Introduction
Osteoarthritis (OA), one of the most frequent
degenerative joint diseases, is characterized by the progressive
degeneration of articular cartilage and leads to limitation of
joint movement, joint deformity, tenderness, inflammation and
severe pain. Cartilage aging and chondrocyte senescence are caused
by the apoptosis of chondrocytes that have been hypothesized to be
a crucial event in the development of OA (1). Chondrocytes, the only cell type
resident in articular cartilage, have a limited ability for
proliferation and self-repair. Therefore, reducing chondrocyte
apoptosis may be a potential way for OA treatment.
Apoptosis is activated by two major pathways; the
extrinsic (death signals are mediated through cell surface
receptors) and intrinsic pathways (death signals are integrated at
the mitochondrial level, which is also referred to as the
mitochondrion-dependent apoptotic signaling pathway). The pathways
eventually lead to the activation of nucleases and caspases,
resulting in cell death (2,3).
The mitochondrial-dependent signaling pathway includes loss of
mitochondrial membrane potential (ΔΨm), regulation of the B-cell
lymphoma 2 (Bcl-2) family of proteins that are the main regulators
of this deadly switch, and activation of caspases, such as
caspase-9 and caspase-3 (4,5).
Mitochondria are associated with the apoptosis of chondrocytes in
OA (6). Therefore, decreasing
apoptosis through regulating the effect of the Bcl-2 family
proteins and caspases on the mitochondrial-dependent signaling
pathway has been the main focus in relieving the progressive
degeneration of articular cartilage.
Currently, the treatments for OA are short-term or
ineffective, and these often have undesirable side-effects that
make treatment unsustainable (7,8).
Natural products, such as traditional Chinese medicine (TCM), have
certain evidence for improving the efficacy for OA through
decreasing joint pain and dysfunction, and preventing and delaying
the cartilage degeneration (9,10).
According to the TCM theory, OA belonging to the category of
BI-syndrome is mainly considered to include liver and kidney
deficiency, retention of cold-damp, phlegm and blood stagnation in
the knees. Duhuo Jisheng decoction (DHJSD), a herbal formula from
TCM, has been proved effective in OA treatment by relieving pain,
reducing joint stiffness and improving mobility and quality of life
(11). A previous study has
proven that DHJSD has potential cooperation and polypharmacology
against OA (12). However, the
mechanisms of DHJSD on the apoptosis of chondrocyte remain to be
elucidated. To extend the clinical treatment of OA with a novel
approach for TCM therapy and to aid in establishing a scientific
foundation for further research, the present study was based on the
SNP-induced chondrocyte apoptosis model to determine whether DHJSD
inhibits the apoptosis of chondrocytes by the
mitochondrial-dependent signaling pathway, thus delaying
degeneration of articular cartilage.
Materials and methods
DHJSD aqueous extract preparation
DHJSD consists of 15 plant species as follows: 9 g
of Radix Angelicae pubescentis, 6 g each of Ramulus Loranthi, Radix
Gentianae Macrophyllae, Radix Saposhnikoviae, Herba Asari, Rhizoma
Chuanxiong, Radix Angelicae Sinensis, Radix Rehmanniae, Radix
Paeoniae Alba, Cortex Cinnamomi, Poria Cocos, Ulmoidis Cortex
Eucommiae, Radix Achyranthis Bidentatae, Panax ginseng and Radix
Glycyrrhizae. The components were mixed and extracted with standard
methods according to the Chinese Pharmacopoeia (13). These were soaked in distilled
water, boiled for 30 min twice and the solution was filtered and
concentrated. The filtrate of DHJSD was dissolved in Dulbecco’s
modified Eagle’s medium containing 10% fetal bovine serum at a
concentration of 10 mg/ml, and subsequently filtered through a
filter and stored at 4°C.
Isolation, identification and treatment
of chondrocytes
Chondrocytes were isolated from knee cartilage of
4-week-old male Sprague Dawley rats (Super-BK Laboratory Animal,
Co. Shanghai, China), cultured and identified as described
previously (14,15). The present study was reviewed and
approved by the Ethics Committee of Science and Technology of
China, and all the animals complied with the guidance suggestions
for the care and use of laboratory animals at Fujian University of
TCM (Fuzhou, China). Chondrocytes were treated with or without 1 mM
SNP (Sigma, Upper Saddle River, NJ, USA), as described previously
(14), and various concentrations
of DHJSD. The images of chondrocytic morphology were captured by
phase-contrast (Olympus, Tokyo, Japan) and scanning electron
microscopes (SEM; Philips XL30; Hitachi, Tokyo, Japan). Apoptosis
was detected using 4′,6-diamidino-2-phenylindole (DAPI) staining
followed by a fluorescent microscope (Olympus), Annexin V/propidium
iodide (PI) staining and JC-staining followed by
fluorescence-activated cell sorting (FACS) using the FACS caliber
(Becton-Dickinson, CA, USA). The mRNA and protein expressions of
Bcl-2, Bax, caspase-9 and caspase-3 by RT-PCR and western blot
analysis, respectively.
MTT assay
Chondrocytes were seeded in a 96-well plates (100
μl/well) at a density of 5×104 cells/ml and cultured for
24 h. They were subsequently treated with or without 1 mM SNP and
various concentrations of DHJSD (200, 300, 400, 500 and 600 μg/ml)
for 12, 24, 36, 48 or 72 h, respectively. After treatment, the
medium was changed by 100 μl 1 mg/ml MTT (Sigma) at 37°C for 4 h,
and replaced with 150 μl dimethyl sulfoxide and agitated for 10
min. The cells were analyzed on an ELISA reader (Model EXL 800;
BioTek Instruments, Inc., Winooski, VT, USA) using a 490-nm wave
length.
SEM observation
After treatment, the chondrocytes underwent
post-fixation with 1% osmium in 0.1 M Na-cacodylate and dehydrated
in ethanol solutions and lyophilized. They were subsequently coated
with gold in a sputtering device. Images were captured under a
Philips XL30 SEM.
Assessment of chondrocyte apoptosis by
DAPI staining
The adherent cells were washed three times with
ice-cold phosphate-buffered saline (PBS), fixed with 4% neutral
formaldehyde for 15 min and washed three times with ice-cold PBS.
The cells were subsequently incubated in 5 μg/ml DAPI for 5 min,
washed three times and observed under a fluorescent microscope.
Detection of apoptosis by flow cytometry
analysis with Annexin V/PI staining and JC-1 staining
The apoptosis rate of the chondrocytes was measured
using an Annexin V/PI kit (KeyGEN BioTECH, Nanjing, China) by FACS.
To evaluate for the loss of ΔΨm, the collected cells were incubated
with the JC-1 kit (KeyGEN BioTECH) and the processed cells were
analyzed by FACS. All the staining was performed according to the
manufacturer’s instructions (14).
RNA extraction and reverse
transcription-polymerase chain reaction (RT-PCR) analysis
Total RNA was extracted from the treated cells
according to the standard instructions using the TRIzol reagent
(Invitrogen, Grand Island, NY, USA). RNA (1 μg) was reverse
transcribed into cDNA according to the instructions provided by the
manufacturer. The cDNA was detected for the mRNA expression of
Bax, Bcl-2, caspase-3, caspase-9 and
β-actin by RT-PCR. Primer sequences were as follows:
Bax forward, 5′-GGC GAT GAA CTG GAC AAC-3′; and reverse,
5′-TCC CGA AGT AGG AAA GGA g-3′; Bcl-2 forward, 5′-CCC TGG
CAT CTT CTC CTT-3′ and reverse, 5′-GGT ACA TCT CCC TGT TGA CG-3′;
caspase-3 forward, 5′-GGA CCT GTG GAC CTG AAA-3′; and
reverse, 5′-GGG TGC GGT AGA GTA AGC-3′; caspase-9 forward,
5′-GCC TCA TCA TCA ACA ACG-3′; and reverse, 5′-CTG GTA TGG GAC AGC
ATC T-3′; and β-actin forward, 5′-GAG AGG GAA ATC GTG CGT
GAC-3′; and reverse, 5′-CAT CTG CTG GAA GGT GGA CA-3′. The DNA
bands were analyzed by gel electrophoresis (1.5% agarose) and were
examined using a Gel Documentation System (Model Gel Doc 2000;
Bio-Rad, Hercules, CA, USA) and β-actin was used as a
reference gene.
Western blot analysis
Total proteins were extracted from the treated cells
using the radioimmunoprecipitation assay lysis buffer (Beyotime
Biotechnology, Shanghai, China) containing 1 mM
phenylmethanesulfony fluoride (Beyotime Biotechnology) and
quantified using the bicinchoninic acid assay. Proteins were
separated by electrophoresis on 12% SDS-polyacrylamide gels and
transferred onto polyvinylidene fluoride membranes. Subsequent to
blocking with 5% skimmed milk, the membranes were incubated with
primary antibodies rabbit anti-Bcl-2, rabbit anti-Bax, rabbit
anti-caspase-3 and rabbit anti-caspase-9 (Cell Signaling
Technology, Inc., Beverly, MA, USA) or rabbit anti-β-actin (Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA) overnight at 4°C,
and incubated with secondary horseradish peroxidase-conjugated
immunoglobulin G antibody (Zhongshan Golden Bridge Biotechnology,
Co., Ltd., Beijing, China). Electrochemiluminescence was used to
make the signal visible, and images were captured using a Bio-Rad
Chemi Doc XRS+ (Bio-Rad), prior to normalizing to that
of β-actin.
Statistical analysis
Data were expressed as the mean ± standard deviation
from at least three independent experiments. Statistical analysis
was performed by one-way analysis of variance or Student’s t-test
using SPSS 19.0 (IBM, Corp., Armonk, NY, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Morphology and identification of
chondrocytes
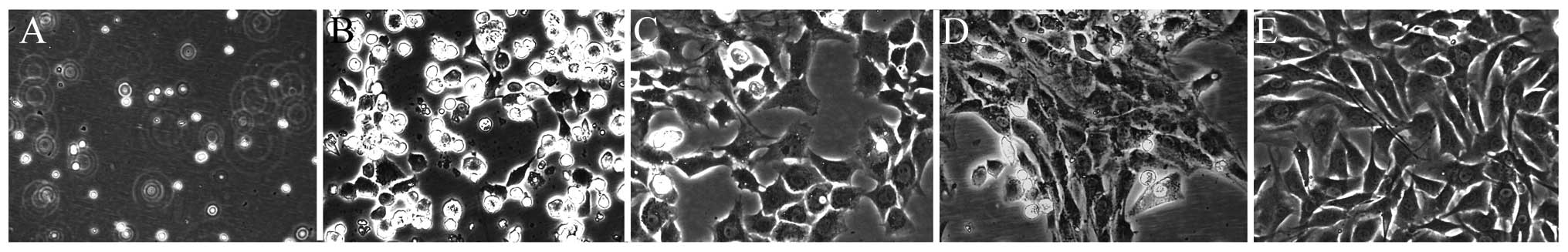
The chondrocyte morphology was as described
previously (Fig. 1) (16,17). Newly-isolated chondrocytes were
known as passage 0 (P0). P2 chondrocytes are rich in extracellular
matrix and optimum with good cell vitality and morphology.
DHJSD enhances SNP-induced chondrocyte
viability
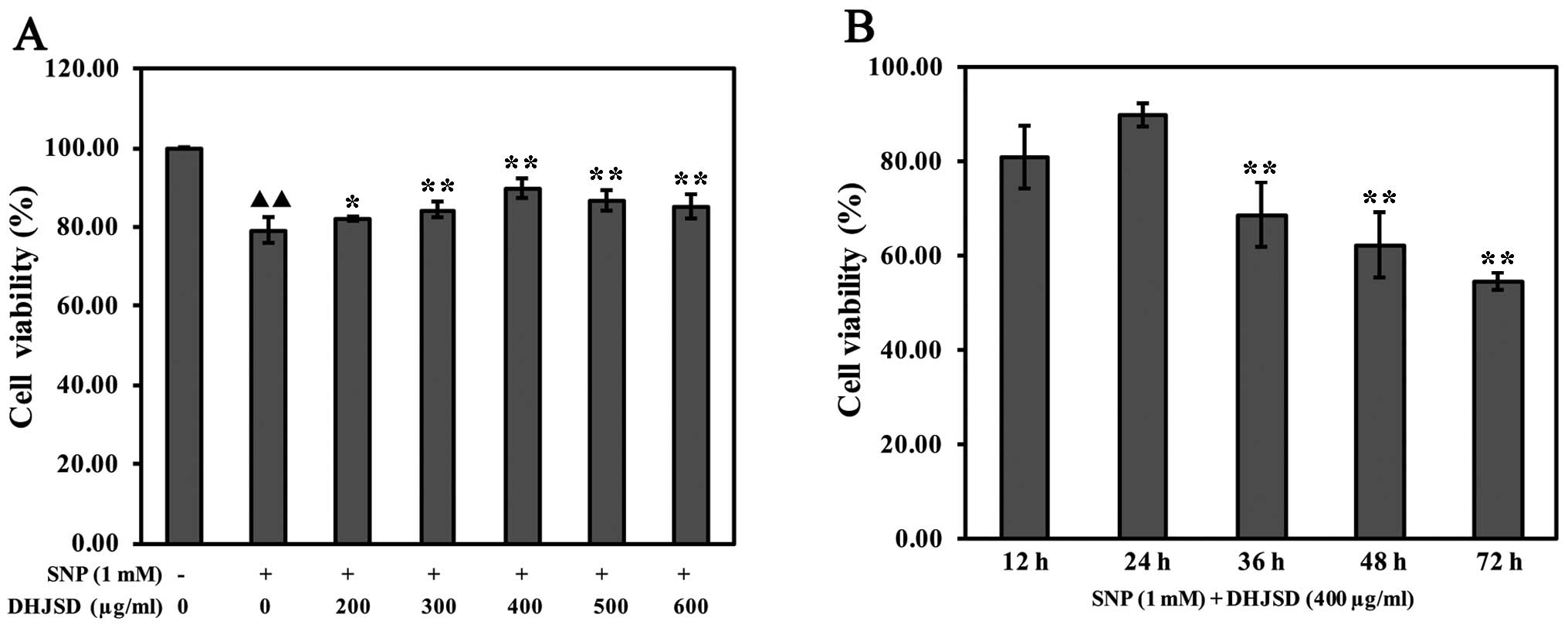
To investigate the effects of DHJSD on cell
viability, SNP-induced chondrocytes were treated with various
concentrations of DHJSD for different times, and subsequently
subjected to the MTT assay. The results showed that the viability
of SNP-induced chondrocytes was significantly lower than that of
the untreated cells (P<0.01) and the viability of SNP-induced
chondrocytes treated by DHJSD was higher compared to the
SNP-induced chondrocytes (P<0.05) (Fig. 2A). The viability of SNP-induced
chondrocytes treated by 400 μg/ml DHJSD for 24 h was higher
compared to 36, 48 and 72 h, respectively (P<0.01) (Fig. 2B), indicating that DHJSD enhanced
SNP-induced chondrocyte viability in a dose- and time-dependent
manner. Therefore, 1 mM SNP and various concentrations (300, 400
and 500 μg/ml) of DHJSD for 24 h were used in the following
experiments.
Effects of DHJSD on the morphology
changes of SNP-induced chondrocytes
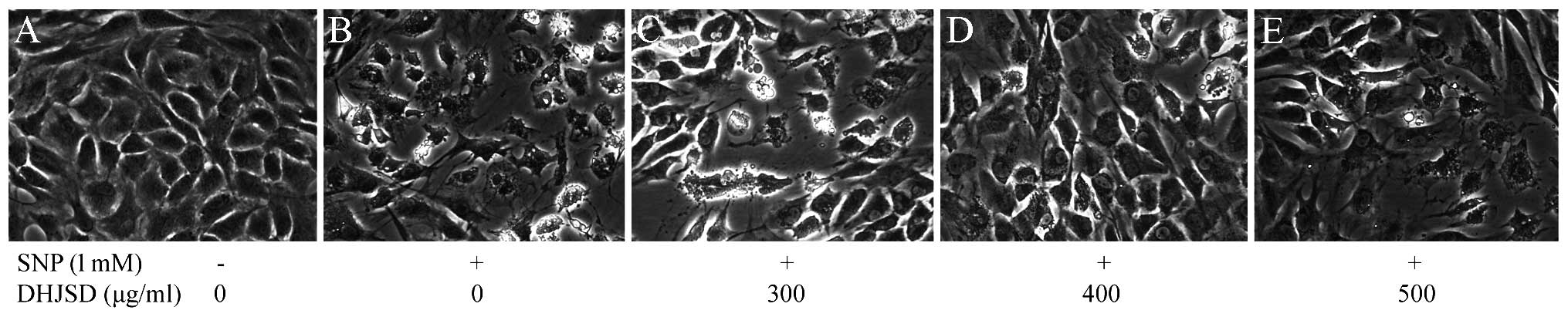
To observe the morphology changes of SNP-induced
chondrocytes treated by DHJSD, the morphology was observed by a
phase-contrast microscope (Figs.
3 and 4). Untreated cells
showed that the morphology of chondrocytes in culture was
indicative of the healthy status of the cells, whereas SNP-induced
chondrocytes exhibited apoptotic characteristic cells that became
rounded, bright and contracted, and detached from each other or
floated in the medium, compared to the SNP-induced chondrocytes
treated by DHJSD.
DHJSD inhibits the apoptosis of
SNP-induced chondrocytes
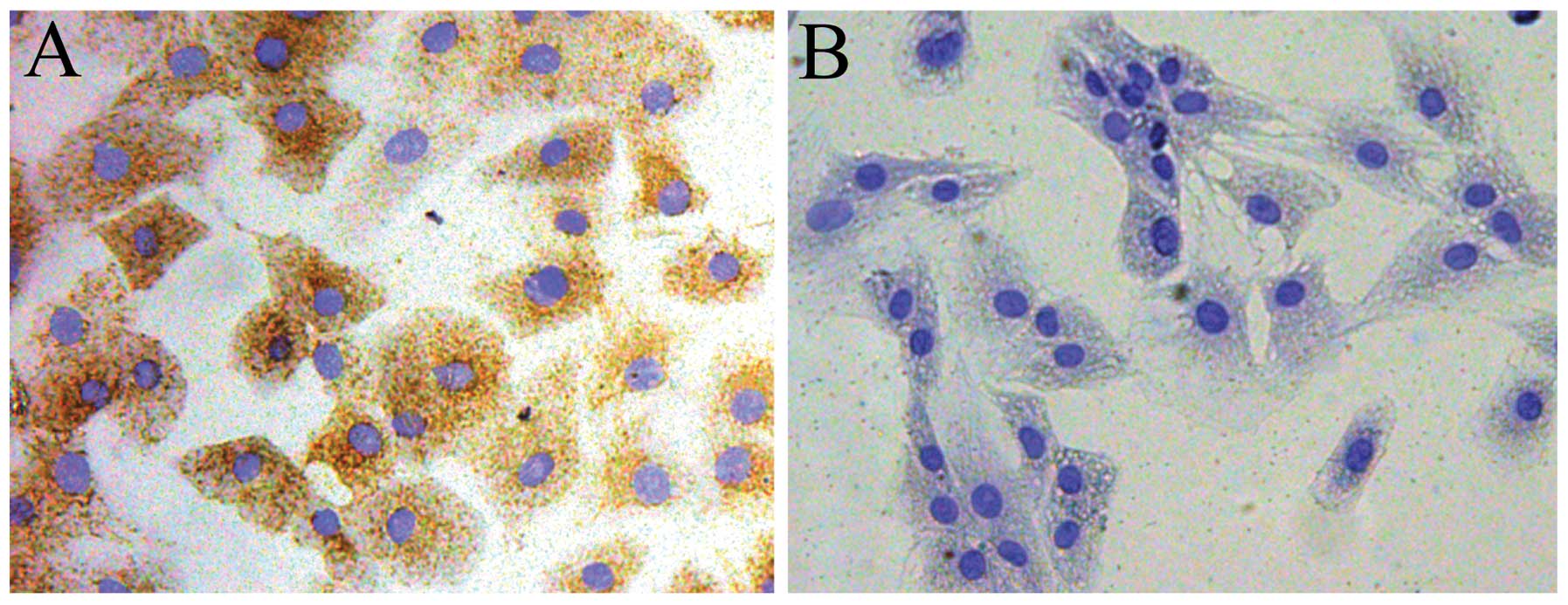
The chondrocytes were identified by collagen type II
immunohistochemistry. The cytoplasm of positive chondrocytes was
stained brown, whereas the negative control failed to stain in the
cytoplasm (Fig. 5A and B). To
examine whether DHJSD enhanced SNP-induced chondrocyte viability by
suppressing apoptosis, the apoptotic cells were determined by DAPI
staining. Apoptotic cells exhibited typical changes, including
reduction of cellular volume, bright blue staining and condensed or
fragmented nuclei. This phenomenon was more clear in SNP-induced
chondrocytes than that of the SNP-induced chondrocytes treated by
DHJSD (Fig. 5C). To further
investigate the effect of DHJSD on the apoptosis of SNP-induced
chondrocyte, chondrocyte apoptosis was measured by Annexin V/PI
staining. Apoptosis is shown in Fig.
5D; upper right quadrant, late apoptotic cells; upper left
quadrant, dead cells; lower left quadrant, normal cells; and lower
right quadrant, early apoptotic cells. The percentage of apoptotic
cells (including the early and late apoptotic cells, and dead
cells) in SNP-induced chondrocytes treated by DHJSD were
significantly lower than that of the SNP-induced chondrocytes
(P<0.01) (Fig. 5F), which
implied that DHJSD inhibited SNP-induced chondrocyte apoptosis.
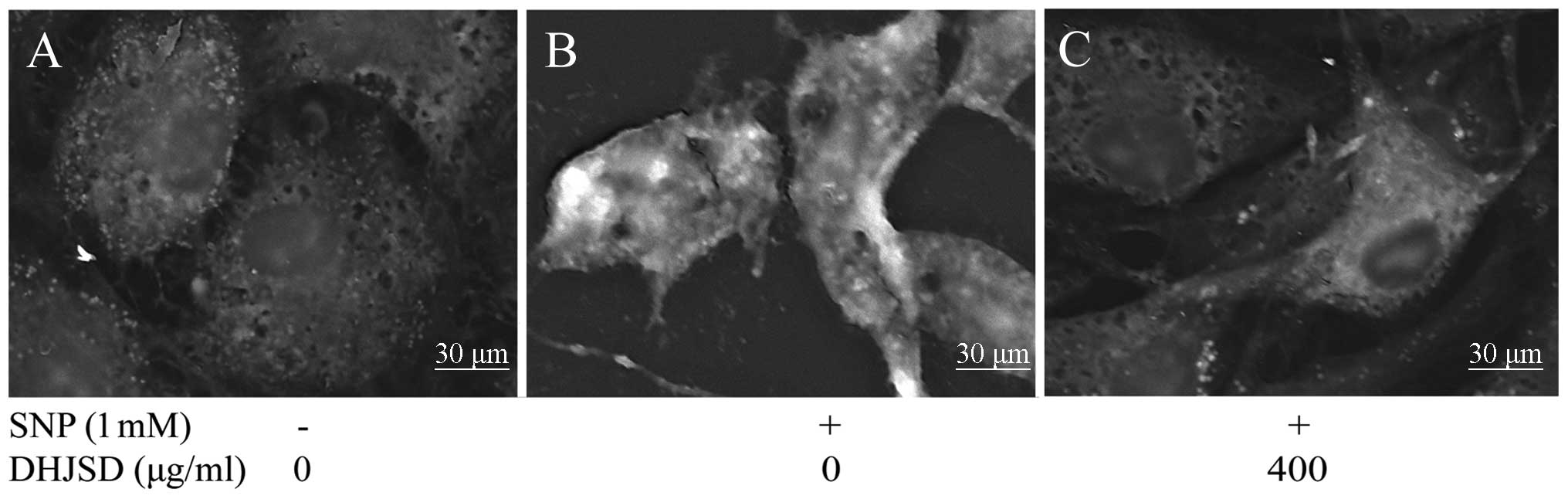
DHJSD decreases the mitochondrial
membrane potential (ΔΨm) of SNP-induced chondrocytes
JC-1 converts to the monomeric form within the
cytoplasm, which is caused by the loss of mitochondrial membrane
potential. To investigate the effect of DHJSD on the loss of ΔΨm, a
typical early event of apoptosis, JC-1 staining analysis was used
to measure the changes of ΔΨm. As shown in Fig. 5E, the cells with a loss of ΔΨm
were revealed by the decrease of red fluorescence (R2). The
percentage of live cells (R2) in SNP-induced chondrocytes treated
by DHJSD had a greater number of cells compared to the SNP-induced
chondrocytes (P<0.01) (Fig.
5G), suggesting that DHJSD decreased the loss of ΔΨm in
SNP-induced chondrocytes.
DHJSD increases Bcl-2 and decreases Bax,
caspase-9 and caspase-3 expression
The mitochondrial-dependent signaling pathway is
controlled by the Bcl-2 family proteins, such as anti-apoptotic
member Bcl-2 and pro-apoptotic member Bax. The anti-apoptotic
protein Bcl-2 suppresses cell apoptosis, and the expression of Bax
results in the release of numerous apoptogenic proteins from the
mitochondria triggering the activation of caspase-9 and caspase-3,
and eventually inducing apoptosis. To further study the mechanism
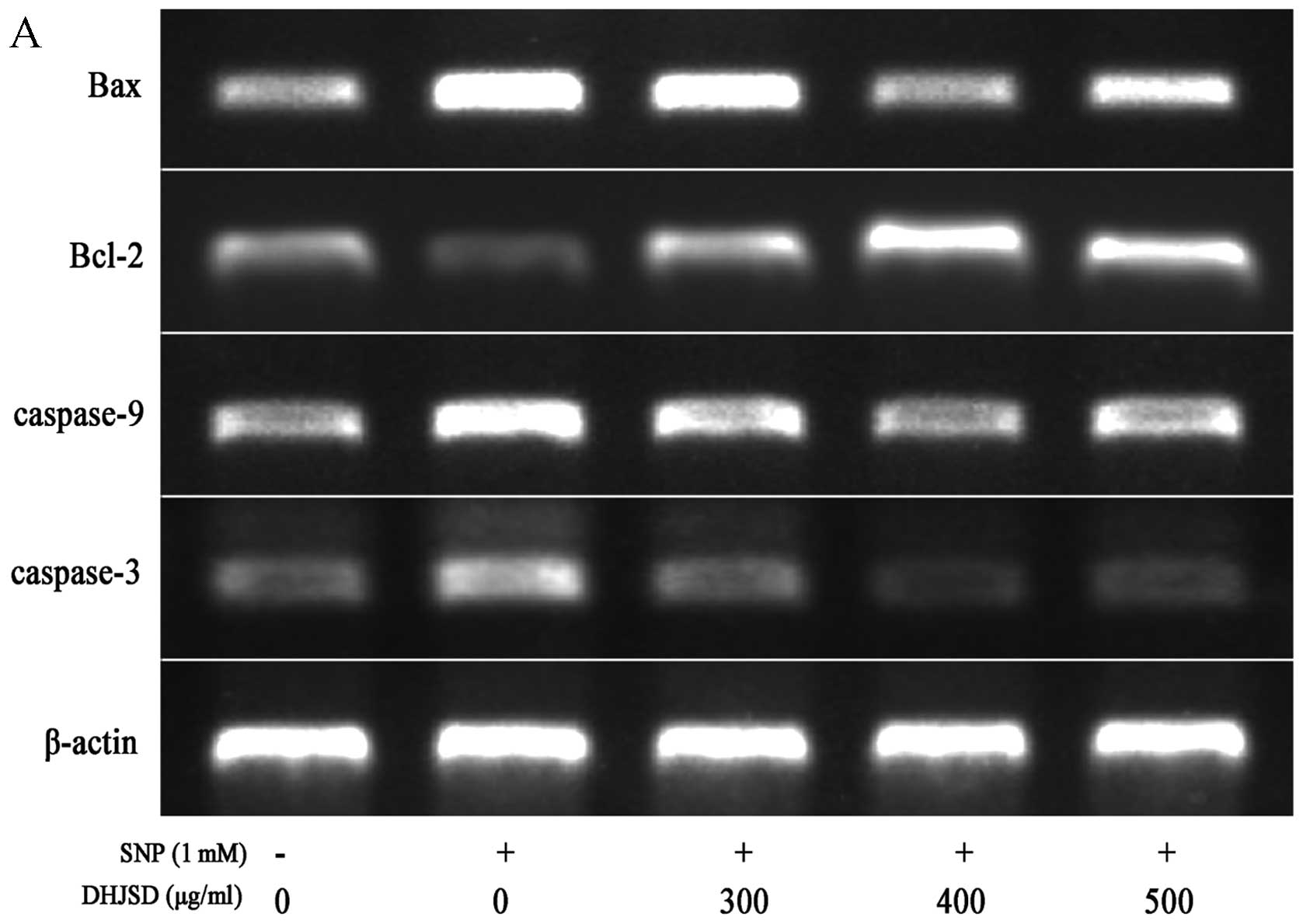
of DHJSD on SNP-induced chondrocyte apoptosis, the expressions of
Bax, Bcl-2, caspase-9 and caspase-3 were detected by RT-PCR and
western blot analysis, respectively. The RT-PCR assay showed that
in the SNP-induced chondrocytes treated by DHJSD, the Bcl-2
expression was extremely increased, whereas the Bax, caspase-9 and
caspase-3 expressions were decreased, compared to that of the
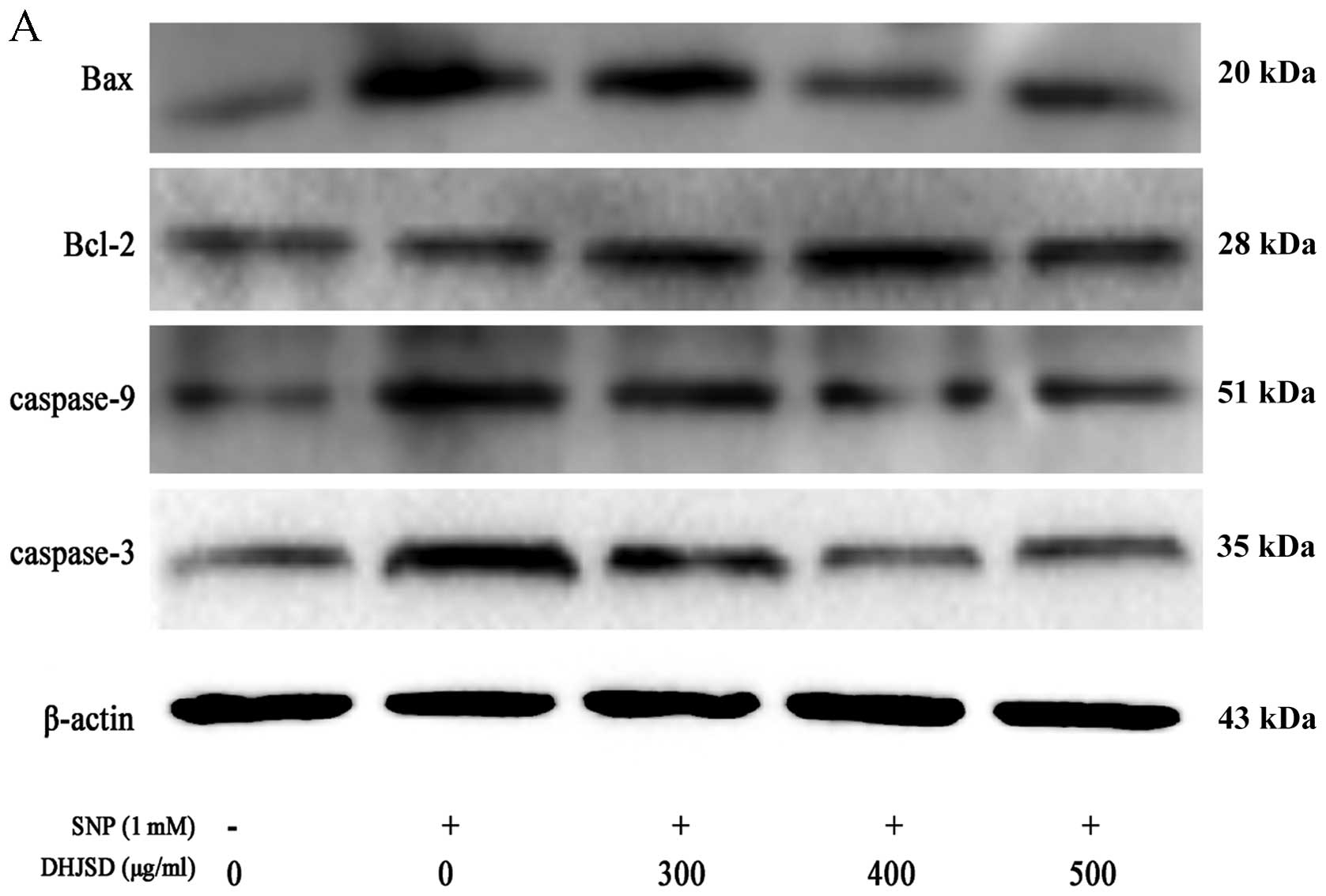
SNP-induced chondrocytes (P<0.01) (Fig. 6). The protein levels of Bax,
Bcl-2, caspase-3 and caspase-9 was similar to their respective mRNA
expression (P<0.01) (Fig. 7).
Taken together, the results indicated that DHJSD inhibits
SNP-induced chondrocyte apoptosis via the mitochondrial-dependent
signaling pathway.
Discussion
Chondrocyte apoptosis leads to cartilage
degeneration (18,19), thus, inhibiting chondrocyte
apoptosis may be an efficient method for the treatment of OA. The
present results showed that DHJSD inhibits SNP-induced chondrocyte
apoptosis by upregulating the protein and mRNA expression of Bcl-2,
whereas downregulating the protein and mRNA expression of Bax,
caspase-9 and caspase-3 indicates that DHJSD may be a potential
agent for treating OA.
OA is a chronic disease and thus far there is no
radical therapy available (20),
and only several drugs, such as non-steroidal anti-inflammatory
drugs and cyclooxygenase-2 inhibitors, are used to treat OA, but
they cause serious side-effects. Therefore, novel therapeutic
methods require development for reducing the disease burden of OA.
Chinese medical herbs, which are safe and cheap, have been used to
alleviate symptoms and delay the pathological progress of OA for
thousands of years. DHJSD has been proved effective in OA treatment
by clinical practice (21).
However, the underlying mechanisms for the management of OA are not
fully understood. In addition, NO has been shown to be a key
inducer of chondrocyte apoptosis, a central pathogenic feature of
OA (22,23). SNP, an NO donor compound, induces
chondrocyte apoptosis via the mitochondrial-dependent signaling
(24). To explore the effects of
DHJSD on SNP-induced chondrocyte, SNP-induced chondrocyte apoptosis
was used as the mitochondrial-dependent apoptotic model.
The MTT assay is based on the reduction of
tetrazolium salt by mitochondrial succinate dehydrogenases in
viable cells yielding purple formazan crystals, whereas dead cells
do not yield crystals. Therefore, the monitoring of alterations in
mitochondrial activity can be detected by the MTT assay. The
concentration of MTT formazan is directly proportional to the
number of viable cells (25). A
screening method was used in the present study to measure
SNP-induced chondrocyte viability by MTT. According to the results
from the MTT assay, DHJSD enhanced SNP-induced chondrocyte
viability in a dose- and time-dependent manner. To study this
further, Annexin V/PI and DAPI staining were used to explore the
effect of DHJSD on apoptosis of SNP-induced chondrocyte. Annexin V
is a 35–36 kDa Ca2+-dependent phospholipid-binding
protein that has a high affinity for membrane phospholipid
phosphatidylserine (PS), which is translocated from the inner to
the outer leaflet of the plasma membrane in apoptotic cells. This
allowed the number of apoptosis-positive cells to be expressed as a
percentage of total cells. Therefore, flow cytometry with Annexin
V/PI staining was used to further confirm the percentage of
apoptosis, which decreased in a dose-dependent manner. The loss of
ΔΨm, a characteristic of apoptosis, is an early event preceding
phosphatidylserine externalization and coincides with caspase
activation. JC-1 is sensitive to ΔΨm and the changes in the ratio
between red and green fluorescence provide information regarding
the mitochondrial membrane potential by forming aggregates that
have a fluorescence emission spectra peak at 590 nm (red) at high
ΔΨm, and becoming a monomer again with a fluorescence emission peak
at 530 nm (green) at low ΔΨm. Therefore, JC-1 staining assay was
used to evaluate mitochondrial membrane potential and data showed
that DHJSD reduced the collapse of ΔΨm.
The mitochondrial-dependent signaling pathway is the
center of apoptosis control, and is relient on mitochondrial outer
membrane permeabilisation (MOMP), which is regulated by the
pro-apoptotic Bcl-2 family proteins, such as Bax and Bcl-2
(26). Bax, a direct
pro-apoptotic effector of MOMP, translocates from the cytosol to
the outer mitochondrial membrane (OMM) and oligomerizes, wherein it
contributes to the formation of pores to release cytochrome
c that stimulates the activation of caspase-9 and caspase-3
(27,28). Caspase activation is usually
regulated by the Bcl-2 family (29). Bcl-2, one of the anti-apoptotic
proteins that are located in the OMM, is a mitochondrial
membrane-associated protein, and exerts its anti-apoptotic effect
by inhibiting Bax expression from mitochondria and reducing the
release of cytochrome c, as well as subsequently inhibiting
the activation of caspase-3 (30,31). Activation of upstream caspases-9
leads to the proteolytic activation of downstream or effector
caspases-3, a marker of apoptosis. Cytosolic caspase-3 is cleaved
and exerts final execution of apoptosis through degeneration of
vital proteins, resulting in cell destruction by apoptosis via the
cleavage of structural and regulatory proteins. Whether DHJSD
inhibits mitochondrial-dependent signaling pathway to regulate
chondrocytes apoptosis was investigated. The mRNA and protein
expression of Bax, Bcl-2, caspase-9 and caspase-3 were detected by
RT-PCR and western blot analysis, respectively. The results showed
that DHJSD upregulates the mRNA and protein expression of Bcl-2,
and downregulates the mRNA and protein expression of Bax, caspase-9
and caspase-3.
In conclusion, DHJSD inhibits SNP-induced
chondrocyte apoptosis by the mitochondrial-dependent signaling
pathway, indicating that DHJSD may be a potential novel therapeutic
agent for the treatment of OA. These results may in part explain
the mechanisms by which DHJSD exerts its beneficial effects in OA,
and have certain limits. Using a mitochondrion-dependent apoptotic
pathway signal inhibitor and exploring in vivo are required
in future studies.
Acknowledgements
The present study was supported by the Key Project
of Fujian Provincial Department of Science and Technology (grant
no. 2012Y0046) and the Young Talent Scientific Research Project of
Fujian Province Universities (grant no. JA12165).
References
|
1
|
Thomas CM, Fuller CJ, Whittles CE and
Sharif M: Chondrocyte death by apoptosis is associated with the
initiation and severity of articular cartilage degradation. Int J
Rheum Dis. 14:191–198. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Usichenko TI, Edinger H, Witstruck T, et
al: Millimetre wave therapy for pain relief after total knee
arthroplasty: a randomised controlled trial. Eur J Pain.
12:617–623. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Adams JM and Cory S: The Bcl-2 apoptotic
switch in cancer development and therapy. Oncogene. 26:1324–1337.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Knudson CM and Brown NM: Mitochondria
potential, bax ‘activation’, and programmed cell death. Methods Mol
Biol. 414:95–108. 2008.
|
|
5
|
Gupta S, Kass GE, Szegezdi E and Joseph B:
The mitochondrial death pathway: a promising therapeutic target in
diseases. J Cell Mol Med. 13:1004–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rego I, Fernández-Moreno M,
Fernández-López C, et al: Role of European mitochondrial DNA
haplogroups in the prevalence of hip osteoarthritis in Galicia,
Northern Spain. Ann Rheum Dis. 69:210–213. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Edmonds S: Therapeutic targets for
osteoarthritis. Maturitas. 63:191–194. 2009. View Article : Google Scholar
|
|
8
|
Altman R and Barkin RL: Topical therapy
for osteoarthritis: clinical and pharmacologic perspectives.
Postgrad Med. 121:139–147. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hua B, Abbas E, Hayes A, Ryan P, Nelson L
and O’Brien K: Reliability of Chinese medicine diagnostic variables
in the examination of patients with osteoarthritis of the knee. J
Altern Complement Med. 18:1028–1037. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang X, Wei S, Liu T, et al:
Effectiveness, medication patterns, and adverse events of
traditional Chinese herbal patches for osteoarthritis: a systematic
review. Evid Based Complement Alternat Med. 2014:1–17. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lai J, Chen H, Chen C, Lin J, Hwang J and
Wang J: Duhuo Jisheng Tang for treating osteoarthritis of the knee:
a prospective clinical observation. Chin Med. 2:1–8. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zheng CS, Xu XJ, Ye HZ, et al:
Computational approaches for exploring the potential synergy and
polypharmacology of Duhuo Jisheng Decoction in the therapy of
osteoarthritis. Exp Ther Med. 7:1163–1168. 2013.PubMed/NCBI
|
|
13
|
Chinese Pharmacopeia Commission.
Pharmacopoeia of the People’s Republic of China. Chinese Medical
Science and Technology Press; Beijing, China: 2010
|
|
14
|
Li X, Du M, Liu X, et al: Millimeter wave
treatment inhibits NO-induced apoptosis of chondrocytes through the
p38MAPK pathway. Int J Mol Med. 25:393–399. 2010.PubMed/NCBI
|
|
15
|
Li X, Du M, Liu X, et al: Millimeter wave
treatment promotes chondrocyte proliferation by upregulating the
expression of cyclin-dependent kinase 2 and cyclin A. Int J Mol
Med. 26:77–84. 2010.PubMed/NCBI
|
|
16
|
Yu F, Li X, Cai L, et al: Achyranthes
bidentata polysaccharides induce chondrocyte proliferation via the
promotion of the G1/S cell cycle transition. Mol Med Rep.
7:935–940. 2013.PubMed/NCBI
|
|
17
|
Li H, Li X, Liu G, et al: Bauhinia
championi (Benth.) Benth polysaccharides upregulate Wnt/β-catenin
signaling in chondrocytes. Int J Mol Med. 32:1329–1336.
2013.PubMed/NCBI
|
|
18
|
Chan BY, Fuller ES, Russell AK, et al:
Increased chondrocyte sclerostin may protect against cartilage
degradation in osteoarthritis. Osteoarthr Cartilage. 19:874–885.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang JG, Xia C, Zheng XP, et al:
17β-Estradiol promotes cell proliferation in rat osteoarthritis
model chondrocytes via PI3K/Akt pathway. Cell Mol Biol Lett.
16:564–575. 2011.
|
|
20
|
Bijlsma JW, Berenbaum F and Lafeber FP:
Osteoarthritis: an update with relevance for clinical practice.
Lancet. 377:2115–2126. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hsieh S, Lai J, Chen P, Chen C, Chen H and
Wang J: Is Duhuo Jisheng Tang containing Xixin safe? A four-week
safety study. Chin Med. 5:1–6. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu GJ, Chen TG, Chang HC, Chiu WT, Chang
CC and Chen RM: Nitric oxide from both exogenous and endogenous
sources activates mitochondria-dependent events and induces insults
to human chondrocytes. J Cell Biochem. 101:1520–1531.
2007.PubMed/NCBI
|
|
23
|
Maneiro E, López-Armada MJ, de Andres MC,
et al: Effect of nitric oxide on mitochondrial respiratory activity
of human articular chondrocytes. Ann Rheum Dis. 64:388–395. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tonomura H, Takahashi KA, Mazda O, et al:
Glutamine protects articular chondrocytes from heat stress and
NO-induced apoptosis with HSP70 expression. Osteoarthr Cartilage.
14:545–553. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Etxeberria A, Mendarte S and Larregla S:
Determination of viability of Phytophthora capsici oospores
with the tetrazolium bromide staining test versus a plasmolysis
method. Rev Iberoam Micol. 28:43–49. 2011.
|
|
26
|
Renault TT, Teijido O, Antonsson B, Dejean
LM and Manon S: Regulation of Bax mitochondrial localization by
Bcl-2 and Bcl-x (L): keep your friends close but your enemies
closer. Int J Biochem Cell Biol. 45:64–67. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Westphal D, Dewson G, Czabotar PE and
Kluck RM: Molecular biology of Bax and Bak activation and action.
Biochim Biophys Acta. 1813:521–531. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Renault TT and Manon S: Bax: Addressed to
kill. Biochimie. 93:1379–1391. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chai WS, Zu XM, Li SH, Fan JX and Chen BY:
Role of Bcl-2 family members in caspase-3/9-dependent apoptosis
during Pseudomonas aeruginosa infection in U937 cells.
Apoptosis. 13:833–843. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lu H, Chie Y, Yang M, et al: Apigenin
induces caspase-dependent apoptosis in human lung cancer A549 cells
through Bax-and Bcl-2-triggered mitochondrial pathway. Int J Oncol.
36:1477–1484. 2010.PubMed/NCBI
|
|
31
|
Autret A and Martin SJ: Emerging role for
members of the Bcl-2 family in mitochondrial morphogenesis. Mol
Cell. 36:355–363. 2009. View Article : Google Scholar : PubMed/NCBI
|