Introduction
Orexin A and orexin B (also called hypocretins) are
evolutionarily-conserved neuropeptides that were initially
discovered by subtractive cDNA cloning (1) and/or orphan receptor technologies
(2). The two peptides originate
from the same precursor synthesized by hypothalamic neurons
(1,2). They trigger diverse facets of
physiology via two subtypes of G-protein coupled receptors, orexin
receptor 1 (OX1R) and OX2R (3).
In addition to the hypothalamus, functions of orexins have also
been described in peripheral tissues, including adrenal gland,
pancreas, adipose tissue, gastrointestinal tract and testis
(4–8). They control a number of important
physiological processes, such as food intake, sleep-wake cycle,
drug addition, energy metabolism, gastrointestinal function,
neuroendocrine regulation and cardiovascular modulation (5,9,10).
Apart from its roles in regulating central and
peripheral actions, orexins have been previously highlighted in
cancer cells (11–14). Orexins, acting at OX1R or OX2R,
result in strong apoptosis and a decrease of cell growth in diverse
cancer cell lines, including human colon cancer cells (11,12), human neuroblastoma cells (11), rat pancreatic tumor cells
(13) and rat C6 glioma cells
(14). Studies have shown that
orexins promote strong apoptosis in human colon cancer cells
through OX1R by inducing the release of cytochrome c from
mitochondria and activation of caspase-3/7 (12). Orexin A can induce apoptosis via
OX2R in rat pancreatic tumor cells involving the caspase-3 and
caspase-9 pathway (13). A study
also found that orexin A induces p38/mitogen-activated protein
kinase (MAPK)- and caspase-dependent cell death in rat C6 glioma
cells (14).
The finding that orexin signaling is capable of
inducing apoptosis in cancer cells, however, is not applicable to
all cell lines. The signaling pathways induced by orexins may be a
possible factor determining their effects on cell survival. The
complexity of orexin physiology and pathology is reflected in the
complicated downstream MAPK cascades being activated, particularly
the extracellular signal-regulated kinase (ERK) and p38 (9). Previous studies have shown
involvement of orexin A-induced ERK1/2 activation in the survival
of Chinese hamster ovary (CHO) cells overexpressing OX1R (15). The ERK1/2-MAPK pathway, which is
frequently activated in human cancers, may be conducive to increase
cell proliferation and viability (16,17). The phosphorylation of the
ERK1/2-MAPK cascade can induce cell proliferation, and inhibition
of ERK1/2 with selective inhibitors may lead to cell apoptosis in
gastric cancer cells (18–20).
Although there is increasing interest in the
biological actions of orexins on cancer cells, little information
is available regarding the potential role of this peptide on
gastric cancer cells. The present study aimed to investigate the
expression of the orexin receptors in the human gastric carcinoma
cell line, SGC-7901, and further examine whether orexin A induces
OX1R and ERK1/2 signaling mediates its effects on the survival of
SGC-7901 cells.
Materials and methods
Reagents
Orexin A was obtained from Sigma-Aldrich (St. Louis,
MO, USA). RPMI 1640 medium and fetal bovine serum were purchased
from Gibco (Grand Island, NY, USA). The ERK1/2 inhibitor, U0126,
was purchased from Cell Signaling Technology (Beverly, MA, USA).
OX1R-specific antagonist SB334867 was obtained from Tocris
Bioscience (Minneapolis, MA, USA). Total/phospho-ERK1/2 polyclonal
antibodies were obtained from Santa Cruz Biotechnology, Inc. (Santa
Cruz, CA, USA). The OX1R and β-actin polyclonal antibodies were all
obtained from Abcam (Cambridge, MA, USA).
Cell culture
Human gastric cancer cells SGC-7901 were obtained
from the Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China) and maintained in RPMI 1640 medium supplemented
with 10% fetal bovine serum, 50 μg/ml penicillin and 100
μg/ml streptomycin (Xianfeng, Shanghai, China). The cells
were grown in a humidified atmosphere containing 5% CO2
at 37°C. Before the experiment, the cells were grown in petri
dishes in a serum-free medium for 24 h. The following day, the
cells were treated with different concentrations of orexin A (0,
10−10, 10−8 and 10−6 M),
10−6 M orexin A plus 10−6 M SB334867 (OX1R
antagonist), 10−6 M orexin A plus 30 μM U0126
(ERK1/2 inhibitor), and 10−6 M orexin A plus U0126 and
SB334867 for 20 min, respectively.
Cell proliferation
SGC-7901 cells were seeded (2×103
cells/well) in 96-well plates and cultured for 24 h. To synchronize
the cell cycles, cells were serum-deprived for 24 h and
subsequently treated with test agents for an additional 24 h. BrdU
solution (10−6 M) was added and cells were incubated for
2.5 h. The BrdU incorporation into the DNA was measured by the cell
proliferation ELISA BrdU colorimetric kit (Roche Diagnostics,
Penzberg, Germany).
Cell viability
SGC-7901 cells were seeded into (2×103
cells/well) well plates and cultured for 24 h. Following incubation
in a serum-free RPMI 1640 supplemented with various concentrations
(0, 10−10, 10−8 and 10−6 M) of
orexin A or 10−6 M orexin A with 10−6 M OX1R
antagonist SB334867 at 37°C, SGC-7901 cell proliferation was
determined by a colorimetric methyl thiazolyl tetrazolium cell
proliferation and viability assay. A total of 50 μl 3-(4,
5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT
Sigma-Aldrich) cell proliferation assay solution was added to each
well. After an additional 3 h, the culture medium was removed and
the formed formazan crystals were dissolved in 100 μl
dimethyl sulfoxide. Optical density was measured by a plate reader
(SpectraMax Plus384 microplate reader; Molecular
Devices, Ismaning, Germany) at 570 nm and 650 nm (reference wave
length). All the experiments were performed in triplicate. The
absorbance 570 nm value of the control was used as a 100% standard
and all the individual measurements were compared to this
standard.
Annexin V/propidium iodide (PI) assays
for apoptosis
For Annexin V/PI assays, cells were stained with
Annexin V-fluorescein isothiocyanate (FITC) and PI, and evaluated
for apoptosis by flow cytometry according to the manufacturer’s
instructions (BD Biosciences Pharmingen, San Diego, CA, USA). Cells
were treated with different concentrations of orexin A in the
absence of serum for 48 h. Briefly, cells (1×105) were
washed twice with phosphate-buffered saline, and stained with 5
μl Annexin V-FITC and 10 μl PI in 500 μl
binding buffer for 15 min at room temperature in the dark.
Quantification of apoptosis was determined by counting the number
of cells stained by FITC-labeled Annexin V. Cell apoptosis was
detected using the Annexin V-FITC/PI apoptosis detection kit
(BestBio, Shanghai, China) by fluorescence-activated cell sorting
analysis. Early apoptotic cells were identified as PI negative and
FITC Annexin V positive, while late apoptotic or dead cells were
considered FITC Annexin V and PI positive.
Activity of caspase-8 and caspase-9 in
SGC-7901 cells
SGC-7901 cells were cultured in a serum-free medium
in 6-well plates (1.5×105 cells/well). Caspase-8 and
caspase-9 activities were assessed using Caspase-8 and Caspase-9
colorimetric assay kit (BioVision Inc., Headquarters, Milpitas, CA,
USA), respectively.
Quantitative polymerase chain reaction
(qPCR)
Total RNA was extracted from SGC-7901 cells using
the TRIzol reagent (Life Technologies Co., Carlsbad, CA, USA).
Following spectrophotometric quantification, 1 μg total RNA
was converted into cDNA using the PrimeScript™ RT reagent kit with
gDNA Eraser (Takara Bio, Otsu, Japan) according to the
manufacturer’s instructions. cDNA aliquots corresponding to equal
amounts of RNA were used for the quantification of mRNA by qPCR
using the LightCycler 96 real-time quantitative PCR detection
system (Roche, Indianapolis, IN, USA). The following specific
primers were used: OX1R forward, 5′-TGCGGCCAACCCTATCATCTA-3′
and reverse, 5′-ACCGGCTCTGCAAGGACAA-3′; and OX2R forward,
5′-ATCGCAGGGTATATCATCGTGTTC-3′ and reverse,
5′-TGACTGTCCTCATGTGGTGGTTC-3′. As an internal control for reverse
transcription (RT) and reaction efficiency, amplification of
glyceraldehydes-3-phosphate dehydrogenase (GAPDH) mRNA was
carried out in parallel for each sample. The following specific
primers were used: GAPDH forward,
5′-GGCACAGTCAAGGCTGAGAATG-3′ and reverse,
5′-ATGGTGGTGAAGACGCCAGTA-3′. The reaction system was 25 μl,
including 2 μl cDNA template, 2 μl forward and
reverse primers, 8.5 μl RNase-free ddH2O, and
12.5 μl SYBR® Premix Ex Taq™ II (Takara). The PCR
reactions were carried out using the following conditions: 95°C for
30 sec, and subsequently 40 cycles of 95°C for 5 sec, 60°C for 30
sec and 95°C for 15 sec. All the primers specific to OX1R,
OX2R and GAPDH were designed using Primer Premier 5.0
software (Premier Biosoft International, Palo Alto, CA, USA).
Protein preparations and western blot
analysis
Total protein was extracted from SGC-7901 cells
using radioimmunoprecipitation assay cell lysis reagent containing
proteinase and phosphatase inhibitors (Solarbio, Beiijng, China).
The cells remained in the medium on ice for 30 min with
re-dispersion every 5 min. Cell lysates were centrifuged at 12,000
× g for 10 min at 4°C, and the protein concentrations of the
supernatants were determined using the bicinchoninic acid protein
assay reagent kit (Beyotime Institute of Biotechnology, Shanghai,
China). The supernatants containing total protein were mixed with a
corresponding volume of 5X SDS loading buffer and were subsequently
denatured by boiling for 10 min. Samples were separated by SDS
polyacrylamide gel electropheresis using 5% stacking and 12%
separating gels. Subsequently, the samples were transferred onto
polyvinylidene difluoride membranes (0.2 μm, Immobilon-P;
Millipore, Billerica, MA, USA) at 60 V for 2.5 h. After being
blocked with skimmed dry milk for 2 h at room temperature, the
membranes were washed three times with Tris-buffered saline with
Tween 20 (TBST) for 30 min. The samples were incubated overnight at
4°C with the appropriate primary antibody. The primary antibodies
and dilutions used were as follows: Rabbit anti-human OX1R (cat.
no. Ab68718; 1:250), rabbit anti-human ERK1/2 (cat. no. sc-292838;
1:1,000), rabbit anti-human phospho-ERK1/2 (cat. no. sc-101760;
1:1,000) and rabbit anti-human β-actin (cat. no. Ab8337; 1:1,000).
The membranes were washed and incubated at room temperature for 1.5
h with the secondary goat anti-rabbit immunoglobulin G (H+L)
antibody (Beyotime, cat. no. A0208; 1:2,000) conjugated with
horseradish peroxidase, and were washed three times with TBST for
30 min. The membranes were subjected to enhanced chemiluminescence
(ECL) using an ECL detection kit (Beyotime) and quantified using
Quantity One software (Bio-Rad Laboratories Inc., Hercules, CA,
USA).
Statistical analysis
All the data are expressed as the means ± standard
error of the mean and differences between the means were analyzed
by one-way analysis of variance. P<0.05 was considered to
indicate a statistically significant difference. Statistical
analysis was performed using the SPSS 15.0 software package (SPSS
Inc., Chicago, IL, USA).
Results
Detection of OX1R expression in SGC-7901
cells
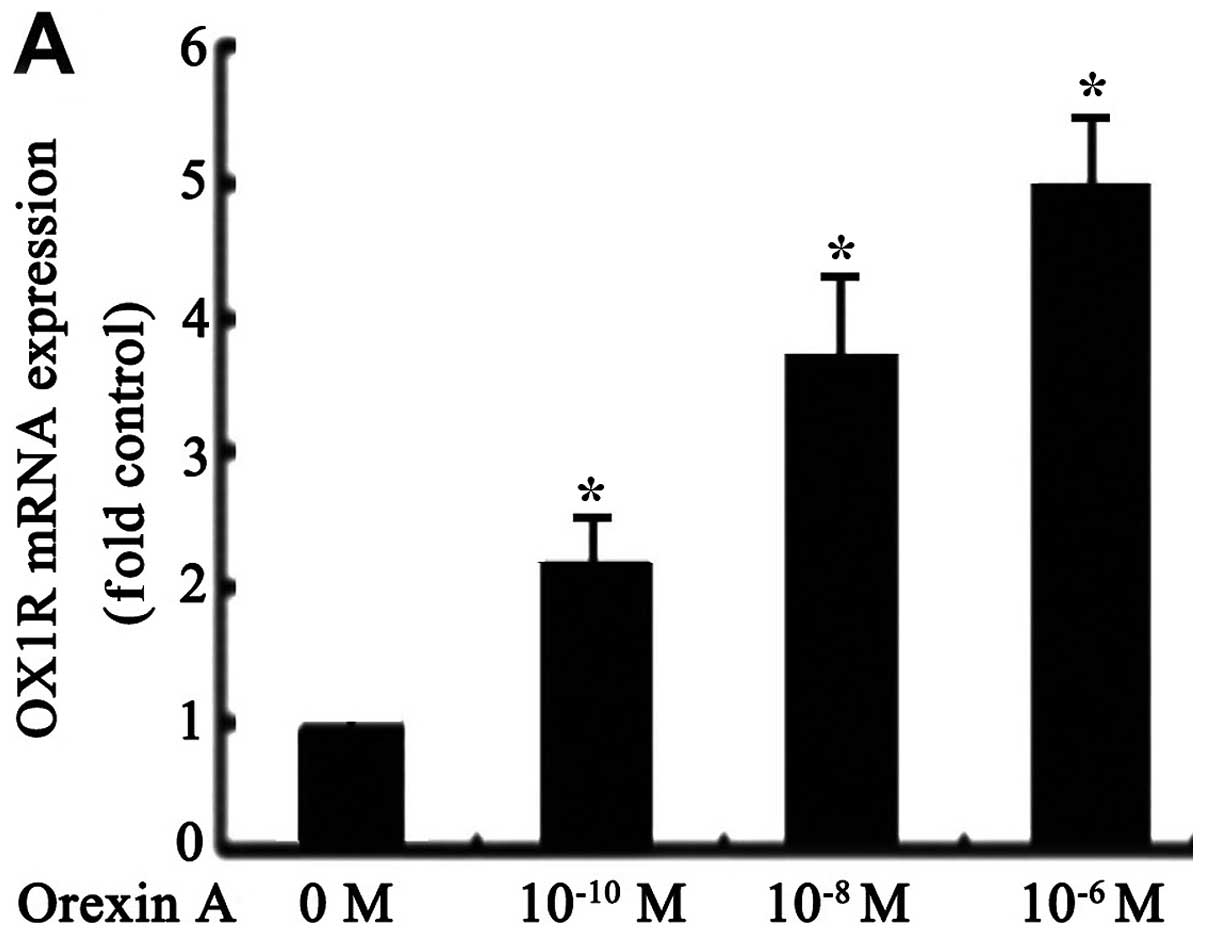
qPCR tests showed that OX1R mRNA was
expressed in SGC-7901 cells (Fig.
1A). However, OX2R mRNA was not detectable under the
same conditions (data not shown). OX1R mRNA and protein
levels were significantly increased in response to orexin A
(10−10, 10−8 and 10−6 M) treatment
compared to the untreated control group. The observed effects were
concentration-dependent, with 10−6 M orexin A being the
most potent (Fig. 1A and B).
However, these effects were blocked with 10−6 M
SB334867, a specific OX1R antagonist (Fig. 1B). These results suggested that
orexin A increased OX1R mRNA and protein levels in SGC-7901
cells.
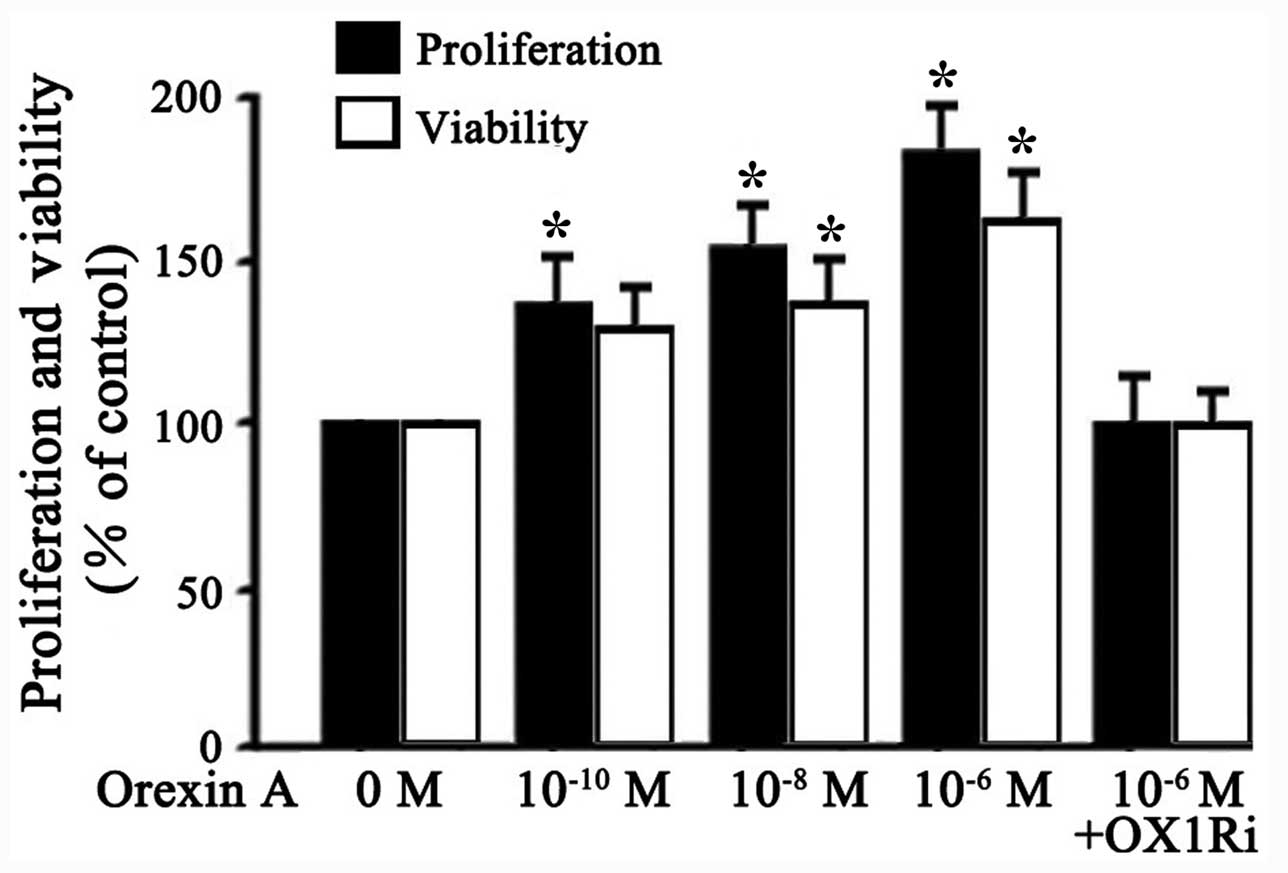
Effects of orexin A on proliferation and
viability of SGC-7901 cells
To characterize the effects of orexin A on the
proliferation and viability of SGC-7901 cells, viable cells were
treated with different concentrations of orexin A (0,
10−10, 10−8 and 10−6 M) or orexin
A (10−6 M) with OX1R antagonist, SB334867
(10−6 M). Results showed that orexin A
(10−10, 10−8 and 10−6 M)
dose-dependently improved the proliferation and viability of
SGC-7901 cells (Fig. 2).
Stimulation by 10−6 M orexin A increased proliferation
and viability of the cells by 80 and 60% over basal, respectively
(Fig. 2). These effects were
blocked by SB334867 (10−6 M) (Fig. 2). These results indicated that
orexin A, acting at OX1R, promoted proliferation and viability of
SGC-7901 cells.
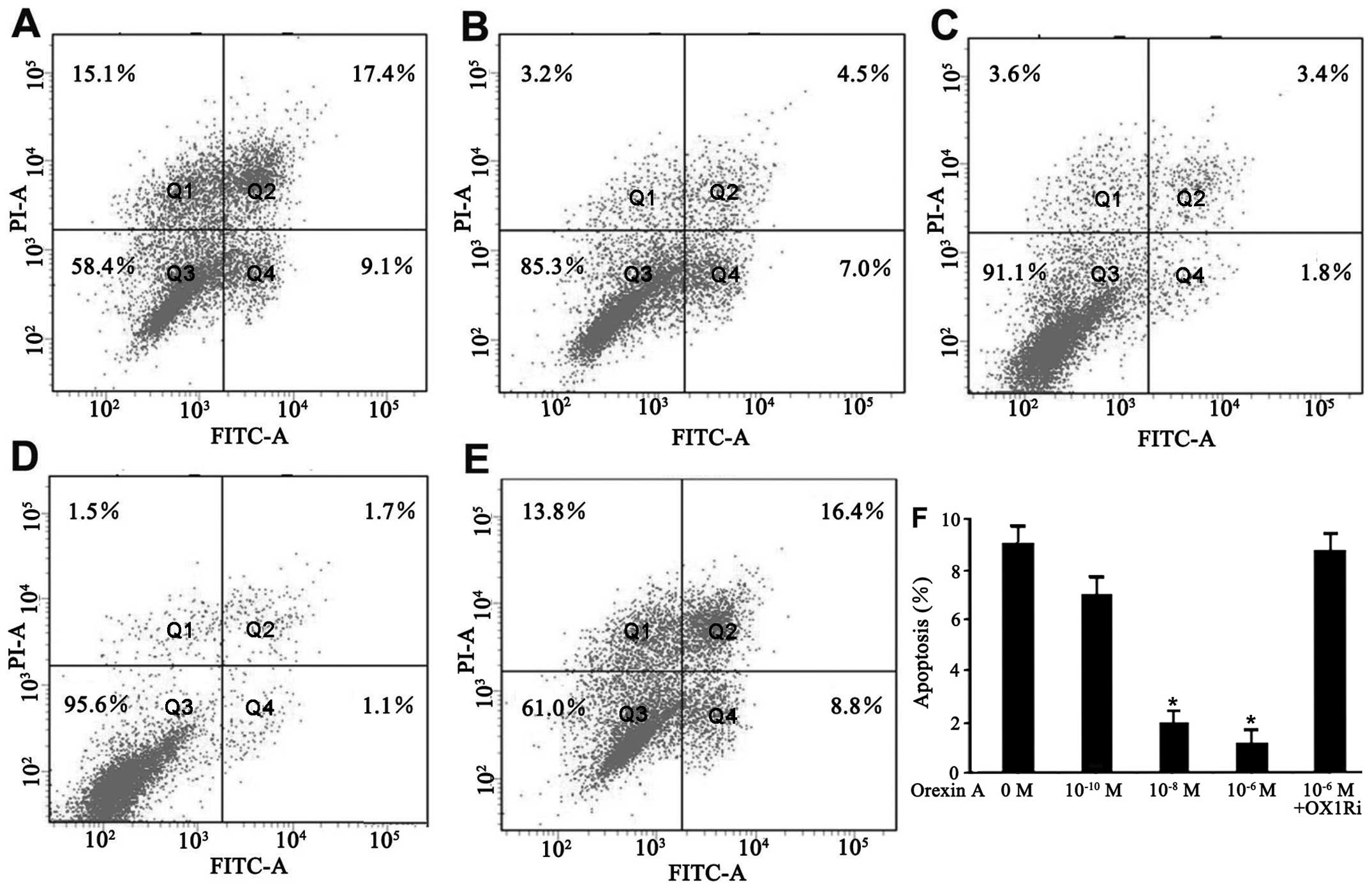
Orexin A protects SGC-7901 cells from
apoptosis
The effect of orexin A on apoptosis of SGC-7901
cells in a serum-deprived culture condition was evaluated by flow
cytometry for Annexin V/PI staining. The cells underwent apoptotic
death in the absence of serum for 48 h (Fig. 3A). However, addition of orexin A
(10−10, 10−8 and 10−6 M)
dose-dependently decreased the number of dead cells. The percentage
of early apoptotic cells (Annexin V+/PI−) was
9.1, 7.0, 1.8 and 1.1% in response to the vehicle,
10−10, 10−8, and 10−6 M orexin A,
respectively, for 48 h (Fig.
3A–D). Orexin A (10−6 M) did not protect the cells
against apoptosis in the presence of SB334867 (Fig. 3E and F). These results showed that
orexin A effectively inhibited serum starvation-induced apoptosis
in SGC-7901 cells.
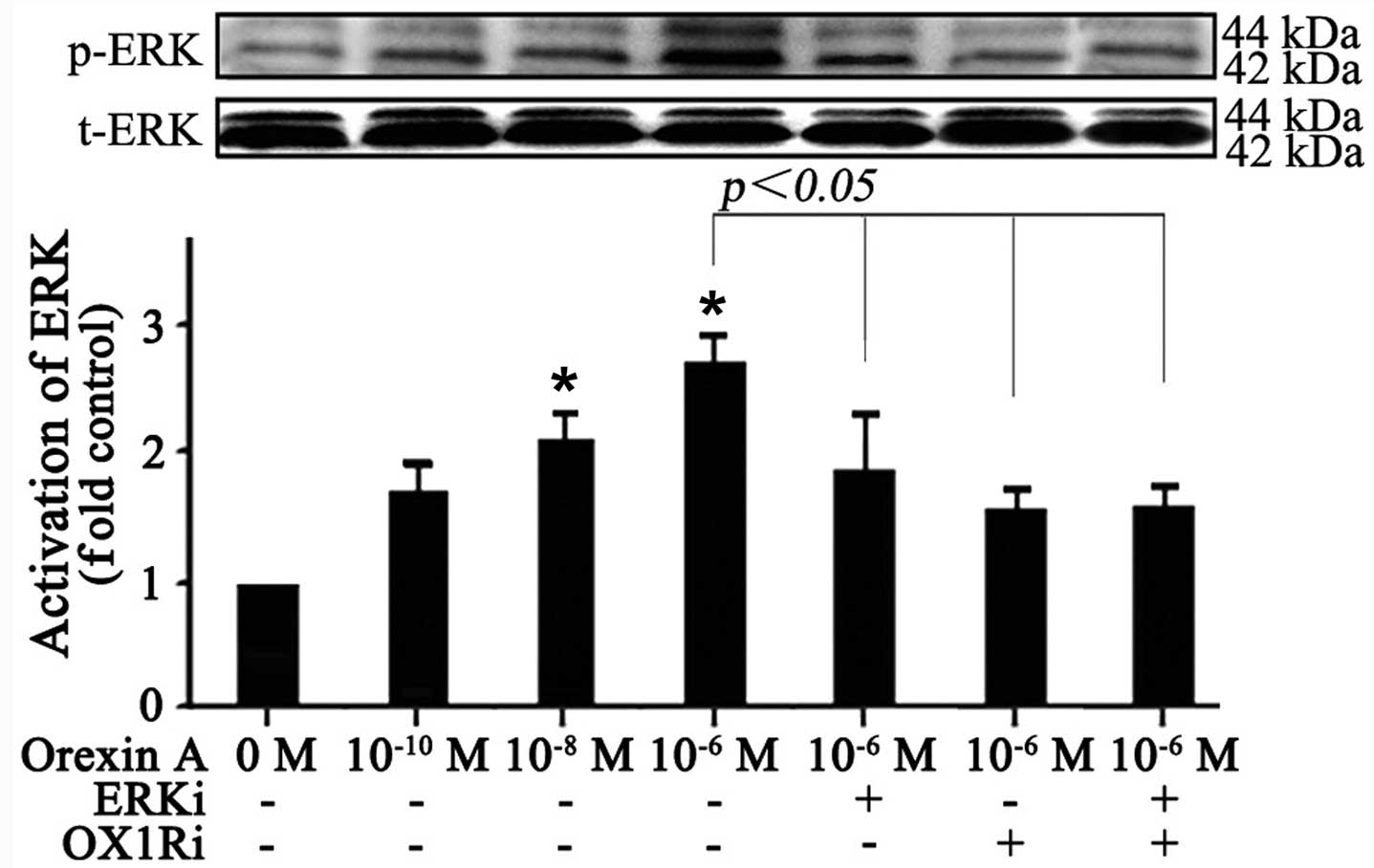
Effect of orexin A on ERK1/2 activation
in SGC-7901 cells
To elucidate the molecular mechanism responsible for
the effects observed with orexin A treatment, the effect of orexin
A was investigated on the ERK1/2-MAPK pathway, which is frequently
activated in human cancers and contributed to increase cell
proliferation and survival. The results showed that no significant
change was observed in total protein expression of ERK1/2 (Fig. 4). The phosphorylation level of
ERK1/2, however, was increased in response to orexin A
(10−10, 10−8 and 10−6 M) treatment
for 20 min in a dose-dependent manner (Fig. 4). Orexin A (10−6 M)
stimulated ERK1/2 phosphorylation by 2.7-fold over basal (Fig. 4). Additionally, the 30 μM
ERK1/2 antagonist U0126, 10−6 M OX1R antagonist SB334867
or the combination of the two antagonists prevented orexin A
(10−6 M) in stimulating ERK1/2 phosphorylation (Fig. 4). Thus, the data suggested that
orexin A treatment resulted in the activation of ERK1/2, which may
participate in orexin A-induced cell growth in SGC-7901 cells.
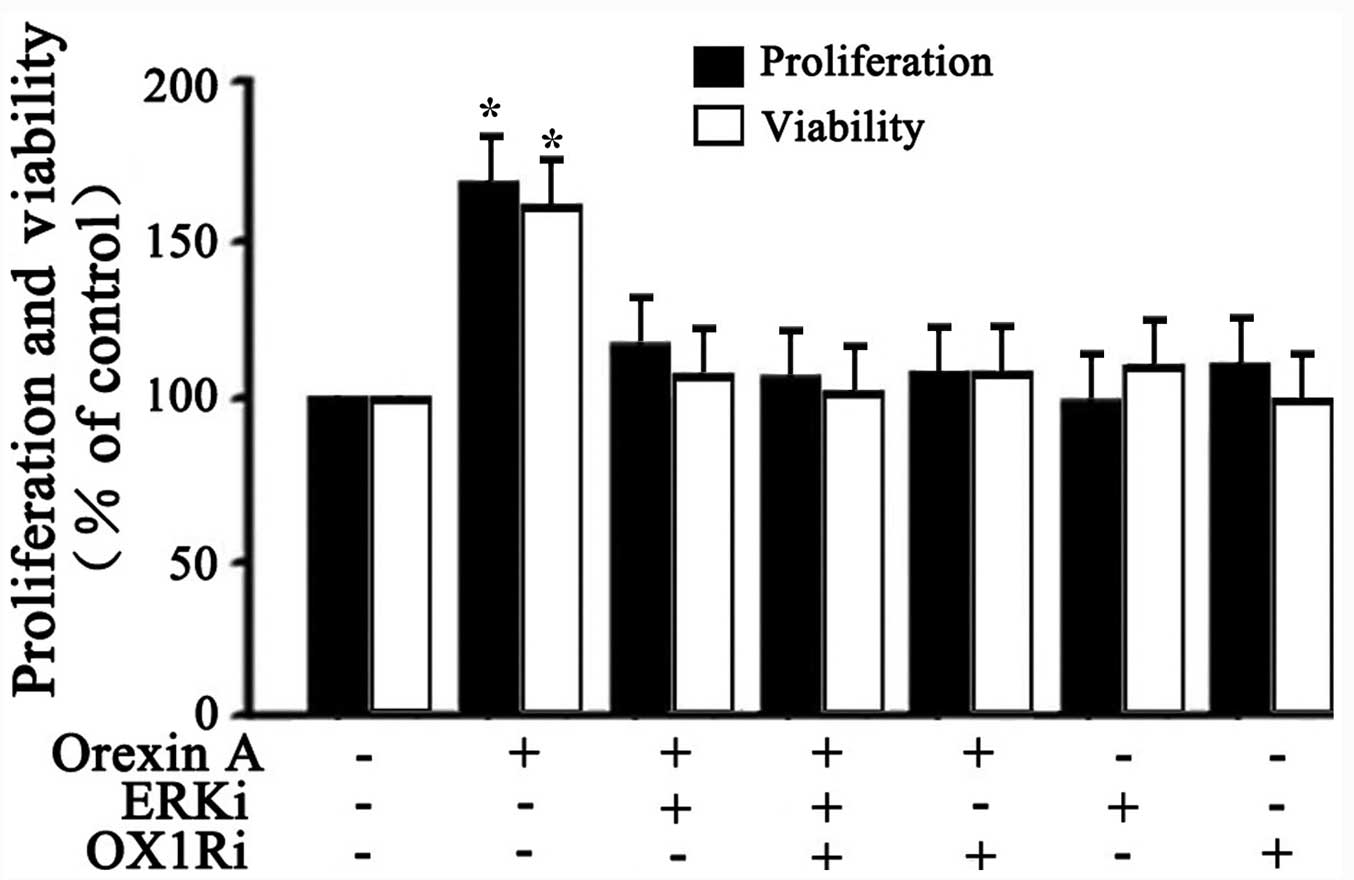
Effects of orexin A on proliferation and
viability of SGC-7901 cells via ERK1/2 signaling pathway
Whether the activation of ERK1/2 signaling was
responsible for orexin A-induced cell proliferation and viability
was further explored. BrdU analysis and MTT analysis were employed
to test cell survival. As shown in Fig. 5, 10−6 M orexin A
stimulation significantly increased the proliferation and viability
of SGC-7901 cells compared to the control. However, these effects
were inhibited with U0126 (30 μM ERKi) co treatment,
SB334867 (10−6 M OX1Ri) or the combination of the two
antagonists (Fig. 5). These data
suggested that ERK1/2 participated in orexin A-induced stimulation
of proliferation and viability in SGC-7901 cells.
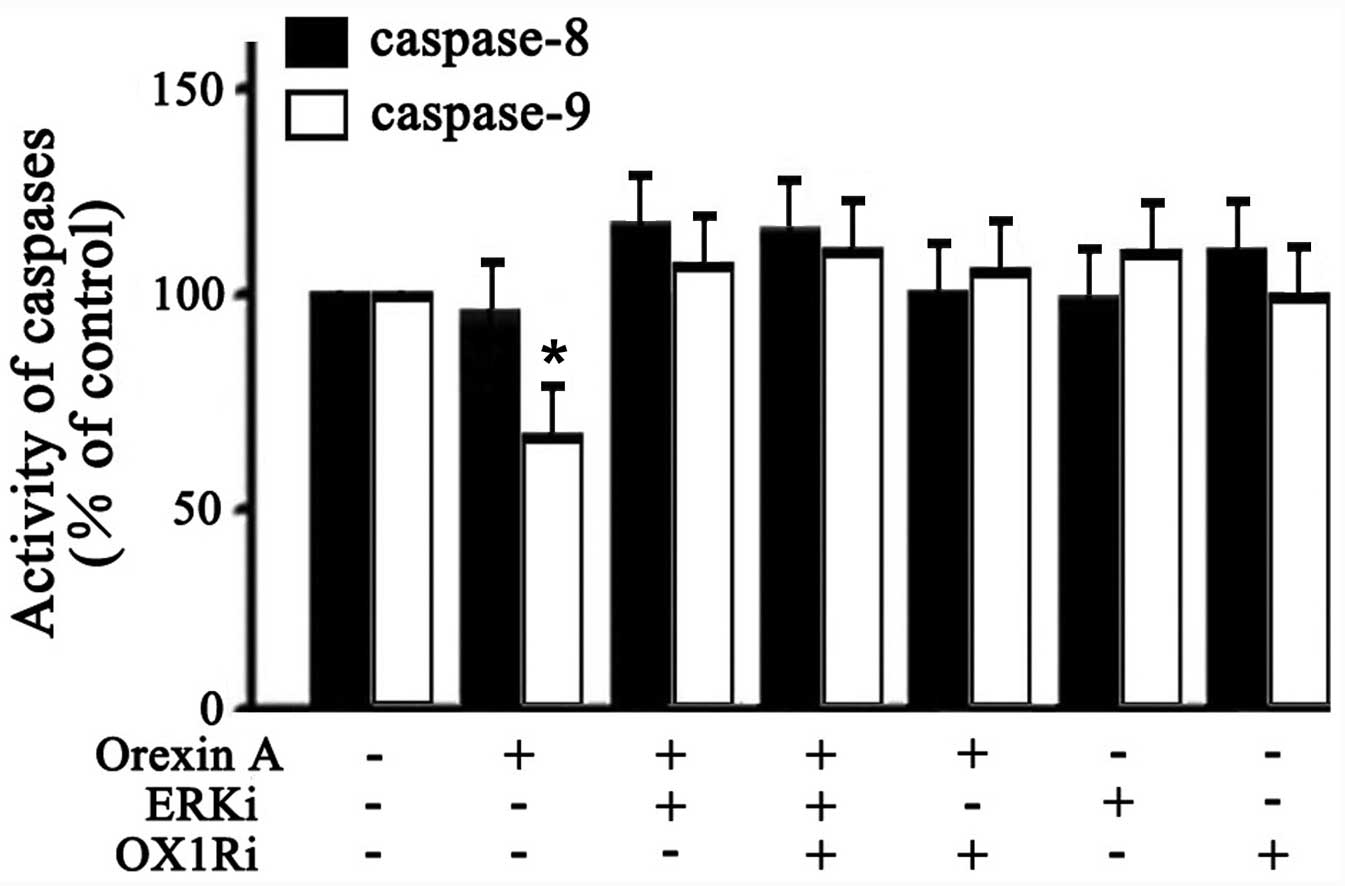
Activities of caspase-8 and caspase-9 in
SGC-7901 cells
The caspase family proteins are critical enzymes
that execute apoptosis. To determine whether the orexin A-induced
anti-apoptotic effect was through extrinsic or intrinsic
mechanisms, caspase-8 and -9 activities was examined. As shown in
Fig. 6, 10−6 M orexin
A treatment caused a significant attenuation in caspase-9 activity
(33% below the control). However, caspase-8 activity in cells was
not significantly changed (Fig.
6). Additionally, the effect of orexin A in caspase-9 activity
was inhibited in the presence of U0126 (30 μM ERKi),
SB334867 (10−6 M OX1Ri), or the combination of these two
antagonists (Fig. 6). These
findings suggested that orexin A protected SGC-7901 cells against
apoptosis through blocking the activation of the pro-apoptotic
executor protease caspase-9 via ERK1/2.
Discussion
To the best of our knowledge, the present study
demonstrated for the first time that OX1R was expressed in
human gastric cancer cells SGC-7901. These results showed that
orexin A promoted proliferation and viability, attenuated caspase-9
activity and protected against apoptotic cell death of SGC-7901
cells. In addition, the pro-survival and anti-apoptotic properties
of orexin A in SGC-7901 cells were associated, at least in part,
with activation of ERK1/2-MAPK pathway.
As an important group of G protein-coupled
receptors, orexin receptors have been identified as expressed in a
number of cancer cell lines. The cell lines expressing OX1R
included human colon carcinoma cells HT29-D4, SW480, LoVo, Caco-2
and human neuroblastoma SK-N-MC (11). OX2R mRNA was expressed in
rat pancreatic acinar tumor line AR42J (13). OX1R and OX2R were detected in rat
C6 glioma cells (14). These cell
lines exhibited decreased cell division and increased cell death
upon orexin exposure. Clinically, recent evidence indicated the
involvement of epigenetic silencing of OX2R in endometrial
endometrioid carcinoma (21).
Compared to normal prostates, expression levels of OX2R were
markedly elevated in adenomatous prostates (22). All these studies point to orexin
receptors as novel therapeutic target in cancer chemotherapy. In
the present study, OX1R was expression was confirmed in
SGC-7901 gastric cancer cells while OX2R mRNA was not
detectable under the same conditions. Orexin A dose-dependently
upregulated the expression of OX1R in SGC-7901 cells.
Instead of causing apoptosis, orexin A exerted survival-promoting
activity through OX1R in SGC-7901 cells, which coincided
with the effects observed in human adrenocortical adenomas
(23) and rat adrenocortical
cells (24). Thus, orexins may
act as a regulatory peptide taking part in cell proliferation and
apoptosis.
MAPK signaling pathways regulate multiple cellular
programs including differentiation, gene expression and
proliferation (25). ERK1/2 is a
main member of the MAPK family and has been well-documented to
associate with cell proliferation and survival (26,27). Several key growth factors and
pro-oncogenes promote cell growth by activating this signaling
cascade (28–31). Previous evidence indicates that
orexins can govern diverse physiological and pharmacological
processes by regulating ERK1/2 activation (32–34). In 3T3-L1 preadipocytes, orexin A
stimulated cell proliferation and viability, and protected the
cells from apoptosis via ERK1/2 signaling pathway (35). Consistent with these observations,
activation of the ERK1/2 in response to orexin A treatment was also
observed in the present study. Furthermore, orexin A-induced cell
proliferation and viability was significantly reduced by
co-treatment with the ERK1/2 antagonist, indicating that the growth
and proliferation of SGC-7901 cells possibly occur through the
ERK1/2 signaling pathway.
Apoptotic signaling is typically mediated by two
main apoptotic pathways, which have been identified as the death
receptor-mediated caspase-8 extrinsic pathway and the
mitochondria-mediated caspase-9 intrinsic pathway (36,37). Caspase-3, characterized as the
downstream executor, cleaves cellular target proteins leading to
cell death. Orexins have been reported to suppress cell growth by
inducing apoptosis through activation of caspase-3 and caspase-9 in
rat pancreatic tumor cells and CHO cells (13). Conversely, orexins can promote
growth of neuronal cells via inhibition of caspase-3 activity
(38). In the present study,
orexin A treatment was show to markedly inhibit serum
starvation-induced apoptosis of SGC-7901 cells, which was further
confirmed by attenuated activity of caspase-9. However, orexin A
had no effect on the activity of caspase-8 under the same
conditions, suggesting that orexin A can inhibit the intrinsic
apoptotic pathway to protect SGC-7901 cells against apoptosis.
Furthermore, activation of ERK signaling is known to inhibit
apoptosis by inactivating the pro-apoptotic proteins (39–41). In the present study, orexin A
treatment failed to inhibit caspase-9 activity in the presence of
the ERK1/2 antagonist. Thus, it can be hypothesized that the
activation of ERK1/2 by orexin A treatment resulted in the
attenuation of caspase-9 activity and subsequent inhibition of
apoptosis in SGC-7901 cells.
Although studies have demonstrated the ability of
orexins to induce apoptosis and subsequently inhibit cell growth in
diverse cancer cells, the effect of orexin signaling appears to be
different in different types of cells. Contrary to expectation,
orexin A stimulated the proliferation and viability of the gastric
cancer cells, SGC-7901, reduced pro-apoptotic activity of caspase-9
and protected the cells from apoptosis. This is similar to a
previous study performed on human adrenocortical adenomas that
express the two orexin receptors, which demonstrated that orexins
stimulated in vitro growth of the tumor cells (23). Orexin A also increased cell
viability and inhibited the activities of caspase-3 and caspase-7
to protect against apoptosis in immortalized primary embryonic rat
hypothalamic R7 cells (42). The
mechanisms underlying dual functions of orexins in the context of
cell growth and death require investigation. Why the peptides raise
proliferative activity in some cells, while in other cells they
induce apoptosis remains to be solved. One possible explanation for
this may be due to the pathways induced by orexins. Studies
performed on CHO cells and human embryonic kidney (HEK-293) stably
expressing human OX1R and OX2R have demonstrated that orexins can
exert the opposite effects on cell survival through activation of
the classical MAPK pathways. The ERK1/2 pathway was central for
cell growth, whereas p38 was important for cell death (15,43). In accordance with these
observations, the orexin A-induced increase in proliferation and
viability of 3T3-L1 preadipocytes was blocked by U0126, an ERK1/2
inhibitor (35), whereas the
suppressive action of orexin A on survival of rat C6 glioma cells
was blocked by SB202190, a specific p38 MAPK inhibitor (14). Another possible explanation may be
the different intrinsic sensitivity of cells to the action of
cytochrome c. In the intrinsic apoptotic pathway, cytochrome
c releases from mitochondria to cytosol, and binds to Apaf-1
resulting in formation of the apoptosome (44). The levels of Apaf-1 may be
different depending on cell type and growth stage (45). However, the understanding of the
function and mechanism of orexin signaling in cancer remains at an
early stage and further studies are required to clarify this novel
field.
In conclusion, the present study demonstrates for
the first time that a physiologically present neuropeptide, orexin
A, has direct pro-survival and anti-apoptotic effects presumably
through OX1R being expressed in SGC-7901 gastric cancer
cells through the ERK1/2 signaling pathway. Overall, these findings
add a new dimension to the biological activities of this
neuropeptide on gastric cancer cells, which may have important
implications in health and disease.
Acknowledgments
The authors are thankful to the China Medical
University Affiliated Hospital Laboratory Center for kindly
providing the equipment required. The present study was supported
by the National Natural Science Foundation of China (grant nos.
30872724, 81071460 and 81271996) and the Natural Science Foundation
of Liaoning Province (grant no. 201202292).
References
|
1
|
De Lecea L, Kilduff TS, Peyron C, et al:
The hypocretins: hypothalamus-specific peptides with
neuroexcitatory activity. Proc Natl Acad Sci USA. 95:322–327. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sakurai T, Amemiya A, Ishii M, et al:
Orexins and orexin receptors: a family of hypothalamic
neuropeptides and G protein-coupled receptors that regulate feeding
behavior. Cell. 92:573–585. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Karteris E and Randeva HS: Orexin
receptors and G-protein coupling: evidence for another
‘promiscuous’ seven transmembrane domain receptor. J Pharmacol Sci.
93:126–128. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nakabayashi M, Suzuki T, Takahashi K, et
al: Orexin-A expression in human peripheral tissues. Mol Cell
Endocrinol. 205:43–50. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Korczynski W, Ceregrzyn M, Matyjek R, et
al: Central and local (enteric) action of orexins. J Physiol
Pharmacol. 57:17–42. 2006.
|
|
6
|
Okumura T and Nozu T: Role of brain orexin
in the pathophysiology of functional gastrointestinal disorders. J
Gastroenterol Hepatol. 26:61–66. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kagerer S, M and Jöhren O: Interactions of
orexins/hypocretins with adrenocortical functions. Acta Physiol.
198:361–371. 2010. View Article : Google Scholar
|
|
8
|
Jöhren O, Neidert S, J, Kummer M, et al:
Prepro-orexin and orexin receptor mRNAs are differentially
expressed in peripheral tissues of male and female rats.
Endocrinology. 142:3324–3331. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu TR, Yang Y, Ward R, Gao L and Liu Y:
Orexin receptors: multi-functional therapeutic targets for sleeping
disorders, eating disorders, drug addiction, cancers and other
physiological disorders. Cell Signal. 25:2413–2423. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Heinonen MV, Purhonen AK, Makela KA and
Herzig KH: Functions of orexins in peripheral tissues. Acta
Physiologica. 192:471–485. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rouet-Benzineb P, Rouyer-Fessard C, Jarry
A, et al: Orexins acting at native OX1 receptor in colon cancer and
neuroblastoma cells or at recombinant OX1 receptor suppress cell
growth by inducing apoptosis. J Biol Chem. 279:45875–45886. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Voisin T, El Firar A, Fasseu M, et al:
Aberrant expression of OX1 receptors for orexins in colon cancers
and liver metastases: an openable gate to apoptosis. Cancer Res.
71:3341–3351. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Voisin T, El Firar A, Avondo V, et al:
Orexin-induced apoptosis: the key role of the seven-transmembrane
domain orexin type 2 receptor. Endocrinology. 147:4977–4984. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bieganska K, Sokolowska P, Johren O and
Zawilska JB: Orexin A suppresses the growth of rat C6 glioma cells
via a caspase-dependent mechanism. J Mol Neurosci. 48:706–712.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ammoun S, Lindholm D, Wootz H, et al:
G-protein-coupled OX1 orexin/hcrtr-1 hypocretin receptors induce
caspase-dependent and-independent cell death through p38
mitogen-/stress-activated protein kinase. J Biol Chem. 281:834–842.
2006. View Article : Google Scholar
|
|
16
|
Roberts PJ and Der CJ: Targeting the
Raf-MEK-ERK mitogen-activated protein kinase cascade for the
treatment of cancer. Oncogene. 26:3291–3310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hong SK, Yoon S, Moelling C, et al:
Noncatalytic function of ERK1/2 can promote Raf/MEK/ERK-mediated
growth arrest signaling. J Biol Chem. 284:33006–33018. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tian PY and Fan XM: The proliferative
effects of ghrelin on human gastric cancer AGS cells. J Dig Dis.
13:453–458. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qian C, Yao J, Wang J, et al: ERK1/2
inhibition enhances apoptosis induced by JAK2 silencing in human
gastric cancer SGC7901 cells. Mol Cell Biochem. 387:159–170. 2014.
View Article : Google Scholar
|
|
20
|
Wu S, Lao XY, Sun TT, et al: Knockdown of
ZFX inhibits gastric cancer cell growth in vitro and in vivo via
down-regulating the ERK-MAPK pathway. Cancer Lett. 337:293–300.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dehan P, Canon C, Trooskens G, et al:
Expression of type 2 orexin receptor in human endometrium and its
epigenetic silencing in endometrial cancer. J Clin Endocr Metab.
98:1549–1557. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Malendowicz W, Szyszka M, Ziolkowska A, et
al: Elevated expression of orexin receptor 2 (HCRTR2) in benign
prostatic hyperplasia is accompanied by lowered serum orexin A
concentrations. Int J Mol Med. 27:377–383. 2011. View Article : Google Scholar
|
|
23
|
Spinazzi R, Rucinski M, Neri G, et al:
Preproorexin and orexin receptors are expressed in
cortisol-secreting adrenocortical adenomas, and orexins stimulate
in vitro cortisol secretion and growth of tumor cells. J Clin
Endocr Metab. 90:3544–3549. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Spinazzi R, Ziolkowska A, Neri G, et al:
Orexins modulate the growth of cultured rat adrenocortical cells,
acting through type 1 and type 2 receptors coupled to the MAPK
p42/p44-and p38-dependent cascades. Int J Mol Med. 15:847–852.
2005.PubMed/NCBI
|
|
25
|
Raman M, Chen W and Cobb MH: Differential
regulation and properties of MAPKs. Oncogene. 26:3100–3112. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ballif BA and Blenis J: Molecular
mechanisms mediating mammalian mitogen-activated protein kinase
(MAPK) kinase (MEK)-MAPK cell survival signals. Cell Growth Differ.
12:397–408. 2001.PubMed/NCBI
|
|
27
|
Junttila MR, Li S-P and Westermarck J:
Phosphatase-mediated crosstalk between MAPK signaling pathways in
the regulation of cell survival. FASEB J. 22:954–965. 2008.
View Article : Google Scholar
|
|
28
|
Yan L, Gu H, Li J, et al: RKIP and 14-3-3ε
exert an opposite effect on human gastric cancer cells SGC7901 by
regulating the ERK/MAPK pathway differently. Dig Dis Sci.
58:389–396. 2013. View Article : Google Scholar
|
|
29
|
Santarpia L, Lippman SM and El-Naggar AK:
Targeting the MAPK-RAS-RAF signaling pathway in cancer therapy.
Expert Opin Ther Targets. 16:103–119. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fang JY and Richardson BC: The MAPK
signaling pathways and colorectal cancer. Lancet Oncol. 6:322–327.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kadowaki Y, Ishihara S, Miyaoka Y, et al:
Reg protein is overexpressed in gastric cancer cells, where it
activates a signal transduction pathway that converges on ERK1/2 to
stimulate growth. FEBS Lett. 530:59–64. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim MK, Park HJ, Kim SR, et al: Angiogenic
role of orexin-A via the activation of extracellular
signal-regulated kinase in endothelial cells. Biochem Biophys Res
Commun. 403:59–65. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ramanjaneya M, Conner AC, Chen J, et al:
Orexin-stimulated MAP kinase cascades are activated through
multiple G-protein signaling pathways in human H295R adrenocortical
cells: diverse roles for orexins A and B. J Endocrinol.
202:249–261. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gorojankina T, Grébert D, Salesse R, et
al: Study of orexins signal transduction pathways in rat olfactory
mucosa and in olfactory sensory neurons-derived cell line Odora:
multiple orexin signaling pathways. Regul Pept. 141:73–85. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Skrzypski M, Kaczmarek P, Le TT, et al:
Effects of orexin A on proliferation, survival, apoptosis and
differentiation of 3T3-L1 preadipocytes into mature adipocytes.
FEBS Lett. 586:4157–4164. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Igney FH and Krammer PH: Death and
anti-death: tumour resistance to apoptosis. Nat Rev Cancer.
2:277–288. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hu W and Kavanagh JJ: Anticancer therapy
targeting the apoptotic pathway. Lancet Oncol. 4:721–729. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sokołowska P, Urbańska A, Namiecińska M,
et al: Orexins promote survival of rat cortical neurons. Neurosci
Lett. 506:303–306. 2012. View Article : Google Scholar
|
|
39
|
Boucher MJ, Duchesne C, Lainé J, et al:
cAMP protection of pancreatic cancer cells against apoptosis
induced by ERK inhibition. Biochem Biophys Res Commun. 285:207–216.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Martin MC, Allan LA, Mancini EJ, et al:
The docking interaction of caspase-9 with ERK2 provides a mechanism
for the selective inhibitory phosphorylation of caspase-9 at
threonine 125. J Biol Chem. 283:3854–3865. 2008. View Article : Google Scholar
|
|
41
|
Allan LA, Morrice N, Brady S, et al:
Inhibition of caspase-9 through phosphorylation at Thr 125 by ERK
MAPK. Nat Cell Biol. 5:647–654. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Butterick TA, Nixon JP, Billington CJ, et
al: Orexin A decreases lipid peroxidation and apoptosis in a novel
hypothalamic cell model. Neurosci Lett. 524:30–34. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tang J, Chen J, Ramanjaneya M, et al: The
signaling profile of recombinant human orexin-2 receptor. Cell
Signal. 20:1651–1661. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Danial NN and Korsmeyer SJ: Cell death:
critical control points. Cell. 116:205–219. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Laburthe M, Voisin T and El Firar A:
Orexins/hypocretins and orexin receptors in apoptosis: a
mini-review. Acta Physiol. 198:393–402. 2010. View Article : Google Scholar
|