1. Preeclampsia as a series of
disorders
Preeclampsia belongs to the group of hypertensive
disorders of pregnancy. It belongs to the same group of disorders,
which also includes gestational hypertension and chronic
hypertension. Preeclampsia is one of the complications of pregnancy
responsible for maternal and perinatal morbidity and mortality
(1), and is as life-threatening
for the mother as it is for the fetus. Scientists and doctors have
been unable to find a means of controling this disorder. The
present review summarizes the facts that have been recently
obtained from various studies, including studies on animals, which
are based on the knowledge of the most experienced scientists. It
discusses the most important results of previously published
studies on preeclampsia, as well as the role of fetal DNA in the
pathogenesis of this disorder.
Preeclampsia is most commonly classified into
early-onset (before 34 weeks) and late-onset preeclampsia (after 34
weeks). Signs of early-onset preeclampsia include abnormal
placentation, fetal growth restriction and the deterioration of
maternal health, whereas late-onset preeclampsia is determined
mostly by the symptoms observed in the mother only (2). In preeclampsia there is also reduced
placental perfusion, which causes complications, such as the
widespread apoptosis of cytotrophoblasts that invade the uterus
(3).
Cytotrophoblasts are abnormally decayed as a result
of the non-standard proliferation and differentiation due to
maternal conditions (4). Most
importantly, the reduced perfusion of the placenta affects the
placenta itself. The preeclamptic placenta is light, thick and not
as circular in shape as the placenta of a normal pregnancy. There
is massive trophoblastic invasion in the center of the placenta,
which decreases towards the periphery (5). Image analysis of histological
sections of the preeclamptic placenta has shown enhanced villus
bifurcation, huge syncytial knots and minor sclerotic villi
(6). In a previous study using a
mouse model of preeclampsia, visible differences were observed
between the placentas from the mice with a normal pregnancy and
those with hypertensive disorders (7).
Endothelial cell activation has been described to
occur during preeclampsia (8).
The altered synthesis and the release of endothelial cells initiate
endothelial dysfunction along with the imbalance between
vasodilation and vasoconstrictor prostaglandins (9). Initially, one of the functions of
the endothelium is the generation of new vessels through the
process of angiogenesis. Therefore, there is an imbalance between
angiogenic and anti-angiogenic factors. The high activity of
anti-angiogenic factors is one of the characteristics of
preeclampsia (10). It has also
been proven that normotensive women with preeclampsia tend to have
increased blood pressure, sometimes even to a critical point. These
women have been found to have high levels of protein in urine after
20 weeks of gestation when afflicted with preeclampsia (11). The high risk group includes women
with chronic hypertension, diabetes mellitus, kidney disease and a
high body mass index, as well as women of advanced maternal age
(12).
It can be stated that preeclampsia can be classified
as an autoimmune disease. It has been shown that the sera from
preeclamptic women contain autoantibodies that react with
angiotensin receptor 1, which activate it and induce immune
responses (13). In animal
experiments, the administration of autoantibodies has been shown to
cause high blood pressure, proteinuria and the production of
soluble factors derived from the placenta (14). All the symptoms of preeclampsia
mentioned above suggest that preeclampsia is a series of disorders,
which manifest mainly during gestation.
2. Inflammation in preeclampsia
Inflammation is characterized as a non-specific
response of an organism to various stimuli which affect all types
of tissues (15). By activating
neutrophils and macrophages, the initiation of acute inflammation
is launched. By the infiltration of T lymphocytes and plasma cells,
the inflammation becomes a non-self-limiting response and
consequently becomes chronic inflammation.
Even though some crucial facts about preeclampsia
are known, its etiology remains clear. One of the facts is that
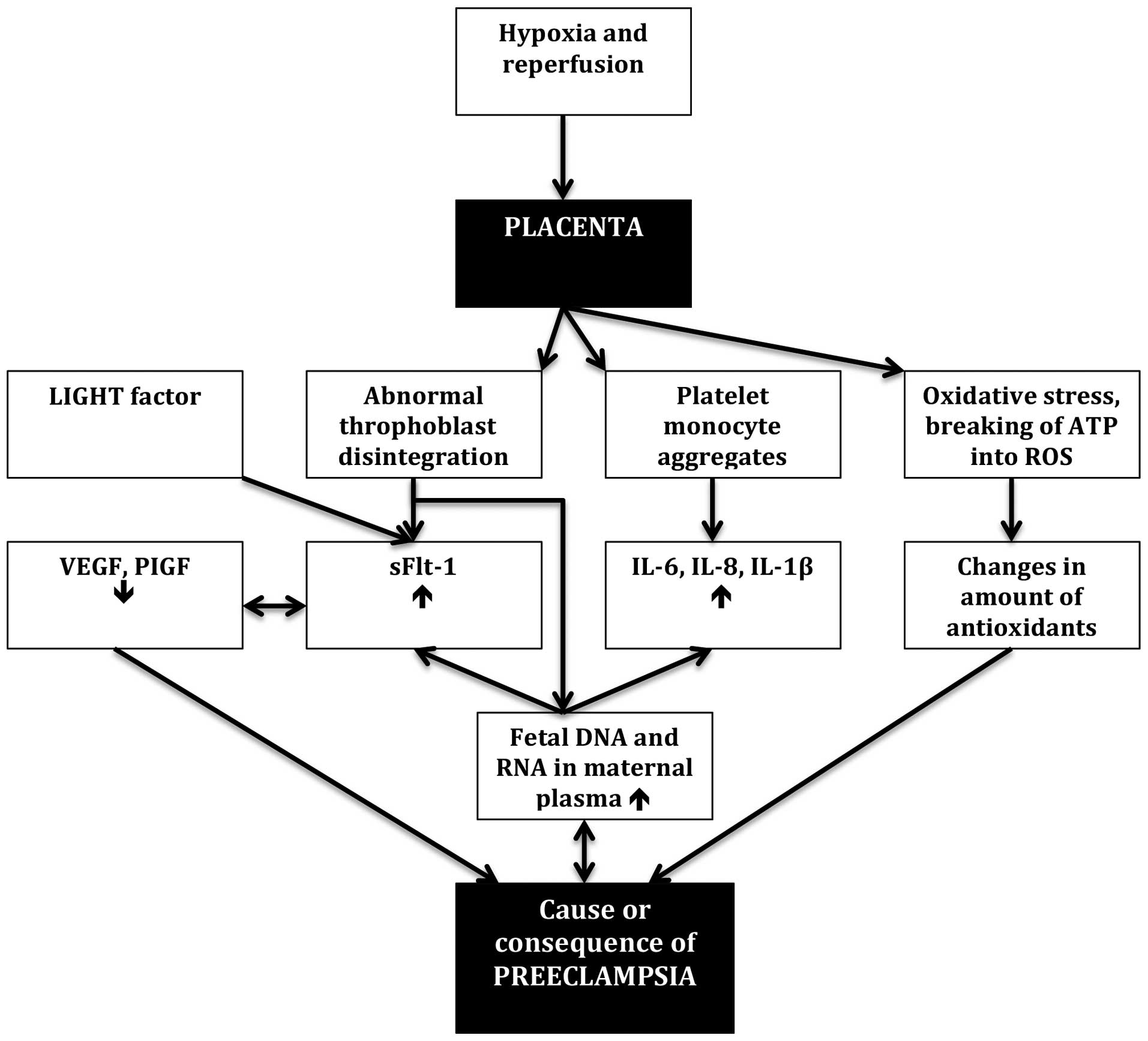
soluble fms-like tyrosine kinase 1 (sFlt-1) expression is increased
during pregnancies complicated by preeclampsia (16). sFlt-1 is an anti-angiogenic
protein that is produced by trophoblasts as a response to placental
hypoxia. However, the main stimulus behind this activation remains
unclear. It has been suggested that the activation of sFlt-1 occurs
through platelet-monocyte aggregates, which are known to induce
signaling pathways in monocytes and may be the cause for the
release of interleukin (IL)-6, IL-8 and IL-1β (17). On the other hand, the secretion of
sFlt-1 may be caused by other factors, such as the LIGHT factor, a
novel tumor necrosis factor superfamily member, the growth level of
which can be observed during preeclampsia; it may also be the cause
of sFlt-1 secretion (18).
Angiogenic stability is secured by the balance
between vascular endothelial growth factor (VEGF) and placental
growth factor (PIGF), which are pro-angiogenic factors, and sFlt-1
and soluble endoglin (sEng), which are both anti-angiogenic
(19). In fact, VEGF and PIGF are
produced by cytotrophoblasts and villous syncytiotrophoblasts in
the placenta (20). It is known
that during pregancies complicated by preeclampsia, the levels of
VEGF and PIGF are reduced; this correlates with the higher levels
of sFlt-1 (21). However, it is
not clear as to whether this decrease is the cause or the
consequence of the hither sFlt-1 levels. The ratio of VEGF to
sFlt-1 has also been shown to be decreased in diseases associated
with endothelial dysfunction, for example in diabetic retinopathy
(22). VEGF polymorphisms,
cooperating with other outer factors and genetics, play a crucial
role in the incidence of HELLP syndrome, which occasionally occurs
in connection with preeclampsia (23). The question is whether VEGF has
any impact on the incidence of preeclampsia. A group of German
researchers measured the sFlt-1/PlGF ratio in order to assess the
clinical validity of preeclampsia. They found that this ratio is
not a decisive factor in predicting preeclampsia, although it can
be useful as an indicator of its severity along with other
co-factors (24) (Fig. 1).
Furthermore, there are several studies focusing on
which genes are expressed in preeclampsia, but the findings differ
widely (25,26). Within a single mesasurment, one
can expect that some genes are upregulated whereas others are
downregulated; however, the outcome varies from sample to sample
(27). Kleinrouweler et al
(28) published a review, the
outcome of which was the identification of 33 eligible genes and
their expression data in placental tissue in preeclampsia. Some of
the transcripts encode proteins that may be potential biomarkers of
the disease.
Not only gene expression but also the amount of
antioxidants fluctuates between the same pathological samples,
which leads to different levels of oxidative stress. The growth of
oxidative stress products may be the result of reperfusion of the
placenta and poor circulation in this organ. Under these
conditions, ATP breaks down to substances which, when metabolized,
produce reactive oxygen species, and the organism needs to use the
stock of antioxidants to deactivate them (29). This shift in antioxidant status
may affect signaling pathways (30). The gene expression levels are
possibly affected by the degree of trophoblastic invasion of the
placenta (27) or by the
dysregulation of the trophoblastic gene expression itself. In spite
of the knowledge of these facts, it should be emphasized again that
the etiology of preeclampsia is not well known. Therefore, no
causal therapy for preeclampsia has been established yet.
3. Role of fetal DNA in preeclampsia
In 1997, Lo et al (31) detected the presence of circulating
fetal DNA in maternal plasma and serum. This finding offered a
unique possibility of access to fetal material using a non-invasive
method i.e., a regular blood test. Until this discovery, fetal
genetic material was obtained through amniocentesis or chorionic
villus sampling. Nowadays, several medical tests, such as testing
for sex-linked disorders, can be performed more easily. The amount
of fetal DNA increases as the delivery date approaches. Increased
fetal DNA concentrations are closely associated with certain
pregnancy disorders, most importantly with preeclampsia and trisomy
21 (32).
A number of studies have been carried out to show
that the levels of fetal DNA are significantly higher in
pregnancies complicated by preeclampsia in comparison with normal
pregnancies. A group of researchers from Switzerland measured
circulating DNA levels in samples from pregnant women with
preeclampsia and women with normal pregnancy by real-time
polymerase chain reaction, and showed that increased fetal DNA
levels were associated with preeclampsia (33). Hahn et al (34) suggested that circulating fetal DNA
levels can be used as a predicting factor of preeclampsia. As
mentioned above, preeclampsia is considered an autoimmune disease.
Based on this and the presence of fetal DNA in maternal plasma,
Vlková et al (35)
suggested that fetal RNA induces an autoimmune reaction by
directing the immune system of the mother against her own antigens
by a complicated process of transfection of maternal monocytes with
fetal fragments of RNA. On the whole, the ratio of fetal to
maternal DNA in the plasma of the mother is very low. The most
important contribution to non-invasive prenatal diagnosis was the
establishment of real-time quantitative polymerase chain reaction,
and the identification of specific fetal genes, rhesus D and Y
chromosome (36,37).
In addition to fetal DNA, other indicators of
preeclampsia include angiogenic factors, soluble endoglin,
P-selectin, pregnancy-associated plasma protein A, and so on.
Despite numerous studies on preeclampsia, it remains unclear
whether these are causes or consequences of preeclampsia (38). Real-time quantitative PCR has
shown that fetal DNA in maternal plasma is hypomethylated before
and after digestion by methylation-sensitive restriction enzymes
(39). The placenta, in
particular, was observed to contain a high percentage of methylated
DNA in comparison with other tissues; therefore, the activity of
DNA methyltransferase inhibitors results in small placentas and, as
shown by a histological evaluation, the labyrinthine part of the
placenta is also severely reduced in animal experiments (40). In 2010, Vora et al
(41) made a correlation between
cell-free fetal DNA and other factors indicating the placental
condition. Lo et al (42)
measured the levels of these factors using real-time PCR for DYS1
(multicopy Y chromosome sequence) and found that total and fetal
DNA levels correlated with the results of the PAPP-A test in the
first trimester of the pregnancy. However, there was no association
between fetal DNA and other factors in the second trimester.
Moreover, fetal DNA rapidly disappears from maternal plasma
following delivery (42).
Nevertheless, it is still not clear whether increased fetal DNA
levels are the cause or consequence of preeclampsia. Scharfe-Nugent
et al (43), who
demonstrated that the injection of high concentrations of fetal DNA
induces inflammation and symptoms similar to preeclampsia in mice,
also discussed this issue. However, other factors may have affected
the outcome observed in their study, and, thus, their conclusion
was that the role of fetal DNA remains unclear. More specifically,
the DNA used by Scharfe-Nugent et al (43) came from human tissue. Therefore,
the outcome of their experiment may have been affected by the use
of DNA from different species (i.e., using human DNA in mice).
4. Biology of fetal DNA
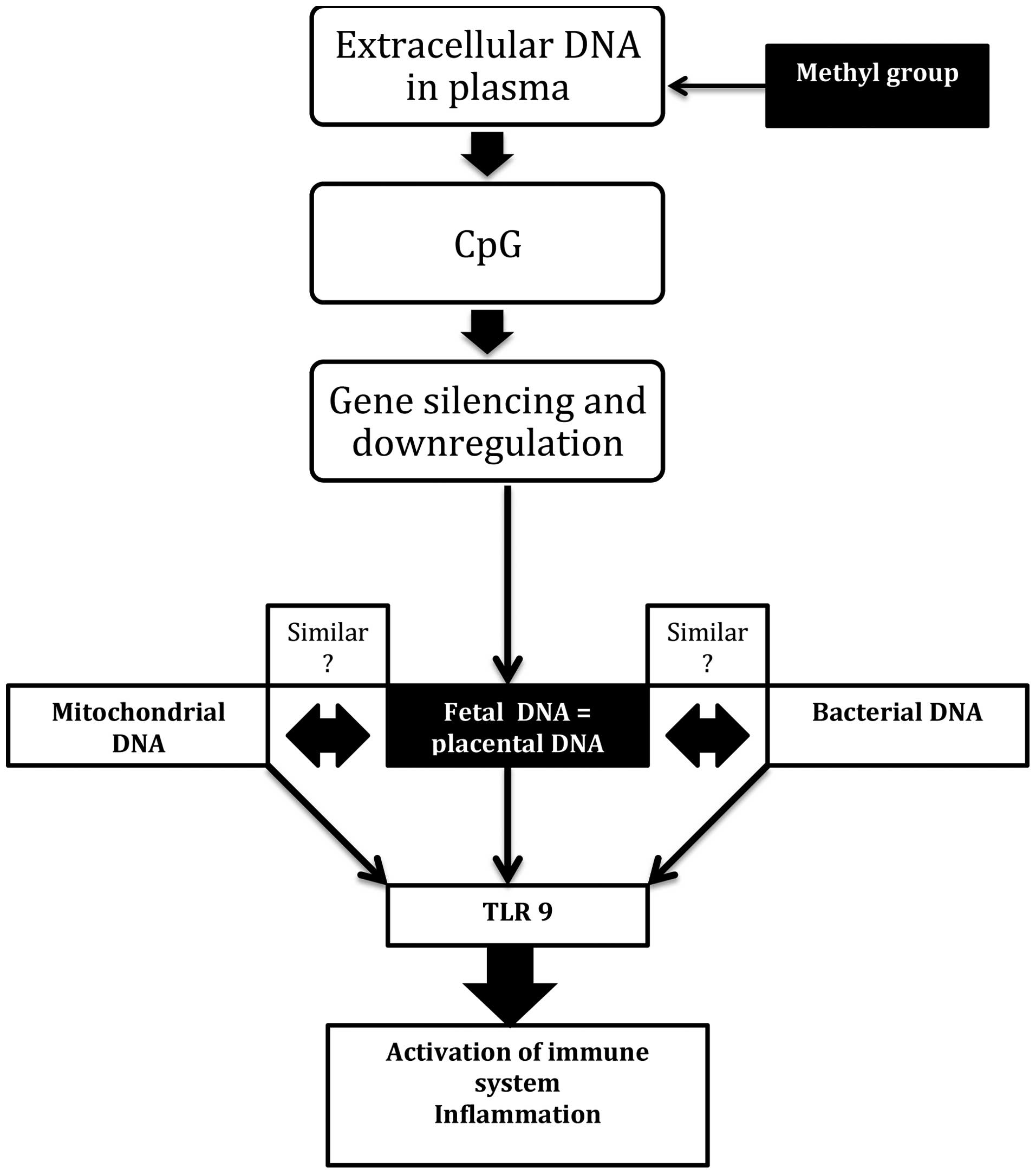
Cell-free DNA, and therefore extracellular and
cytosolic DNA is recognized by cell receptors and induces cell
reactions. As mentioned earlier, cell-free fetal DNA circulating
freely in the maternal bloodstream is hypomethylated in
preeclampsia. This means that the methyl group is added to the DNA
molecule at the cytosine-guanine residue and forms the so-called
CpG. This addition has such an effect that it blocks transcription
factors followed by gene silencing and downregulation (44,56). Furthermore, the increased
apoptosis of trophoblasts in placentas from pregnancies complicated
by preeclampsia produces circulating fetal DNA containing
mitochondrial DNA (mtDNA) (45).
Toll-like receptors (TLRs) are sensitive to this type of molecule
in the way that they are sensitive to bacterial or viral ones. The
reason is that mtDNA is structurally similar to bacterial DNA and
covers CpG sides as well. Consequently, after sensing these
molecules, the TLR9 receptor activates the immune system and
initiates inflammation (46,47) (Fig.
2).
Sepsis, i.e., the systemic inflammatory response
syndrome secondary to bacterial infection (48), is associated with mtDNA.
Mitochondrial dysfunction plays a crucial role in the pathogenesis
of sepsis. Reactive oxygen species and reactive nitrogen species
may cause the dysfunction of several organelles, such as
mitochondria, and inflict inflammation (49). Jiménez-Sousa et al
(50) studied the potential
association between mtDNA and severe sepsis and found that
different mitochondrial haplogroups affected the development of
sepsis.
Under certain circumstances, tumor necrosis factor-α
(TNF-α) and lipopolysaccharides stimulate cellular cyclic guanosine
monophosphate-adenosine monophosphate (cGAMP), which is responsible
for DNA synthesis (51). In
pregnancies complicated by preeclampsia, the growth of cGAMP is the
result of high levels of circulating natriuretic peptides (52,53). When cytosolic DNA is transfected,
cGAMP is triggered and binds to the endoplasmic-reticulum-resident
protein STING, followed by the activation of interferon 3 and
interferon β (54). Yet, cGAMP
produces a nucleotide second messenger and belongs to the family of
oligoadenylate synthetases, which can produce unique
2′-5′-phosphodiester bonds (55).
Taking into account all of the above, it can be hypothesized that
circulating fetal DNA in pregnancies complicated by preeclampsia
behave in a similar manner to bacterial DNA or mtDNA, and may thus
activate cGAMP and induce inflammation. If this is the case, then
fetal DNA is one of the possible causes of preeclampsia.
5. Causes and prevention of
preeclampsia
Due to the increasing number of cases of pre-term
births and the danger this poses by causing life-long handicaps, a
number of studies have focused on determining the causes of and
finding a solution to this issue. The association between fetal DNA
and preterm birth in pregnancies complicated by preeclampsia has
already been demonstrated by an Irish research group who
demonstrated, through animal experiments, that circulating free DNA
induces inflammation, leading to pre-term delivery (43). Their study was based on injecting
either human fetal DNA or CpG into mice. The authors suggested that
the TLR9 receptor detects hypomethylated fetal DNA and provokes
inflammation, leading to preeclampsia (43). Their outcome was that the
application of human fetal DNA infiltrated in the placenta induced
inflammation in pregnant mice, causing fetal resorption and
pre-term delivery. Either way, the pregnancy status is closely
associated with TLR9. When both human fetal DNA and TLR9 inhibitor
chloroquine were injected into mice, the pregnancies had a better
outcome. Chloroquine is known to cross the placenta when applied;
thus, its metabolites have been found in the urine of neonates
delivered from mothers treated with this medication (57). In conclusion, Scharfe-Nugent et al
(43) reported the impact of
human fetal DNA on mouse fetuses and their delivery status only.
However, information on the impact on the mothers and basic
preeclampsia parameters, such as blood pressure and proteinuria was
not provided. Moreover, it should also be noted that interspecies
differences may affect the induction of an immune response similar
to that induced by bacterial DNA.
6. Conclusions
The majority of available evidence suggests that
fetal DNA is either the cause or a consequence of preeclampsia. As
already mentioned, in a previous study, Lo et al (31) demonstrated that fetal DNA was
significantly increased in maternal plasma in preeclamptic
patients; thus, the possibility of prenatal diagnosis was no longer
a doubt for patients. In comparison with the past, when acquiring
fetal material was an invasive intervention (through amniocentesis
or chorionic villi sampling), today fetal material can be obtained
safely through the collection of maternal blood. Moreover, it has
been demostrated that the levels of fragmentation of fetal DNA are
different in maternal plasma (58). As previously demonstrated, short
fragments of fetal DNA are more suitable for PCR amplification and
further prenatal analysis (58).
Overall, the diagnosis has focused on the detection of Down
syndrome and other fetal chromosomal aneuploidies (59), single gene disorders which are
paternally inherited, and on simply decoding the fetal genome for
various purposes. However, due to the increasing incidence of
preeclampsia cases associated with conditions, such as inherited
hypertension and obesity, prenatal diagnosis now also focuses on
the prevention of preeclampsia.
The issue of whether preeclampsia can be prevented
in pregnant women remains unresloved. One of the assumptions is
that in pregnancies complicated by preeclampsia, DNase activity is
lowered due to inflammation. An alternative suggestion is that the
function of DNase is normal, but fetal DNA are somehow protected
from its lytic activity. After delivery, circulating DNA is cleared
from the maternal plasma rapidly (42). However, the exact mechanisms
involved are not clear. Certain studies have reported the use of
magnesium sulphate in preeclampsia and that its application was
effective in the management of severe preeclampsia in terms of
seizure prevention (60–62); however, it is not always
effective. Another possibility is the use of chloroquine, which may
act as a TLR9 inhibitor. as demonstrated by Scharfe-Nugent et
al (43).
Further research is required in order to determine
the principal cause of preeclampsia and the role of fetal DNA in
its pathogenesis, as well as the ways that the condition can be
prevented. Most importantly, more studies should be directed
towards the overall health throughout the gestational period.
Acknowledgments
The present study was supported by the Slovak
Research and Development Agency under contract APVV-0754_10.
References
|
1
|
Steegers EA, von Dadelszen P, Duvekot JJ
and Pijnenborg R: Pre-eclampsia. Lancet. 376:631–644. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Trogstad L, Magnus P and Stoltenberg C:
Pre-eclampsia: Risk factors and causal models. Best Pract Res Clin
Obstet Gynaecol. 25:329–342. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Genbacev O, DiFederico E, McMaster M and
Fisher SJ: Invasive cytotrophoblast apoptosis in pre-eclampsia. Hum
Reprod. 14(Suppl 2): S59–S66. 1999. View Article : Google Scholar
|
|
4
|
Roberts JM and Escudero C: The placenta in
preeclampsia. Pregnancy Hypertens. 2:72–83. 2012.PubMed/NCBI
|
|
5
|
Kajantie E, Thornburg KL, Eriksson JG,
Osmond C and Barker DJ: In preeclampsia, the placenta grows slowly
along its minor axis. Int J Dev Biol. 54:469–473. 2010. View Article : Google Scholar
|
|
6
|
Roberts DJ and Post MD: The placenta in
pre-eclampsia and intrauterine growth restriction. J Clin Pathol.
61:1254–1260. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ma RQ, Sun MN and Yang Z: Effects of
preeclampsia-like symptoms at early gestational stage on
feto-placental outcomes in a mouse model. Chin Med J (Engl).
123:707–712. 2010.
|
|
8
|
Roberts JM: Endothelial dysfunction in
preeclampsia. Semin Reprod Endocrinol. 16:5–15. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mustafa R, Ahmed S, Gupta A and Venuto RC:
A comprehensive review of hypertension in pregnancy. J Pregnancy.
2012:1059182012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Escudero C, Roberts JM, Myatt L and
Feoktistov I: Impaired adenosine-mediated angiogenesis in
preeclampsia: potential implications for fetal programming. Front
Pharmacol. 5:1342014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sibai BM: Maternal and uteroplacental
hemodynamics for the classification and prediction of preeclampsia.
Hypertension. 52:805–806. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Duckitt K and Harrington D: Risk factors
for pre-eclampsia at antenatal booking: systematic review of
controlled studies. BMJ. 330:5652005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wallukat G, Homuth V, Fischer T, Lindschau
C, Horstkamp B, Jüpner A, Baur E, Nissen E, Vetter K, Neichel D,
Dudenhausen JW, Haller H and Luft FC: Patients with preeclampsia
develop agonistic autoantibodies against the angiotensin AT1
receptor. J Clin Invest. 103:945–952. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou CC, Ahmad S, Mi T, Abbasi S, Xia L,
Day MC, Ramin SM, Ahmed A, Kellems RE and Xia Y: Autoantibody from
women with preeclampsia induces soluble Fms-like tyrosine kinase-1
production via angiotensin type 1 receptor and calcineurin/nuclear
factor of activated T-cells signaling. Hypertension. 51:1010–1019.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ferrero-Miliani L, Nielsen OH, Andersen PS
and Girardin SE: Chronic inflammation: importance of NOD2 and NALP3
in interleukin-1beta generation. Clin Exp Immunol. 147:227–235.
2007.PubMed/NCBI
|
|
16
|
Levine RJ, Maynard SE, Qian C, Lim KH,
England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein
FH, Sibai BM, Sukhatme VP and Karumanchi SA: Circulating angiogenic
factors and the risk of preeclampsia. N Engl J Med. 350:672–683.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Major HD, Campbell RA, Silver RM, Branch
DW and Weyrich AS: Synthesis of sFlt-1 by platelet-monocyte
aggregates contributes to the pathogenesis of preeclampsia. Am J
Obstet Gynecol. 210:547.e541–e547. 2014. View Article : Google Scholar
|
|
18
|
Wang W, Parchim NF, Iriyama T, Luo R, Zhao
C, Liu C, Irani RA, Zhang W, Ning C, Zhang Y, Blackwell SC, Chen L,
Tao L, Hicks MJ, Kellems RE and Xia Y: Excess LIGHT contributes to
placental impairment, increased secretion of vasoactive factors,
hypertension, and proteinuria in preeclampsia. Hypertension.
63:595–606. 2014. View Article : Google Scholar
|
|
19
|
Ehrig JC, Horvat D, Allen SR, Jones RO,
Kuehl TJ and Uddin MN: Cardiotonic steroids induce anti-angiogenic
and anti-proliferative profiles in first trimester extravillous
cytotrophoblast cells. Placenta. 35:932–936. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Weed S, Bastek JA, Anton L, Elovitz MA,
Parry S and Srinivas SK: Examining the correlation between
placental and serum placenta growth factor in preeclampsia. Am J
Obstet Gynecol. 207:140.e141–e146. 2012. View Article : Google Scholar
|
|
21
|
Bills VL, Varet J, Millar A, Harper SJ,
Soothill PW and Bates DO: Failure to up-regulate VEGF165b in
maternal plasma is a first trimester predictive marker for
pre-eclampsia. Clin Sci (Lond). 116:265–272. 2009. View Article : Google Scholar
|
|
22
|
Zhai YL, Zhu L, Shi SF, Liu LJ, Lv JC and
Zhang H: Elevated soluble VEGF receptor sFlt-1 correlates with
endothelial injury in IgA nephropathy. PLoS One. 9:e1017792014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Haram K, Mortensen JH and Nagy B: Genetic
aspects of preeclampsia and the HELLP syndrome. J Pregnancy.
2014:9107512014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lehnen H, Mosblech N, Reineke T, Puchooa
A, Menke-Möllers I, Zechner U and Gembruch U: Prenatal clinical
assessment of sFlt-1 (soluble fms-like tyrosine kinase-1)/PlGF
(placental growth factor) ratio as a diagnostic tool for
preeclampsia, pregnancy-induced hypertension, and proteinuria.
Geburtshilfe Frauenheilkd. 73:440–445. 2013. View Article : Google Scholar
|
|
25
|
Sitras V, Paulssen RH, Gronaas H, Leirvik
J, Hanssen TA, Vartun A and Acharya G: Differential placental gene
expression in severe preeclampsia. Placenta. 30:424–433. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Reimer T, Koczan D, Gerber B, Richter D,
Thiesen HJ and Friese K: Microarray analysis of differentially
expressed genes in placental tissue of pre-eclampsia: up-regulation
of obesity-related genes. Mol Hum Reprod. 8:674–680. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ma K, Lian Y, Zhou S, Hu R, Xiong Y, Ting
P, Xiong Y, Li X and Wang X: Microarray analysis of differentially
expressed genes in preeclamptic and normal placental tissues. Clin
Exp Obstet Gynecol. 41:261–271. 2014.PubMed/NCBI
|
|
28
|
Kleinrouweler CE, van Uitert M, Moerland
PD, Ris-Stalpers C, van der Post JA and Afink GB: Differentially
expressed genes in the pre-eclamptic placenta: a systematic review
and meta-analysis. PLoS One. 8:e689912013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Roberts JM and Hubel CA: Oxidative stress
in preeclampsia. Am J Obstet Gynecol. 190:1177–1178. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bilodeau JF: Review: maternal and
placental antioxidant response to preeclampsia - impact on
vasoactive eicosanoids. Placenta. 35(Suppl): S32–S38. 2014.
View Article : Google Scholar
|
|
31
|
Lo YM, Corbetta N, Chamberlain PF, Rai V,
Sargent IL, Redman CW and Wainscoat JS: Presence of fetal DNA in
maternal plasma and serum. Lancet. 350:485–487. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lo YM: Fetal DNA in maternal plasma. Ann
NY Acad Sci. 906:141–147. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhong XY, Laivuori H, Livingston JC,
Ylikorkala O, Sibai BM, Holzgreve W and Hahn S: Elevation of both
maternal and fetal extracellular circulating deoxyribonucleic acid
concentrations in the plasma of pregnant women with preeclampsia.
Am J Obstet Gynecol. 184:414–419. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hahn S, Rusterholz C, Hösli I and Lapaire
O: Cell-free nucleic acids as potential markers for preeclampsia.
Placenta. 32(Suppl): S17–S20. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Vlková B, Szemes T, Minarik G, Turna J and
Celec P: Circulating free fetal nucleic acids in maternal plasma
and preeclampsia. Med Hypotheses. 74:1030–1032. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yu H, Shen Y, Ge Q, He Y, Qiao D, Ren M
and Zhang J: Quantification of maternal serum cell-free fetal DNA
in early-onset preeclampsia. Int J Mol Sci. 14:7571–7582. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chiu RW and Lo YM: Clinical applications
of maternal plasma fetal DNA analysis: translating the fruits of 15
years of research. Clin Chem Lab Med. 51:197–204. 2013.
|
|
38
|
Grill S, Rusterholz C, Zanetti-Dällenbach
R, Tercanli S, Holzgreve W, Hahn S and Lapaire O: Potential markers
of preeclampsia - a review. Reprod Biol Endocrinol. 7:702009.
View Article : Google Scholar
|
|
39
|
Kim MJ, Kim SY, Park SY, Ahn HK, Chung JH
and Ryu HM: Association of fetal-derived hypermethylated RASSF1A
concentration in placenta-mediated pregnancy complications.
Placenta. 34:57–61. 2013. View Article : Google Scholar
|
|
40
|
Koukoura O, Sifakis S and Spandidos DA:
DNA methylation in the human placenta and fetal growth (review).
Mol Med Rep. 5:883–889. 2012.PubMed/NCBI
|
|
41
|
Vora NL, Johnson KL, Lambert-Messerlian G,
Tighiouart H, Peter I, Urato AC and Bianchi DW: Relationships
between cell-free DNA and serum analytes in the first and second
trimesters of pregnancy. Obstet Gynecol. 116:673–678. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lo YM, Zhang J, Leung TN, Lau TK, Chang AM
and Hjelm NM: Rapid clearance of fetal DNA from maternal plasma. Am
J Hum Genet. 64:218–224. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
43
|
Scharfe-Nugent A, Corr SC, Carpenter SB,
Keogh L, Doyle B, Martin C, Fitzgerald KA, Daly S, O’Leary JJ and
O’Neill LA: TLR9 provokes inflammation in response to fetal DNA:
mechanism for fetal loss in preterm birth and preeclampsia. J
Immunol. 188:5706–5712. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tost J: DNA methylation: an introduction
to the biology and the disease-associated changes of a promising
biomarker. Mol Biotechnol. 44:71–81. 2010. View Article : Google Scholar
|
|
45
|
Colleoni F, Lattuada D, Garretto A,
Massari M, Mandò C, Somigliana E and Cetin I: Maternal blood
mitochondrial DNA content during normal and intrauterine growth
restricted (IUGR) pregnancy. Am J Obstet Gynecol.
203:365.e361–e366. 2010. View Article : Google Scholar
|
|
46
|
Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal
T, Junger W, Brohi K, Itagaki K and Hauser CJ: Circulating
mitochondrial DAMPs cause inflammatory responses to injury. Nature.
464:104–107. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Goulopoulou S, Matsumoto T, Bomfim GF and
Webb RC: Toll-like receptor 9 activation: a novel mechanism linking
placenta-derived mitochondrial DNA and vascular dysfunction in
pre-eclampsia. Clin Sci (Lond). 123:429–435. 2012. View Article : Google Scholar
|
|
48
|
Levy MM, Fink MP, Marshall JC, Abraham E,
Angus D, Cook D, Cohen J, Opal SM, Vincent JL and Ramsay G: 2001
SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions
Conference. Crit Care Med. 31:1250–1256. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Garrabou G, Morén C, López S, Tobías E,
Cardellach F, Miró O and Casademont J: The effects of sepsis on
mitochondria. J Infect Dis. 205:392–400. 2012. View Article : Google Scholar
|
|
50
|
Jiménez-Sousa MA, Tamayo E,
Guzmán-Fulgencio M, Heredia M, Fernández-Rodríguez A, Gómez E,
Almansa R, Gómez-Herreras JI, García-Álvarez M, Gutiérrez-Junco S,
Bermejo-Martin JF and Resino S; the Spanish Sepsis Group (SpSG):
Mitochondrial DNA haplogroups are associated with severe sepsis and
mortality in patients who underwent major surgery. J Infect. Jul
17–2014.Epub ahead of print. View Article : Google Scholar
|
|
51
|
Tantini B, Flamigni F, Pignatti C,
Stefanelli C, Fattori M, Facchini A, Giordano E, Clô C and
Caldarera CM: Polyamines, NO and cGMP mediate stimulation of DNA
synthesis by tumor necrosis factor and lipopolysaccharide in chick
embryo cardiomyocytes. Cardiovasc Res. 49:408–416. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sandrim VC, Palei AC, Sertório JT, Amaral
LM, Cavalli RC and Tanus-Santos JE: Alterations in cyclic GMP
levels in preeclampsia may reflect increased B-type natriuretic
peptide levels and not impaired nitric oxide activity. Clin
Biochem. 44:1012–1014. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Okuno S, Hamada H, Yasuoka M, Watanabe H,
Fujiki Y, Yamada N, Sohda S and Kubo T: Brain natriuretic peptide
(BNP) and cyclic guanosine monophosphate (cGMP) levels in normal
pregnancy and preeclampsia. J Obstet Gynaecol Res. 25:407–410.
1999. View Article : Google Scholar
|
|
54
|
Wu J, Sun L, Chen X, Du F, Shi H, Chen C
and Chen ZJ: Cyclic GMP-AMP is an endogenous second messenger in
innate immune signaling by cytosolic DNA. Science. 339:826–830.
2013. View Article : Google Scholar
|
|
55
|
Ablasser A, Goldeck M, Cavlar T, Deimling
T, Witte G, Röhl I, Hopfner KP, Ludwig J and Hornung V: cGAS
produces a 2′-5′-linked cyclic dinucleotide second messenger that
activates STING. Nature. 498:380–384. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Yuen RK, Peñaherrera MS, von Dadelszen P,
McFadden DE and Robinson WP: DNA methylation profiling of human
placentas reveals promoter hypomethylation of multiple genes in
early-onset preeclampsia. Eur J Hum Genet. 18:1006–1012. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Essien EE and Afamefuna GC: Chloroquine
and its metabolites in human cord blood, neonatal blood, and urine
after maternal medication. Clin Chem. 28:1148–1152. 1982.PubMed/NCBI
|
|
58
|
Koide K, Sekizawa A, Iwasaki M, Matsuoka
R, Honma S, Farina A, Saito H and Okai T: Fragmentation of
cell-free fetal DNA in plasma and urine of pregnant women. Prenat
Diagn. 25:604–607. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Evans MI and Kilpatrick M: Noninvasive
prenatal diagnosis: 2010. Clin Lab Med. 30:655–665. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kassie GM, Negussie D and Ahmed JH:
Maternal outcomes of magnesium sulphate and diazepam use in women
with severe pre-eclampsia and eclampsia in Ethiopia. Pharm Pract
(Granada). 12:4002014.
|
|
61
|
Tannirandorn Y: Is magnesium sulfate for
prevention or only therapeutic in preeclampsia? J Med Assoc Thai.
88:1003–1010. 2005.PubMed/NCBI
|
|
62
|
von Dadelszen P and Magee LA:
Pre-eclampsia:an update. Curr Hypertens Rep. 16:4542014. View Article : Google Scholar
|