point (h) | Vehicle | Control | sACM | sACM HMGB1
shRNA | sACM control
shRNA | HMGB1 |
|---|
| 24 | 0.197±0.036 | 0.196±0.033 | 0.175±0.027 | 0.196±0.032 | 0.179±0.026 | 0.180±0.033 |
| 48 | 0.294±0.041 | 0.298±0.048 | 0.319±0.050 | 0.313±0.039 | 0.310±0.046 | 0.312±0.031 |
| 72 | 0.404±0.047 | 0.413±0.049 | 0.490±0.041 | 0.423±0.030 | 0.487±0.041 | 0.479±0.045 |
| 96 | 0.765±0.059 | 0.745±0.071 | 0.966±0.056 | 0.848±0.051 | 0.980±0.053 | 0.984±0.073 |
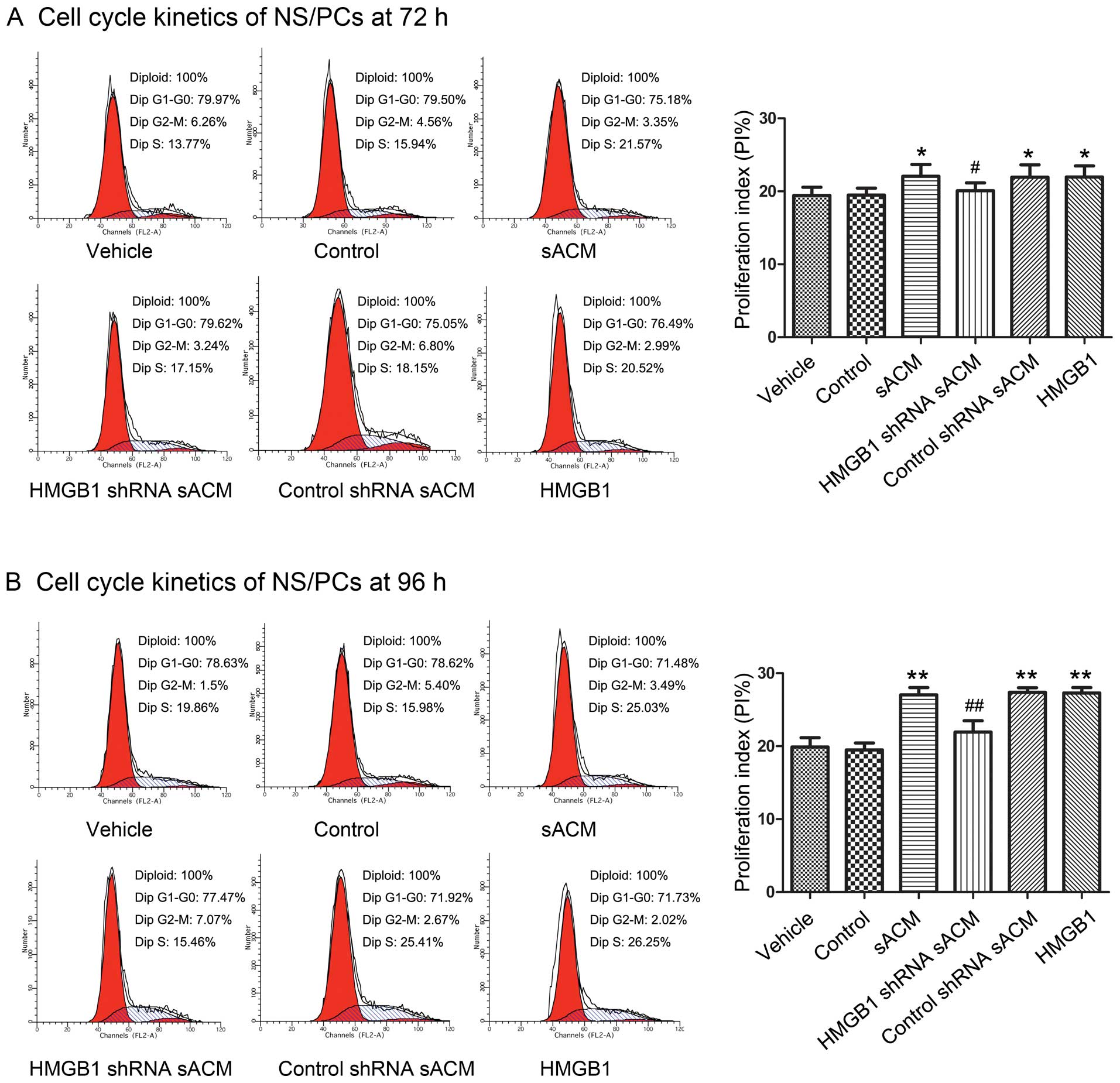
HMGB1 released by astrocytes promotes
NS/PC proliferation in vitro by regulating the cell cycle
To further investigate the effects of
astrocyte-derived HMGB1 on NS/PC proliferation, we analyzed the
cell cycle of NS/PCs by flow cytometry in a time course experiment
using the different culture media. The PI was selected to reflect
the proliferative ability of the NS/PCs, using the following
formula: PI = (S + G2/M)/(G0/G1 + S + G2/M), as previously
described (28). The PI in the
NS/PC proliferation experiments is shown in Table III. There was no statistically
significant difference in the PI following culture for 24 or 48 h
(P>0.05) with the different culture media. Compared with the PI
of the vehicle group, the PI of the sACM group, control shRNA sACM
group and HMGB1 group showed a significant increase after 72 and 96
h (Fig. 5). Compared with the
sACM group, the PI of the HMGB1 shRNA sACM group exhibited a
statistically significant decrease after 72 and 96 h (Fig. 5). There was no significant
difference in the PI between the vehicle group and the control
group at all 4 time points (P>0.05).
 | Table IIIEffects of astrocyte-conditioned
medium on the proliferation index (PI) of NS/PCs (n=9, values
indicate the means ± SD). |
Table III
Effects of astrocyte-conditioned
medium on the proliferation index (PI) of NS/PCs (n=9, values
indicate the means ± SD).
| Time point (h) | Vehicle | Control | sACM | sACM HMGB1
shRNA | sACM control
shRNA | HMGB1 |
|---|
| 24 h | 17.87±0.92 | 17.87±0.86 | 17.69±0.97 | 17.72±0.96 | 17.84±1.09 | 17.59±1.02 |
| 48 h | 18.95±0.96 | 18.58±1.00 | 18.81±0.96 | 19.09±1.07 | 18.65±1.07 | 18.58±0.92 |
| 72 h | 19.45±1.12 | 19.48±0.97 | 22.07±1.61 | 20.06±1.00 | 21.95±1.69 | 21.96±1.53 |
| 96 h | 19.89±1.27 | 19.48±0.96 | 27.04±1.00 | 21.13±1.09 | 27.38±0.63 | 27.82±0.76 |
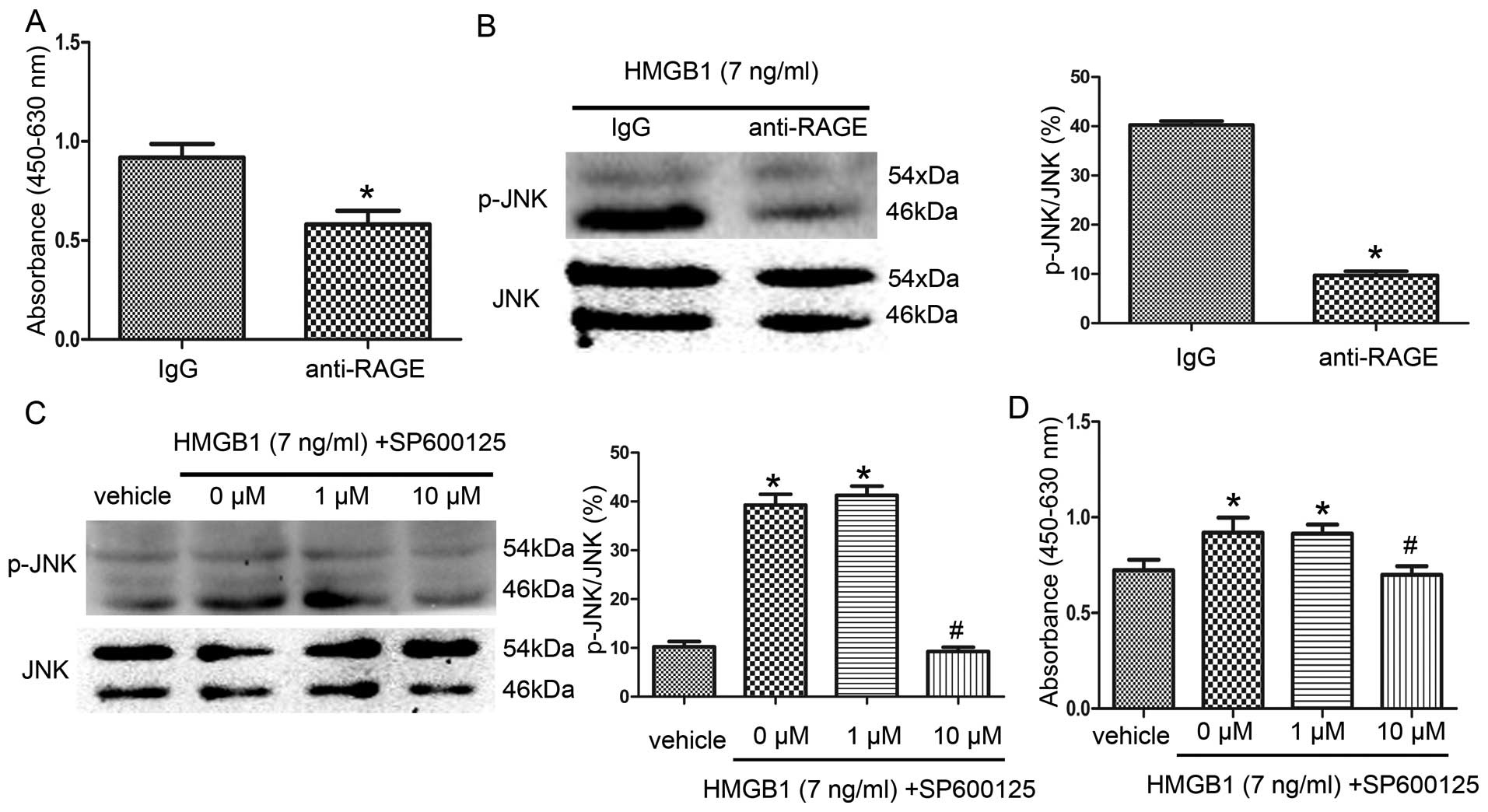
HMGB1 promotes NS/PC proliferation
through the activation of the RAGE-dependent JNK signaling
pathway
To explore the mechanisms behind HMGB1-mediated
NS/PC proliferation, we investigated the RAGE receptor pathway, a
critical receptor for HMGB1, and the JNK signaling pathway, a
signaling pathway involved in HMGB1-RAGE signaling (30). First, we performed an in
vitro CCK-8 assay in which the NS/PCs were placed in HMGB1
culture medium for 96 h with or without anti-RAGE antibodies. As
shown in Fig. 6A, the NS/PC
proliferation rates in the anti-RAGE group (absorbance,
0.581±0.068) decreased significantly compared with the IgG group
(absorbance, 0.919±0.069). Correspondingly, the p-JNK protein
levels were significantly attenuated in the anti-RAGE group
compared to the IgG group (Fig.
6B).
To further determine the effects of the JNK
signaling pathway on HMGB1-mediated NS/PC proliferation, the NS/PCs
were incubated for 96 h in HMGB1 culture medium with various
concentrations of the JNK inhibitor, SP600125. As shown in Fig. 6C, compared with the vehicle group,
the p-JNK levels in the 0 μM group and 1 μM group were markedly
increased, while the p-JNK levels significantly decreased in the 10
μM group compared to the 0 μM group. Correspondingly, the NS/PC
proliferation rates significantly increased in the 0 μM group
(absorbance, 0.920±0.078) and 1 μM group (absorbance, 0.914±0.047),
compared with the vehicle group (absorbance, 0.723±0.055) (Fig. 6D), and the NS/PC proliferation
rates were significantly decreased in the 10 μM group (absorbance,
0.700±0.045) relative to the 0 μM group.
Discussion
In the present study, the CCK-8 proliferation assay
and PI were selected to reflect the proliferative capacity of the
NS/PCs. NS/PCs treated with IL-1β-stimulated ACM (sACM) displayed
increased proliferation rates in vitro. When endogenous
HMGB1 in the IL-1β-stimulated ACM was suppressed using shRNA, the
ability of the sACM to evoke in vitro NS/PC proliferation
was decreased. The addition of exogenous HMGB1 to the NS/PC
cultures also increased the NS/PC proliferation rates in
vitro. Furthermore, treatment of the HMGB1-induced NS/PCs with
an anti-RAGE antibody significantly decreased both the NS/PC
proliferation rates and p-JNK protein levels. Finally, treatment of
the HMGB1-induced NS/PCs with various concentrations of the JNK
inhibitor, SP600125, revealed that p-JNK levels decreased
concomitantly with the proliferation rates of NS/PCs. These results
suggest that astrocyte-derived HMGB1 promotes NS/PC proliferation
in vitro, and that HMGB1-stimulated NS/PC proliferation may
be mediated through the RAGE-dependent JNK signaling pathway.
As major components of the neurogenic niche,
astrocytes play a crucial role in regulating neural stem cell
proliferation and differentiation (1–4).
However, following injury to the central nervous system,
traditional thinking presumes that astrocytes are activated to form
glial scars and promote inflammation, which is detrimental to
neuronal recovery (31–34). However, emerging data suggest that
the role of reactive astrocytes may be more complex than previously
appreciated. It has been reported that reactive astrocytes
expressing HMGB1 in the peri-infarct cortex may promote
neurovascular remodeling and functional recovery after stroke,
suggesting that these cells may in fact also possess beneficial
effects (7,19). In the present study, we found that
reactive ACM containing low levels of HMGB1 stimulated NS/PC
proliferation in vitro. HMGB1 is a non-histone DNA-binding
protein which stabilizes nucleosome formation and regulates gene
expression (13,14). HMGB1 is also a multifunctional
molecule that can act as a vital pro-inflammatory cytokine to
trigger inflammation by stimulating the secretion of
pro-inflammatory cytokines and chemokines, including tumor necrosis
factor-α (TNF-α), IL-2, IL-6 and other cytokines (13,14). Hence, studies have focused on
blocking HMGB1 signaling in order to reduce damage following brain
injury (15,16).
In contrast to these negative effects, a growing
body of literature indicates that HMGB1 may also have the potential
to promote tissue regeneration. It has been reported that following
a tissue lesion, HMGB1 can stimulate certain populations of cells
to proliferate, such as mesoangioblasts (vessel-associated stem
cells), myocardial cells and EPCs (7,35,36). It has also been reported that low
levels of exogenous HMGB1 (1–10 ng/ml) promote EPC and neuronal
precursor cell (NPC) proliferation in vitro (7,29).
Previous studies have suggested that stimulated astrocytes can
produce and release HMGB1 into the extracellular medium (8,9),
and the release of HMGB1 from astrocytes has been shown to increase
EPC proliferation both in vitro and in vivo (7). In this context, HMGB1 may also
provide a potential missing link between reactive astrocytes and
NS/PC proliferation, and the results presented in this study are
consistent with this hypothesis. Firstly, when the expression of
astrocytic HMGB1 was inhibited using shRNA, the ability of the sACM
to promote NS/PC proliferation was blocked. Secondly, when
exogenous HMGB1 was added to the NS/PC culture medium, the HMGB1
culture medium also enhanced the NS/PC proliferation rates. Taken
together, these results suggest that it was HMGB1 in the sACM that
increased NS/PC proliferation in vitro.
These experiments identified that astrocytic HMGB1
had a relatively modest and slow effect on NS/PC proliferation
in vitro. Previous studies have identified that
concentrations of HMGB1 ranging from 1 to 10 ng/ml significantly
increase neural progenitor cell proliferation in vitro
(29). Hayakawa et al
(7) used multiple doses of HMGB1
and showed biphasic effects on proliferation, where low levels of
HMGB1 (1–10 ng/ml) increased the proliferation rates of EPCs, but
higher concentrations (100–1,000 ng/ml) appeared to have no effect,
suggesting that high concentrations of HMGB1 may be deleterious by
promoting endothelial inflammation and inducing neurotoxicity. In
the experiments presented in this study, the concentration of HMGB1
in the sACM (~7 ng/ml) was within the limits of low levels of HMGB1
(1–10 ng/ml). We further identified that low levels of endogenous
HMGB1 secreted from astrocytes can also increase NS/PC
proliferation in vitro. In our experiments, the NS/PC
proliferation rates exhibited a relatively stationary phase for up
to 48 h and a statistically significant increased rate from 72 to
96 h. The time scales are consistent with previous findings
reported by Meneghini et al (29) that HMGB1 produced a relatively
stationary phase up to 48 h and a statistically significant
increase at 72 h. In our experiments, we stimulated astrocytes with
low levels of IL-1β in vitro to mimic the in vivo
inflammatory microenviroment. Since others have reported that as
little as 0.8 ng/ml IL-1β inhibits the proliferation of NPCs
(37), and that astrocytes may
release certain cytokines that affect NPC proliferation, we used a
control group in which nACM was supplemented with IL-1β (0.1
ng/ml). We found that there was no effect on the proliferation of
NS/PCs. In these experiments, we used ACM, but not NS/PC-astrocyte
co-cultures; therefore, it is clear that molecules secreted by
astrocytes, but not cell surface molecules on astrocytes, regulate
NS/PC proliferation. All the findings in these in vitro
experiments demonstrate that HMGB1 released from reactive
astrocytes promotes the proliferation of NS/PCs.
We also provided evidence of the presence of a
functional RAGE-JNK axis in HMGB1-mediated NS/PC proliferation. As
a receptor of HMGB1, RAGE plays an important role in regulating
stem cell proliferation and differentiation. Meneghini et al
(29) reported that HMGB1
interacted with RAGE and stimulated both proliferation and neuronal
differentiation of subventricular zone (SVZ)-derived NS/PCs in
vitro. Additionally, Kim et al (38) demonstrated that HMGB1 may interact
with RAGE and have important functions during the neuronal
differentiation of NT2/D1 cells. In our in vitro NS/PC
culture system, after blocking the activation of RAGE with a
neutralizing antibody on the NS/PC surface, the ability of HMGB1 to
enhance NS/PC proliferation was prevented. These findings suggest
that RAGE also has an important function in the process of
HMGB1-mediated NS/PC proliferation. Taguchi et al (30) reported that blocking
RAGE-HMGB1-mediated cellular stimulation decreased the levels of
p-JNK, and further inhibited the proliferation of rat C6 glioma
cells. In our experiments, blocking RAGE with an anti-RAGE antibody
in HMGB1 cultures also decreased p-JNK. To further demonstrate that
the JNK signaling pathway is involved in HMGB1-induced NS/PC
proliferation, we used the potent JNK inhibitor, SP600125. We found
that HMGB1 culture medium increased p-JNK levels and the rate of
NS/PC proliferation. When the p-JNK levels in the NS/PCs were
reduced by SP600125, the HMGB1 cultured NC/PC proliferation rates
decreased correspondingly. All these results indicated that after
binding to RAGE, HMGB1 increased NS/PC proliferation by promoting
JNK phosphorylation.
Nevertheless, the present study also has certain
limitations. Firstly, in these experiments, we stimulated
astrocytes with low levels of IL-1β in vitro to mimic an
in vivo inflammatory microenviroment, and confirmed that
HMGB1 released from astrocytes promoted NS/PC proliferation in
vitro. However, the actual inflammatory microenviroment is far
more intricate, and whether this mechanism also exists in
vivo requires further investigation. Secondly, astrocytes may
also release other cytokines, such as nerve growth factor,
neurotrophic factor, ciliary neurotrophic factor and vascular
endothelial growth factor (39–42). The elucidation of the mechanisms
through which HMGB1 interacts with these other networks and
pathways requires further investigation. Thirdly, there is no doubt
that the mechanism through which HMGB1 promotes NS/PC proliferation
is very complex, and whether other signaling pathways, such as the
ERK and p38 MAPK signaling pathways (43) are also involved in this process
requires more detailed investigation in future studies.
In conclusion, our study provides evidence that
HMGB1 released from reactive astrocytes is sufficient to promote
NS/PC proliferation, and the mechanisms through which HMGB1
promotes NS/PC proliferation may involve the activation of the
RAGE-dependent JNK signaling pathway. Since the NS/PCs afford the
plasticity to generate, repair and change nervous system function
(44), the proliferation of
NS/PCs may have beneficial effects in repairing brain damage. Our
collective results support a previously described mechanism of a
crosstalk between astrocytes and NS/PCs, and provide an
experimental basis that reactive astrocyte-derived HMGB1 can
promote NS/PC proliferation, which may be one of the reasons that
NS/PCs spontaneously proliferate following brain injuries, and
suggest that HMGB1 may be a very important factor for the
regeneration of injured brain tissue. Further studies are required
to investigate whether astrocyte-derived HMGB1 can induce NS/PCs to
differentiate into neurons and to promote functional recovery
following brain injury.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (no. 30470606) and the Program
of the Traditional Chinese Medicine Research of Chongqing Municipal
Health Bureau (no. 2003-B-16).
References
|
1
|
Go HS, Shin CY, Lee SH, et al: Increased
proliferation and gliogenesis of cultured rat neural progenitor
cells by lipopolysaccharide-stimulated astrocytes.
Neuroimmunomodulation. 16:365–376. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee C, Hu J, Ralls S, et al: The molecular
profiles of neural stem cell niche in the adult subventricular
zone. PLoS One. 7:e505012012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cao X, Li LP, Qin XH, et al: Astrocytic
adenosine 5′-triphosphate release regulates the proliferation of
neural stem cells in the adult hippocampus. Stem Cells.
31:1633–1643. 2013.
|
|
4
|
Guo Y, Wei Q, Huang Y, Xia W, Zhou Y and
Wang S: The effects of astrocytes on differentiation of neural stem
cells are influenced by knock-down of the glutamate transporter,
GLT-1. Neurochem Int. 63:498–506. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhao W, Wang Y, Shi W, et al: The
expression of FBP1 after traumatic brain injury and its role in
astrocyte proliferation. J Mol Neurosci. 51:687–694. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sukumari-Ramesh S, Alleyne CH Jr and
Dhandapani KM: Astrocyte-specific expression of survivin after
intracerebral hemorrhage in mice: a possible role in reactive
gliosis? J Neurotrauma. 29:2798–2804. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hayakawa K, Pham LD, Katusic ZS, Arai K
and Lo EH: Astrocytic high-mobility group box 1 promotes
endothelial progenitor cell-mediated neurovascular remodeling
during stroke recovery. Proc Natl Acad Sci USA. 109:7505–7510.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim JB, Lim CM, Yu YM and Lee JK:
Induction and subcellular localization of high-mobility group box-1
(HMGB1) in the postischemic rat brain. J Neurosci Res.
86:1125–1131. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hayakawa K, Arai K and Lo EH: Role of ERK
map kinase and CRM1 in IL-1β-stimulated release of HMGB1 from
cortical astrocytes. Glia. 58:1007–1015. 2010.PubMed/NCBI
|
|
10
|
Sawa H, Ueda T, Takeyama Y, et al:
Blockade of high mobility group box-1 protein attenuates
experimental severe acute pancreatitis. World J Gastroenterol.
12:7666–7670. 2006.PubMed/NCBI
|
|
11
|
Valdes-Ferrer SI, Rosas-Ballina M,
Olofsson PS, et al: High-mobility group box 1 mediates persistent
splenocyte priming in sepsis survivors: evidence from a murine
model. Shock. 40:492–495. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Achouiti A, van der Meer AJ, Florquin S,
et al: High-mobility group box 1 and the receptor for advanced
glycation end products contribute to lung injury during
Staphylococcus aureus pneumonia. Crit Care. 17:R2962013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Agnello D, Wang H, Yang H, Tracey KJ and
Ghezzi P: HMGB-1, a DNA-binding protein with cytokine activity,
induces brain TNF and IL-6 production, and mediates anorexia and
taste aversion. Cytokine. 18:231–236. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu H, Yao YM, Ding LH, et al: High
mobility group box-1 protein acts as a coactivator of nuclear
factor of activated T cells-2 in promoting interleukin-2
transcription. Int J Biochem Cell Biol. 41:641–648. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim JB, Sig Choi J, Yu YM, et al: HMGB1, a
novel cytokine-like mediator linking acute neuronal death and
delayed neuroinflammation in the postischemic brain. J Neurosci.
26:6413–6421. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lei C, Lin S, Zhang C, et al:
High-mobility group box1 protein promotes neuroinflammation after
intracerebral hemorrhage in rats. Neuroscience. 228:190–199. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Laird MD, Shields JS, Sukumari-Ramesh S,
et al: High mobility group box protein-1 promotes cerebral edema
after traumatic brain injury via activation of toll-like receptor
4. Glia. 62:26–38. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim SW, Lim CM, Kim JB, et al:
Extracellular HMGB1 released by NMDA treatment confers neuronal
apoptosis via RAGE-p38 MAPK/ERK signaling pathway. Neurotox Res.
20:159–169. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hayakawa K, Nakano T, Irie K, et al:
Inhibition of reactive astrocytes with fluorocitrate retards
neurovascular remodeling and recovery after focal cerebral ischemia
in mice. J Cereb Blood Flow Metab. 30:871–882. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hayakawa K, Miyamoto N, Seo JH, et al:
High-mobility group box 1 from reactive astrocytes enhances the
accumulation of endothelial progenitor cells in damaged white
matter. J Neurochem. 125:273–280. 2013. View Article : Google Scholar
|
|
21
|
Guazzi S, Strangio A, Franzi AT and
Bianchi ME: HMGB1, an architectural chromatin protein and
extracellular signalling factor, has a spatially and temporally
restricted expression pattern in mouse brain. Gene Expr Patterns.
3:29–33. 2003. View Article : Google Scholar
|
|
22
|
Zhao X, Kuja-Panula J, Rouhiainen A, Chen
YC, Panula P and Rauvala H: High mobility group box-1 (HMGB1;
amphoterin) is required for zebrafish brain development. J Biol
Chem. 286:23200–23213. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Meneghini V, Bortolotto V, Francese MT, et
al: High-mobility group box-1 protein and β-amyloid oligomers
promote neuronal differentiation of adult hippocampal neural
progenitors via receptor for advanced glycation end
products/nuclear factor-κB axis: relevance for Alzheimer’s disease.
J Neurosci. 33:6047–6059. 2013.
|
|
24
|
Nakagomi T, Taguchi A, Fujimori Y, et al:
Isolation and characterization of neural stem/progenitor cells from
post-stroke cerebral cortex in mice. Eur J Neurosci. 29:1842–1852.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shen J, Xie L, Mao X, et al: Neurogenesis
after primary intracerebral hemorrhage in adult human brain. J
Cereb Blood Flow Metab. 28:1460–1468. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Itoh T, Imano M, Nishida S, et al:
Exercise increases neural stem cell proliferation surrounding the
area of damage following rat traumatic brain injury. J Neural
Transm. 118:193–202. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Louis SA, Mak CK and Reynolds BA: Methods
to culture, differentiate, and characterize neural stem cells from
the adult and embryonic mouse central nervous system. Methods Mol
Biol. 946:479–506. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen J, Guo Y, Cheng W, et al: High
glucose induces apoptosis and suppresses proliferation of adult rat
neural stem cells following in vitro ischemia. BMC Neurosci.
14:242013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Meneghini V, Francese MT, Carraro L and
Grilli M: A novel role for the Receptor for Advanced Glycation
End-products in neural progenitor cells derived from adult
SubVentricular Zone. Mol Cell Neurosci. 45:139–150. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Taguchi A, Blood DC, del Toro G, et al:
Blockade of RAGE-amphoterin signalling suppresses tumour growth and
metastases. Nature. 405:354–360. 2000. View Article : Google Scholar
|
|
31
|
Bao Y, Qin L, Kim E, et al: CD36 is
involved in astrocyte activation and astroglial scar formation. J
Cereb Blood Flow Metab. 32:1567–1577. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jeong SR, Kwon MJ, Lee HG, et al:
Hepatocyte growth factor reduces astrocytic scar formation and
promotes axonal growth beyond glial scars after spinal cord injury.
Exp Neurol. 233:312–322. 2012. View Article : Google Scholar
|
|
33
|
Kawano H, Kimura-Kuroda J, Komuta Y, et
al: Role of the lesion scar in the response to damage and repair of
the central nervous system. Cell Tissue Res. 349:169–180. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Brambilla R, Morton PD, Ashbaugh JJ,
Karmally S, Lambertsen KL and Bethea JR: Astrocytes play a key role
in EAE pathophysiology by orchestrating in the CNS the inflammatory
response of resident and peripheral immune cells and by suppressing
remyelination. Glia. 62:452–467. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Palumbo R, Sampaolesi M, De Marchis F, et
al: Extracellular HMGB1, a signal of tissue damage, induces
mesoangioblast migration and proliferation. J Cell Biol.
164:441–449. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Limana F, Germani A, Zacheo A, et al:
Exogenous high-mobility group box 1 protein induces myocardial
regeneration after infarction via enhanced cardiac
C-kit+cell proliferation and differentiation. Circ Res.
97:e73–e83. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang X, Fu S, Wang Y, et al:
Interleukin-1β mediates proliferation and differentiation of
multipotent neural precursor cells through the activation of
SAPK/JNK pathway. Mol Cell Neurosci. 36:343–354. 2007.
|
|
38
|
Kim J, Wan CK, S JOC, Shaikh SB and
Nicholson LF: The role of receptor for advanced glycation end
products (RAGE) in neuronal differentiation. J Neurosci Res.
90:1136–1147. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yoshida H, Mimura J, Imaizumi T, et al:
Edaravone and carnosic acid synergistically enhance the expression
of nerve growth factor in human astrocytes under
hypoxia/reoxygenation. Neurosci Res. 69:291–298. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Koyama Y, Maebara Y, Hayashi M, Nagae R,
Tokuyama S and Michinaga S: Endothelins reciprocally regulate
VEGF-A and angiopoietin-1 production in cultured rat astrocytes:
implications on astrocytic proliferation. Glia. 60:1954–1963. 2012.
View Article : Google Scholar
|
|
41
|
Kuric E, Wieloch T and Ruscher K: Dopamine
receptor activation increases glial cell line-derived neurotrophic
factor in experimental stroke. Exp Neurol. 247:202–208. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Modi KK, Sendtner M and Pahan K:
Up-regulation of ciliary neurotrophic factor in astrocytes by
aspirin: implications for remyelination in multiple sclerosis. J
Biol Chem. 288:18533–18545. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tian Y, Liu Y, Chen X, et al: AMN082
promotes the proliferation and differentiation of neural progenitor
cells with influence on phosphorylation of MAPK signaling pathways.
Neurochem Int. 57:8–15. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gage FH and Temple S: Neural stem cells:
generating and regenerating the brain. Neuron. 80:588–601. 2013.
View Article : Google Scholar : PubMed/NCBI
|