Introduction
Osteosarcoma is the most common human primary
malignant bone tumor in children and adolescents (1). It is associated with a high risk of
local relapse or distant metastasis even after curative resection
of the primary tumor and intensive chemotherapy. The 5-year
survival rate for patients with osteosarcoma has improved
significantly over the past few decades, reaching rates of
approximately 60–70% since the introduction of combination
chemotherapy (2). Although
treatment with a combination of chemotherapy and aggressive
surgical resection has markedly improved the prognosis of patients
with osteosarcoma, approximately 80% of patients develop metastatic
disease following surgery. Therefore, the identification of
metastasis-associated molecules and the elucidation of the
mechanisms contributing to invasion in osteosarcoma are
essential.
microRNAs (miRNAs or miRs) are short, non-coding
RNAs that regulate mRNA stability and protein translation by
binding to their target mRNAs (3). Increasing evidence suggests that
miRNAs play a role in the regulation of diverse biological
processes (4). Since their
initial identification, approximately 1,000 miRNA sequences have
been identified in mammals (5).
In humans, miRNAs regulate up to 30% of human genes representing
the majority of genetic pathways (6). miRNAs are often deregulated in human
malignancies and they have been implicated in the regulation of a
number of cellular processes, including proliferation,
differentiation, apoptosis and metastasis (7). The aberrant expression of miRNAs
occurs in many types of cancer (8–10).
miRNAs have been characterized as oncogenes, tumor suppressors or
as components of regulatory pathways critical for tumorigenesis,
supporting an important role for miRNAs in tumorigenesis and
metastasis. However, the evaluation of deregulated miRNAs and their
roles in cancer development, particularly in osteosarcoma, is an
ongoing process (11).
The role of miRNAs in the development of
osteosarcoma has attracted increasing attention. Certain miRNAs,
including miR-143, miR-133a and miR-376c play a role in the
initiation and progression of osteosarcoma and modulate the
biological properties of cancer cells (12–14). Recently, the miR-144/miR-451
cluster was found to be downregulated in osteosarcoma cells
(15); however, its potential
role in osteosarcoma carcinogenesis and progression remains
unknown.
In the present study, we investigated the
differential expression of miR-144 in osteosarcoma cells and in
clinically resected human osteosarcoma tissue samples. We
demonstrate that miR-144 is downregulated in osteosarcoma cells and
tissue, identifying TAGLN as a target gene for miR-144. We examined
the correlation between the deregulation of miR-144 and the
expression of TAGLN in osteosarcoma to elucidate their role in the
growth and invasion of osteosarcoma cells. Our data indicate that
miR-144 may regulate osteosarcoma cell proliferation and invasion
by downregulating TAGLN, suggesting that miR-144 is a potential
therapeutic target for the treatment of osteosarcoma.
Materials and methods
Materials
A total of 60 pairs of cancer tissue and
corresponding normal tissue samples were collected from patients
with primary osteosarcoma who received surgery between 2009 and
2011 at Jinshan Hospital, Fudan University School of Medicine
(Shanghai, China). Surgically removed tissues were snap frozen in
liquid nitrogen overnight and kept at −80°C before use. Each
specimen was verified histopathologically and scored according to
the TNM staging system. The study protocol was approved by the
Ethics Committee of Fudan University, Shanghai, China. All samples
were collected after obtaining informed consent from all
patients.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was isolated from the osteosarcoma cell
lines and tumor tissue samples using the miRNeasy kit (Qiagen,
Valencia, CA, USA). To detect miRNA expression, RT-qPCR was
performed using the SYBR RT-PCR kit (Takara, Shiga, Japan). The
primers for TAGLN were as follows: 5′-AATGGCG TGATTCTGAGCAA-3′
(forward) and 5′-CGATGCCTGCTG GAGCCGTCTA-3′ (reverse). The relative
expression level of TAGLN was normalized to the internal control,
β-actin. Stem-loop RT primers for miR-144 were as follows:
5′-GCTGGGA TATCATCATATACTG-3′ (forward) and 5′-CGGACTAGTA
CATCATCTATACTG-3′ (reverse), as previously described (16). The primers used for quantitative
PCR were as follows: 5′-ACACTCCAGCTGGGTCATGTA GTAGATA-3′ (forward)
and 5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAG TTGAGATGTCATA-3′ (reverse).
The relative expression level of miR-144 was normalized to that of
the internal control U6 by using the 2−ΔΔCt cycle
threshold method, as previously described (17).
Cell culture and transfection
The human normal osteoblastic cell line, hFOB1.19,
and the human osteosarcoma cell lines, MG63, U2OS and HOS, were
obtained from the American Type Culture Collection (ATCC, Manassas,
VA, USA) and cultured in Dulbecco’s modified Eagle’s medium (DMEM)
(Invitrogen, Carlsbad, CA, USA) supplemented with heat-inactivated
10% fetal bovine serum (FBS) at 37°C in a humidified incubator with
5% CO2.
The procedure used for miR-144 eukaryotic expression
vector construction was as previously described (7). The following primers were used for
PCR amplification of the pri-miR-144 and native flanking sequence:
5′-GGATCCCAC AGTGCTTTTCAAGCCATG-3′ and 5′-AAGCTTAGTGCC
CTGGCAGTCAGTAGG-3′. The amplified fragments were then cloned into
the pcDNA3.1 vector (Invitrogen) and verified by sequencing.
Transfection was performed using Lipofectamine 2000
transfection reagent (Invitrogen) according to the manufacturer’s
instructions. For establishing stable transfectants, the miR-144
mimics or negative control (NC) RNA-tansfected HOS and U2OS cells
were selected for 6 weeks in the presence of G418 (400 μg/ml).
Control cells were transfected with empty vector. The expression of
miR-144 in the stably transfected cells was evaluated by RT-qPCR.
TAGLN shRNA Lentivirus (TAGLN-shRNA) and Negative Control shRNA
Lentivirus (shRNA-NC) purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA) were used to tansfect the HOS and U2OS cells
following the manufacturer’s instructions and stably tansfected
cells were selected by puromycin (Sigma, St. Louis, MO, USA).
Cell growth and invasion assay
The in vitro proliferation of the HOS and
U2OS cells transfected with miR-NC, miR-144 mimics, TAGLN-shRNA or
shRNA-NC was measured by 3-(4,5-dimethythiazol-2-yl)-2,5-diphenyl
tetrazolium bromide (MTT) assay as previously described (12). Briefly, the cells were seeded onto
96-well plates and at the indicated periods to time, 100 μl of
medium was removed and replaced with fresh medium containing 0.5
mg/ml MTT solution. The plates were incubated at 37°C for 4 h and
then the medium was replaced by 100 μl DMSO and the plates were
shaken at room temperature for 10 min. Absorbance was measured at
570 nm.
The invasive potential of the cells was evaluated
using Transwell inserts with 8 mm pores (Corning Inc., Corning, NY,
USA). For invasion assay, at 24 h after transfection,
3.0×105 cells in serum-free medium were added to each
upper insert pre-coated with Matrigel matrix (BD Biosciences,
Bedford, MA, USA). Approximately 500 ml of 10% FBS medium was added
to the matched lower chamber. After 48 h of incubation, the
non-invading cells were removed from the upper surface of the
Transwell membrane with a cotton swab, and the invading cells on
the lower membrane surface were fixed in methanol, stained with
0.1% crystal violet, photographed and counted. Six random fields at
×100 magnification for each insert were counted. The inserts were
analyzed in triplicate in 3 separate experiments.
3′ Untranslated region (3′ UTR)
luciferase reporter assay
The micro-RNA database (www.microRNA.org)
was used to find the putative miR-144 binding site in the 3′ UTR of
TAGLN (433 bp). The psiCHECK-2-TAGLN-3′UTR-wt vector or the
psiCHECK-2-TAGLN-3′UTR-mut vector were co-transfected with control,
miR-NC or miR-144 into the HOS cells using Lipofectamine 2000
(Invitrogen). Subsequently, reporter gene assays were performed
48-h post-transfection using the Dual Luciferase Reporter assay
system (Promega, Madison, WI, USA) according to the manufacturer’s
instructions. The normalized firefly luciferase activity was
obtained by measuring firefly luciferase activity/Renilla
luciferase activity. All experiments were performed at least 3
times.
Tumorigenicity assay in nude mice
A total of 54 BALB/c athymic nude mice were
purchased from Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai,
China). All animal protocols and procedures were approved by the
Institutional Animal Care Utilization Committee, and were in
accordance with the Johns Hopkins Animal Care and Use Committee
guidelines. HOS cells stably transfected with miR-144 mimics,
negative control (NC) RNA and control (1×106) were
suspended in 0.1 ml PBS and then injected subcutaneously into
either side of the posterior flank of the same BALB/c athymic nude
mice at 4 weeks of age. Tumor growth was measured using calipers
weekly, and tumor volume was calculated according to the following
formula: volume = length × width2 × 0.5.
Western blot analysis
Lysates obtained from the cells and tissue samples
were extracted using the M-PER Protein Extraction Reagent (Pierce,
Rockford, IL, USA), and the protein content was quantified using
the BCA assay (Pierce). Equal amounts of extracts were subjected to
SDS-PAGE, transferred onto polyvyniledene difluoride membranes and
probed with primary antibodies against TAGLN (Sigma), matrix
metalloproteinase 2 (MMP2; Abcam, Cambridge, MA, USA) and β-actin
followed by the appropriate secondary antibodies (Cell Signaling
Technology). Densitometric analysis was performed using LabWorks
image acquisition and analysis software (UVP, Upland, CA, USA).
Statistical analysis
Data are presented as the means ± SD. Statistical
comparisons between groups were analyzed using a two-tailed
Student’s t-test and a P-value <0.05 was considered to indicate
a statistically significant difference. The correlation between
miR-144 expression and clinical osteosarcoma stages was analyzed
using the Spearman’s rank correlation assay with SPSS 17.0 software
(SPSS Inc., Chicago, IL, USA). The correlation between miR-144
expression and TAGLN mRNA levels was analyzed using the Spearman’s
correlation coefficient assay with SPSS 17.0 software.
Results
miR-144 is downregulated in
osteosarcoma
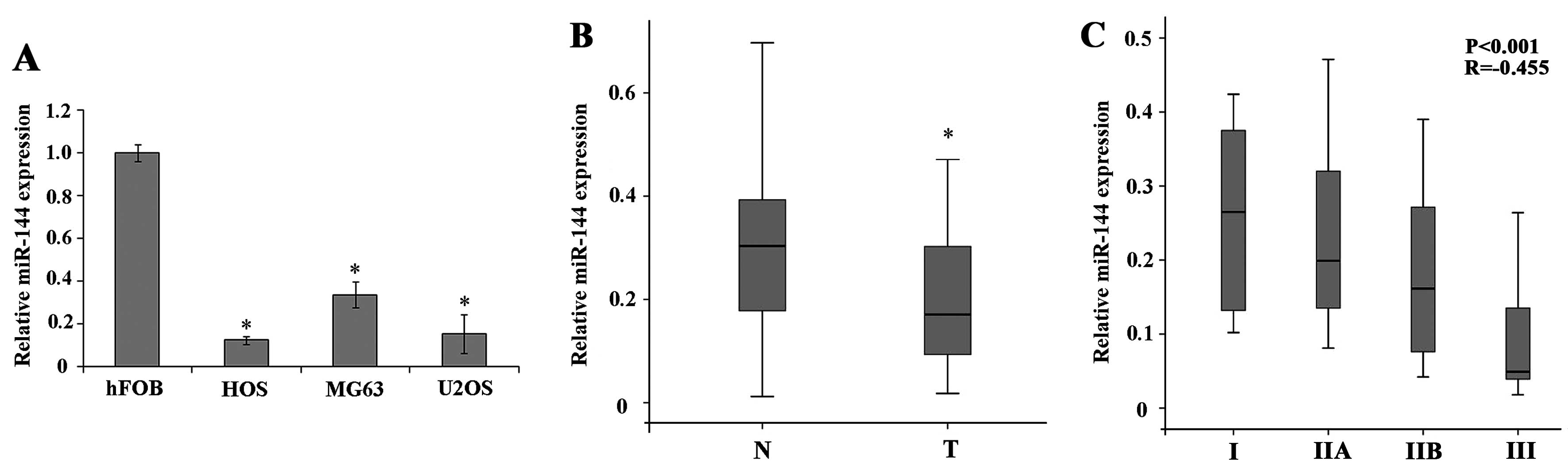
To determine the role of miR-144 in the development
of human osteosarcoma, we analyzed miR-144 expression in the human
osteosarcoma cell lines, MG63, U2OS and HOS, by RT-qPCR and
compared it to that in the normal human osteoblastic cell line,
hFOB1.19. The results revealed that miR-144 was significantly
downregulated in the osteosarcoma cell lines compared to the normal
osteoblastic cells (Fig. 1A;
P<0.05). We then analyzed miR-144 expression in the paired human
osteosarcoma tumor tissue and adjacent normal tissue samples; the
results revealed a significant downregulation of miR-144 expression
in tumor tissues compared to adjacent normal tissues (Fig. 1B; P<0.05). To determine whether
the downregulation of miR-144 is correlated with the progression
and development of osteosarcoma, we used Spearman’s rank
correlation, which revealed a significant negative correlation
between miR-144 expression in tumor tissue and clinical stage
(Fig. 1C). These results suggest
that the downregulation of miR-144 expression plays a role in the
progression of osteosarcoma.
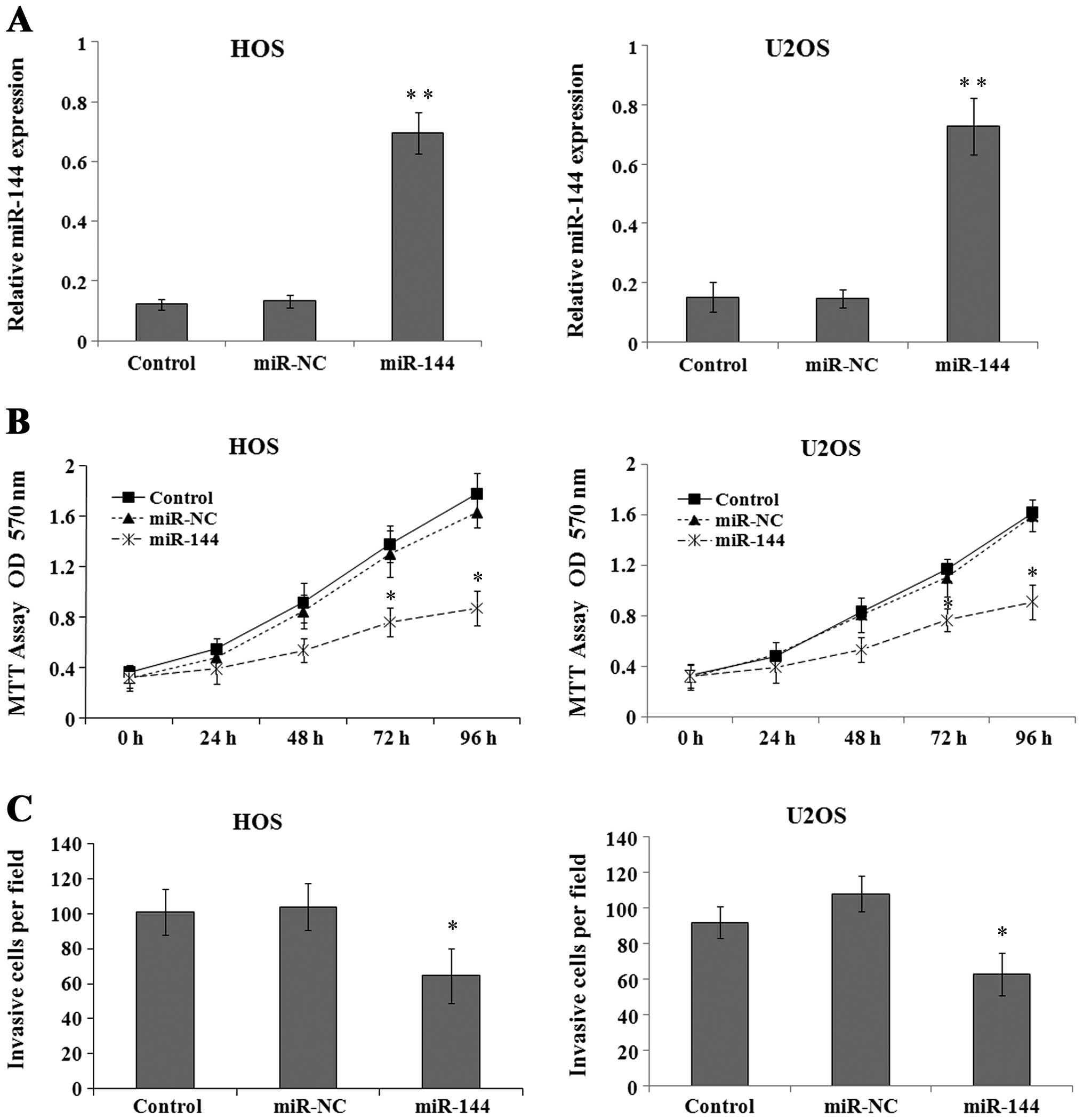
miR-144 inhibits osteosarcoma cell growth
and invasion
To further investigate the biological role of
miR-144 in osteosarcoma tumor progression, the HOS and U2OS cell
lines were infected with lentivirus carrying a negative control miR
(miR-NC) or miR-144 mimics and miR-144 expression was analyzed by
RT-qPCR 72 h after infection. The results revealed that miR-144
expression was significantly upregulated in the miR-144-infected
cells compared to the cells infected with miR-NC (Fig. 2A) (P<0.01). To determine the
effects of miR-144 on osteosarcoma cell growth, the viability of
the HOS and U2OS cells treated as described above was assessed by
MTT assay at the indicated time points. The results revealed that
transfection with miR-144 mimics significantly decreased the growth
of osteosarcoma cells compared to the control- or
miR-NC-transfected cells (Fig.
2B; P<0.05 at 72 and 96 h). Furthermore, the assessment of
cell invasion by Transwell assay revealed a significant impairment
in the invasive ability of the cells expressing miR-144 mimics
compared to that of the control- or miR-NC-transfected cells
(Fig. 2C; P<0.05).
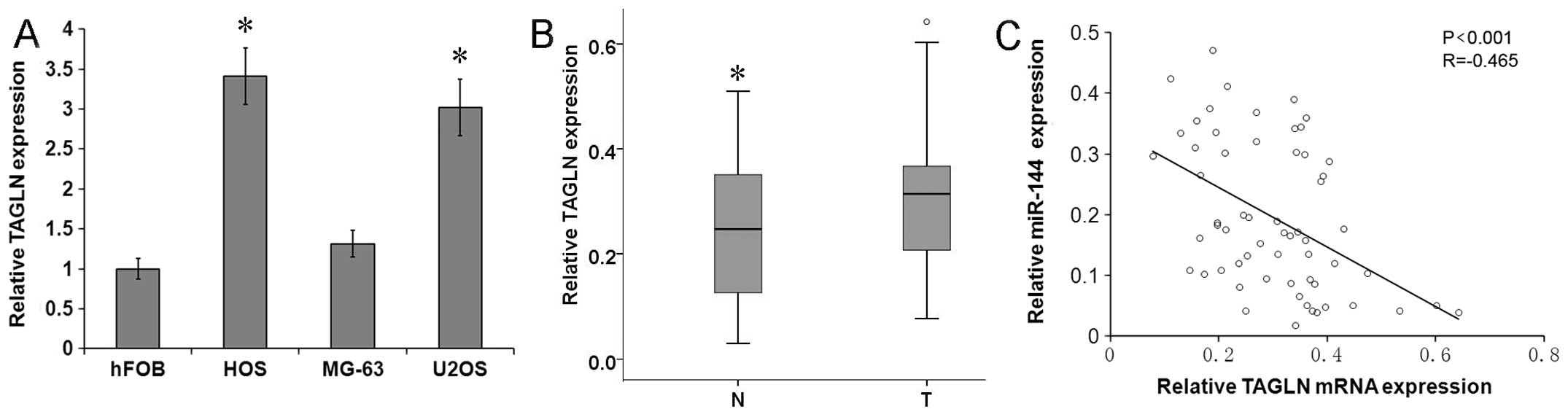
TAGLN expression is upregulated in
osteosarcoma and correlates with miR-144 expression
To examine the correlation between miR-144 and TAGLN
in osteosarcoma, the expression of TAGLN was analyzed by RT-qPCR in
the osteosarcoma cell lines and primary tumor tissue samples. The
results revealed that the TAGLN mRNA level was significantly higher
in the osteosarcoma cell lines, U2OS and HOS, than in the control
human osteoblast cell line, hFOB1.19 (Fig. 3A; P<0.05), and it was
significantly higher in the human primary osteosarcoma tumor tissue
samples than in the adjacent normal tissue samples (Fig. 3B; P<0.05). To identify a
potential correlation between the expression of miR-144 and TAGLN
in osteosarcoma tissue, we used Spearman’s correlation, which
revealed a significant inverse correlation between miR-144 and
TAGLN mRNA expression (Fig. 3C;
R=−0.465, P<0.001), confirming that the downregulation of
miR-144 is associated with the upregulation of TAGLN in
osteosarcoma.
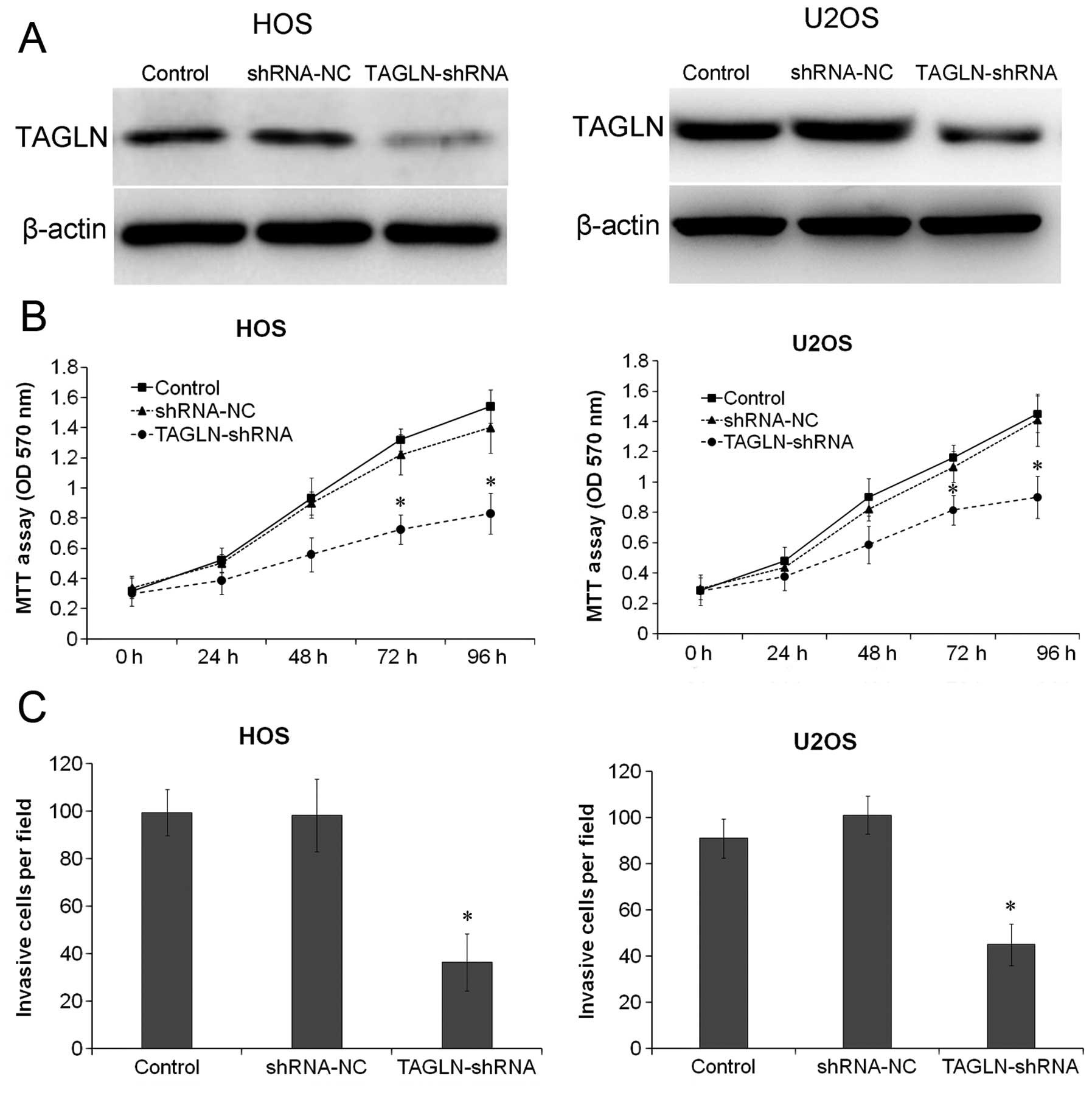
TAGLN silencing inhibits osteosarcoma
cell growth and invasion
To further determine whether TAGLN is the primary
regulator of cell invasion and migration in osteosarcoma, TAGLN was
stably silenced in the HOS and U2OS cells. The cells were
transfected with TAGLN-shRNA and stable transfectants were obtained
after 48 h. The expression of TAGLN was determined by western blot
analysis, which revealed that the protein levels of TAGLN were
significantly decreased in the HOS and U2OS cells (both P<0.05).
HOS and U2OS cells stably depleted of TAGLN were cultured and cell
viability and invasion were assessed by MTT and Transwell assays.
As shown in Fig. 4B, the
depletion of TAGLN significantly (P<0.05) reduced the viability
of the HOS and U2OS cells compared with the respective negative
control shRNA-transfected cells. The results of Transwell assay
revealed that the silencing of TAGLN significantly inhibited the
invasive ability of the HOS and U2OS cells compared to that of the
control- or negative control shRNA-transfected cells (Fig. 4C; P<0.05).
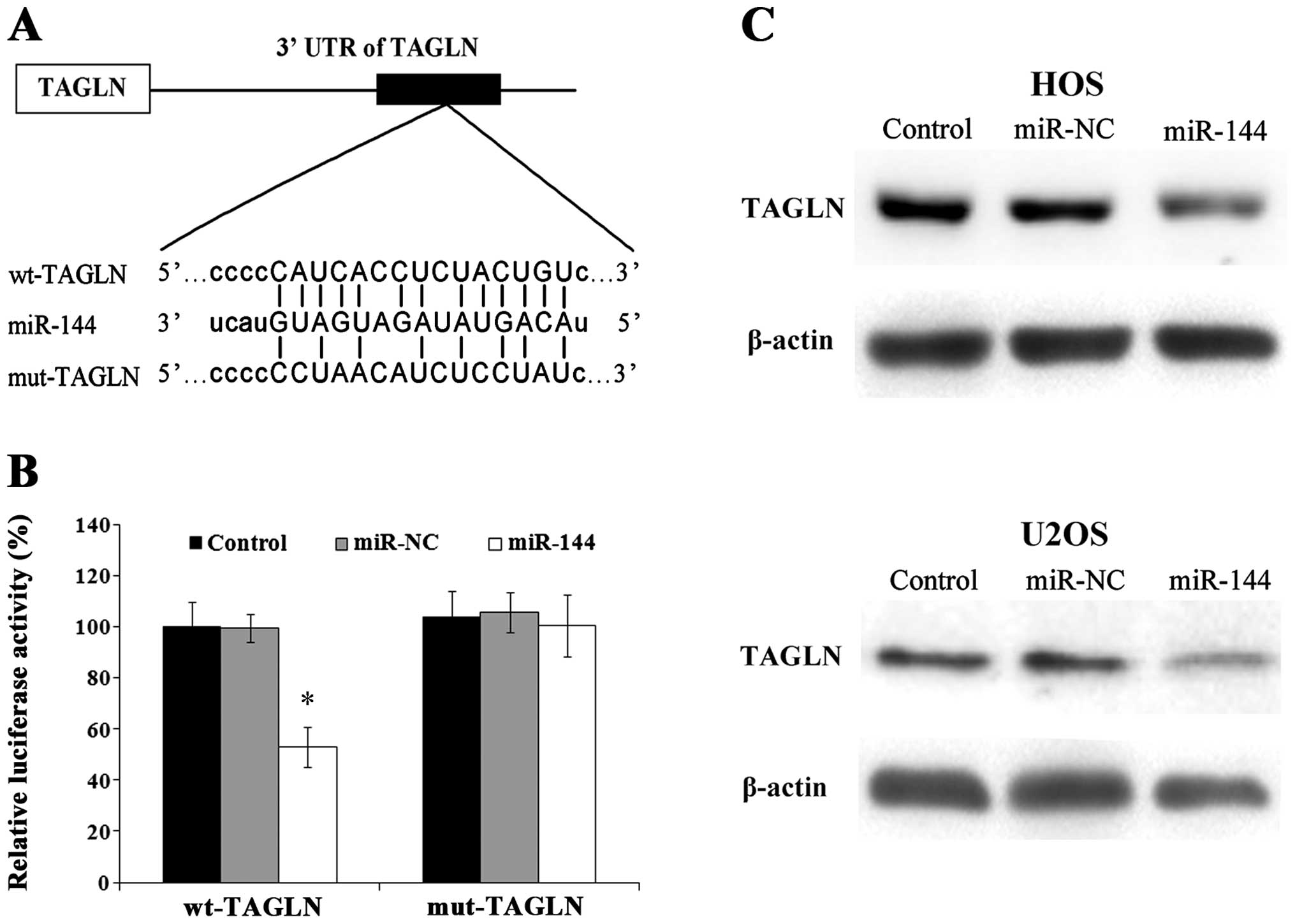
miR-144 directly targets TAGLN
Potential miR-144 binding sites in the 3′ UTR of
TAGLN were predicted using micro-RNA.org (www.microRNA.org). A sequential replacement of a 5
base pair region was performed to produce a mutant vector (Fig. 5A). To further investigate whether
the predicted binding site of miR-144 in the 3′ UTR of TAGLN is
responsible for the interaction, the 3′ UTR of TAGLN was cloned
downstream to a luciferase reporter gene (wt-TAGLN), and a mutant
version (mut-TAGLN) was generated by binding site mutagenesis. The
wt-TAGLN vector or mut-TAGLN with control, negative control or
miR-144 mimics was cloned into the HOS cells and the luciferase
activity was determined. The results revealed that the luciferase
activity of the miR-144-transfected cells was significantly reduced
compared with that of the negative control-transfected cells
(Fig. 5B). To further confirm
that TAGLN is a direct target of miR-144, the protein levels of
TAGLN were assessed by western blot anlaysis in the cells
transfected with miR-144 mimics, which indicated that the exogenous
expression of miR-144 significantly downregulated TAGLN expression
in the HOS and U2OS cells (Fig.
5C).
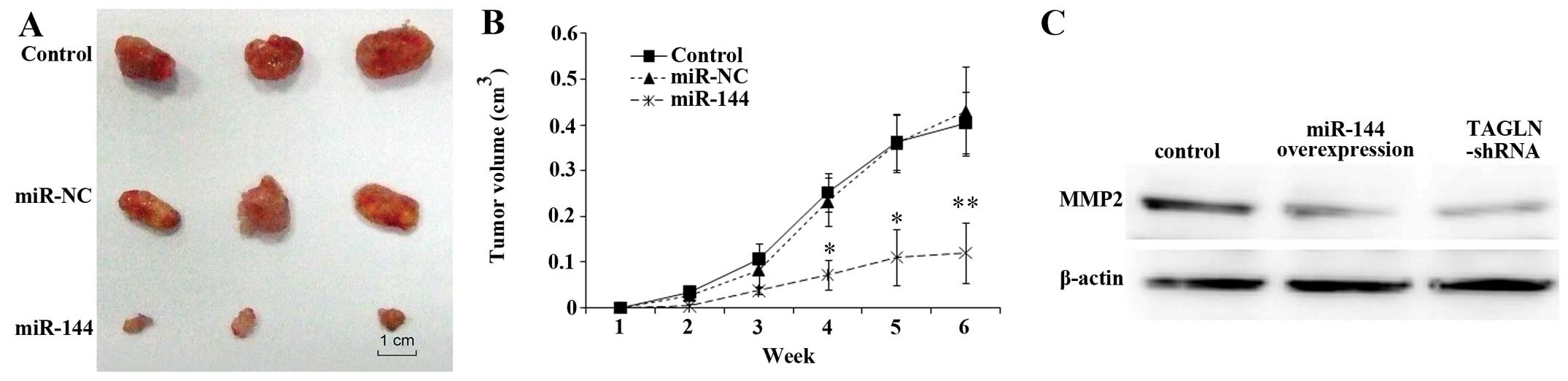
miR-144 overexpression inhibits
tumorigenicity
The osteosarcoma cell line, HOS, was stably
transfected with miR-144 mimics and negative control (NC) RNA, as
previously described (18) and
implanted subcutaneously into nude mice to examine tumorigenicity
in vivo. The effects were most prominent in the first week
after inoculation, in which a significant suppression of
tumorigenicity by miR-144 was observed as determined by the
significantly smaller tumors formed in the mice (Fig. 6A). Fig. 6B shows the reduction in tumor
volume in mice carrying miR-144-overexpressing tumors compared to
the control mice or mice transplanted with negative
control-transfected cells. To confirm the effects of miR-144 and
its target, TAGLN, on invasion in vivo, the expression of
proteins involved in cell adhesion and migration was determined.
The results revealed that the expression of MMP2 was downregulated
by TAGLN-siRNA or by miR-144 overexpression (Fig. 6C). Taken together, these results
indicate that miR-144 functions as a tumor suppressor in
osteosarcoma through the modulation of its target gene, TAGLN.
Discussion
Osteosarcoma is the most common human primary
malignant bone tumor and is associated with an aggressive clinical
course. In recent years, the investigation of the signaling
pathways that regulate osteosarcoma carcinogenesis, invasion and
metastasis has gained increasing attention (19,20), in particular the identification of
deregulated miRNAs and their role in the development of
osteosarcoma (12–14). Recent accumulating evidence
suggests that the aberrant expression of miRNAs in cancer is not a
random event, but rather plays an important role in tumorigenic
processes (6,10). Studies on tumor invasion and
metastasis have revealed the critical role of miRNAs (7,21–24) in these processes, and have
provided potential therapeutic targets for anti-metastatic
therapies.
The TAGLN gene codes for transgelin, an actin stress
fiber binding protein that plays a role in cell growth,
differentiation, migration invasion and matrix remodeling by
stabilizing the cytoskeleton through actin binding (25–27). Previous studies have demonstrated
that the expression of TAGLN is significantly reduced in tissues of
the urinary bladder, renal cell carcinoma and colorectal carcinoma
compared to matched normal tissues (28–30). In colorectal carcinoma, the
overexpression of TAGLN has been shown to decrease proliferation
and invasion (29), supporting a
role for transgelin as a tumor suppressor. However, the increased
expression of transgelin has been reported in colorectal
adenocarcinoma, prostate carcinoma, hepatocellular carcinoma,
gastric, pancreatic and colon cancer (26,31–35). Elevated levels of transgelin have
been shown to significantly increase the invasiveness of tumor
cells (26), whereas reduced
transgelin expression markedly interferes with invasiveness
(36). In gastric carcinoma, the
upregulation of TAGLN expression promotes tumor metastasis, and the
effects of increased TAGLN expression on enhancing tumor cell
invasion and migration are mediated by the upregulation of the
expression of MMP2 (37).
Previous studies have shown that MMP2 regulates cell migration and
invasion in cancer and its activity is, therefore, linked to the
process of metastasis (38–40).
In the present study, we hypothesized that miR-144
may be involved in the metastatic process in osteosarcoma. We first
identified TAGLN as a direct target of miR-144 and confirmed their
interaction. We then demonstrated that miR-144 inhibited
osteosarcoma cell invasion by targeting TAGLN. Our RT-qPCR results
revealed that miR-144 was downregulated in osteosarcoma cell lines,
which was consistent with the results obtained in 60 osteosarcoma
tissue samples. Conversely, we showed that TAGLN was upregulated in
osteosarcoma cell lines and tissue samples. The exogenous
expression of miR-144 or the knockdown of TAGLN expression in the
HOS and U2OS cells resulted in the inhibition of cell growth and
invasion as determined by MTT and Transwell assays. Furthermore,
miR-144 levels negatively correlated with the TAGLN mRNA levels in
osteosarcoma tissue samples, confirming the importance of the
molecular association between miR-144 and TAGLN. The results of a
luciferase reporter assay confirmed that miR-144 directly targets
the TAGLN gene by binding to a specific complementary site within
its 3′ UTR, resulting in the suppression of TAGLN expression by
miR-144. Furthermore, the expression of MMP2 was downregulated by
TAGLN-siRNA or by miR-144 overexpression. Despite our findings
showing an association between miR-144 and TAGLN and their effects
on the expression of invasion-related molecules, further
investigation is required to elucidate the specific mechanisms
underlying their effects on the metastatic potential of
osteosarcoma cells. Nevertheless, our findings provide clear
evidence that miR-144 plays a tumor suppressive role, inhibiting
cellular proliferation and invasion, at least in part through the
downregulation of TAGLN expression.
In the present study, we identified TAGLN as a
direct target of miR-144 in osteosarcoma, suggesting that miR-144
exerts its anti-metastatic effects by inhibiting TAGLN expression.
Previous studies have indicated that human zinc finger protein,
X-linked (ZFX), enhancer of zeste homolog 2 (EZH2) and phosphatase
and tensin homolog (PTEN) are molecular targets of miR-144 and they
all participate in cancer development (41–44). Our data suggest that the
anti-metastatic effects of miR-144 in osteosarcoma cells are
mediated mainly through the inhibition of its target, TAGLN.
However, a single miRNA can target multiple mRNAs to modulate gene
expression (45). Hence, other
miR-144 targets may exist that have not yet been identified, and
their effects on osteosarcoma carcinogenesis and progression remain
to be elucidated. Future studies are required to identify the
targetome and roles of miR-144 in cancer development.
In conclusion, to the best of our knowledge, we
describe for the first time a miR-144/TAGLN link and provide a
potential mechanism for TAGLN deregulation and its contribution to
osteosarcoma cell proliferation and invasion. Further studies are
required focusing on elucidating the precise regulatory mechanisms
of miR-144 and its contribution to the proliferation and invasion
of osteosarcoma cells.
Acknowledgements
The present study was supported by grants from the
Shanghai Municipal Health Bureau youth Foundation (2012-138,
2012-167), the Nature Science Foundation (81202160), the Shanghai
Municipal Health Bureau Key Project (2011-19) and the Shanghai
Young Physician Training Program (2012-30, 2012-31).
References
|
1
|
Bielack SS, Kempf-Bielack B, Delling G, et
al: Prognostic factors in high-grade osteosarcoma of the
extremities or trunk: an analysis of 1,702 patients treated on
neoadjuvant cooperative osteosarcoma study group protocols. J Clin
Oncol. 20:776–790. 2002. View Article : Google Scholar
|
|
2
|
Marina N, Gebhardt M, Teot L and Gorlick
R: Biology and therapeutic advances for pediatric osteosarcoma.
Oncologist. 9:422–441. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bushati N and Cohen SM: microRNA
functions. Annu Rev Cell Dev Biol. 23:175–205. 2007. View Article : Google Scholar
|
|
5
|
Griffiths-Jones S: miRBase: the microRNA
sequence database. Methods Mol Biol. 342:129–138. 2006.PubMed/NCBI
|
|
6
|
Hwang HW and Mendell JT: MicroRNAs in cell
proliferation, cell death, and tumorigenesis. Br J Cancer.
94:776–780. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yan K, Gao J, Yang T, et al: MicroRNA-34a
inhibits the proliferation and metastasis of osteosarcoma cells
both in vitro and in vivo. PloS One. 7:e337782012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kumar MS, Lu J, Mercer KL, Golub TR and
Jacks T: Impaired microRNA processing enhances cellular
transformation and tumorigenesis. Nat Genet. 39:673–677. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang B, Pan X, Cobb GP and Anderson TA:
microRNAs as oncogenes and tumor suppressors. Dev Biol. 302:1–12.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kobayashi E, Hornicek FJ and Duan Z:
MicroRNA involvement in osteosarcoma. Sarcoma. 2012:359732012.
View Article : Google Scholar
|
|
12
|
Zhang H, Cai X, Wang Y, Tang H, Tong D and
Ji F: microRNA-143, down-regulated in osteosarcoma, promotes
apoptosis and suppresses tumorigenicity by targeting Bcl-2. Oncol
Rep. 24:1363–1369. 2010.PubMed/NCBI
|
|
13
|
Ji F, Zhang H, Wang Y, et al:
MicroRNA-133a, downregulated in osteosarcoma, suppresses
proliferation and promotes apoptosis by targeting Bcl-xL and Mcl-1.
Bone. 56:220–226. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jin Y, Peng D, Shen Y, et al:
MicroRNA-376c inhibits cell proliferation and invasion in
osteosarcoma by targeting to transforming growth factor-alpha. DNA
Cell Biol. 32:302–309. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Namlos HM, Meza-Zepeda LA, Baroy T, et al:
Modulation of the osteosarcoma expression phenotype by microRNAs.
PloS One. 7:e480862012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sureban SM, May R, Mondalek FG, et al:
Nanoparticle-based delivery of siDCAMKL-1 increases microRNA-144
and inhibits colorectal cancer tumor growth via a Notch-1 dependent
mechanism. J Nanobiotechnology. 9:402011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT Method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Diaz-Prado S, Cicione C, Muinos-Lopez E,
et al: Characterization of microRNA expression profiles in normal
and osteoarthritic human chondrocytes. BMC Musculoskelet Disord.
13:1442012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guo YS, Zhao R, Ma J, et al: βig-h3
promotes human osteosarcoma cells metastasis by interacting with
integrin α2β1 and activating PI3K signaling pathway. PloS One.
9:e902202014.
|
|
20
|
Lin CH, Guo Y, Ghaffar S, et al: Dkk-3, a
secreted wnt antagonist, suppresses tumorigenic potential and
pulmonary metastasis in osteosarcoma. Sarcoma.
2013:1475412013.PubMed/NCBI
|
|
21
|
Duan Z, Choy E, Harmon D, et al:
MicroRNA-199a-3p is downregulated in human osteosarcoma and
regulates cell proliferation and migration. Mol Cancer Ther.
10:1337–1345. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fan L, Wu Q, Xing X, Wei Y and Shao Z:
MicroRNA-145 targets vascular endothelial growth factor and
inhibits invasion and metastasis of osteosarcoma cells. Acta
Biochim Biophys Sin. 44:407–414. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bao YP, Yi Y, Peng LL, et al: Roles of
microRNA-206 in osteosarcoma pathogenesis and progression. Asian
Pac J Cancer Prev. 14:3751–3755. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jin J, Cai L, Liu ZM and Zhou XS:
miRNA-218 inhibits osteosarcoma cell migration and invasion by
down-regulating of TIAM1, MMP2 and MMP9. Asian Pac J Cancer Prev.
14:3681–3684. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang Z, Chang YJ, Miyamoto H, et al:
Transgelin functions as a suppressor via inhibition of
ARA54-enhanced androgen receptor transactivation and prostate
cancer cell growth. Mol Endocrinol. 21:343–358. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee EK, Han GY, Park HW, Song YJ and Kim
CW: Transgelin promotes migration and invasion of cancer stem
cells. J Proteome Res. 9:5108–5117. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Thompson O, Moghraby JS, Ayscough KR and
Winder SJ: Depletion of the actin bundling protein SM22/transgelin
increases actin dynamics and enhances the tumourigenic phenotypes
of cells. BMC Cell Biol. 13:12012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Assinder SJ, Stanton JA and Prasad PD:
Transgelin: an actin-binding protein and tumour suppressor. Int J
Biochem Cell Biol. 41:482–486. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li Q, Shi R, Wang Y and Niu X: TAGLN
suppresses proliferation and invasion, and induces apoptosis of
colorectal carcinoma cells. Tumour Biol. 34:505–513. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chunhua L, Donglan L, Xiuqiong F, et al:
Apigenin up-regulates transgelin and inhibits invasion and
migration of colorectal cancer through decreased phosphorylation of
AKT. J Nutr Biochem. 24:1766–1775. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin Y, Buckhaults PJ, Lee JR, et al:
Association of the actin-binding protein transgelin with lymph node
metastasis in human colorectal cancer. Neoplasia. 11:864–873.
2009.PubMed/NCBI
|
|
32
|
Shi YY, Wang HC, Yin YH, et al:
Identification and analysis of tumour-associated antigens in
hepatocellular carcinoma. Br J Cancer. 92:929–934. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li N, Zhang J, Liang Y, et al: A
controversial tumor marker: is SM22 a proper biomarker for gastric
cancer cells? J Proteome Res. 6:3304–3312. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Huang Q, Huang Q, Chen W, et al:
Identification of transgelin as a potential novel biomarker for
gastric adenocarcinoma based on proteomics technology. J Cancer Res
Clin Oncol. 134:1219–1227. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mikuriya K, Kuramitsu Y, Ryozawa S, et al:
Expression of glycolytic enzymes is increased in pancreatic
cancerous tissues as evidenced by proteomic profiling by
two-dimensional electrophoresis and liquid chromatography-mass
spectrometry/mass spectrometry. Int J Oncol. 30:849–855. 2007.
|
|
36
|
Yu H, Konigshoff M, Jayachandran A, et al:
Transgelin is a direct target of TGF-beta/Smad3-dependent
epithelial cell migration in lung fibrosis. FASEB J. 22:1778–1789.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yu B, Chen X, Li J, et al: Stromal
fibroblasts in the microenvironment of gastric carcinomas promote
tumor metastasis via upregulating TAGLN expression. BMC Cell Biol.
14:172013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang H, Zhu Y, Zhao M, et al: miRNA-29c
suppresses lung cancer cell adhesion to extracellular matrix and
metastasis by targeting integrin β1 and matrix metalloproteinase2
(MMP2). PloS One. 8:e701922013.PubMed/NCBI
|
|
39
|
Wang XX, Cheng Q, Zhang SN, et al:
PAK5-Egr1-MMP2 signaling controls the migration and invasion in
breast cancer cell. Tumour Biol. 34:2721–2729. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yu G, Li H, Wang X, et al: MicroRNA-19a
targets tissue factor to inhibit colon cancer cells migration and
invasion. Mol Cell Biochem. 380:239–247. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Akiyoshi S, Fukagawa T, Ueo H, et al:
Clinical significance of miR-144-ZFX axis in disseminated tumour
cells in bone marrow in gastric cancer cases. Br J Cancer.
107:1345–1353. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zha W, Cao L, Shen Y and Huang M: Roles of
Mir-144-ZFX pathway in growth regulation of non-small-cell lung
cancer. PloS One. 8:e741752013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Guo Y, Ying L, Tian Y, et al: miR-144
downregulation increases bladder cancer cell proliferation by
targeting EZH2 and regulating Wnt signaling. FEBS J. 280:4531–4538.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang LY, Ho-Fun Lee V, Wong AM, et al:
MicroRNA-144 promotes cell proliferation, migration and invasion in
nasopharyngeal carcinoma through repression of PTEN.
Carcinogenesis. 34:454–463. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Selbach M, Schwanhausser B, Thierfelder N,
Fang Z, Khanin R and Rajewsky N: Widespread changes in protein
synthesis induced by microRNAs. Nature. 455:58–63. 2008. View Article : Google Scholar : PubMed/NCBI
|