Introduction
Quantitative polymerase chain reaction (qPCR) is the
most fundamental, sensitive and common method used for quantitative
analysis of DNA. However, its accuracy is influenced by a number of
external and internal factors, including the amount of starting
samples, the quality of templates and PCR efficiency (1). At present, using control genes as a
standard normalizer is the most common method to minimize the
effects (2). Control genes are
commonly defined as genes that ubiquitously exist at stable levels
in various biological contexts and are used to confirm the presence
and content of DNA, as well as quantitatively measure the total DNA
in each sample (3,4). However, accumulating evidence
indicates that content levels of widely used control genes vary
significantly in different independent studies (5,6).
Since the presence of cell-free fetal DNA in
maternal plasma and serum was confirmed by the Lo et al
(7) study in 1997, there are
increasing studies focusing on the utilization of plasma DNA for
non-invasive prenatal diagnosis (NIPD). Thus far, plasma DNA
analysis is widely studied in numerous NIPD, including fetal gender
detection, Rhesus blood group, D antigen (RhD) status
determination, monogenic diseases and chromosomal aneuploidies
prenatal diagnosis. To the best of our knowledge, the commonly used
control genes for plasma DNA analysis are frequently chosen
empirically and without any preliminary evaluation of their
suitability. Thus, it is essential to compare and evaluate the
content stability of each control gene prior to use for
normalization in quantitative analysis of plasma DNA.
The focus of the present study is on the content
stability of six commonly used control genes (HBB,
TERT, GAPDH, ALB, ACTB and TRG)
in pregnant and non-pregnant plasma DNA using qPCR. The candidate
control genes were selected from previous studies on pregnant
plasma DNA (8–11). Three common programs, geNorm
(12), NormFinder (13) and BestKeeper (14), were used for data analysis. In
order to confirm the analysis results, each of the candidate
control genes was used as a normalizer to quantitatively measure
the DSCR3 gene. The DSCR3 region only exists in
chromosome 21 and it is supposed to have the same relative quantity
in pregnant and non-pregnant groups of normal females (15,16). The result may reveal the optimal
control gene selections for further quantitative studies on plasma
DNA from pregnant females.
Materials and methods
Plasma sample collection and DNA
extraction
A total of 2 ml peripheral blood donated from 18
pregnant females (gestational age, 12.87±1.25 weeks) and 18
non-pregnant volunteers was collected. A form of consent was
obtained from each volunteer and the experiment was approved by the
Ethical Committee of Second Hospital, Jilin University (Jilin,
China). The blood samples were anti-coagulated by EDTA. The plasma
supernatant was separated from the entire blood by centrifugation
at 2,000 × g for 20 min at room temperature, followed by further
centrifugation at 12,000 × g for 5 min to remove the residual
intact cells. The supernatant was collected carefully. DNA was
extracted from 350 μl plasma from each sample by the QIAamp DNA
mini kit (Qiagen, Hilden, Germany) following the manufacturer’s
protocol. The whole process was performed within 4 h of the
withdrawal time.
qPCR analysis
qPCR was carried out using Roche LightCycler 480
[Roche Diagnostics (Schweiz) AG, Risch, Switzerland]. The primers
of the control (HBB, TERT, GAPDH, ALB,
ACTB and TRG) and two target genes (DSCR3 and
SRY) were synthesized by Sangon Biotech Shanghai Co., Ltd.,
(Shanghai, China) (Table I).
 | Table IPrimer sequences, product sizes and
PCR efficiency. |
Table I
Primer sequences, product sizes and
PCR efficiency.
| Symbol | Gene name | Primers sequences
(5′→3′) | Amplicon size
(bp) | PCR efficiency |
|---|
| HBB | β-globin |
F-GTGCACCTGACTCCTGAGGAGA
R-CCTTGATACCAACCTGCCCAG | 101 | 2.58 |
| TERT | Telomerase |
F-GGTGAACCTCGTAAGTTTATGCAA
R-GGCACACGTGGCTTTTCG | 97 | 2.00 |
| GAPDH |
Glyceraldehyde-3-phosphate
dehydrogenase |
F-GGACTGAGGCTCCCACCTTT
R-GCATGGACTGTGGTCTGCAA | 157 | 1.72 |
| ALB | Albumin |
F-TGAAACATACGTTCCCAAAGAGTTT
R-CTCTCCTTCTCAGAAAGTGTGCATAT | 80 | 1.79 |
| ACTB | β-actin |
F-CCTGTACGCCAACACAGTGC
R-ATACTCCTGCTTGCTGATCC | 211 | 2.08 |
| TRG | T cell receptor
γ |
F-AGGGTTGTGTTGGAATCAGG
R-CGTCGACAACAAGTGTTGTTCCAC | 160 | 1.82 |
| DSCR3 | Down syndrome
critical region-3 |
F-CAGTGCAATGACAGCAGTAT
R-TGGGATCACATCAAGCTAA | 141 | 2.11 |
| SRY | Gender-determining
region Y |
F-AAAGGCAACGTCCAGGATAGAG
R-CCACTGGTATCCCAGCTGCT | 137 | 2.19 |
The reactions were performed in a 20 μl volume
containing 8 ng DNA using the All-in-One qPCR Mix kit (GeneCopodia,
Inc., Rockville, MD, USA) following the protocol. The amplification
was as follows: An initial step of 95°C for 10 min, 50 cycles of
95°C for 15 sec, 58°C for 15 sec and 72°C for 30 sec. Each assay
was performed in triplicate. qPCR results were subjected to 1%
agarose gel electrophoresis. To estimate the efficiencies of
amplification, a standard curve was generated for each primer pair
based on four points of serial 2-fold DNA dilution. The
efficiencies were calculated using the slope of the calibration
curve following the equation: E=2−1/slope.
Data analysis
Microsoft Excel was used to calculate the mean and
standard deviation (SD) values. The content stabilities of the six
candidate-control genes were assessed by three commonly used
programs: geNorm, NormFinder and BestKeeper, as described in their
manuals. geNorm calculates a gene content stability measure (M) and
pairwise variation (V) parameter. Lower M values represent higher
content stability. V is calculated to determine the minimal number
of control genes required. When V<0.15, the number of control
genes is enough for valid normalization. NormFinder computes inter-
and intra-group content stability values by an analysis of
variance-based model. Lower value indicates higher content
stabilities. BestKeeper analyses content stability based on SD and
coefficient of correlation (r) of all the candidate control genes.
The genes with SD >1.00 are suggested to be considered
unreliable as a stable control gene and the remaining genes are
ranked according to their r values, with a higher r value
indicating higher stability. All the analyses were performed
separately for the following three groups: Pregnant, non-pregnant
and total sample (pregnant and non-pregnant) groups.
Control gene validation
DSCR3 was used as the target gene in order to
validate the control genes for normalization of relative quantity
in the pregnant and non-pregnant groups (17). The relative quantity in each
sample was normalized by each of the six control genes and the most
stable combination recommended by geNorm and NormFinder
independently, using the 2−ΔΔCt method (18). SRY is only presented in
pregnant females carrying male fetuses (19), and was used to detect whether or
not the extracted DNA was contaminated with exogenous DNA.
Results
Amplification performance of primers
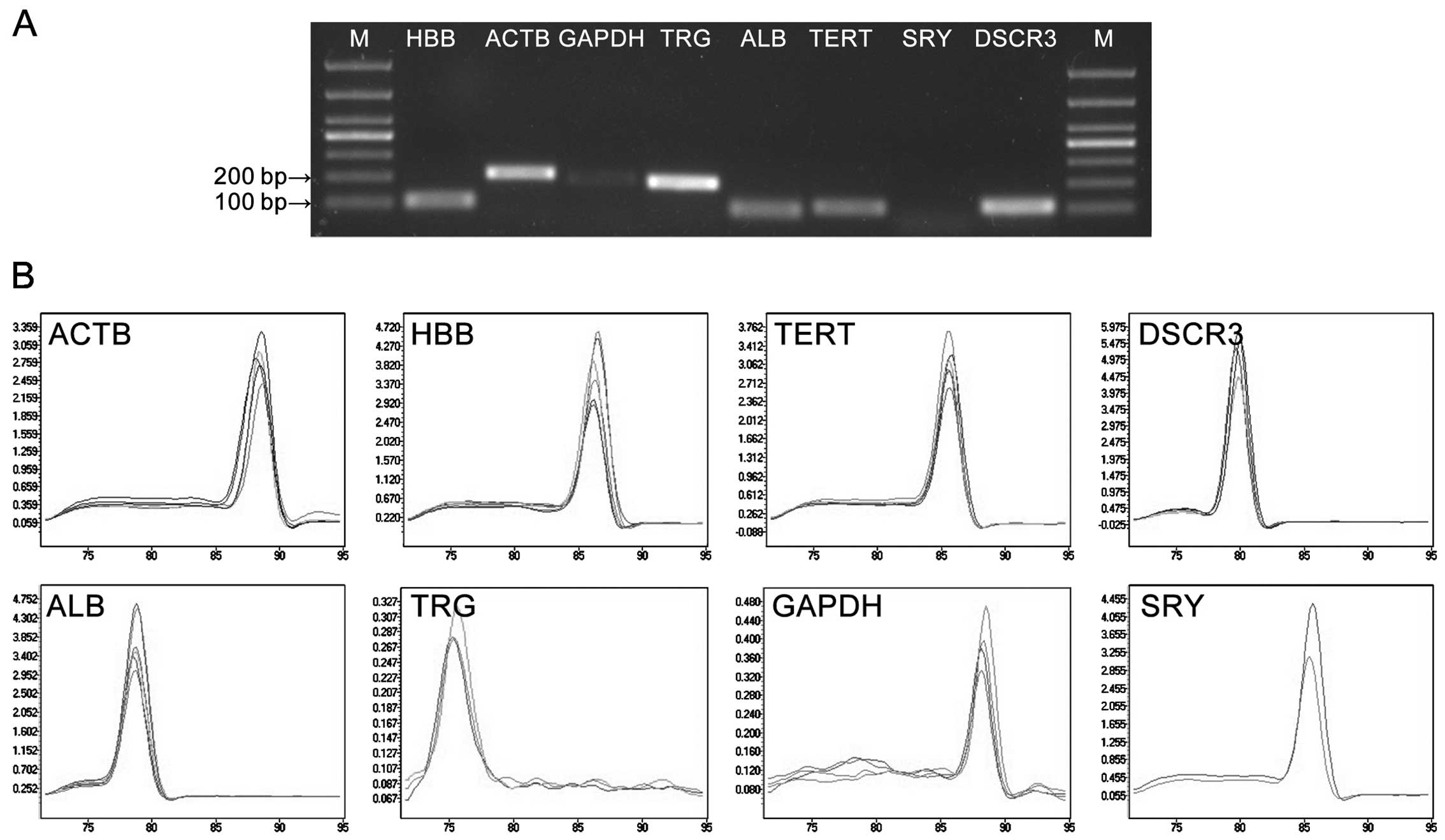
The qPCR amplification product was detected in 1%
agarose gel electrophoresis. The results showed clear bands with
expected size and no primer dimers (Fig. 1A). One single peak was obtained in
each amplification reaction during the analysis of the melting
curves; this confirmed the specific amplification of primers
(Fig. 1B). The efficiencies of
the primers are listed in Table
I. SRY was only amplified in the pregnant group,
indicating that there was no exogenous DNA contamination.
Amplification profile of candidate
control genes
The amplification profile of the candidate control
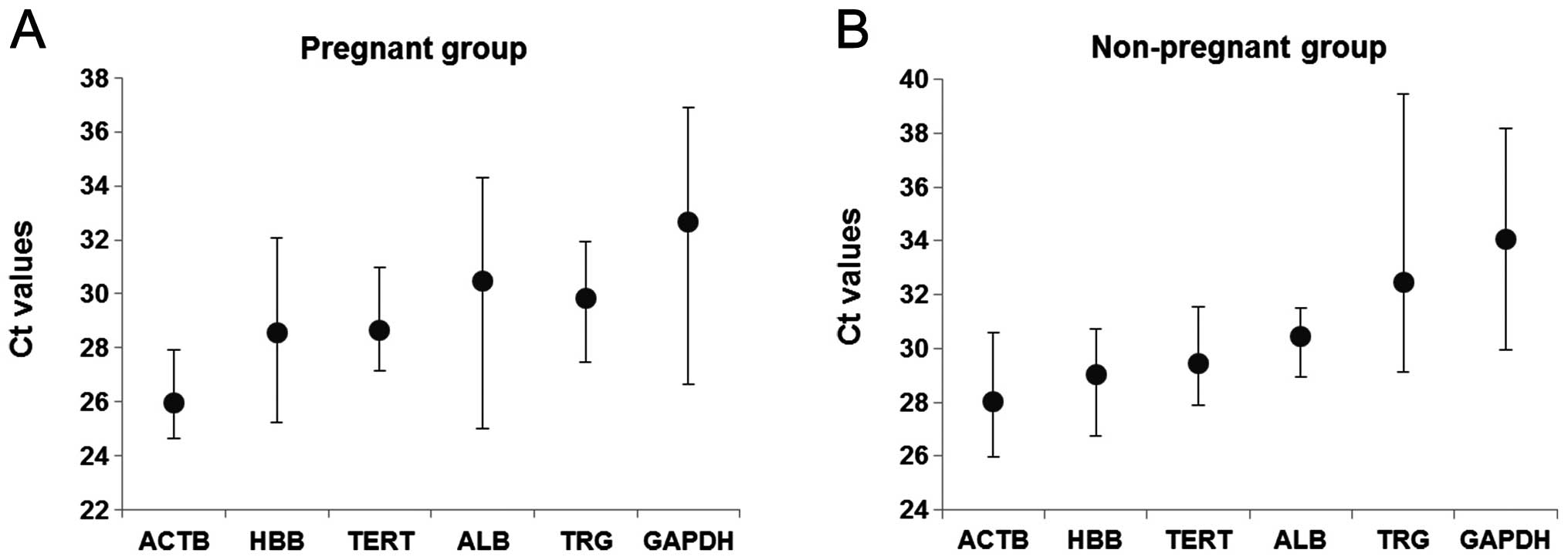
genes was estimated as Ct values. Fig. 2 shows the mean Ct values of each
gene in the pregnant and non-pregnant groups. The Ct values of the
groups varied between 25.99 to 32.66 for the pregnant group
(Fig. 2A) and 28.02 to 34.09 for
the non-pregnant group (Fig. 2B).
ACTB exhibited the lowest Ct value (mean ± SD is 25.99±0.99
and 28.02±1.86) and GAPDH exhibited the highest Ct value
(mean ± SD is 32.66±3.21 and 34.09±2.92) in the two groups
respectively. In the pregnant group, GAPDH is the most
variable with a high SD value (3.21), whereas ACTB had the
lowest SD values (0.99). In the non-pregnant group, TRG was
the most variable with a high SD value (4.13), whereas ALB
had the lowest SD values (1.19). There was no significant
difference of the Ct values between maternal- and fetal-derived DNA
in each gene.
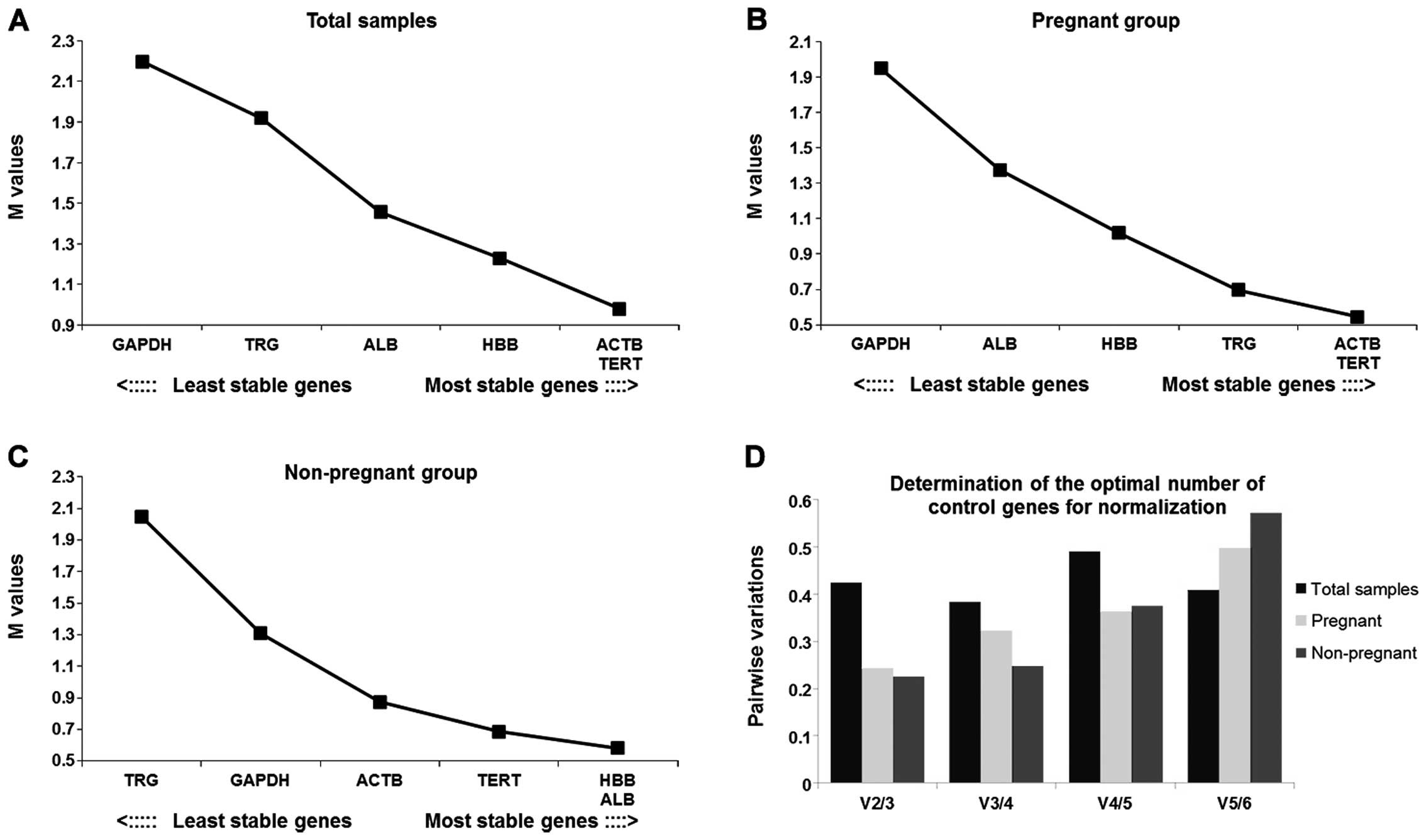
geNorm analysis
The geNorm analysis result revealed that ACTB
and TERT were the most stable genes and GAPDH was the
least stable among the total samples (Fig. 3A). Similar results were obtained
in the pregnant group (Fig. 3B).
In the non-pregnant group, HBB and ALB were the most
stable genes and TRG was the least stable (Fig. 3C). None of the V values were below
the cut-off value (Fig. 3D)
indicating that there was no optimal combination number of control
genes for normalization.
NormFinder analysis
The results of the NormFinder analysis showed that
ACTB and TERT were the top two content stable genes
in the total and pregnant groups, whereas HBB and ALB
were the top two genes in the non-pregnant group (Table II). GAPDH and TRG
were the least stable genes in the pregnant and non-pregnant
groups, respectively, and TRG was also considered as the
least stable in the total group. The best combination of two genes
for total sample analysis was ACTB and TERT, and the
stability value (0.224) was lower than TERT (0.340). This
indicated that the combination of these two genes provide higher
stability than using TERT alone.
 | Table IIRank of six candidate control genes
in order of their quantity stability calculated by NormFinder. |
Table II
Rank of six candidate control genes
in order of their quantity stability calculated by NormFinder.
| Total sample | Pregnant group | Non-pregnant
group |
|---|
|
|
|
|
|---|
| Rank | Gene | Stability
value | Gene | Stability
value | Gene | Stability
value |
|---|
| 1 | TERT | 0.340 | ACTB | 0.115 | HBB | 0.318 |
| 2 | ACTB | 0.418 | TERT | 0.299 | ALB | 0.419 |
| 3 | HBB | 0.462 | TRG | 0.439 | TERT | 0.782 |
| 4 | GAPDH | 0.552 | ALB | 0.928 | ACTB | 0.820 |
| 5 | ALB | 0.577 | HBB | 1.218 | GAPDH | 0.930 |
| 6 | TRG | 0.771 | GAPDH | 2.042 | TRG | 2.360 |
BestKeeper analysis
According to BestKeeper analysis, when considering
the total samples, TERT was found to be the optimal control
gene with SD<1.00 and the highest r value (0.870). In the
pregnant group, ACTB and TERT were acceptable with
SD<1.00, whereas ACTB had a higher r value (0.954) and
was considered to be the most optimal control gene. In the
non-pregnant group, TERT was the only gene with SD<1.00,
but ALB had the highest r value (0.951). Although the SD of
ALB was higher than 1.00 (SD=1.07), it was still considered
to be a reliable control gene, similar to TERT (Table III). GAPDH and TRG
were the least stable genes as shown by the results of geNorm and
NormFinder in the pregnant and non-pregnant groups.
 | Table IIIDescriptive statistics of six
candidate control genes based on their cycle threshold values as
calculated by BestKeeper. |
Table III
Descriptive statistics of six
candidate control genes based on their cycle threshold values as
calculated by BestKeeper.
| Group | CP data | ACTB | HBB | TERT | ALB | TRG | GAPDH |
|---|
| Total samples | geo Mean (CP) | 26.74994 | 28.67918 | 28.94395 | 30.42784 | 30.74505 | 33.09142 |
| Min (CP) | 24.63311 | 25.21569 | 27.16459 | 25.00278 | 27.47232 | 26.64072 |
| Max (CP) | 30.58614 | 32.06806 | 31.57339 | 34.2869 | 39.44698 | 38.16773 |
| SD (± CP) | 1.310503 | 1.699849 | 0.999535 | 1.369684 | 1.955511 | 2.533179 |
| coeff. of corr.
(r) | 0.853 | 0.754 | 0.870 | 0.776 | 0.784 | 0.774 |
| Pregnant | geo Mean (CP) | 25.96939 | 28.4677 | 28.61777 | 30.40847 | 29.79071 | 32.51344 |
| Min (CP) | 24.63311 | 25.21569 | 27.16459 | 25.00278 | 27.47232 | 26.64072 |
| Max (CP) | 27.91232 | 32.06806 | 30.97218 | 34.28690 | 31.92536 | 36.89494 |
| SD (± CP) | 0.76351 | 1.804439 | 0.877834 | 1.575063 | 1.160367 | 2.65516 |
| coeff. of corr.
(r) | 0.954 | 0.711 | 0.887 | 0.888 | 0.852 | 0.644 |
| Non-pregnant | geo Mean (CP) | 27.96496 | 28.99936 | 29.44021 | 30.45691 | 32.23419 | 33.97771 |
| Min (CP) | 25.98068 | 26.73664 | 27.9093 | 28.95515 | 29.13656 | 29.93401 |
| Max (CP) | 30.58614 | 30.70807 | 31.57339 | 31.51242 | 39.44698 | 38.16773 |
| SD (± CP) | 1.545731 | 1.419053 | 0.983466 | 1.066481 | 3.279869 | 2.360286 |
| coeff of corr
(r) | 0.814 | 0.920 | 0.815 | 0.951 | 0.785 | 0.948 |
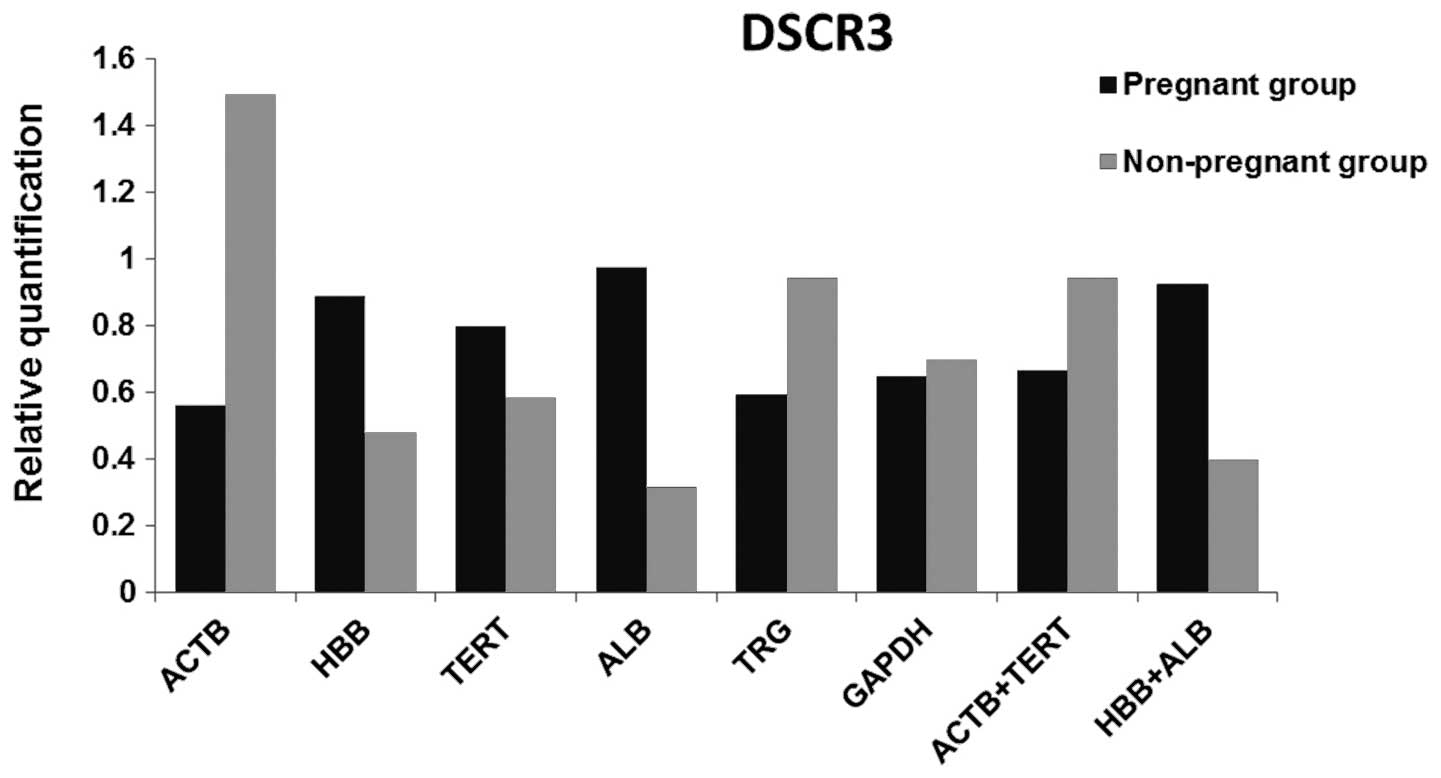
Validation of control genes
In order to verify the results of the control genes
from geNorm, NormFinder and BestKeeper, the relative quantities of
DSCR3 were determined using six candidate control genes and
the combinations recommended by geNorm (ACTB + TERT)
and NormFinder (HBB + ALB) were the normalizers
(Fig. 4). GAPDH had the
minimum difference between the two groups, followed by TERT.
ACTB had the largest difference, although it was ranked as
one of the top two in all three algorithms. TERT combined
with ACTB provided a smaller difference between the two
groups compared to ALB + HBB.
Discussion
The discovery of cell-free fetal DNA in maternal
plasma has become a primary target for NIPD (7). In healthy gravidae, fetal DNA can be
detected in maternal plasma as early as the seventh week after
conception (20), and it
increases with the pregnancy progresses (10), reaches the plateau in the ensuing
three months, is promptly cleared from maternal plasma and
disappears within 2 h of delivery (21). These properties caused plasma DNA
to be the optimal material for NIPD. Thus far, qPCR is the most
fundamental, sensitive and accurate method, widely used in studies
of maternal plasma DNA. Due to its low cost and ease of use, a
number of diseases, including gender determination (22–24), β-thalassemia (25–27), rhesus fetal blood group genotyping
(28–30) and aneuploidies diseases (31), have been successfully diagnosed by
qPCR. Although it is an extremely useful technique, there are
challenges concerned with its use. The most important is
normalization with an accurate, reliable control gene. To avoid the
incorrect analysis results caused by pipetting errors, inhibitory
compounds, quality of starting material or other systematic errors
in qPCR (32), control genes
should be stably contained in pregnant and non-pregnant female
plasma. Ideally, control genes in plasma should not be influenced
or regulated by pregnancy conditions, stress response, stimulation
or any other physiological or pathological states between different
individuals (4,33). However, there is accumulating
evidence indicating that content levels of widely used control
genes varies significantly in different independent studies, for
example, B2M, ACTB and SDHA showed significant
variation in expression levels in human epileptogenic brain tissues
(34), and the single-copy DNA
control gene HBB, which is used for representing the cell
number, has been proved to not be the most reliable control gene
(3). Therefore, it has become
indispensable to normalize the control gene quantity levels and
determine reliable control genes prior to any qPCR relative
quantitative analysis. To the best of our knowledge, this is the
first study to evaluate the content stability of control genes
commonly used in the plasma DNA from pregnant and non-pregnant
females. In the present study, the samples in the second trimester
of the gestational age were selected, as in this stage the content
of plasma DNA is stable. Six commonly used control genes
(HBB, TERT, GAPDH, ALB, ACTB and
TRG) were analyzed by qPCR of the plasma DNA from pregnant
and non-pregnant females. Three common statistical algorithms
(geNorm, NormFinder and BestKeeper) were used for data analysis and
DSCR3 was used to confirm the data analysis results.
On the basis of the results obtained from the three
algorithms, the rank of the candidate genes stability was slightly
different. These variations were possibly caused by different
calculation algorithms (35,36) and indicated different features of
the correlations between these control genes. Among the six
candidate control genes, ACTB and TERT in the total
samples and pregnant group, and HBB and ALB in the
non-pregnant group showed the highest stability. This conclusion is
consistent with the Ct values. ACTB, TERT and
TRG had the lowest SD (0.99, 1.16 and 1.43) of the Ct values
in the pregnant group; ALB, TERT and HBB had
the lowest SD (1.19, 1.25 and 1.64) in the non-pregnant group. By
contrast, GAPDH was unanimously affirmed as the least stable
gene by the three algorithms in the pregnant group, as was
TRG in the non-pregnant group. This corresponded to their
high SD (3.21 and 4.13, respectively) for the Ct values, which
means that they clearly vary.
In order to evaluate the exactitude of the control
genes recommended by the three algorithms, the candidate control
genes were used as a normalizer to detect the relative levels of
the DSCR3 gene. The DSCR3 region only exists in
chromosome 21, which is supposed to have the same relative quantity
in the plasma DNA from pregnant and non-pregnant females. The
content variance between the pregnant and non-pregnant groups was
performed at maximum when using ACTB as the control gene,
but minimum when using GAPDH. There is a slight discrepancy
between the DSCR3 evaluation and algorithm results. When
using TERT as the normalizer, the content variance is within
the tolerable range. When combining more than one control gene as
the normalizer, the best combination chosen was ACTB +
TERT, suggested by geNorm, and HBB + ALB from
NormFinder. The result reveals that ACTB + TERT
obtain an improved result compared to using TERT alone.
The optimal number of control genes for
normalization was indicated by the V value below the cut-off of
0.15, as shown in geNorm (12).
However, as the result from geNorm showed, there was no optimal
combination of the selected control genes below the cut-off value.
It has been suggested that when conditions permit, three of the
most stable control genes could be used instead of a single gene
(37,38).
Furthermore, it is of note that the concentration of
plasma DNA in plasma is extremely low (21) and highly originates from the
apoptosis process (39,40). These characteristics influence the
amplification efficiency of plasma DNA. For example, firstly, the
amplificon sizes should be short enough, as the longer the template
of the target gene is, the more opportunities there are to be
digested in the apoptosis process, which reduces the effective
templates. Secondly, the succesful amplification of every single
target gene from plasma DNA cannot be guaranteed. There are
increasing studies focusing further on the clinical application of
plasma DNA, which is required for NIPD. However, to the best of our
knowledge, all the control genes used in plasma DNA analysis are
chosen by experience, and no evaluation has been performed to
confirm the content stability of these control genes in the plasma
DNA from pregnant and non-pregnant females. The present study
validated the most content stable control genes at the second
trimester of gestational age, which can be used as a criterion in
subsequent studies.
In conclusion, the present study indicated that the
content stability of control genes used for DNA showed significant
variation in pregnant and non-pregnant plasma DNA. Every qPCR DNA
study should commence with the selection of an appropriate control
gene individually. According to the study, TERT and the
combination of ACTB and TERT permit an efficient
normalization for DNA quantitation using qPCR in pregnant and
non-pregnant plasma, whereas GAPDH and TRG were
proved to be the least reliable control genes.
Acknowledgements
The present study was supported by the Project
supported by the Key Foundation of Jilin Provincial Science and
Technology Department, China (nos. 20130727038YY and 20100942) and
the Jilin Provincial Development and Reform Commission, China (no.
20101928).
References
|
1
|
Zhong Q, Zhang Q, Wang Z, et al:
Expression profiling and validation of potential reference genes
during Paralichthys olivaceus embryogenesis. Mar Biotechnol
(NY). 10:310–318. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dheda K, Huggett JF, Bustin SA, Johnson
MA, Rook G and Zumla A: Validation of housekeeping genes for
normalizing RNA expression in real-time PCR. Biotechniques.
37:112–114. 116118–119. 2004.PubMed/NCBI
|
|
3
|
Steinau M, Rajeevan MS and Unger ER: DNA
and RNA references for qRT-PCR assays in exfoliated cervical cells.
J Mol Diagn. 8:113–118. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Radonic A, Thulke S, Mackay IM, Landt O,
Siegert W and Nitsche A: Guideline to reference gene selection for
quantitative real-time PCR. Biochem Biophys Res Commun.
313:856–862. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cordoba EM, Die JV, González-Verdejo CI,
Nadal S and Román B: Selection of reference genes in Hedysarum
coronarium under various stresses and stages of development.
Anal Biochem. 409:236–243. 2011.PubMed/NCBI
|
|
6
|
Guénin S, Mauriat M, Pelloux J, Van
Wuytswinkel O, Bellini C and Gutierrez L: Normalization of qRT-PCR
data: the necessity of adopting a systematic, experimental
conditions-specific, validation of references. J Exp Bot.
60:487–493. 2009.PubMed/NCBI
|
|
7
|
Lo YM, Corbetta N, Chamberlain PF, et al:
Presence of fetal DNA in maternal plasma and serum. Lancet.
350:485–487. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Alizadeh M, Bernard M, Danic B, et al:
Quantitative assessment of hematopoietic chimerism after bone
marrow transplantation by real-time quantitative polymerase chain
reaction. Blood. 99:4618–4625. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bianchi DW, Avent ND, Costa JM and van der
Schoot CE: Noninvasive prenatal diagnosis of fetal Rhesus D: ready
for Prime (r) Time. Obstet Gynecol. 106:841–844. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lo YM, Tein MS, Lau TK, et al:
Quantitative analysis of fetal DNA in maternal plasma and serum:
implications for noninvasive prenatal diagnosis. Am J Hum Genet.
62:768–775. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Picchiassi E, Coata G, Fanetti A, Centra
M, Pennacchi L and Di Renzo GC: The best approach for early
prediction of fetal gender by using free fetal DNA from maternal
plasma. Prenat Diagn. 28:525–530. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vandesompele J, De Preter K, Pattyn F, et
al: Accurate normalization of real-time quantitative RT-PCR data by
geometric averaging of multiple internal control genes. Genome
Biol. 3:RESEARCH00342002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Andersen CL, Jensen JL and Ørntoft TF:
Normalization of real-time quantitative reverse transcription-PCR
data: a model-based variance estimation approach to identify genes
suited for normalization, applied to bladder and colon cancer data
sets. Cancer Res. 64:5245–5250. 2004. View Article : Google Scholar
|
|
14
|
Pfaffl MW, Tichopad A, Prgomet C and
Neuvians TP: Determination of stable housekeeping genes,
differentially regulated target genes and sample integrity:
BestKeeper-Excel-based tool using pair-wise correlations.
Biotechnol Lett. 26:509–515. 2004. View Article : Google Scholar
|
|
15
|
Helmy SM, Ismail S, Bassiouni R and Gaber
KR: Sensitivity of DCSR3/GAPDH ratio using quantitative real-time
PCR in the rapid prenatal diagnosis for down syndrome. Fetal Diagn
Ther. 25:220–223. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu Y, Zheng M, Xu Z, Wang X and Cui H:
Quantitative real-time PCR technique for rapid prenatal diagnosis
of Down syndrome. Prenat Diagn. 24:704–707. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Papageorgiou EA, Karagrigoriou A, Tsaliki
E, Velissariou V, Carter NP and Patsalis PC: Fetal-specific DNA
methylation ratio permits noninvasive prenatal diagnosis of trisomy
21. Nat Med. 17:510–513. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Honda H, Miharu N, Ohashi Y, et al: Fetal
gender determination in early pregnancy through qualitative and
quantitative analysis of fetal DNA in maternal serum. Hum Genet.
110:75–79. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Galbiati S, Smid M, Gambini D, et al:
Fetal DNA detection in maternal plasma throughout gestation. Hum
Genet. 117:243–248. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lo YM, Zhang J, Leung TN, Lau TK, Chang AM
and Hjelm NM: Rapid clearance of fetal DNA from maternal plasma. Am
J Hum Genet. 64:218–224. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Aghanoori MR, Vafaei H, Kavoshi H,
Mohamadi S and Goodarzi HR: Sex determination using free fetal DNA
at early gestational ages: a comparison between a modified mini-STR
genotyping method and real-time PCR. Am J Obstet Gynecol.
207:e1–e8. 2012.PubMed/NCBI
|
|
23
|
Fernández-Martínez FJ, Galindo A,
Garcia-Burguillo A, et al: Noninvasive fetal sex determination in
maternal plasma: a prospective feasibility study. Genet Med.
14:101–106. 2012.PubMed/NCBI
|
|
24
|
Lim JH, Park SY, Kim SY, et al: Effective
detection of fetal sex using circulating fetal DNA in
first-trimester maternal plasma. FASEB J. 26:250–258. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yenilmez ED, Tuli A and Evruke IC:
Noninvasive prenatal diagnosis experience in the Çukurova Region of
Southern Turkey: detecting paternal mutations of sickle cell anemia
and β-thalassemia in cell-free fetal DNA using high-resolution
melting analysis. Prenat Diagn. 33:1054–1062. 2013.
|
|
26
|
Gao T, Nie Y and Guo J: Hypermethylation
of the gene LARP2 for noninvasive prenatal diagnosis of
β-thalassemia based on DNA methylation profile. Mol Biol Rep.
39:6591–6598. 2012.PubMed/NCBI
|
|
27
|
Gao T, Nie Y, Hu H and Liang Z:
Hypermethylation of IGSF4 gene for noninvasive prenatal diagnosis
of thalassemia. Med Sci Monit. 18:BR33–BR40. 2012.PubMed/NCBI
|
|
28
|
Scheffer PG, van der Schoot CE,
Page-Christiaens GC and de Haas M: Noninvasive fetal blood group
genotyping of rhesus D, c, E and of K in alloimmunised pregnant
women: evaluation of a 7-year clinical experience. BJOG.
118:1340–1348. 2011.PubMed/NCBI
|
|
29
|
Chinen PA, Nardozza LM, Martinhago CD, et
al: Noninvasive determination of fetal rh blood group, D antigen
status by cell-free DNA analysis in maternal plasma: experience in
a Brazilian population. Am J Perinatol. 27:759–762. 2010.
View Article : Google Scholar
|
|
30
|
Gutensohn K, Müller SP, Thomann K, et al:
Diagnostic accuracy of noninvasive polymerase chain reaction
testing for the determination of fetal rhesus C, c and E status in
early pregnancy. BJOG. 117:722–729. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Della Ragione F, Mastrovito P, Campanile
C, et al: Differential DNA methylation as a tool for noninvasive
prenatal diagnosis (NIPD) of X chromosome aneuploidies. J Mol
Diagn. 12:797–807. 2010.PubMed/NCBI
|
|
32
|
Bustin S, Benes V, Nolan T and Pfaffl MW:
Quantitative real-time RT-PCR-a perspective. J Mol Endocrinol.
34:597–601. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Peters IR, Peeters D, Helps CR and Day MJ:
Development and application of multiple internal reference
(housekeeper) gene assays for accurate normalisation of canine gene
expression studies. Vet Immunol Immunopathol. 117:55–66. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wierschke S, Gigout S, Horn P, et al:
Evaluating reference genes to normalize gene expression in human
epileptogenic brain tissues. Biochem Biophys Res Commun.
403:385–390. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chang E, Shi S, Liu J, et al: Selection of
reference genes for quantitative gene expression studies in
Platycladus orientalis (Cupressaceae) Using real-time PCR.
PLoS One. 7:e332782012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Brugè F, Venditti E, Tiano L, Littarru G
and Damiani E: Reference gene validation for qPCR on normoxia-and
hypoxia-cultured human dermal fibroblasts exposed to UVA: Is
β-actin a reliable normalizer for photoaging studies? J Biotechnol.
156:153–162. 2011.PubMed/NCBI
|
|
37
|
Liman M, Wenji W, Conghui L, et al:
Selection of reference genes for reverse transcription quantitative
real-time PCR normalization in black rockfish (Sebastes
schlegeli). Mar Genomics. 11:67–73. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xu Y, Zhu X, Gong Y, Xu L, Wang Y and Liu
L: Evaluation of reference genes for gene expression studies in
radish (Raphanus sativus L.) using quantitative real-time
PCR. Biochem Biophys Res Commun. 424:398–403. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Alberry M, Maddocks D, Jones M, et al:
Free fetal DNA in maternal plasma in anembryonic pregnancies:
confirmation that the origin is the trophoblast. Prenat Diagn.
27:415–418. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chan KC, Zhang J, Hui AB, et al: Size
distributions of maternal and fetal DNA in maternal plasma. Clin
Chem. 50:88–92. 2004. View Article : Google Scholar : PubMed/NCBI
|