Introduction
Renal cell carcinoma (RCC), a common malignant tumor
in the urinary system with a low rate of early diagnosis and high
rate of recurrence, accounts for ~90% of renal neoplasms (1,2).
One-third of RCC patients have distant metastasis when initially
diagnosed and <30% of patients who underwent radical surgery
have tumor recurrence (3). Clear
cell RCC (ccRCC), the main histological type of RCC, accounts for
>80% of all the RCC patients, is more aggressive than other
types and has higher rate of metastasis and a poorer prognosis
among common renal malignancies (4,5).
Previously, increasing evidence has demonstrated
that microRNA (miRNA), a class of small (~22 nucleotides)
endogenous non-coding RNAs, plays a significant role in various
biological processes, including cell metabolism, proliferation,
differentiation and apoptosis (6,7).
Different from the mechanism of small interfering RNA (siRNA),
mature miRNAs usually inhibit genes expression at the
post-transcriptional level by binding to the 3′-untranslated
regions (3′-UTRs) of target mRNAs, leading to mRNA degradation or
depressing translation (8). As
miRNA plays important roles in the biological activities of living
cells, increasing studies have discovered that certain aberrantly
expressed miRNAs in various human cancers were involved in the
oncogenesis, progression and metastasis (9). Considerable miRNAs have been
characterized as oncogenes or tumor suppressors by targeting
downstream genes (10–13).
Numerous studies have discovered that
miR-886-3p was unconventionally expressed in certain cancers
(8,14–17). However, the role of
miR-886-3p in ccRCC has not been characterized. Whether
miR-886-3p is involved in the initiation, progression and
metastasis of ccRCC remains indistinct. Our previous sequencing for
miRNA expression in ccRCC tissues revealed that miR-886-3p
was overexpressed compared to the normal kidney tissues (20). Based on these sequencing results,
reverse transcription quantitative polymerase chain reaction
(RT-qPCR) was performed to quantify miR-886-3p levels in
ccRCC tissues, as well as functional experiments to evaluate the
effects of miR-886-3p on cell migration, proliferation and
apoptosis. Bioinformatics, immunohistochemistry, western blot
analysis and luciferase reporter assay were performed to identify
the downstream targets of miR-886-3p.
Paired-like homeodomain 1 (PITX1), located in human
chromosome 5, has been identified as a tumor suppressor gene in a
number of human malignances (21,22). Thus, we wished to identify whether
PITX1 is a target of miR-886-3p.
Materials and methods
Tissue collection
The ccRCC and paired normal tissues were collected
from Guangdong (Anhui, Hunan province, China) and written informed
consent was obtained from each patient. Fresh tumor and adjacent
normal tissues (located 2.0 cm outside the visible ccRCC lesions)
were frozen in liquid nitrogen once dissected, reviewed and
classified with hematoxylin and eosin staining. The characteristics
of 36 paired tissues used for qPCR of miR-886-3p in the
study are shown in Table I. The
age range of the patients was 20–76 years, with a median age of 53
years. The study was reviewed and approved by the Hospital Ethics
Committees (Peking University Shenzhen Hospital, Shenzhen,
China).
 | Table IClinicopathological characteristics
of 36 patients with ccRCC. |
Table I
Clinicopathological characteristics
of 36 patients with ccRCC.
| Variables | No. of cases |
|---|
| Age, years |
| ≥53 | 22 |
| <53 | 14 |
| Gender |
| Male | 19 |
| Female | 17 |
| pT-stage |
| T1 | 17 |
| T2 | 18 |
| T3 and T4 | 1 |
| AJCC clinical
stages |
| I | 17 |
| II | 16 |
| III+IV | 3 |
Cell culture and transfection
The cell lines used in the study were human renal
carcinoma 786-O and ACHN, human embryo kidney cell 293T (HEK-293T)
and cervical cancer cell line HeLa, cultured in Dulbecco’s modified
Eagle’s medium (DMEM) (Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% fetal bovine serum, at 37°C in a
humidified incubator containing 5% CO2. The level of
miR-886-3p in cells was down- or upregulated by transfecting
the synthesized miR-886-3p inhibitor or miR-886-3p
mimics (GenePharma Co., Ltd., Shanghai, China) into cells using
Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the
manufacturer’s instructions. Random sequences (provided by
GenePharma) were used as the negative control. The fold changes of
miR-886-3p were determined by RT-qPCR in 786-O and ACHN
cells 24 h after transfection.
RNA extraction and RT-qPCR
Total RNA of each sample (tissues and cells) was
extracted with TRIzol Reagent (Invitrogen) and purified with the
RNeasy Maxi kit (Qiagen, Hilden, Germany) according to the
manufacturer’s instructions. For the detection of
miR-886-3p, miScript reverse transcription (Qiagen) was used
to obtain the cDNA templates. The qPCR was performed using the
miScript SYBR-Green PCR kit (Qiagen) and U6 was the internal
control. For the mRNA detection, the RevertAid First Strand cDNA
Synthesis kit (MBI Fermentas, Inc., Burlington, ON, Canada) was
used to obtain the cDNA templates and SYBR® Premix Ex
Taq™ II (Tli RNaseH Plus) (Takara Bio Inc., Otsu, Japan) was used
for the quantitation. Human GAPDH was used as the reference
gene. The primers (Invitrogen) are shown in Table II and qPCR reaction was performed
in the LightCycler® 480 real-time PCR system
(Hoffmann-La Roche, Basel, Switzerland) according to the
manufacturer’s instructions. The expression levels were calculated
using the ΔΔCt method (18).
 | Table IIPrimers for RT-qPCR. |
Table II
Primers for RT-qPCR.
| Name | Forward
(5′→3′) | Reverse
(5′→3′) |
|---|
|
miR-886-3pa |
CGCGGGTGCTTACTGACCCTT | |
| U6 |
CTCGCTTCGGCAGCACA |
ACGCTTCACGAATTTGCGT |
| PITX1 |
GTTCAGCGGCCTAGTGCAG |
CGGGCTCATGGAGTTGAAGAA |
| GAPDH |
AGAAGGCTGGGGCTCATTTG |
AGGGGCCATCCACAGTCTTC |
Migration assay
To assess the influence of miR-886-3p on the
migratory ability of renal cancer cells (786-O and ACHN) in
vitro, the wound scratch assay was performed. Approximately 24
h after seeding in the 12-well plates, the cells (~150,000
cells/well) were transfected with the miR-886-3p inhibitor
(80 pmol) or negative control (80 pmol) using Lipofectamine 2000
and cultured in DMEM medium without additional fetal bovine serum.
A vertical horizontal wound was made using a sterile 10-μl pipette
tip at 5 h after transfection. Markers were made to allow for
observation of cell migration at the same points. Subsequent to
rinsing with phosphate-buffered saline (PBS) to remove the floating
cells, the adherent cells were cultured in the incubator at 37°C.
At 0 and 24 h after the wounds were generated, wound widths (μm)
were measured using a standard caliper. The wound experiments were
performed in triplicate and repeated at least three times.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
The MTT assay was carried out to measure cell
proliferation of the 786-O and ACHN cells at 0, 24, 48 and 72 h
after transfection with the miR-886-3p inhibitor or negative
control. Approximately 5,000 cells were plated in each well of the
96-well culture plates following transfection. At the time of
detection, 20 μl of MTT (5 mg/ml; Sigma, St. Louis, MO, USA) was
added to each well and incubated at 37°C for 4 h. Subsequently, the
MTT medium was replaced by 150 μl dimethylsulfoxide. After shaking
for 15 min at room temperature, the optical density (OD) of each
well at the wavelength of 490 nm was measured using the enzyme
immunoassay instrument (Model 680; Bio-Rad, Hercules, CA, USA).
Flow cytometry
Flow cytometry was performed to calculate the
apoptosis rates of renal cancer cells transfected with the
miR-886-3p inhibitor or negative control. The cells (786-O
and ACHN) were cultured in 6-well plates at 37°C and transfection
was conducted at a cell confluence of ~60%. At 48 h after
transfection, adherent and floating cells in each well were
harvested and washed twice with cold PBS, resuspended in 1X binding
buffer, and 5 μl Annexin V-FITC (Invitrogen) and 10 μl propidium
iodide were added. Within 30 min of staining, according to the
manufacturer’s instructions, the fluorescence of each sample was
detected by flow cytometry (Beckman Coulter, Inc., Brea, CA, USA)
using 488 nm excitation.
Bioinformatics
The potential targets of miR-886-3p were
created by combining four public algorithms, which were TargetScan
(http://www.targetscan.org/), miRanda
(http://www.targetscan.org/), miRWalk
(http://www.umm.uni-heidelberg.de/apps/zmf/mirwalk/)
and PicTar (http://pictar.mdc-berlin.de/). The putative genes that
were predicted by at least three algorithms were accepted and the
candidates were chosen based on the gene function.
Plasmid construction and luciferase
reporter assay
The 3′-UTR of PITX1 (930 base pair)
containing putative binding site (5′-CACCCGC-3′) for
miR-886-3p was cloned into the empty psiCHECK-2 Vector
(Promega Corporation, Madison, WI, USA), generating the wide type
of psiCHECK2-3′UTR (Wt). The mutant type (Mt) was generated by
changing the putative binding site to 5′-ACGCCGC-3′ in the
complementary site for the seed region of miR-886-3p. All
the constructed plasmids were sequenced-verified by DNA sequencing
analysis.
For the luciferase reporter assay, HEK-293T and HeLa
cells were seeded in 12-well plates, co-transfected with 0.5-μg
constructed plasmids and 40 pmol miR-886-3p mimics or
control mimics using Lipofectamine 2000 (Invitrogen). At 24 h after
transfection, firefly and renilla Luciferase of the cells were
detected using the Dual-Luciferase Reporter Assay system (Promega)
on the Modulus™ Single Tube Multimode Reader (Bio-Systems
International, Beloit, WI, USA). All the wells were performed in
triplicate and repeated at least three times.
Immunohistochemistry and western blot
analysis of PITX1
The immunohistochemical assay of PITX1 was performed
in 20 ccRCC paraffin-embedded tissues according to standard
procedures. The 5-μm sections were dewaxed in xylene, rehydrated in
descending ethanol series, incubated in 3% hydrogen peroxide
solution for 20 min, boiled in 0.01 M citrate buffer (pH 6.0) for
antigen retrieval and treated in 10% bovine serum albumin for 30
min in 37°C to block non-specific protein binding. Subsequently,
the sections were incubated in diluted (1:100) PITX1 rabbit
polyclonal antibody (NBP1-19686; Novus International, Saint
Charles, MO, USA) overnight at 4°C. The sections were rinsed with
PBS and treated with the anti-rabbit IHC kit (Maixin Bio, Fuzhou,
China) at 37°C for 30 min, followed by staining with a DAB kit
(Maixin Bio) for 4 min and counterstained with hematoxylin. The
negative controls were performed with omission of the primary
antibodies.
For western blot analysis, protein was extracted
from 16 cases of paired ccRCC tissues and 786-O cells that were
transfected with miR-886-3p mimics or inhibitors. The
samples were homogenised in lysis buffer on ice and the protein
concentration was quantified using the Pierce BCA Protein assay kit
(Thermo Fisher Scientific). Following separation by 10% SDS-PAGE
and transferring onto nitrocellulose membranes, the protein samples
(100-μg) were blocked with 10% skimmed milk at room temperature for
2 h and incubated in primary antibodies overnight at 4°C. Primary
antibodies of PITX1 and β-actin (internal control) were rabbit
polyclonal anti-PITX1 (1:1,000, NBP1-19686) and rabbit polyclonal
anti-β-actin (1:10,000, NB600-532) (Novus International),
respectively. Subsequent to washing three times with Tris-buffered
saline with Tween, the membranes were treated with horseradish
peroxidase (HRP) AffiniPure goat anti-rabbit immunoglobulin G (H+L)
(E030120-01; EarthOx Life Science, Millbrae, CA, USA) for 2 h,
followed by detection of the protein bands using the Immun-Star™
HRP Chemiluminescence kit (Bio-Rad). Each assay was repeated ≥3
times.
Statistical analysis
The data are presented as the means ± standard
deviation and statistical significance was determined with t-test
or Mann-Whitney U test, as appropriate. All the statistical
analysis was performed with SPSS 17.0 (SPSS, Inc., Chicago, IL,
USA) and values of P<0.05 were considered to indicate a
statistically significant difference.
Results
Verification of miR-886-3p upregulation
in ccRCC tissues by qPCR
By means of miRNA sequencing, previous studies have
discovered that miR-886-3p was upregulated in ccRCC tissues
(19,20). To validate the sequencing result,
qPCR was used to quantify the miR-886-3p levels in 36 paired
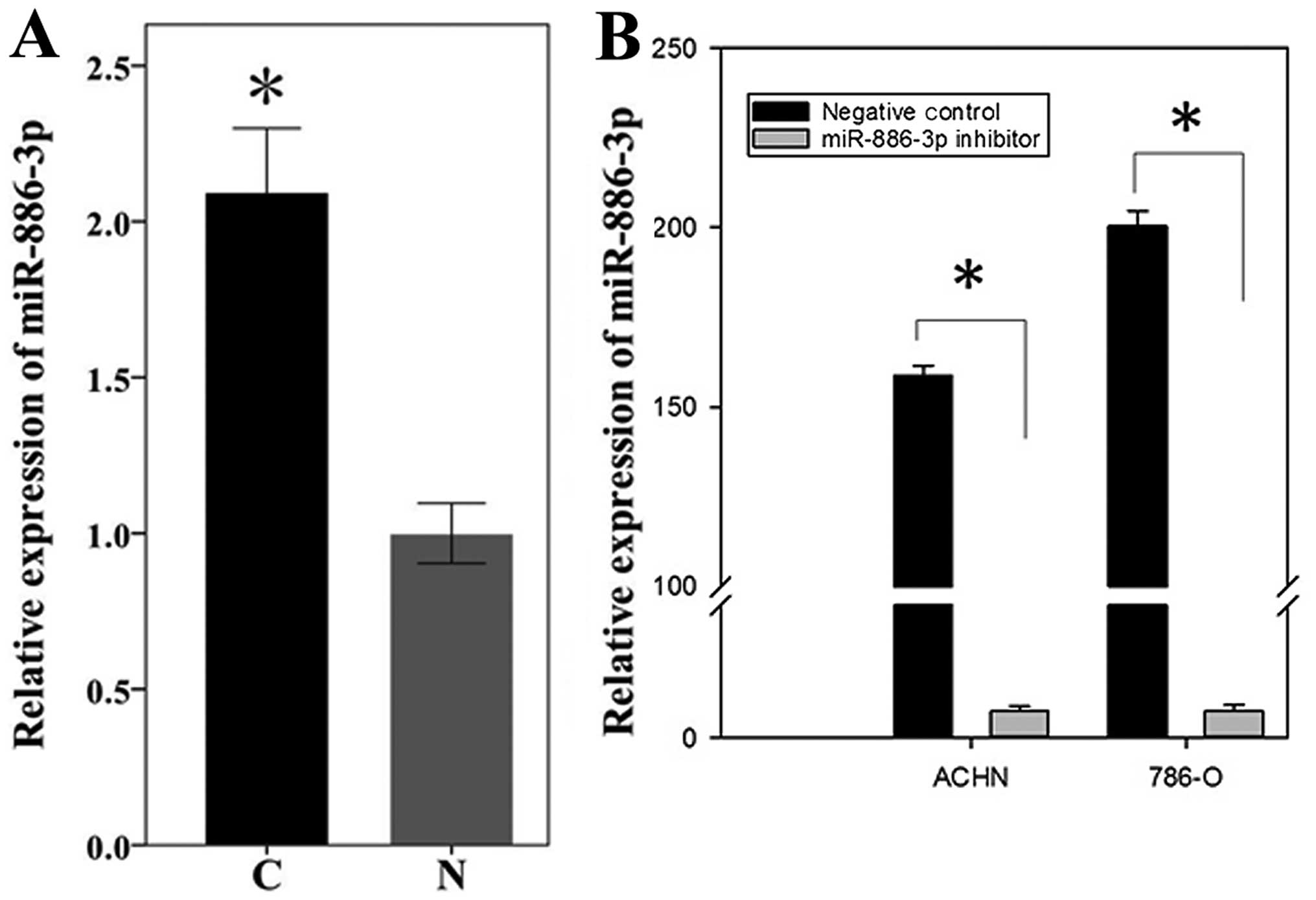
ccRCC and adjacent normal tissues. As shown in Fig. 1A, miR-886-3p was
significantly higher in ccRCC tissues compared to adjacent normal
tissues (P=0.001, paired-t test), which was identical to the
sequencing result.
Downregulation of miR-886-3p inhibits
cell migration, proliferation and induces cell apoptosis in
vitro
To explore the role of upregulated miR-886-3p
in biological behavior of renal cancer, the miR-886-3p
levels in 786-O and ACHN cells were downregulated by transfecting
the synthetic miR-886-3p inhibitor (Fig. 1B). The wound scratch assay, MTT
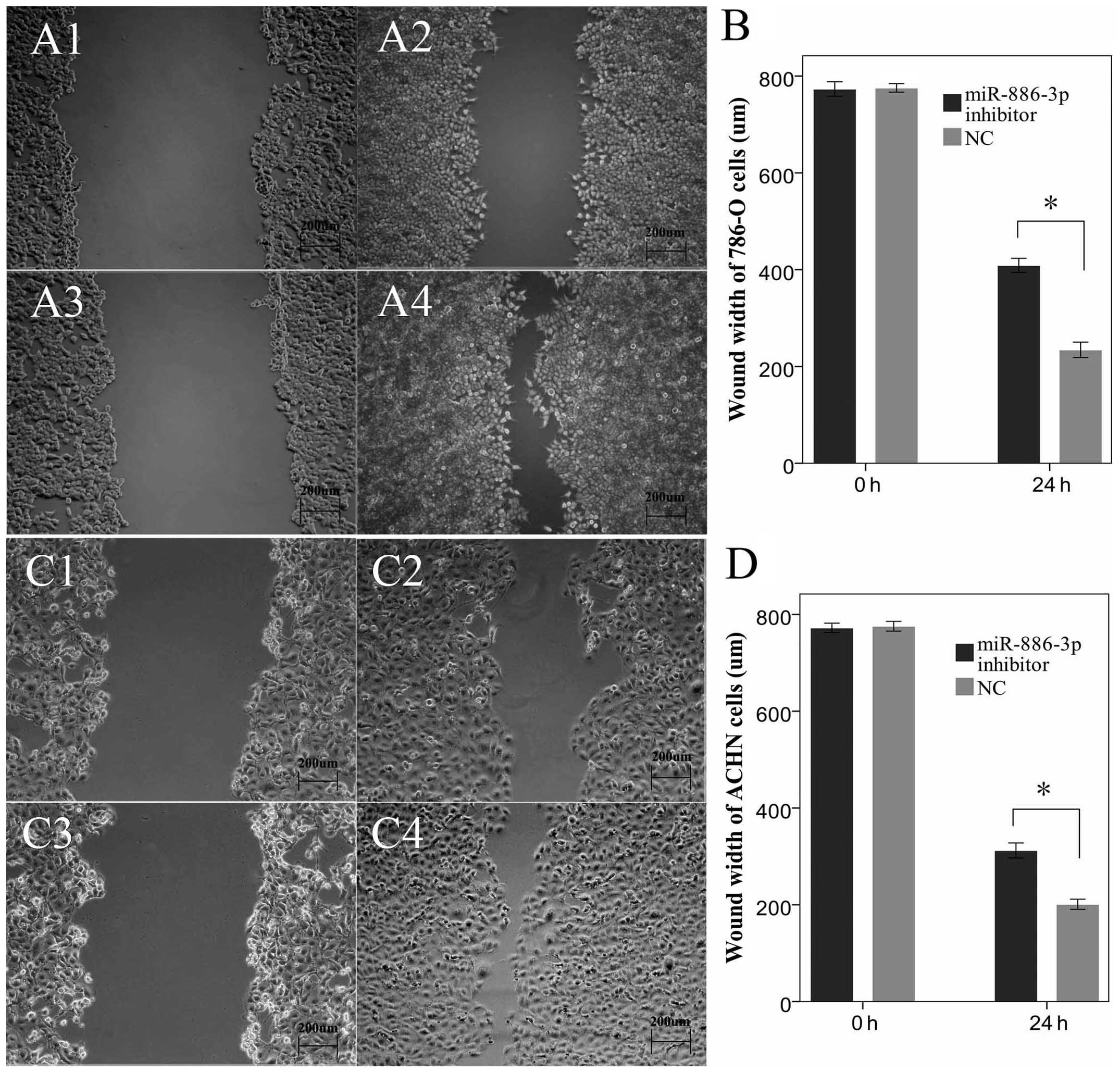
assay and flow cytometry were performed. The results of the wound
scratch assay showed the wound widths of the experimental group
(miR-886-3p inhibitor group) at 24 h after transfection were
much more spacious compared to the negative control group
(P<0.05). In particular, the cells transfected with
miR-886-3p migrated less distance, prompting the
downregulation of miR-886-3p, which inhibited the migratory
ability of the renal cancer cells (Fig. 2).
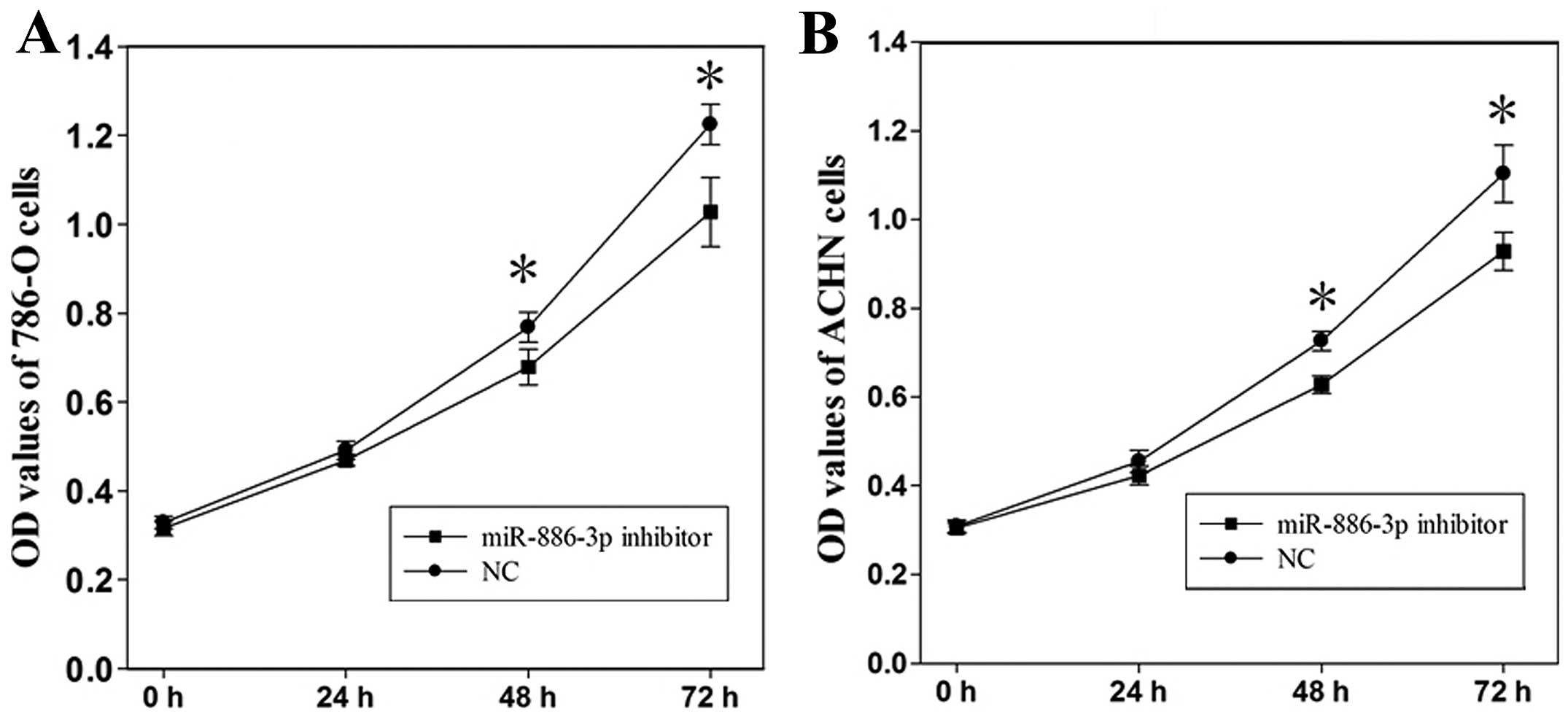
The MTT assay was used to determine the change of
cell proliferation at 24, 48 and 72 h after the level of
miR-886-3p was downregulated. Compared to the control group,
the OD values of the 786-O cells transfected with miR-886-3p
were decreased by 4.6 (P>0.05), 11.7 (P<0.05) and 16.1%
(P<0.05), whereas the OD values of the ACHN cells were decreased
by 7.0 (P>0.05), 13.6 (P<0.05) and 15.8%(P<0.05) at 24, 48
and 72 h after transfection, respectively (Fig. 3). The results demonstrated that
abatement of miR-886-3p depressed cell proliferation of
ccRCC.
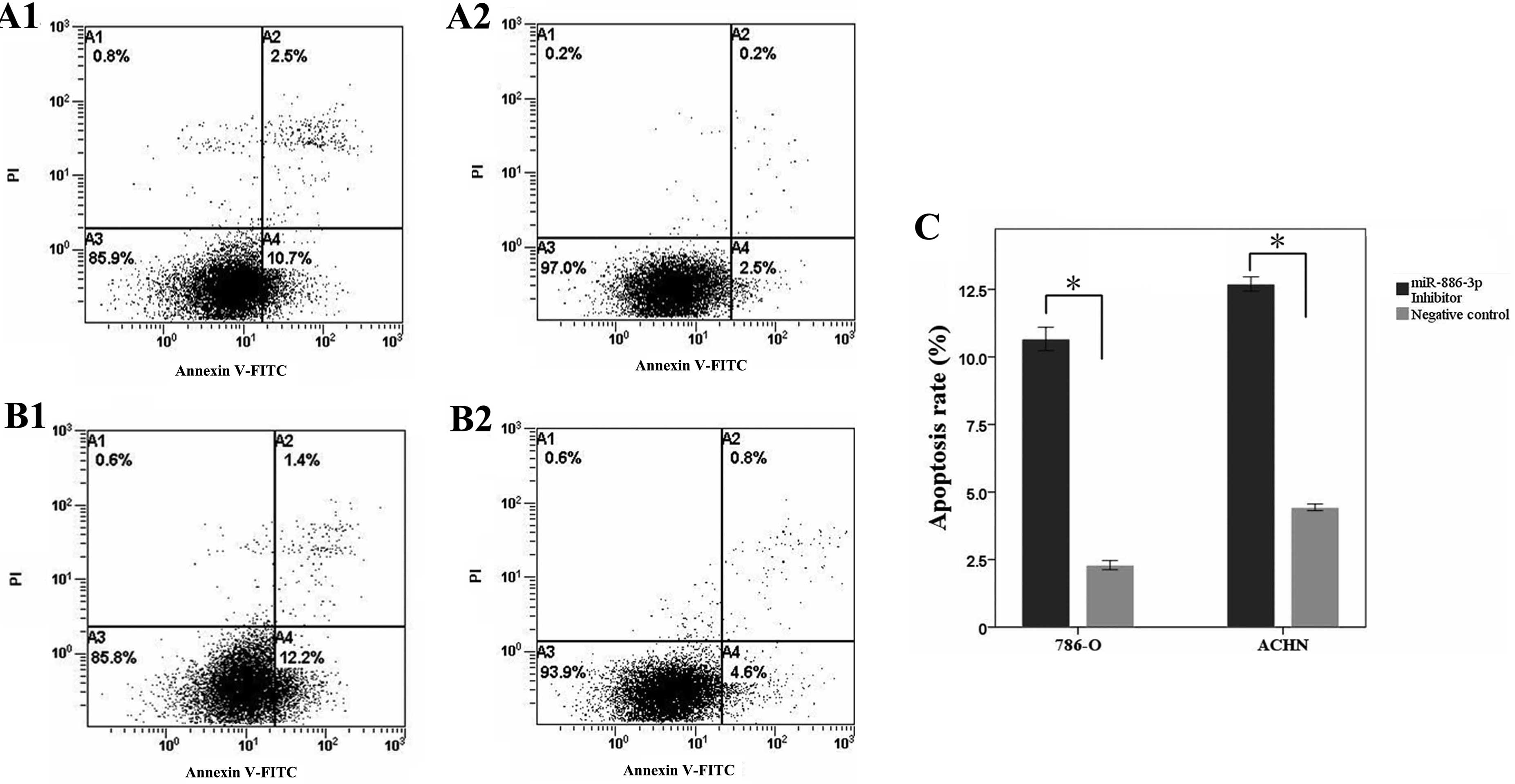
To evaluate the effect of reduced miR-886-3p
expression on renal cancer cell apoptosis, flow cytometry was used
to calculate the apoptosis rates of 786-O and ACHN cells following
transfection. The results showed that the apoptosis rates of 786-O
cells transfected with the miR-886-3p inhibitor and negative
control groups were 10.7 and 2.5% (P<0.05), while the apoptosis
rates of the ACHN cells were 12.2 and 4.6% (P<0.05),
respectively, as shown in Fig. 4.
The experiments were repeated three times and identical results
were obtained, indicating that the reduction of miR-886-3p
promoted renal cancer cell apoptosis.
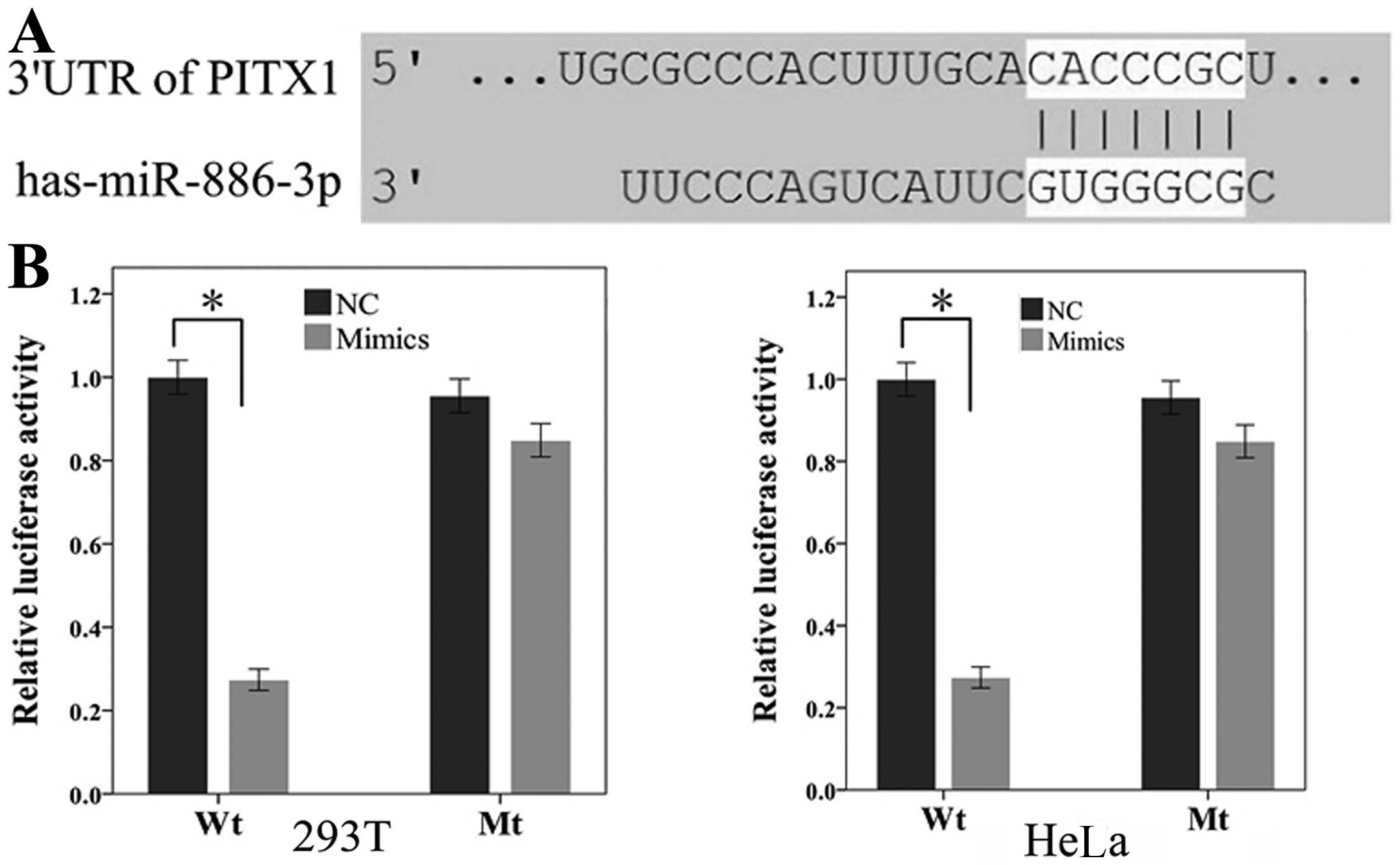
miR-886-3p targets the tumor suppressor
gene, PITX1
miRNAs are believed to control gene expression at
the post-transcription level by targeting the 3′-UTRs of the
downstream genes. To explore the downstream targets of
miR-886-3p, bioinformatics was performed to identify the
potential targets. PITX1 was one of the putative genes by
three algorithms (TargetScan, miRanda and miRWalk) simultaneously,
of which the mRNA contained a complementary site for the seed
region of miR-886-3p (Fig.
5A). Furthermore, PITX1 has been identified as a tumor
suppressor and is associated with the progression and development
in various cancers (21–25).
To determine whether PITX1 was directly
regulated by miR-886-3p, the 3′-UTR fragment of PITX1
containing the putative or mutant binding site were cloned into
psiCHECK-2 to construct recombinant plasmids (Wt and Mt) and the
luciferase reporter assay was performed in 293T and HeLa cells. As
shown in Fig. 5B, the relative
luciferase activity of wide-type plasmids containing the putative
binding site was significantly decreased when transfected with
miR-886-3p mimics in 293T cells (P<0.05), whereas no
notable reduction was observed in the mutant groups. An identical
result was also obtained in Hela cells, indicating that
miR-886-3p may restrain the expression of PITX1 by
targeting the putative binding site in the 3′-UTR.
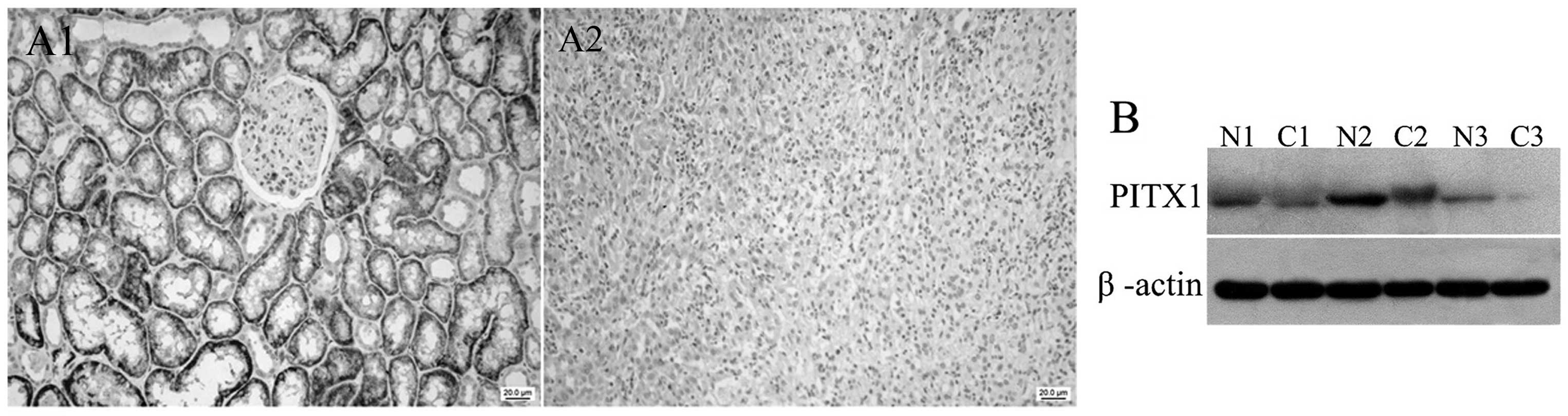
Protein expression of PITX1 in ccRCC
tissues and adjacent normal tissues
PITX1 was discovered to be downregulated in a number
of types of human cancer, including gastric cancer, colorectal
carcinoma and lung cancer. However, thus far the protein expression
of PITX1 in renal cancer is unclear. Immunohistochemistry and
western blot analysis were performed to determine the protein
expression of PITX1 in 20 pairs of ccRCC sections and 16 pairs of
fresh tissues, respectively. The results of the immunohistochemical
assay showed that PITX1 reduction was observed in 90% (18/20) of
the ccRCC sections (Fig. 6A),
while western blot analysis also showed that PITX1 was
downregulated in the ccRCC tissues (Fig. 6B). The association between the
expression level of PITX1 and clinicopathological variables was not
evaluated due to limited cases.
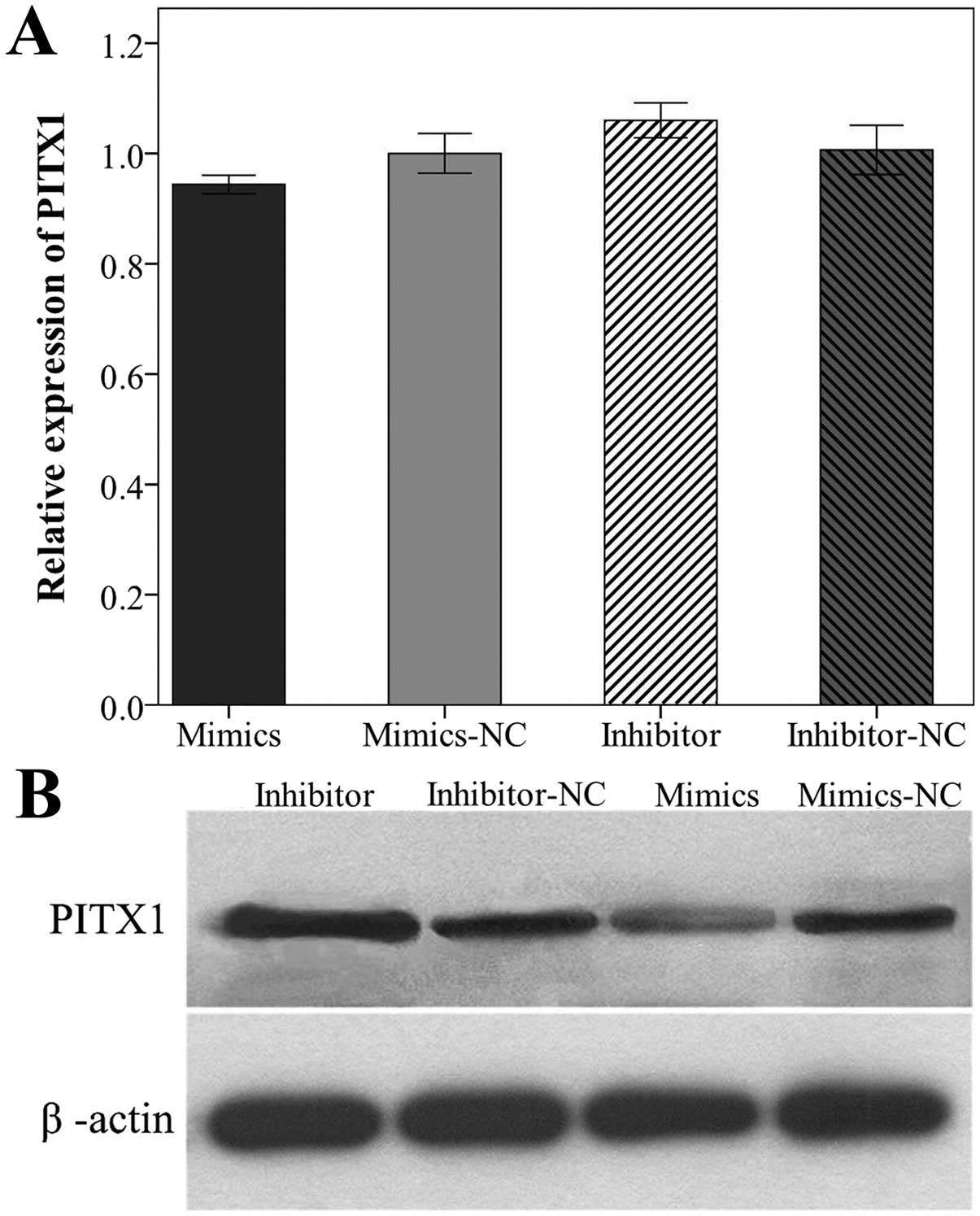
Regulation of PITX1 by miR-886-3p
To validate the results of the luciferase reporter
assay and to evaluate the association between PITX1
expression and the miR-886-3p level, qPCR and western blot
analysis were performed to quantify the mRNA and protein level of
PITX1 in 786-O cells 48 h after transfection with miR-886-3p
mimics or inhibitor. Notably, the mRNA level of PITX1 in
786-O cells was not significantly decreased or increased following
transfection (P<0.05, Fig.
7A). By contrast, the PITX1 protein level was significantly
upregulated when 786-O cells were transfected with the
miR-886-3p inhibitor and downregulated when transfected with
the miR-886-3p mimics (P<0.05, Fig. 7B). These results indicated that
miR-886-3p directly regulates protein expression of PITX1
and the regulation is on post-transcriptional level.
Discussion
Since the first miRNA (miRNA-lin-4) was
identified, miRNAs have been demonstrated to play a significant
role in diverse cellular activities, including cell proliferation,
differentiation and apoptosis (6,26,27). A large number of miRNAs have been
found to be aberrantly expressed in certain types of cancer and are
associated with the initiation, progression, invasion and
metastasis of tumors (10–12).
In general, mature miRNAs bind to the 3′-UTRs of target mRNAs,
leading to mRNA degradation or suppressed translation to proteins.
Therefore, a specific miRNA can act as a tumor suppressor or an
oncogene by inhibiting the expression of downstream oncogenes or
anti-oncogenes (8). Even though
potential applications of miRNAs remain restricted to experiments
in laboratories, miRNAs have provided novel thinking and methods in
the early diagnosis, treatment and prognosis of cancer (28,29).
miR-886-3p has been identified as a tumor
suppressor in familial non-medullary thyroid cancer. The study
discovered that miR-886-3p overexpression in thyroid cancer
cell lines significantly inhibited cellular proliferation, the
number and size of spheroids and cellular migration and increased
the number of cells in the S phase (8). miR-886-3p has also been
discovered to be downregulated in lung cancer. A study demonstrated
that downregulated miR-886-3p was closely correlated with a
shorter survival rate of small cell lung cancer and
miR-886-3p potently repressed cell proliferation, migration
and invasion of cancer cells in vitro via targeting genes,
PLK1 and TGF-β1, at the post-transcription level
(14). In addition,
miR-886-3p was found to be downregulated greater than 2-fold
in primary squamous cell lung carcinoma (15). However, different results were
observed in certain studies. A study discovered miR-886-3p
was significantly overexpressed in extranodal NK/T cell lymphoma
nasal type, and considered to play crucial roles in hemopoiesis,
cellular proliferation and apoptosis (16). Using miRNA microarrays,
miR-886-3p was also observed to be upregulated in
malignantly transformed oral leukoplakia tissues compared to oral
leukoplakia (17), indicating
that miR-886-3p may act as an oncogene in certain other
cancers.
PITX1 was discovered to be downregulated in a
number of types of human cancer and is correlated with a poor
prognosis (21,22,25). Furthermore, studies have
demonstrated that PITX1 suppressed the expression of
telomerase reverse transcriptase (TERT) through direct
binding to the TERT promoter, thus regulating the activity
of telomerase, which is crucial for cellular immortalization and
cancer progression (30). In
addition, PITX1 was revealed to suppress tumorigenicity by
downregulating the RAS pathway through targeting a RAS guanosine
triphosphate-activating factor, RASAL1 (23).
In the present study, RT-qPCR was used to validate
the miR-886-3p levels in ccRCC and adjacent normal tissues
based on previous miRNAs sequencing. Consistent with the previous
results, miR-886-3p was significantly upregulated in ccRCC
tissues (19). Further functional
analysis revealed that forced downregulation of miR-886-3p
inhibited cell migration, proliferation and induced cell apoptosis,
indicating that miR-886-3p may be characterized as an
oncogene in ccRCC. To determine the downstream genes of
miR-886-3p, bioinformatics and experimental verification
were performed, and PITX1 was identified. The downregulation of
PITX1 in ccRCC was determined using immunohistochemistry and
western blot analysis. Furthermore, the PITX1 protein, and not the
mRNA, was discovered to be directly regulated by miR-886-3p,
indicating that the regulation was at the post-transcriptional
level. Although the specific mechanisms of PITX1 as a tumor
suppressor in ccRCC remain largely unknown, miR-886-3p was
identified as an upstream regulator. The association between
PITX1 expression and prognoses of ccRCC patients should be
further explored, as well as the molecular mechanisms of PITX1 in
the tumorigenicity, immortalization and progression of ccRCC.
The role of miR-886-3p in cancer appears
controversial as it was identified as a tumor suppressor in certain
cancers and an oncogene in others (8,14–17). Removing experimental error, this
contradiction may be explained by the ‘imperfect’ complementary
interactions between miRNAs and target genes. Different from siRNA,
the interactions between miRNAs and 3′-UTRs of target genes are not
always completely complementary (particularly in mammals), leading
to the relative specifity rather than absolute specifity between
miRNAs and target genes (31).
Precise interactions between miRNAs and target genes may be further
dictated by cell types and micro-environment, contributing to the
bipolar regulations of specific miRNAs (6,15).
In conclusion, to the best of our knowledge, this is
the first study to reveal that miR-886-3p was upregulated in
ccRCC. Forced downregulation of miR-886-3p inhibited
cellular migration, proliferation and induced cell apoptosis by
targeting the tumor suppressor PITX1 in ccRCC.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81101922), Medical
Scientific Research Foundation of Guangdong Province of China
(grant no. A2012584 and A2013606) and Science and Technology
Development Fund Project of Shenzhen (grant no.
JCYJ20130402114702124).
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
2
|
Patel C, Ahmed A and Ellsworth P: Renal
cell carcinoma: a reappraisal. Urol Nurs. 32:182–191.
2012.PubMed/NCBI
|
|
3
|
Rouvière O, Bouvier R, Négrier S, Badet L
and Lyonnet D: Nonmetastatic renal-cell carcinoma: is it really
possible to define rational guidelines for post-treatment
follow-up? Nat Clin Pract Oncol. 3:200–213. 2006.PubMed/NCBI
|
|
4
|
McLaughlin JK, Lipworth L and Tarone RE:
Epidemiologic aspects of renal cell carcinoma. Semin Oncol.
33:527–533. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cairns P: Renal cell carcinoma. Cancer
Biomark. 9:461–473. 2010.
|
|
6
|
Jiang L, Liu X, Chen Z, et al: MicroRNA-7
targets IGF1R (insulin-like growth factor 1 receptor) in tongue
squamous cell carcinoma cells. Biochem J. 432:199–205. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Soeda S, Ohyashiki JH, Ohtsuki K, et al:
Clinical relevance of plasma miR-106b levels in patients with
chronic obstructive pulmonary disease. Int J Mol Med. 31:533–539.
2013.PubMed/NCBI
|
|
8
|
Xiong Y, Zhang L, Holloway AK, et al:
miR-886–3p regulates cell proliferation and migration, and is
dysregulated in familial non-medullary thyroid cancer. PLoS One.
6:e247172011.
|
|
9
|
Rosén A, Bergh AC, Gogok P, et al:
Lymphoblastoid cell line with B1 cell characteristics established
from a chronic lymphocytic leukemia clone by in vitro EBV
infection. Oncoimmunology. 1:18–27. 2012.PubMed/NCBI
|
|
10
|
Shibuya H, Iinuma H, Shimada R, Horiuchi A
and Watanabe T: Clinicopathological and prognostic value of
microRNA-21 and microRNA-155 in colorectal cancer. Oncology.
79:313–320. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Akao Y, Noguchi S, Iio A, et al:
Dysregulation of microRNA-34a expression causes drug-resistance to
5-FU in human colon cancer DLD-1 cells. Cancer Lett. 300:197–204.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tili E, Michaille JJ, Wernicke D, et al:
Mutator activity induced by microRNA-155 (miR-155) links
inflammation and cancer. Proc Natl Acad Sci USA. 108:4908–4913.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kong X, Li G, Yuan Y, et al: MicroRNA-7
inhibits epithelial-to-mesenchymal transition and metastasis of
breast cancer cells via targeting FAK expression. PLoS One.
7:e415232012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu G, Keeler BE, Zhukareva V and Houlé
JD: Cycling exercise affects the expression of apoptosis-associated
microRNAs after spinal cord injury in rats. Exp Neurol.
226:200–206. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kharaziha P, Ceder S, Li Q and Panaretakis
T: Tumor cell-derived exosomes: a message in a bottle. Biochim
Biophys Acta. 1826:103–111. 2012.PubMed/NCBI
|
|
16
|
Xu F, Zhang X, Lei Y, et al: Loss of
repression of HuR translation by miR-16 may be responsible for the
elevation of HuR in human breast carcinoma. J Cell Biochem.
111:727–734. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lennox KA and Behlke MA: A direct
comparison of anti-microRNA oligonucleotide potency. Pharm Res.
27:1788–1799. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Z, Zhang J, Luo H, et al: Screening
and confirmation of microRNA markers for forensic body fluid
identification. Forensic Sci Int Genet. 7:116–123. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li X, Chen J, Hu X, et al: Comparative
mRNA and microRNA expression profiling of three genitourinary
cancers reveals common hallmarks and cancer-specific molecular
events. PLoS One. 6:e225702011. View Article : Google Scholar
|
|
20
|
Zhou L, Chen J, Li Z, et al: Integrated
profiling of microRNAs and mRNAs: microRNAs located on Xq27.3
associate with clear cell renal cell carcinoma. PLoS One.
5:e152242010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen Y, Knosel T, Ye F, Pacyna-Gengelbach
M, Deutschmann N and Petersen I: Decreased PITX1 homeobox gene
expression in human lung cancer. Lung Cancer. 55:287–294. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen YN, Chen H, Xu Y, Zhang X and Luo Y:
Expression of pituitary homeobox 1 gene in human gastric
carcinogenesis and its clinicopathological significance. World J
Gastroenterol. 14:292–297. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kolfschoten IG, van Leeuwen B, Berns K, et
al: A genetic screen identifies PITX1 as a suppressor of RAS
activity and tumorigenicity. Cell. 121:849–858. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qi DL, Ohhira T, Fujisaki C, et al:
Identification of PITX1 as a TERT suppressor gene located on human
chromosome 5. Mol Cell Biol. 31:1624–1636. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Knosel T, Chen Y, Hotovy S, Settmacher U,
Altendorf-Hofmann A and Petersen I: Loss of desmocollin 1–3 and
homeobox genes PITX1 and CDX2 are associated with tumor progression
and survival in colorectal carcinoma. Int J Colorectal Dis.
27:1391–1399. 2012.
|
|
26
|
Zhai Q, Zhou L, Zhao C, et al:
Identification of miR-508-3p and miR-509-3p that are associated
with cell invasion and migration and involved in the apoptosis of
renal cell carcinoma. Biochem Biophys Res Commun. 419:621–626.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu M, Tang Q, Qiu M, et al: miR-21
targets the tumor suppressor RhoB and regulates proliferation,
invasion and apoptosis in colorectal cancer cells. FEBS Lett.
585:2998–3005. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang GK, Zhu JQ, Zhang JT, et al:
Circulating microRNA: a novel potential biomarker for early
diagnosis of acute myocardial infarction in humans. Eur Heart J.
31:659–666. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Guo LJ and Zhang QY: Decreased serum
miR-181a is a potential new tool for breast cancer screening. Int J
Mol Med. 30:680–686. 2012.PubMed/NCBI
|
|
30
|
Sendt W, Rippe V, Flor I, Drieschner N and
Bullerdiek J: Monosomy and ring chromosome 13 in a thyroid nodular
goiter-do we underestimate its relevance in benign thyroid lesions?
Cancer Genet. 205:128–130. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim VN: MicroRNA biogenesis: coordinated
cropping and dicing. Nat Rev Mol Cell Biol. 6:376–385. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Guinan P, Sobin LH, Algaba F, et al: TNM
staging of renal cell carcinoma: Workgroup No. 3. Union
International Contre le Cancer (UICC) and the American Joint
Committee on Cancer (AJCC). Cancer. 80:992–993. 1997. View Article : Google Scholar : PubMed/NCBI
|