Introduction
The two major features of hepatic cirrhosis are
portal hypertension and fibrosis. Portal hypertension is due partly
to a fibrosis-induced narrowing of hepatic venules and partly to an
increased responsiveness of these venules to vasoconstricting
substances (1,2). Currently, the only medications
available for the treatment of portal hypertension are
non-selective β blockers or vasodilators, such as nitrates
(3). Non-selective β blockers
decrease blood flow into the portal system through splanchnic
vasoconstriction, but do not delay the development of portal
hypertension (4,5). Vasodilators can cause arterial
hypotension as they exert dilating effects on systemic, as well as
portal circulation (4). The
current therapeutic approaches do not address the underlying
issues, as well as the fibrosis and hypersensitivity to contractile
agents.
The RhoA/Rho-kinase pathway in hepatic stellate
cells (HSCs) is a potential novel therapeutic target. The number of
activated HSCs is increased in cirrhosis (5–8);
HSCs produce the increased extracellular matrix responsible for
fibrosis (1) and regulate hepatic
vascular resistance (9) and
sinusoidal tone (10–14). Activated HSCs express high levels
of Rho-kinase, and two downstream effects of the RhoA/Rho-kinase
pathway are the increase in extracellular matrix formation
(1) and vascular
hyperreactivity.
The RhoA/Rho-kinase pathway is activated by the
binding of a vasoconstrictor to membrane-bound RhoA, a small GTPase
protein on the cell surface. However, before binding to a
vasoconstrictor can take place, RhoA must be attached to
geranylgeranyl pyrophosphate (GGPP), a by-product of cholesterol
synthesis, in order to ‘lipidize’ it so that it can be inserted
into the cell membrane (15–17). Therefore a drug that blocks
cholesterol synthesis at a site upstream from GGPP formation may
have the potential to block the RhoA/Rho-kinase pathway and thus
attenuate portal hypertension.
Sodium ferulate (SF) is such a drug. It decreases
cholesterol synthesis by inhibiting mevalonate 5-pyrophosphate
dehydrogenase, an action that prevents the conversion of mevalonite
to GGPP and the subsequent activation of the RhoA/Rho-kinase
pathway (18).
Based on these data, we hypothesized that SF may
decrease fibrosis and portal pressure by inhibiting the
RhoA/Rho-kinase pathway. In this study, we administered SF to rats
in a bile duct ligation (BDL) model of portal hypertension and
examined its effects by measuring indicators of liver function,
serum and tissue indicators of fibrosis, immunohistological
evidence of RhoA, Rho-kinase and endothelial nitric oxide synthase
(eNOS) abundance, as well as responsiveness to the α-adrenergic
agonist, methoxamine by in situ liver perfusion. In
addition, the effects of SF on the apoptosis of in vitro
cultured rat HSCs and a human hepatic stellate cell line were
examined.
Materials and methods
Animals and animal model of portal
hypertension
Male Wistar rats (180–200 g; n=73) were purchased
from the Center for Disease Control of Hubei province, and raised
in the Laboratory Animal Centre of Tongji Medical College, Wuhan,
China. The study was approved by the Ethics Committee of Tongji
Medical College. To induce portal hypertension, the animals were
anesthetized intraperitoneally with chloral hydrate, a median
laparotomy was performed, the common bile duct was ligated twice
and cut between the ligatures, and the abdomen was sutured. The
rats were randomly divided into 3 groups. The first group [BDL +
normal saline (NS) group, n=28] was subjected to BDL and
administered an NS injection via the tail vein for 1 week during
the 4th week after ligation. The second group (BDL + SF group,
n=21), was subjected to BDL, and a middle dosage of SF (50
mg/kg/day) was injected each day for 1 week during the 4th week
after surgery. The third group was the control group [sham-operated
(SHAM) + NS group, n=24] which was subjected to a laparotomy
without ligation, and an NS injection was administered for 1 week
during the 4th week after surgery. At the end of the 4th week, the
rats were anesthetized for liver perfusion experiments (described
in a later section), or sacrificed after blood collection and the
livers and spleens were sampled.
Serum biochemical parameters and sample
preparation
Blood collected from the vena cava was centrifuged
for 5 min at 12,000 rpm at 4°C and the serum was then sent to the
Clinical Laboratory of Wuhan Tongji Hospital for the measurement of
the following substances: alanine aminotransferase (ALT), aspartate
aminotransferase (AST), albumin, total bilirubin (TBIL), direct
bilirubin (DBIL), γ-glutamyl transferase (GGT); and the
fibrogenesis-related compounds hyaluronic acid (HA), laminin (LN),
collagen type IV (IV-C), and procollagen type III peptide
(PCIII).
The livers and spleens were weighed, and 3–4 liver
fragments (0.5×0.5×0.5 cm3) were fixed in formalin. The
remaining fragments were placed into tubes and quick-frozen in
liquid nitrogen. The tissues were stored at −80°C for future
analyses.
Hepatic hydroxyproline content
The hepatic hydroxyproline content was measured
using an assay kit (Nanjing Jiancheng Bioengineering Institute,
Nanjing, China), according to the manufacturer’s instructions.
Briefly, 80–100 mg liver tissue fragments were homogenized,
precipitated using trichloroacetic acid and hydrolyzed for 24 h at
110°C in 6 N HCl solution. After hydrolysis was completed, the
samples were neutralized with 10 N NaOH, oxidized with
chloramine-T, and incubated in Ehrlich’s perchloric acid solution
at 65°C for 20 min. The hydroxyproline content was determined
photometrically by measuring the absorbance at 560 nm.
Pathological analysis
Liver tissue, following formalin fixation, was
embedded in paraffin and cut into slices (4 μm in thickness). The
sections underwent hematoxylin and eosin (H&E) staining, after
which the pathological characteristics of the 3 groups were
observed under an optical microscope (Olympus, Tokyo, Japan). For
ultra microstructure observation by electron microscopy, the rats
were anesthetized intraperitoneally with chloral hydrate and the
livers were rapidly and gently removed. The liver tissues were then
cut into 2×2×3 mm3 sections and fixed in 2.5%
glutaraldehyde buffer for 5–10 min. Following fixation, the liver
became harder, and was then cut into 1 mm3 sections or
into tissue strips (cross-sectional area, 1 mm2; length,
5 mm). After being fixed in 1% osmium tetroxide for 1 h and washed
twice with 0.1 M phosphate-buffered saline (PBS) (20 min for each
wash), the tissues were treated sequentially in 50% ethanol, 70%
ethanol, 90% ethanol, 90% ethanol-acetone, 90% acetone, and then
twice in 100% acetone (5 min in each solution). They were then
embedded in an epoxy-acetone solution (epoxy:acetone, 1:1) for 2 h,
followed by embedding in epoxy for 2 h. After being heated at 80°C
for 10 h, the tissues were cut into ultra-thin sections and stained
with uranyl acetate and lead citrate (10 min for each). The
ultra-structure of the liver was observed under an electron
microscope (FEI Tecnai G2 12; FEI Tecnai, Eindhoven, The
Netherlands), and images were captured and stored for further
analysis.
Immunohistochemistry
Paraffin-embedded liver tissue was cut into 4
μm-thick slices for staining. Rho-kinase, eNOS and α-smooth muscle
actin (α-SMA) (a marker for fibrosis) were stained with a
streptavidin peroxidase 3 kit, and RhoA was stained with a
Histostain-Plus kit (both kits from Zymed Laboratories, Inc., San
Francisco, CA, USA). A diaminobenzidine (DAB) kit (Wuhan Boster
Bio-Engineering Ltd., Wuhan, China) was used for color development.
The primary antibodies and dilutions used were the following: α-SMA
(Wuhan Boster Bio-Engineering Ltd.), 1:100 dilution; Rho-kinase
(Abcam, Cambridge, UK), 1:100 dilution; eNOS (Wuhan Boster
Bio-Engineering Ltd.), 1:50 dilution; RhoA (Abcam), 1:100
dilution.
For α-SMA, Rho-kinase and eNOS staining, the
sections were first deparaffinized in xylene and rehydrated in a
graded series of ethanol. Quenching of endogenous peroxidase
activity was performed for all specimens in 0.1% hydrogen peroxide
diluted in methanol (H2O for laminin-5 γ2 chain
immunohistochemistry) and non-specific binding was blocked by
incubating specimens in 20% fetal calf serum diluted in PBS.
Diluted primary antibodies were added to the sections and incubated
overnight at 4°C. All specimens were then overlaid with the
suitable secondary antibody followed by assay. DAB was used for
color reaction, hematoxylin was used for counterstaining. All steps
were followed by washes with PBS. Negative controls for all
immunostainings were obtained by substituting PBS for the primary
antibody. A DAB kit (Wuhan Boster Bio-Engineering Ltd.) was used to
visualize positive immunoreaction. In some experiments, nuclei were
counterstained with 4′,6-diamidino-2-phenylindole (DAPI; Wuhan
Boster Bio-Engineering Ltd.).
For RhoA staining, briefly, endogenous peroxidase
activity was blocked by incubation with 3%
H2O2 for 15 min. Antigen retrieval was
performed using a microwave. Diluted primary antibodies were added
to the sections following by overnight incubation at 4°C. The
sections were then incubated in secondary antibody and visualized
using the DAB kit. In the control group, the sections were treated
as described above but PBS was added instead of the primary
antibody.
The sections were visualized under an optical
microscope (Olympus). Five fields of vision per section at ×200
magnification were taken blindly. Positive immunostaining was
quantified as integrated optical density (IOD) using the Image-Pro
Plus analysis system 6.0 (19–21).
Cell isolation, cultivation and
identification
HSC isolation was performed as previously described
(22–24). Briefly, male Wistar rats with
biliary cirrhosis (4 weeks after surgery; weighing 350–450 g; n=49)
were used. Heparin (1,000 units) was injected via the vena cava
following anesthesia and laparotomy. A balanced salt solution
containing Na+ and K+ was then injected
through the isolated portal vein, and the vena cava was immediately
severed. The liver was then perfused with solution containing 0.05
g/100 ml collagenase (Gibco-Invitrogen, Carlsbad, CA, USA) and 0.03
g/100 ml pronase (Roche, Basel, Switzerland). Following perfusion,
the liver was cut into sections and incubated with shaking in
digestive solution containing DNase (Sigma, St. Louis, MO, USA) at
37°C, after which Dulbecco’s modified Eagle’s medium (DMEM;
Gibco-Invitrogen) containing 10% fetal bovine serum (FBS) (Thermo
Scientific HyClone, Beijing, China) was used to terminate the
reaction. The homogenate was filtered with nylon gauze and
centrifuged twice at 50 × g for 4 min. The supernatant was then
centrifuged at 500 × g for 7 min. The precipitate was resuspended
and the HSCs were isolated by discontinuous Percoll gradient
centrifugation at 1,400 × g for 17 min, as previously described
(24,25). The cell cluster was collected and
resuspended, then centrifuged at 500 × g for 8 min. The cells were
then cultured in DMEM containing 15% FBS, 100 U/ml penicillin and
100 U/ml streptomycin under a humidified atmosphere containing 5%
CO2 and 95% air at 37°C. The medium was replaced after
48 h, then once every other day. Two weeks later, HSC activation
was identified by immunofluorescence using primary antibodies to
desmin (Wuhan Boster Bio-Engineering Ltd.) and α-SMA.
Cytoimmunofluorescence revealed that the proportion of activated
HSCs in the cells following culture for 2 weeks was >99%.
Assessment of apoptosis by flow
cytometry
SF powder was pre-dissolved in dimethyl sulfoxide
(DMSO) (Sigma), then dissolved in DMEM medium and stored at 4°C
away from light. The activated HSCs were divided into the following
3 groups: i) the ‘SF’ group, in which the HSCs were cultured in
medium containing SF (40, 120, 360 μg/ml) for 48 h; ii) the‘SF +
GGPP’ group, in which the HSCs were treated with 10 μmol/l GGPP and
various concentrations of SF for 48 h; iii) the control group, in
which the HSCs were cultured in medium alone. For these
incubations, medium contained only 2% FBS to support growth. Due to
the low concentration of FBS, non-apoptotic cells were in the
quiescent phase, instead of a growth phase. The cells that died
were identified by flow cytometry, and the proportion of dead cells
was <5%. An Annexin V-FITC apoptosis detection kit (KeyGen
Biotech, Nanjing, China) was used to detect apoptosis according to
the manufacturer’s instructions. Apoptotic cells were detected by
flow cytometry.
A human hepatic stellate cell line (LX-2) was
purchased from Xiangya Central Experiment Laboratory of Xiangya
Medical College, Hunan, China. LX-2 was cultured and cell apoptosis
was detected as described above.
In situ liver perfusion
Perfusion system
BL-420E Bio-experimental system software (Chengdu
Aimeng Technology Ltd., Chengdu, China) was used to record
perfusion pressure in the rat livers. In brief, Krebs-Henseleit
bicarbonate buffer [previously described (26)] containing heparin (2 U/ml) was
kept in a thermostatic water bath at 37°C and saturated with 95%
oxygen and 5% carbon dioxide, as previously described (27,28). A BT100-2J Peristaltic Pump (Lange
Peristaltic Pump Inc., Baoding, China) was linked with the T-branch
pipe of the BL-420E Bio-experimental system. After making certain
that no air was in the working system, the pipe of the peristaltic
pump was placed into the perfusate and the basal pressure was set
to zero.
Rat preparation
Three groups of rats were used for this experiment:
the BDL + NS, BDL + SF and the SHAM + NS group. Each group
comprised 10 rats. The rats were fasted overnight, but water was
supplied as usual. Following anesthetization, a median laparotomy
was performed. A PE-50 catheter was introduced into the portal vein
and secured. This size catheter was selected as it can be smoothly
inserted into the portal vein, but has a large enough diameter so
that it has no influence on the resistance of the circulation
system. The peristaltic pump was then turned on and the vena cava
immediately severed to allow the perfusate to escape. The thoracic
cavity was exposed and another catheter was inserted into the right
atrium and advanced into the superior vena cava. The basal
perfusion pressure was recorded after continuous perfusion for 20
min at a constant flow rate (30 ml/min), as previously described
(2). The basal hepatic resistance
was calculated according to the following formula: ‘pressure = flow
× resistance’, as prevoiusly described (29,30).
When setting up the perfusion process, the following
conditions were adhered to in order to ensure the viability and
stability of the process: accurate and quick intubation, a
sufficiently-oxygenated perfusate with stable pH (7.4±0.1) and a
constant temperature of 37°C and no air or impurities in the
perfused channel.
Effects of methoxamine hydrochloride on
portal perfusion pressure
After a period of continuous perfusion and allowing
the system to stabilize, increasing concentrations (0.1, 1, 10 and
100 μM) of the α1-adrenoreceptor agonist, methoxamine
hydrochloride, were added to the perfusate. Each concentration was
sustained for 3 min, and the pressure recorded. Changes in
intrahepatic resistance were calculated, and differences between
groups as regards the effects of methoxamine hydrochloride on
intrahepatic resistance were indicated by cumulative
concentration-response curves.
Statistical analysis
Continuous variables are presented as the means ±
standard deviation. Comparisons between the 3 experimental groups
(SHAM + NS, BDL + NS and BDL + SF groups) were performed by one-way
analysis of variance (ANOVA). When a significant difference between
groups was observed, multiple comparisons were performed using the
Bonferroni procedure with type I error adjustment. The association
between the methoxamine concentration and portal perfusion pressure
(or intrahepatic resistance) was assessed using the Pearson
correlation coefficient. Statistical analyses were performed using
SAS software version 9.2 (SAS Institute Inc., Cary, NC, USA). A
two-sided P-value <0.05 was considered to indicate a
statistically significant difference.
Results
General characteristics and serum
biochemical parameters
Table I shows the
general characteristics and serum biochemistry of the 3 groups.
Animals with biliary cirrhosis had a lower body weight, higher
liver and spleen weights, lower levels of albumin and higher levels
of other indicators of liver damage and fibrosis than the
sham-operated rats (all P<0.05). The addition of SF caused no
normalization of any of these parameters.
 | Table IGeneral characteristics and serum
biochemical parameters of the different experimental groups. |
Table I
General characteristics and serum
biochemical parameters of the different experimental groups.
| SHAM + NS | BDL + NS | BDL + SF | P-value |
|---|
| General
characteristics | (n=15) | (n=15) | (n=15) | |
| Body weight
(g) | 284.3±29.4 | 237.0±32.6a | 240.7±33.0a | <0.001b |
| Liver weight
(g) | 11.8±1.4 | 17.4±3.0a | 17.0±3.5a | <0.001b |
| Spleen weight
(g) | 0.9±0.2 | 1.9±0.6a | 1.7±0.6a | <0.001b |
| Liver function | (n=15) | (n=10) | (n=10) | |
| ALT (U/l) | 35.7±13.7 | 96.5±42.2a | 89.7±20.3a | <0.001b |
| AST (U/l) | 115.5±54.3 | 364.6±231.2a | 344.8±106.3a | <0.001b |
| ALB (g/l) | 38.7±3.6 | 25.2±3.8a | 26.6±2.8a | <0.001b |
| TBIL (μmol/l) | 0.5±0.2 | 113.9±22.3a | 107.9±50.4a | <0.001b |
| DBIL (μmol/l) | 0.5±0.7 | 81.5±40.3a | 62.4±47.1a | <0.001b |
| γ-GT (U/l) | 1.8±1.0 | 82.8±30.1a | 81.4±38.1a | <0.001b |
| Fibrogenesis | (n=15) | (n=10) | (n=10) | |
| HA (ng/ml) | 110.4±18.2 | 530.0±57.2a | 512.9±64.7a | <0.001b |
| LN (ng/ml) | 65.3±2.5 | 76.1±2.5a | 76.6±1.6a | <0.001b |
| IV-C (ng/ml) | 37.3±3.2 | 45.3±2.3a | 44.5±1.7a | <0.001b |
| PCIII (ng/ml) | 34.6±1.4 | 45.0±1.8a | 44.5±2.8a | <0.001b |
The mean body weight of the BDL + NS and BDL + SF
groups was significantly lower and the liver and spleen weights
were significantly higher than those of the SHAM + NS group (all
P≤0.001). The BDL + NS and BDL + SF groups had significantly higher
ALT, AST, TBIL, DBIL and γ-GT concentrations than the SHAM + NS
group (all P<0.001). However, the albumin level in the BDL + NS
and BDL + SF groups was significantly lower than that of the SHAM +
NS group (both P<0.001). The BDL + NS and BDL + SF groups had
significantly higher HA, LN, IV-C and PCIII than the SHAM + NS
group (all P<0.001).
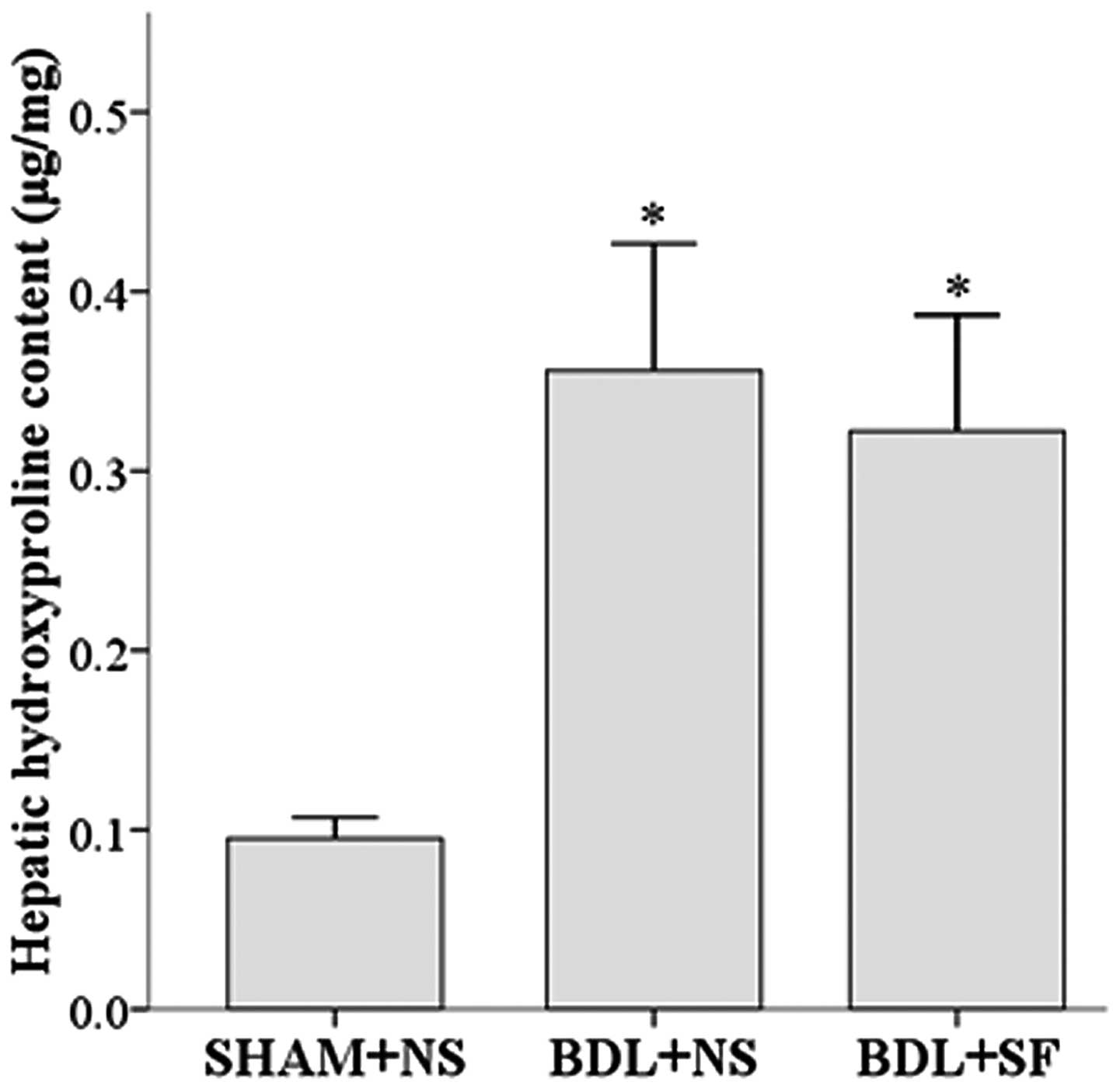
Hepatic hydroxyproline content
The addition of SF had no effect on the high hepatic
hydroxyproline content of the rats with hepatic cirrhosis. The
hepatic hydroxyproline content of both the BDL + NS and the BDL +
SF groups was significantly higher than that of the SHAM + NS group
(both P<0.001) (Fig. 1).
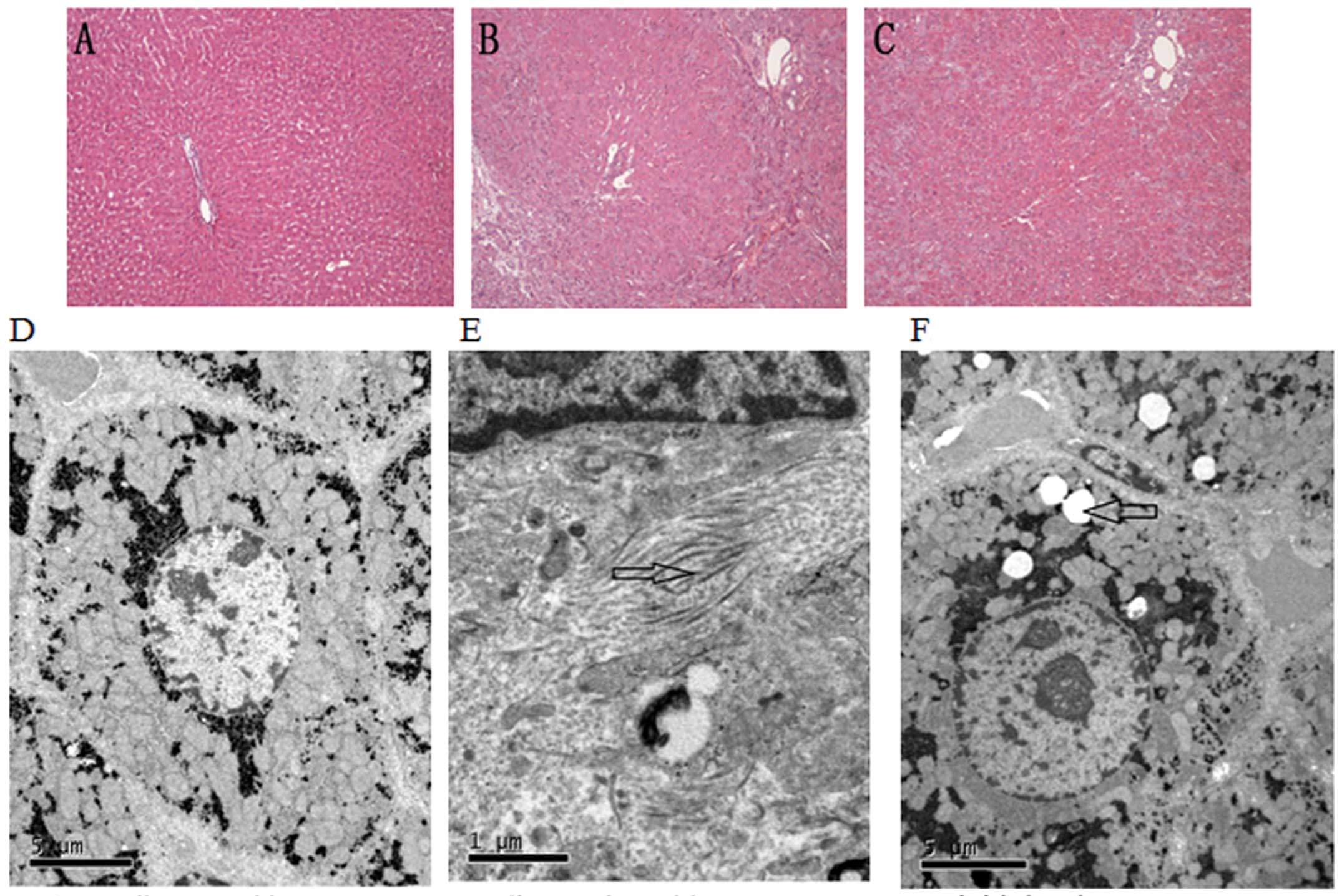
Pathological analysis
The histological observations of H&E-stained
sections between the different groups were as follows: the
sham-operated rats had a normal structure of hepatic lobules and
sinusoids, as well as ordered hepatic cords (Fig. 2A). In the cirrhotic rats, hepatic
lobules with normal structure were absent, and the proliferation of
fibrous tissue, pseudolobule formation and a diffuse distribution
of lymphocytes were observed (Fig.
2B). Compared to the untreated cirrhotic rats, the livers of
SF-treated rats appeared to have a decreased degree of
inflammation, fibrosis and necrosis (Fig. 2C).
Observations using a transmission electron
microscope indicated that the hepatocytes in the sham-operated
group had abundant organelles, an intact cellular morphology and no
fibrous deposition in the perisinusoidal area (Fig. 2D). In the untreated rats subjected
to BDL, the organelles were destroyed, the hepatocytes were
severely damaged and a large number of collagen fibrils was
deposited in the perisinusoidal space (Fig. 2E). Compared to the untreated
cirrhotic rats, the SF-treated rats appeared to have less
hepatocyte necrosis and fibrous deposition; lipid droplets were
also found in the hepatocytoplasm (Fig. 2F).
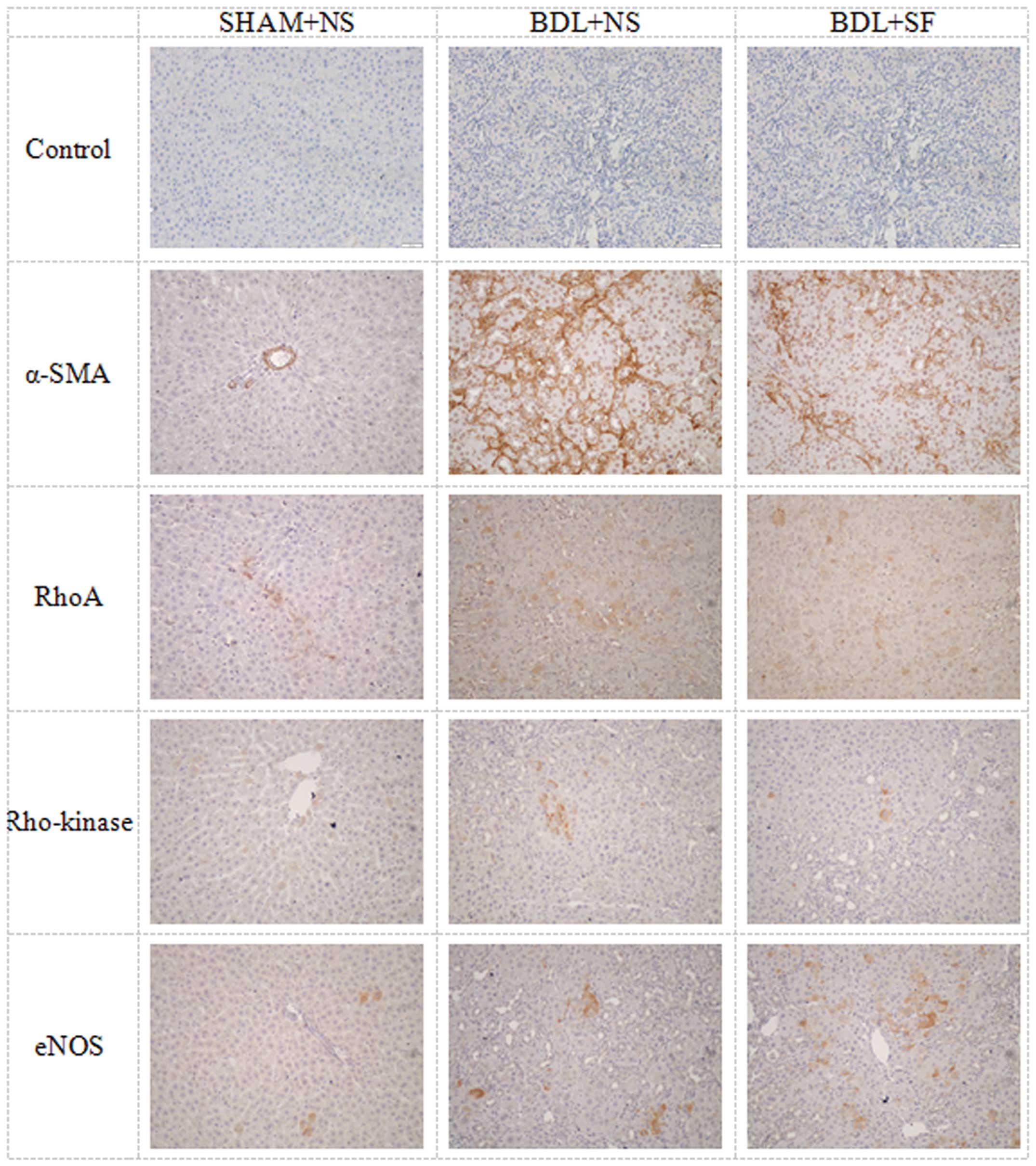
Immunohistochemistry for hepatic
expression of α-SMA, RhoA, Rho-kinase and eNOS
The histological results for antibody staining for
α-SMA, RhoA, Rho-kinase and eNOS in the hepatic cells are shown in
Fig. 3, and the corresponding
semi-quantitative data are shown in Fig. 4.
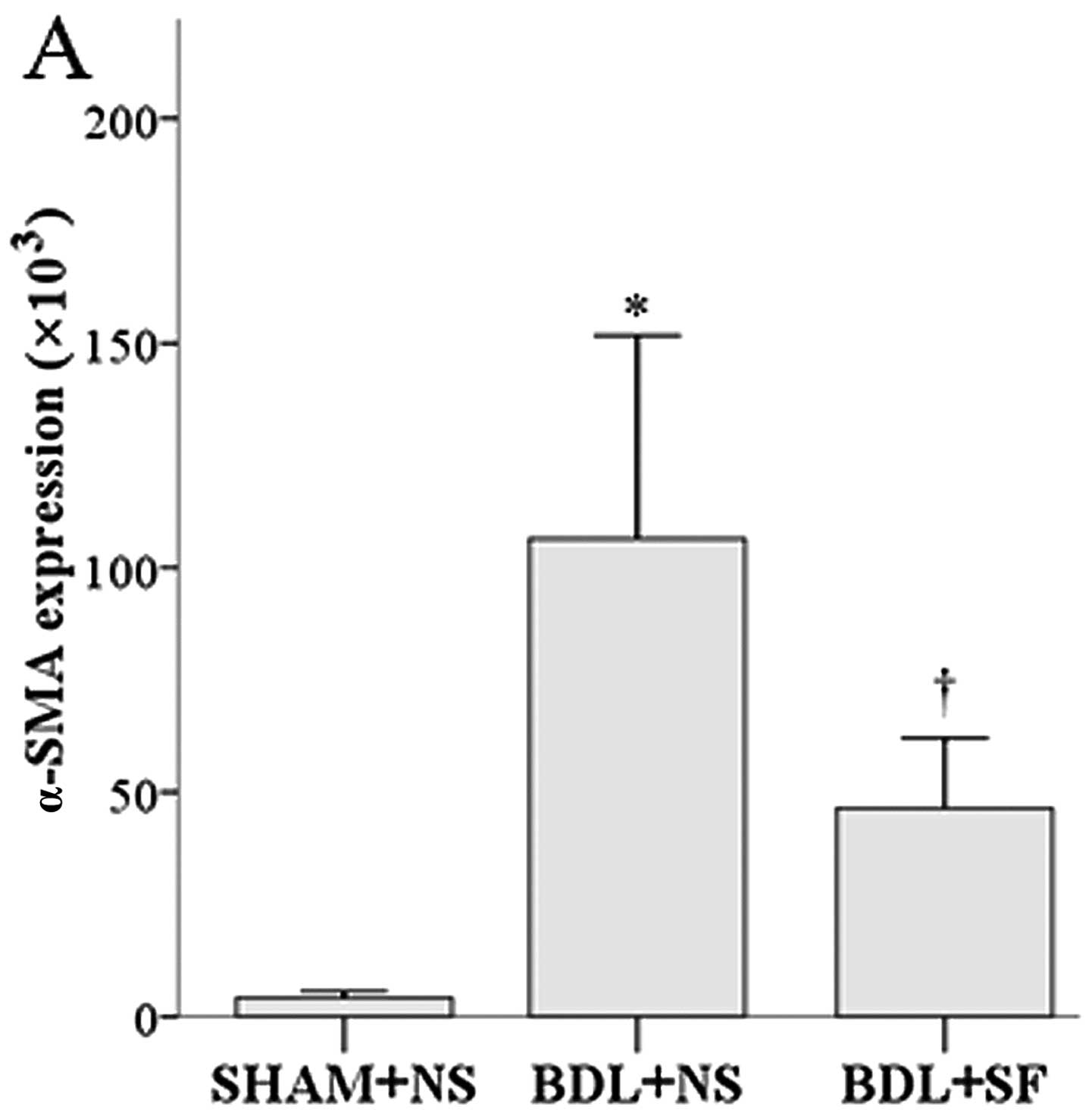
SF reduced the high levels of α-SMA and Rho-kinase
observed in the cirrhotic rats, but had no significant effect on
the high expression of RhoA observed in these animals.
The α-SMA and Rho-kinase expression in the rats in
the BDL + NS group was significantly higher than that in the rats
in the SHAM + NS and BDL + SF groups (all P≤0.004) (Fig. 4A and C). RhoA expression in the
BDL + NS and BDL + SF groups was significantly higher than that in
the SHAM + NS group (both P<0.001) (Fig. 4B).
eNOS expression was increased in the cirrhotic rats,
and treatment with SF increased eNOS expression even further. eNOS
expression in the BDL + NS and BDL + SF groups was significantly
higher than that in the SHAM + NS group (both P≤0.001), and that of
the BDL + SF group was significantly higher than that of the BDL +
NS group (P=0.006) (Fig. 4D).
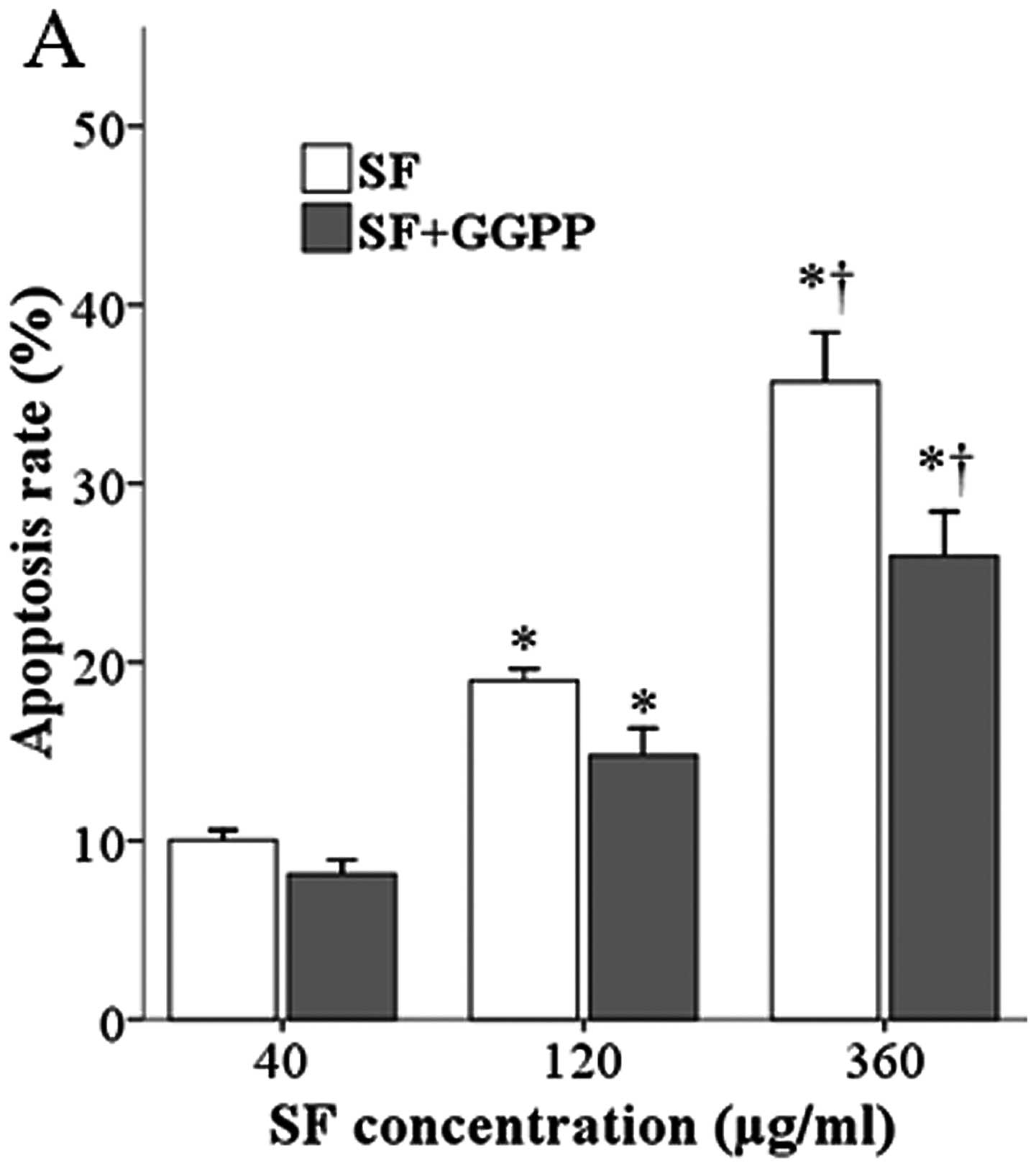
Effect of SF/GGPP on the apoptosis of
primary HSCs and LX-2 cells
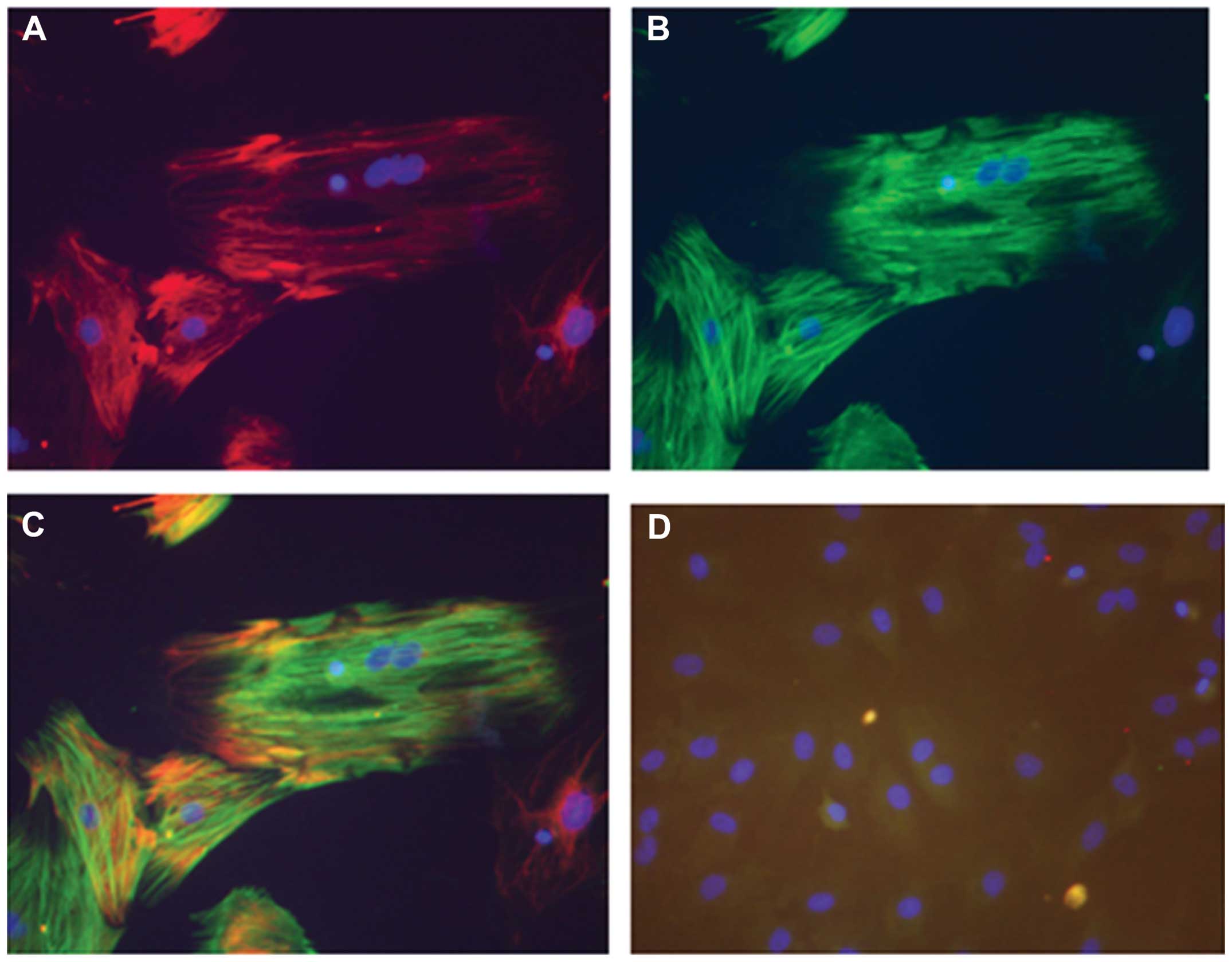
Fig. 5 illustrates
the DAPI, desmin and α-SMA staining of the rat HSCs in culture,
illustrating the activation of these cells. SF produced a
concentration-dependent increase in apoptosis in both the rat and
human HSCs (Fig. 6) compared to
the controls not treated with SF (4.6±0.9 and 4.7±1.4 for rat and
human HSCs, respectively; control data not shown). The addition of
GGPP, the substance that enables RhoA to translocate from the
cytoplasm to the cell membrane and become activated, partly blocked
the ferulate-induced increase.
In situ liver perfusion and the perfusion
pressure response to methoxamine hydrochloride
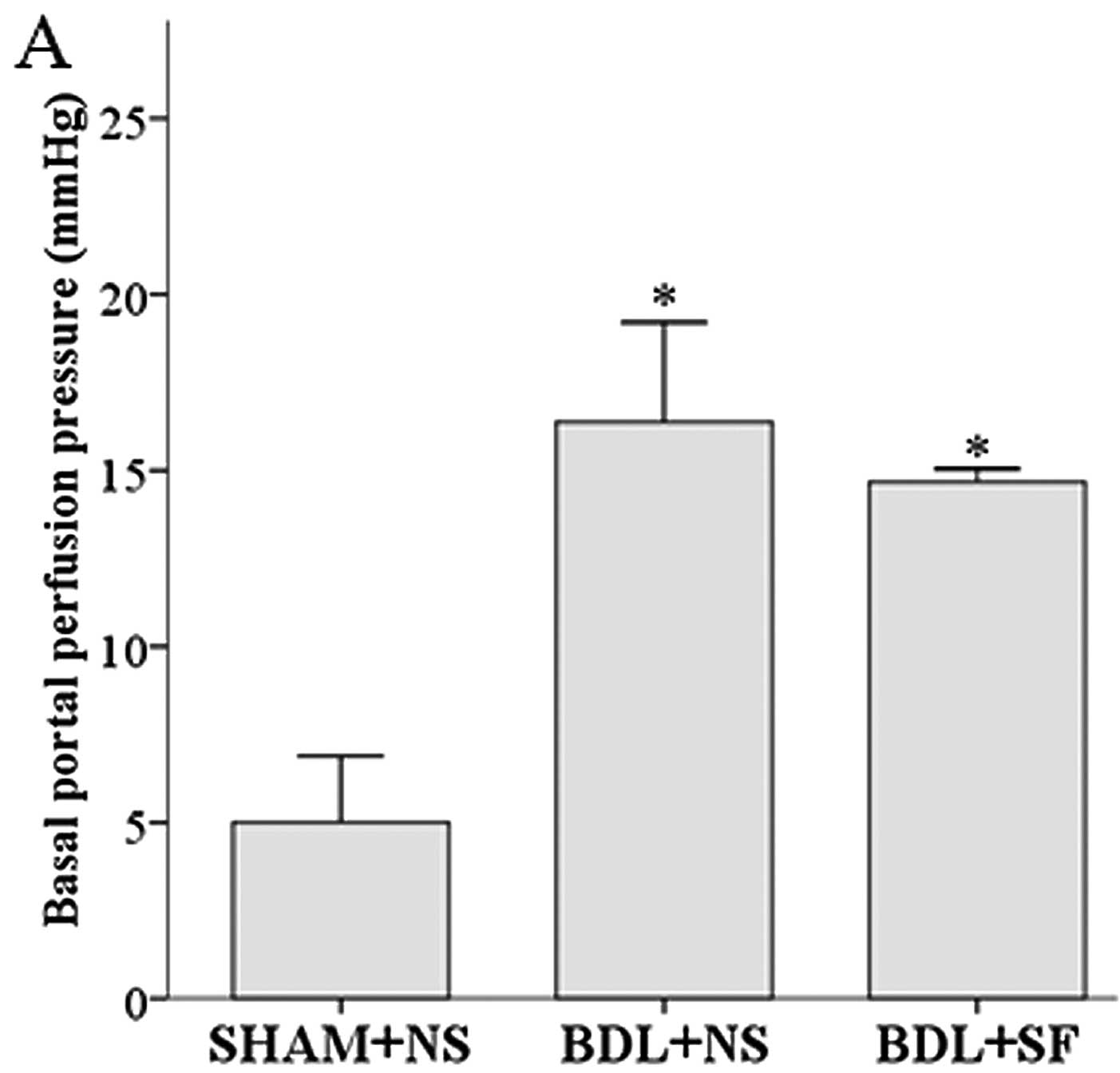
Basal perfusion pressure and intrahepatic resistance
were significantly increased in the cirrhotic rats, and SF had no
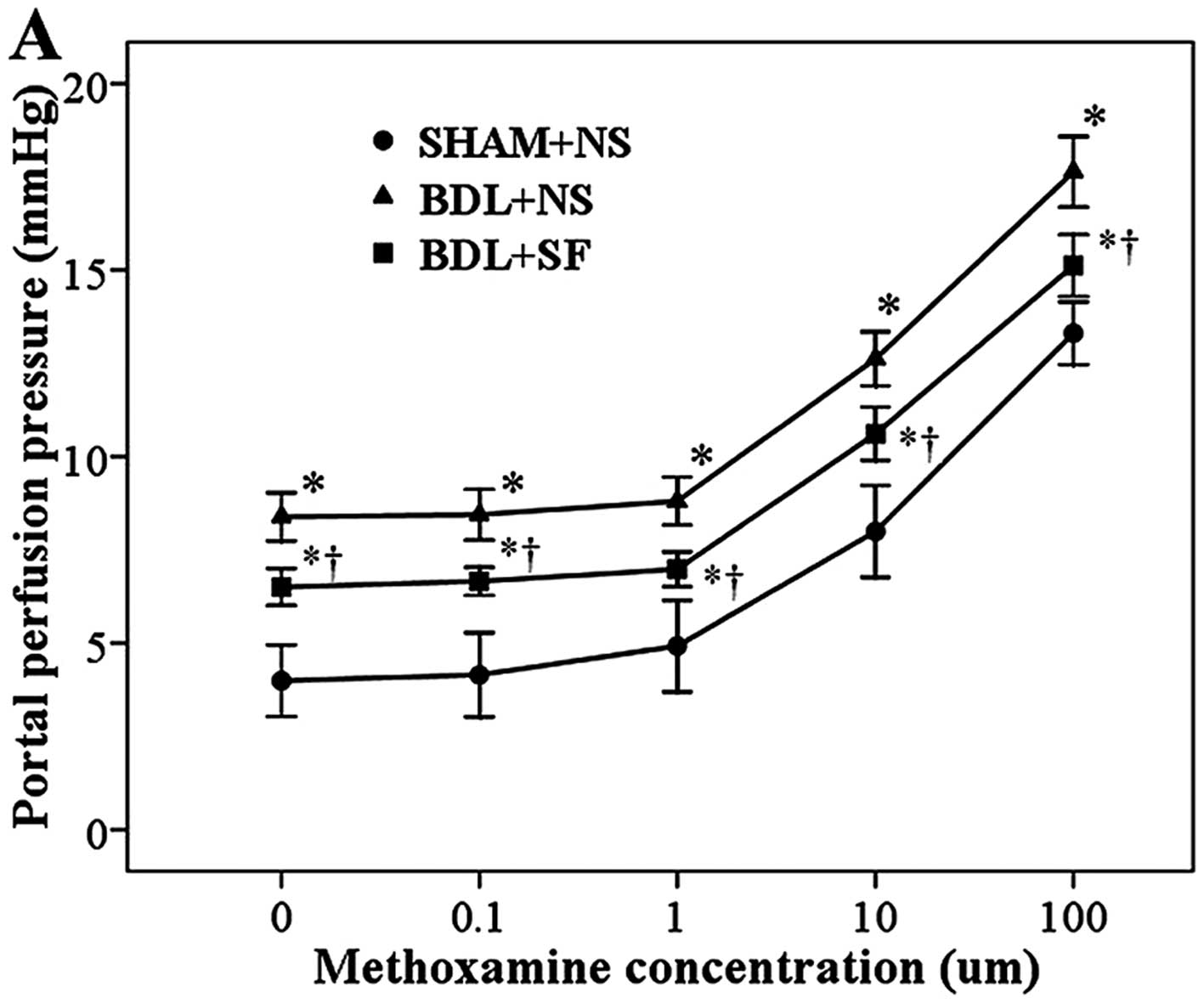
significant effect on these increases (Fig. 7). SF did, however, significantly
decrease the hyperresponsiveness to methoxamine observed in these
rats (Fig. 8).
Basal portal perfusion pressure and basal
intrahepatic resistance were significantly higher in the BDL + NS
and BDL + SF groups compared to the SHAM + NS group (all
P<0.001). In all the experimental groups, methoxamine induced a
concentration-dependent increase in portal perfusion pressure.
Under identical methoxamine concentrations, portal perfusion
pressure in the BDL + NS and BDL + SF groups was significantly
higher than that of the SHAM + NS group (all P<0.001), and that
of the BDL + NS group was significantly higher than that of the BDL
+ SF group (all P<0.001).
Discussion
In the present study, we hypothesized that SF, which
decreases the synthesis of GGPP, a compound essential for RhoA
activation and initiation of the RhoA/Rho-kinase pathway in HSCs,
would ameliorate fibrosis and portal hypertension in rats with
secondary biliary cirrhosis. SF did decrease fibrosis and the
elevated portal pressure, possibly through the inhibition of the
Rho-kinase pathway by decreasing Rho-kinase expression. SF also
increased the apoptosis of HSCs, and this action may also be
mediated through the Rho-kinase pathway, as the addition of the
missing initiator of this pathway, GGPP, decreased the
ferulate-induced HSC apoptosis.
Portal hypertension, the major factor causing high
mortality in cirrhotic patients, is responsible for serious
complications such as ascites, esophagogastric varices, hepatorenal
syndrome and hepatic encephalopathy (31), and is determined by intrahepatic
vascular resistance and portal blood flow (portal pressure =
resistance × flow). Activated HSCs, in addition to their role in
fibrogenesis, are responsible for the increased vascular resistance
obseved in the hepatic sinusoid in cirrhosis, and these actions are
thought to be mediated through the RhoA/Rho-kinase pathway. Our
results revealed that SF decreased the expression of Rho-kinase,
but not that of RhoA in hepatic tissue from cirrhotic rats. RhoA, a
member of the GTP-binding protein-Rho GTPase family, exists in both
activated GTP-RhoA and stationary GDP-RhoA states, and in the
membrane-bound activated state, it activates Rho-kinase. The
activation of Rho-kinase exerts two effects that increase portal
pressure. One is the inhibition of myosin light chain phosphatase,
causing the downstream effect of increasing smooth muscle
contraction (9,32,33). The other is the decrease in
hepatic eNOS activity, thus increasing the sensitivity of the
venules to vasoconstrictors, such as methoxamine. Zhou et al
(2) previously found that the
expression of RhoA and Rho-kinase in the livers of experimental
cirrhotic rats was significantly higher than in normal rats. In
addition, salvianolic acid, a compound that similar to SF,
decreases GGPP synthesis (34),
has been reported to lower portal pressure and inhibit HSC
contraction by downregulating the RhoA/Rho-kinase pathway (35). Although ferulic acid has
previously been reported to decrease blood pressure in hypertensive
rats (36) and lower portal
pressure in patients with liver cirrhosis (37), the mechanisms responsible for
these actions have not yet been elucidated. Therefore, in this
study, we examined the effects of SF on the portal pressure of
cirrhotic rats by investigating the RhoA/Rho-kinase signaling
pathway.
We first examined the effect of SF on the other
endpoint of the RhoA/Rho-kinase pathway, fibrosis. We investigated
the effects on fibrosis using three methods: i) by comparing
fibrous hyperplasia in H&E-stained liver sections, ii) by
electron microscopic examination of hepatocyte ultrastructure and
the deposition of collagen in the perisinusoidal and intracellular
space; and iii) by the semi-quantitative determination of α-SMA. SF
markedly decreased both the histological evidence of fibrosis and
the elevated α-SMA levels observed in cirrhotic rats. SF had no
effect on the serum biochemical indicators of liver fibrosis or on
the hepatic hydroxyproline content in bile duct-ligated rats. This
discrepancy requires further investigation. In addition, SF
promoted HSC apoptosis, and the contribution of decreasing HSC
numbers through apoptosis to the decrease in α-SMA and histological
evidence of decreased liver injury requires further
investigation.
We then assessed the effects of SF on the
RhoA/Rho-kinase pathway and found that SF attenuated the increased
expression of Rho-kinase observed in cirrhotic rats, but had no
effect on the increased expression of RhoA. RhoA must be both
membrane-bound and GTP-bound in order to activate Rho-kinase and
cause downstream effects. Our assay for RhoA included both GTP-RhoA
and GDP-RhoA, and thus could not detect whether or not SF caused a
decrease in the activated form of RhoA. GDP-RhoA and GTP-RhoA are
two kinds of mutual conversion states; only GTP-RhoA, which
transfers to the cell membrane can activate Rho-kinase and cause
downstream effects. However, the decrease in the expression of
hepatic Rho-kinase in response to SF was an indication that the
downstream effects caused by GTP-RhoA were inhibited.
We did not measure the effect of SF on myosin light
chain phosphatase, but we did measure its effect on eNOS activity,
the other downstream target of the RhoA/Rho-kinase pathway involved
in regulating portal pressure. eNOS is broadly distributed and
found in myocardial cells, endothelial cells, mast cells and blood
cells. eNOS activity causes vascular smooth muscle relaxation and
plays a key role in maintaining the steady state of the vascular
wall (38). In this study, eNOS
activity was increased in the cirrhotic rats, and SF increased the
expression of hepatic eNOS even further. Therefore, SF possibly has
a positive effect on intrahepatic vascular relaxation through an
increase in eNOS expression (9,19,39) and this may be mediated through
blocking Rho-kinase activation, for Rho-kinase decreases eNOS and
its activated, phosphorylated form (10,19). However, the explanation may not be
so simple, as the regulation of eNOS in HSC is complex. Rho-kinase
signaling is elevated in HSCs in cirrhosis, but part of the
activation is caused by the release of ET-1 from the spleen
(40). The farnesoid receptor, a
bile-acid-responsive transcription factor, decreases eNOS activity
in cirrhosis, but does so through the Rho-kinase receptor in
thioacetamide-induced cirrhosis, but not BDL-induced cirrhosis. In
BDL-induced cirrhosis, it acts, instead, through DDAH-2, an enzyme
responsible for the degradation of asymmetrical dimethylarginine
(ADMA), an endogenous inhibitor of eNOS (41). The increase in eNOS in the rats
subjected to BDL in this study is inexplicable at this time, as a
decrease in hepatic eNOS activity in cirrhosis is considered to be
one of the mechanisms responsible for the vascular hyperreactivity
to vasoconstrictors observed under this condition.
To examine the effects of SF on intrahepatic
vascular resistance, we performed in situ perfusion to
record the perfusion pressure. SF did not significantly decrease
the high basal perfusion pressure observed in cirrhotic rats.
However, it partly decreased the hyperresponsiveness to methoxamine
observed in cirrhotic livers, possibly through its inhibition of
the RhoA/Rho-kinase pathway. The results supported our hypothesis:
SF decreased fibrosis and portal hypertension possibly through the
inhibition of the Rho-kinase pathway. Further studies are required
to explore the role of eNOS and HSC apoptosis in portal
pressure.
The parent compound, ferulic acid, has been used to
treat a number of clinical conditions (38), but has rarely been used in liver
diseases. Ferulic acid inhibits renal tubulointerstitial fibrosis
in rats (42), protects against
liver injury in mice (10,43–46),
downregulates blood pressure in hypertensive rats (36,47) and decreases portal pressure in
cirrhotic patients with portal hypertension (37). These studies, and our data showing
that SF decreases fibrosis and portal hypertension, suggest that
this compound may have potential for use in the treatment of liver
disease.
Statins, through their inhibition of cholesterol
synthesis, block the same pathway as ferulic acid, are protective
against hepatic fibrosis and have been used in clinical practice
(48). Ferulic acid is a Chinese
herb, and its pharmacological activities have been extensively
investigated (49). It has
advantages over statins as it also exerts anti-inflammatory and
antioxant effects. In addition, statins have side-effects, such as
gastrointestinal discomfort, and, as they cause an increase in
aminotransferase levels, are not suitable for the treatment of
active liver disease (50).
In conclusion, we demonstrate that SF inhibits the
hepatic RhoA/Rho-kinase signaling pathway, thus decreasing the
activation of HSCs. It also increases eNOS synthesis, ultimately
causing hepatic portal pressure decrease in cirrhotic rats. It
decreases fibrosis and increases the apoptosis of HSCs. To date,
the expression of RhoA, Rho-kinase and eNOS has been studied using
a semiquantitative immunohistochemical method. Experiments on these
substances at the protein and RNA levels are required. Our
preliminary research indicates that ferulic acid may be an
effective novel therapeutic agent for the treatment of patients
with hepatic cirrhosis with portal hypertension.
Acknowledgements
The authors would like to thank Minhua Liang from
the Department of Histology and Embryology, Tongji Medical College,
Huazhong University of Science and Technology and Xingxing He from
the Institute of Liver Diseases, Tongji Hospital, Tongji Medical
College, Huazhong University of Science and Technology for their
guidiance and technical support of this study. This study was
supported by National Natural Science Foundation of China, no.
81070342.
References
|
1
|
Hennenberg M, Biecker E, Trebicka J, et
al: Defective RhoA/Rho-kinase signaling contributes to vascular
hypocontractility and vasodilation in cirrhotic rats.
Gastroenterology. 130:838–854. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhou Q, Hennenberg M, Trebicka J, et al:
Intrahepatic upregulation of RhoA and Rho-kinase signalling
contributes to increased hepatic vascular resistance in rats with
secondary biliary cirrhosis. Gut. 55:1296–1305. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bari K and Garcia-Tsao G: Treatment of
portal hypertension. World J Gastroenterol. 18:1166–1175. 2012.
View Article : Google Scholar
|
|
4
|
Miñano C and Garcia-Tsao G: Clinical
pharmacology of portal hypertension. Gastroenterol Clin North Am.
39:681–695. 2010.
|
|
5
|
van Beuge MM, Prakash J, Lacombe M, et al:
Reduction of fibrogenesis by selective delivery of a Rho kinase
inhibitor to hepatic stellate cells in mice. J Pharmacol Exp Ther.
337:628–635. 2011.PubMed/NCBI
|
|
6
|
Ikeda H, Nagashima K, Yanase M, et al:
Involvement of Rho/Rho kinase pathway in regulation of apoptosis in
rat hepatic stellate cells. Am J Physiol Gastrointest Liver
Physiol. 285:G880–G886. 2003.PubMed/NCBI
|
|
7
|
Sohail MA, Hashmi AZ, Hakim W, et al:
Adenosine induces loss of actin stress fibers and inhibits
contraction in hepatic stellate cells via Rho inhibition.
Hepatology. 49:185–194. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
van Beuge MM, Prakash J, Lacombe M, Post
E, Reker-Smit C, Beljaars L and Poelstra K: Increased liver uptake
and reduced hepatic stellate cell activation with a cell-specific
conjugate of the Rho-kinase inhibitor Y27632. Pharm Res.
28:2045–2054. 2011.PubMed/NCBI
|
|
9
|
Trebicka J, Hennenberg M, Laleman W, et
al: Atorvastatin lowers portal pressure in cirrhotic rats by
inhibition of RhoA/Rho-kinase and activation of endothelial nitric
oxide synthase. Hepatology. 46:242–253. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Anegawa G, Kawanaka H, Yoshida D, et al:
Defective endothelial nitric oxide synthase signaling is mediated
by rho-kinase activation in rats with secondary biliary cirrhosis.
Hepatology. 47:966–977. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brandão DF, Ramalho LN, Ramalho FS,
Zucoloto S, de Martinelli AL and de Silva OC: Liver cirrhosis and
hepatic stellate cells. Acta Cir Bras. 21(Suppl 1): S54–S57.
2006.
|
|
12
|
Chakraborty JB, Oakley F and Walsh MJ:
Mechanisms and biomarkers of apoptosis in liver disease and
fibrosis. Int J Hepatol. 2012:6489152012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cichoz-Lach H, Celinski K, Slomka M and
Kasztelan-Szczerbinska B: Pathophysiology of portal hypertension. J
Physiol Pharmacol. 59(Suppl 2): S231–S238. 2008.
|
|
14
|
Friedman SL: Mechanisms of hepatic
fibrogenesis. Gastroenterology. 134:1655–1669. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Charlton-Menys V and Durrington PN: Human
cholesterol metabolism and therapeutic molecules. Exp Physiol.
93:27–42. 2008. View Article : Google Scholar
|
|
16
|
Lee MH, Cho YS and Han YM: Simvastatin
suppresses self-renewal of mouse embryonic stem cells by inhibiting
RhoA geranylgeranylation. Stem Cells. 25:1654–1663. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schmidmaier R, Baumann P, Simsek M,
Dayyani F, Emmerich B and Meinhardt G: The HMG-CoA reductase
inhibitor simvastatin overcomes cell adhesion-mediated drug
resistance in multiple myeloma by geranylgeranylation of Rho
protein and activation of Rho kinase. Blood. 104:1825–1832. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Martinez-Sales V, Vila V, Ferrando M and
Reganon E: Atorvastatin neutralises the thrombin-induced tissue
factor expresion in endothelial cells via geranylgeranyl
pyrophosphate. Cytotechnology. 63:1–5. 2011. View Article : Google Scholar
|
|
19
|
Luo W, Meng Y, Ji HL, et al:
Spironolactone lowers portal hypertension by inhibiting liver
fibrosis, ROCK-2 activity and activating NO/PKG pathway in the
bile-duct-ligated rat. PLoS One. 7:e342302012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Piao RL, Brigstock DR, Zhu J, Zhang ML and
Gao RP: Clinical significance of connective tissue growth factor in
hepatitis B virus-induced hepatic fibrosis. World J Gastroenterol.
18:2280–2286. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu Q, Liu X, Chen W and Zhang Z:
Inhibiting adenoid cystic carcinoma cells growth and metastasis by
blocking the expression of ADAM 10 using RNA interference. J Transl
Med. 8:1362010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jiroutová A, Majdiaková L, Cermáková M,
Köhlerová R and Kanta J: Expression of cytoskeletal proteins in
hepatic stellate cells isolated from normal and cirrhotic rat
liver. Acta Medica (Hradec Kralove). 48:137–144. 2005.PubMed/NCBI
|
|
23
|
Kim KY, Choi I and Kim SS: Purification
and characterization of a novel inhibitor of the proliferation of
hepatic stellate cells. J Biochem. 127:23–27. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Olaso E, Arteta B, Benedicto A, Crende O
and Friedman SL: Loss of discoidin domain receptor 2 promotes
hepatic fibrosis after chronic carbon tetrachloride through altered
paracrine interactions between hepatic stellate cells and
liver-associated macrophages. Am J Pathol. 179:2894–2904. 2011.
View Article : Google Scholar
|
|
25
|
March S, Graupera M, Rosa Sarrias M,
Lozano F, Pizcueta P, Bosch J and Engel P: Identification and
functional characterization of the hepatic stellate cell CD38 cell
surface molecule. Am J Pathol. 170:176–187. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vairetti M, Richelmi P, Bertè F, Currin
RT, Lemasters JJ and Imberti R: Role of pH in protection by low
sodium against hypoxic injury in isolated perfused rat livers. J
Hepatol. 44:894–901. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kukan M, Szatmáry Z, Lutterová M, Kuba D,
Vajdová K and Horecký J: Effects of sizofiran on endotoxin-enhanced
cold ischemia-reperfusion injury of the rat liver. Physiol Res.
53:431–437. 2004.PubMed/NCBI
|
|
28
|
Vairetti M, Ferrigno A, Carlucci F, et al:
Subnormothermic machine perfusion protects steatotic livers against
preservation injury: a potential for donor pool increase? Liver
Transpl. 15:20–29. 2009. View
Article : Google Scholar
|
|
29
|
Kim MY, Baik SK and Lee SS: Hemodynamic
alterations in cirrhosis and portal hypertension. Korean J Hepatol.
16:347–352. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Reynaert H, Urbain D and Geerts A:
Regulation of sinusoidal perfusion in portal hypertension. Anat Rec
(Hoboken). 291:693–698. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Al-Busafi SA, McNabb-Baltar J, Farag A and
Hilzenrat N: Clinical manifestations of portal hypertension. Int J
Hepatol. 2012:2037942012.PubMed/NCBI
|
|
32
|
Bishop AL and Hall A: Rho GTPases and
their effector proteins. Biochem J. 348:241–255. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang Y, Zheng XR, Riddick N, Bryden M,
Baur W, Zhang X and Surks HK: ROCK isoform regulation of myosin
phosphatase and contractility in vascular smooth muscle cells. Circ
Res. 104:531–540. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ho JH and Hong CY: Salvianolic acids:
small compounds with multiple mechanisms for cardiovascular
protection. J Biomed Sci. 18:302011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xu H, Zhou Y, Lu C, Ping J and Xu LM:
Salvianolic acid B lowers portal pressure in cirrhotic rats and
attenuates contraction of rat hepatic stellate cells by inhibiting
RhoA signaling pathway. Lab Invest. 92:1738–1748. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ardiansyah, Ohsaki Y, Shirakawa H, Koseki
T and Komai M: Novel effects of a single administration of ferulic
acid on the regulation of blood pressure and the hepatic lipid
metabolic profile in stroke-prone spontaneously hypertensive rats.
J Agric Food Chem. 56:2825–2830. 2008. View Article : Google Scholar
|
|
37
|
Huang Z, Wei W and Zhong Q: Effect of
sodium ferulate on hemodynamics in hepatic cirrhosis patients with
portal hypertension. Zhongguo Zhong Xi Yi Jie He Za Zhi.
28:640–642. 2008.(In Chinese).
|
|
38
|
Dudzinski DM and Michel T: Life history of
eNOS: partners and pathways. Cardiovasc Res. 75:247–260. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shiga N, Hirano K, Hirano M, Nishimura J,
Nawata H and Kanaide H: Long-term inhibition of RhoA attenuates
vascular contractility by enhancing endothelial NO production in an
intact rabbit mesenteric artery. Circ Res. 96:1014–1021. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Uehara H, Akahoshi T, Kawanaka H, et al:
Endothelin-1 derived from spleen-activated Rho-kinase pathway in
rats with secondary biliary cirrhosis. Hepatol Res. 42:1039–1047.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Verbeke L, Farre R, Trebicka J, et al:
Obeticholic acid, a farnesoid-X receptor agonist, improves portal
hypertension by two distinct pathways in cirrhotic rats.
Hepatology. Nov 20–2013.(Epub ahead of print). View Article : Google Scholar
|
|
42
|
Meng LQ, Tang JW, Wang Y, et al:
Astragaloside IV synergizes with ferulic acid to inhibit renal
tubulointerstitial fibrosis in rats with obstructive nephropathy.
Br J Pharmacol. 162:1805–1818. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Alam MA, Sernia C and Brown L: Ferulic
acid improves cardiovascular and kidney structure and function in
hypertensive rats. J Cardiovasc Pharmacol. 61:240–249. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kim HY, Park J, Lee KH, Lee DU, Kwak JH,
Kim YS and Lee SM: Ferulic acid protects against carbon
tetrachloride-induced liver injury in mice. Toxicology.
282:104–111. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Rukkumani R, Aruna K, Suresh Varma P and
Padmanabhan Menon V: Hepatoprotective role of ferulic acid: a
dose-dependent study. J Med Food. 7:456–461. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Xu X, Xiao H, Zhao J and Zhao T:
Cardioprotective effect of sodium ferulate in diabetic rats. Int J
Med Sci. 9:291–300. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang BH and Ou-Yang JP: Pharmacological
actions of sodium ferulate in cardiovascular system. Cardiovasc
Drug Rev. 23:161–172. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lowyck I and Fevery J: Statins in
hepatobiliary diseases: effects, indications and risks. Acta
Gastroenterol Belg. 70:381–388. 2007.PubMed/NCBI
|
|
49
|
Lin Z, Gu J, Xiu J, Mi T, Dong J and
Tiwari JK: Traditional chinese medicine for senile dementia. Evid
Based Complement Alternat Med. 2012:6926212012.PubMed/NCBI
|
|
50
|
Holmberg B, Brännström M, Bucht B,
Crougneau V, Dimeny E, Ekspong A, Granroth B, Gröntoft KC, Hadimeri
H, Ingman B, Isaksson B, Johansson G, Lindberger K, Lundberg L,
Mikaelsson L, Olausson E, Persson B, Welin D, Wikdahl AM and
Stegmayr BG: Safety and efficacy of atorvastatin in patients with
severe renal dysfunction. Scand J Urol Nephrol. 39:503–510. 2005.
View Article : Google Scholar : PubMed/NCBI
|