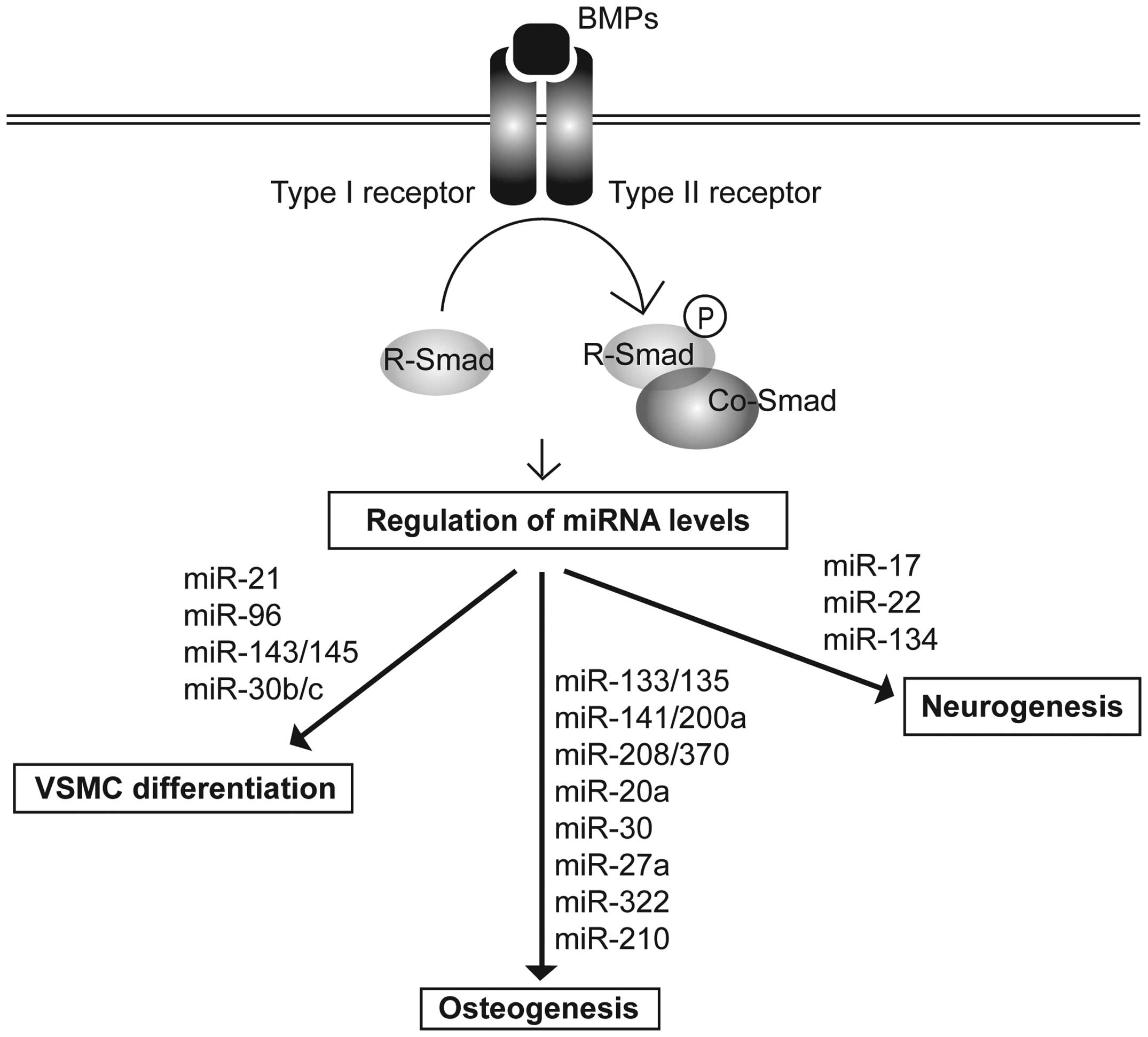
1. Introduction
Bone morphogenetic proteins (BMPs) are members of
the transforming growth factor (TGF)-β superfamily, acting as
potent regulators during embryogenesis and controlling such events
as vascular development, bone formation and neuronal
differentiation (1). In response
to the binding of BMP ligands, a membrane-bound heterotetrameric
complex of type I and type II BMP receptors becomes activated. The
active type II receptor kinase phosphorylates the type I receptor,
which in turn activates the catalytic activity of the type I
receptor. Consequently, the Smad signal transducers are
phosphorylated, and the downstream signal is propagated. In the
canonical signaling pathway, BMPs activate receptor-specific Smads
(R-Smads), Smad1, Smad 5 and Smad 8. The phosphorylation of these
R-Smads promotes their association with the common Smad (co-Smad),
Smad4, in a complex that translocates to the nucleus and regulates
gene transcription either positively or negatively (2). Recently, BMP signaling was
demonstrated to directly control the processing of a cohort of
miRNAs through the non-canonical role of R-Smads (3).
MicroRNAs (miRNAs or miRs) are small non-coding RNA
molecules evolutionarily conserved from plants to humans (4). miRNAs are transcribed by RNA
polymerase II as long primary transcripts known as pri-miRNAs,
which encode either single or multiple miRNAs (5). Hairpin-structured pri-miRNAs are
processed into 60–80 nucleotide (nt) precursor molecules
(pre-miRNAs) by the p68-Drosha microprocessor complex (6). Pre-miRNAs are then exported from the
nucleus to the cytoplasm by exportin 5. In the cytoplasm, the
pre-miRNAs associate with Dicer, which cleaves the pre-miRNA into a
miRNA (mature miRNA) approximately 18–24 nt in size (7,8).
The miRNA duplex is then loaded onto Argonaute proteins and
presented to the RNA-induced silencing complex (RISC) for the
recognition of target mRNAs (9).
The mature miRNA guides the RISC to partially complementary
sequences within the target mRNAs to regulate target gene
expression. The mature miRNAs generally repress protein-coding
genes by promoting the degradation of mRNAs or repressing their
translation (9). Individual
miRNAs have tissue-specific or developmental stage-specific
expression patterns and exhibit a broad range of roles in a wide
range of developmental processes (10).
In the present review, we discuss and summarize the
miRNAs that are mediated by the BMP signaling pathway in essential
biological processes involving vascular smooth muscle cell (VSMC)
differentiation, osteogenesis and neuronal development (Fig. 1).
2. VSMC differentiation
The inactivation of the BMP signaling pathway has
been shown to result in the development of vascular disorders
(11). For example, cells
isolated from patients with heritable pulmonary artery hypertension
(PAH) exhibit mutations of the type II BMP receptor (BMPRII) or
Smad9 (12). Of note, although
the loss of Smad9 function in the canonical BMP signaling pathway
is largely compensated by Smad1 and Smad5, the mutation of Smad9
completely abrogates miRNA induction. This result suggests that the
regulation of miRNAs by BMP signaling is implicated in normal
vascular development and homeostasis. During vascular development,
BMP signaling increases the expression of smooth muscle cell
(SMC)-specific contractile genes and inhibits cell proliferation
and migration, leading to the differentiation of VSMCs (13). The differentiated state of VSMCs
is termed the ‘contractile phenotype’, and VSMCs can switch between
the differentiated and dedifferentiated state in response to
various environmental stimuli (14). Multiple miRNAs have been found to
be regulated by BMP signaling and are responsible for VSMC
differentiation and proliferation under physiological or
pathological conditions.
miRNA-21
Upon TGF-β and BMP signaling, Smads interact with
p68 in the Drosha microprocessor complex and promote the cleavage
of pri-miRNA-21 into pre-miRNA-21, leading to an increase in the
levels of mature miRNA-21 in VSMCs (15). The increased miRNA-21 expression
suppresses the expression of proteins, such as programmed cell
death protein-4 (PDCD4) and multiple members of the dedicator of
cytokinesis (DOCK) family, promoting the contractile phenotype of
VSMCs (16).
In addition to miRNA-21, TGF-β and BMP signals
modulate the expression of a subset of miRNAs through Smad-mediated
post-transcriptional regulation (17). Notably, these miRNAs contain a
conserved sequence (5′-CAGAC-3′) toward the center of the mature
miRNA region that is identical to the consensus sequence for DNA
binding by Smads.
miRNA-96
The regulation of miRNA-96 expression by BMP
signaling is critical for the modulation of the VSMC phenotype
(18). miRNA-96 is downregulated
by BMP4 in VSMCs, which results in the suppression of a novel
target, Tribbles-like protein 3 (Trb3). Trb3 is an essential
positive regulator of the BMP signaling pathway and promotes the
contractile phenotype in VSMCs (19). The BMP-miRNA-96-mediated
upregulation of Trb3 in VSMCs leads to an increase in SMC-specific
gene expression. Unlike the regulation of miRNA-21 by BMP4, the
downregulation of miRNA-96 by BMP4 is dependent on the signal
transducer of the BMP signaling pathway, Smad4 (18).
miRNA-302
BMP signaling also downregulates transcription of
the miRNA-302~367 gene cluster in various types of cells, including
VSMCs (20). This transcriptional
repression of miRNA-302 by BMP signaling is mediated by Smads.
Smad4 associates with the miRNA-302 promoter and recruits histone
deacetylase (HDAC) to repress the transcription of miRNA-302.
BMPRII has been identified as a novel target of miRNA-302. The
functional consequence of the miRNA-302c-dependent downregulation
of BMPRII on the BMP signaling pathway is the inhibition of the
contractile phenotype of VSMCs. Therefore, the regulatory loop of
BMP4-miRNA-302-BMPRII is an essential mechanism for the maintenance
and fine-tuning of the BMP signaling pathway for the modulation of
the VSMC phenotype (20).
miRNA-143/145
miRNA-143 or miRNA-145 knockout mice exhibit an
abnormal vascular tone and reduced SMC-specific gene expression in
VSMCs, suggesting that miRNA-143 and miRNA-145, which are encoded
as a gene cluster, play a critical role in the regulation of the
VSMC phenotype (21). BMP signals
activate the transcription of the miRNA-143/145 gene cluster
through a consensus sequence termed the CArG box by serum response
factor (SRF) and myocardin/myocardin-related transcription factor
(MRTF)-A. miRNA-143/145 promote the contractile phenotype of VSMCs
by regulating the expression of SMC-specific genes and cytoskeletal
dynamics and by inhibiting the proliferation of VSMCs.
miRNA-143/145 also repress multiple targets, including Kruppel-like
factor 4 (KLF4), which is antagonistic to VSMC differentiation
(22).
miRNA-30b/c
miRNA-30b has been shown to be downregulated in
human coronary artery atherosclerosis in calcified atherosclerotic
vessels (23). An increase in
BMP2 expression and a concomitant decrease in miRNA-30b expression
were detected by in situ hybridization with vessels,
suggesting that BMP signaling plays a role in VSMC calcification by
regulating miRNAs. Indeed, a microarray analysis demonstrated that
BMP2 decreases miRNA-30b and miRNA-30c expression, leading to the
promotion of VSMC calcification (23). This downregulation of miRNA-30b
and miRNA-30c is mediated by a Smad-independent pathway.
Runt-related transcription factor 2 (Runx2) was identified as a
target of miRNA-30b and miRNA-30c. Runx2 is a master transcription
factor of the calcification process that induces the
differentiation of osteoblasts and chondrocytes. The downregulation
of miRNA-30b/c by BMP signaling is sufficient to increase Runx2
expression, which in turn results in the increased expression of
the Runx2-dependent genes, osteopontin and osteocalcin, increased
intracellular calcium deposition and the calcification of VSMCs
(23).
3. Osteogenesis
Osteoblast differentiation is a key step in skeletal
development, and precise control is necessary for the prevention of
bone-related diseases. The activation of the TGF-β and BMP
signaling pathways is involved in the differentiation of
mesenchymal stem cells (MSCs) into the osteogenic lineage (24).
BMP2, 4 and 7 act as potential differentiators
through the Smad-mediated activation of osteoblast essential genes,
such as Runx2 (25). Recently,
several miRNAs that are modulated by BMP signaling have been
reported to regulate osteoblast differentiation either positively
or negatively (26).
miRNA-133/135
miRNA microarray analysis has revealed that
miRNA-133 and miRNA-135 are downregulated during the BMP2-induced
osteogenesis of C2C12 mesenchymal cells (27). Both miRNAs functionally inhibit
the differentiation of osteoprogenitors by attenuating the Runx2
and Smad5 pathways. The BMP2-mediated downregulation of miRNA-133
is essential for the induction of Runx2 and osteogenic BMP2
signaling. The downregulation of miRNA-135 by BMP2 also permits BMP
signaling through the derepression of its target, Smad5. Both Runx2
and Smad5 are essential for osteogenesis and synergize for the
activation of bone-specific genes (28). Therefore, BMP2 controls bone cell
determination by downregulating miRNA-133 and miRNA-135 expression,
thereby releasing components required for osteogenic lineage
commitment.
miRNA-141/200a
miRNA expression in BMP-2-treated mouse
pre-osteoblast MC3T3-E1 cells was previously investigated and the
downregulation of miRNA-141 and miRNA-200a was observed (29). miRNA-141 and miRNA-200a modulate
the BMP-2-stimulated pre-osteoblast differentiation. Transfection
experiments with miRNA-141 or miRNA-200a have revealed the
significant suppression of alkaline phosphatase (ALP) activity,
which is widely accepted as a potential osteoblast differentiation
marker. Both miRNA-141 and miRNA-200a target distal-less homeobox 5
(Dlx5). Dlx5 is an osteogenic transcriptional factor that modulates
the expression of BMP2-induced osteogenic transcriptional master
factors, such as Runx2 and Osterix (Osx) (30).
miRNA-208/370
The expression levels of miRNA-208 and miRNA-370
have also been shown to be significantly decreased in BMP2- treated
MC3T3-E1 cells (31,32). In cells transfected with miRNA-208
or miRNA-370, ALP activity and mineralization, as determined by
Alizarin red staining, were suppressed. Moreover, the
overexpression of miRNA-208 or miRNA-370 in primary murine
osteoblast cells significantly attenuated BMP2-induced osteoblast
differentiation (31,32). These results suggest that the
downregulation of miRNA-208 and miRNA-370 is an important common
phenomenon for osteoblast differentiation. miRNA-208 targets an
osteogenic transcriptional factor, V-ets erythroblastosis virus E26
oncogene homolog 1 (Ets1). Ets1 activates the transcription of
osteogenic genes, such as osteopontin (OPN), parathyroid
hormone-related protein (PTHrP), Runx2 and
tenascin-C and type I procollagen (33). Furthermore, Ets1 is highly
expressed during the proliferation stages in BMP2-treated MC3T3-E1
cells (33). Therefore, the
enhanced expression of Ets1 through the downregulation of miRNA-208
and miRNA-370 upon BMP signals may be critical for osteoblast
differentiation.
miRNA-20a
miRNA-20a is a member of the miRNA-17–92 cluster,
which is one of the most extensively studied families of miRNAs.
The members of this family play important roles in tissue and organ
development. During the course of osteogenic differentiation, the
expression of endogenous miRNA-20a has been shown to be increased
(34). Consistently, the
transfection of miRNA-20a mimics or lentiviral miRNA-20a expression
vectors into human MSCs promoted osteogenic differentiation.
Notably, both the transcriptional and translational levels of BMP2,
BMP4 and Runx2 were significantly elevated by miRNA-20a, but were
decreased by anti-miRNA-20a (34). Moreover, miRNA-20a targets
peroxisome proliferator-activated receptor γ (PPARγ), Bambi and
Crim1, the negative regulators of BMP signaling (35). Therefore, miRNA-20a is an
essential positive regulator that activates BMP signaling during
osteogenic differentiation.
miRNA-30
Emdogain is a clinical mixture of enamel matrix
proteins that can induce biomineralization and osteogenesis
(36). The expression profiles of
miRNAs in MC3T3-E1 cells treated with Emdogain were previously
investigated. The data indicated that the expression levels of
miRNA-30 family members, such as miRNA-30a, -30b, -30c and -30d,
were significantly downregulated during emdogain-induced osteoblast
differentiation (37). miRNA-30a
and miRNA-30d have been shown to be downregulated during the
BMP2-induced osteogenesis of C2C12 mesenchymal cells as well
(27), suggesting that the
miRNA-30 family members function as negative regulators of
osteoblastic differentiation. Runx2 and Smad1 were
identified as common target genes of miRNA-30 family members
(27). Smad1 is an immediate
downstream transducing molecule of the BMP receptor and plays an
important role in mediating BMP signaling (2). Therefore, the miRNA-30 family
members affect osteogenesis by modulating BMP signaling.
miRNA-27a
Special AT-rich sequence-binding protein 2 (Satb2)
is a potent transcription factor that promotes osteoblast
differentiation and bone regeneration. Satb2 functions as a protein
scaffold to increase the activity of two essential osteogenic
transcription factors, Runx2 and activating transcription factor 4
(ATF4) (38). The differentially
expressed miRNAs induced by Satb2 overexpression in murine bone
marrow stromal cells were previously investigated using miRNA
microarray, and the downregulation of miRNA-27a was observed during
osteoblast differentiation (39).
miRNA-27a targets BMP2, bone morphogenetic protein receptor, type
IA (BMPR1a) and Smad9, which are involved in the TGF-β/BMP
signaling pathway (39). These
results suggest that the negative regulatory role of miRNA-27a in
Satb2-induced osteogenic differentiation is mediated by directly
targeting positive regulators of the TGF-β/BMP signaling
pathway.
miRNA-322
miRNA-322 has been identified as a regulator of
osteoblast differentiation (40).
miRNA-322 gain- and loss-of-function experiments using C2C12,
MC3T3-E1 cells and primary cultures of murine bone marrow-derived
mesenchymal stem cells (BMMSCs) have demonstrated that miRNA-322
enhances the BMP2 response, increasing the expression of Osx
and other osteogenic genes (40).
The transducer of ERBB2 (Tob2) is characterized as a target of
miRNA-322. Tob2 is a negative regulator of osteogenesis that binds
and mediates the degradation of Osx mRNA (41). Therefore, miRNA-322 decreases
Tob2 mRNA and protein expression, leading to an increase in
Osx expression. The lentivirus-mediated overexpression of miRNA-322
in BMMSCs repressed Smad7, as well as Tob2 and
induced Osx mRNA levels significantly (41).
miRNA-210
The expression profiles of miRNAs during the
osteoblastic differentiation of mouse ST2 mesenchymal stem cells
were obtained by miRNA microarray analyses, and miRNA-210 was found
to be highly expressed in these cells (42). Exogenous miRNA-210 positively
regulates the osteoblastic differentiation of ST2 cells by
targeting activin A receptor type 1B (AcvR1b). AcvR1b is a type I
receptor known to transmit signals to R-Smad, Smad2 and 3, but not
Smad1, 5 or 8, resulting in the transcription of genes that
function as inhibitory regulators of cell proliferation (43). BMP signals, however, are
transmitted through other receptors, such as AcvR1 (Alk2), BMPRIa
(Alk3) and BMPRIb (Alk6), and their signals are transmitted to
Smad1, 5 and 8, thereby initiating osteoblastic differentiation.
Smad2/3 and Smad1/5/8 signaling has been reported to interfere with
each other by competitive binding to a co-Smad, Smad4 (44). Therefore, miRNA-210 acts as a
positive regulator of osteoblastic differentiation by inhibiting
the Smad2/3 signaling pathway by targeting AcvR1b, resulting in the
acceleration of Smad1/5/8-mediated osteoblastic
differentiation.
4. Neurogenesis
In addition to well-characterized roles in bone
development, BMP signaling is crucial during the development of the
nervous system (45). BMP
signaling is involved in the generation of the neural crest and the
induction of both neuronal and glial fates from neural stem cells
or neural precursors in the cortex, hippocampus, midbrain,
hindbrain and spinal cord. By contrast, the inhibition of BMP
signaling is required for the formation of the neural plate
(45). The regulatory mechanism
of BMP signaling is likely to be dependent on spatial and temporal
factors, such as miRNAs. Indeed, the conditional knockout of Dicer
in cortical neural progenitor cells (NPCs) impairs initial neuronal
differentiation and later induces cell death, suggesting that
miRNAs are necessary for appropriate cortical development or
neuronal survival (46). Several
miRNAs have been identified that can modulate the neural cell
lineage during differentiation (47).
miRNA-17
miRNA expression levels in the mouse cortex at
different developmental stages have been investigated. The
expression levels of miRNA-17 have been shown to be decreased in
the developing cortex (48). As
miRNA-17 represses the expression of BMPRII, the downregulation of
miRNA-17 activates the BMP signaling pathway, which facilitates
astrocytogenesis during differentiation (48). However, miRNA-17 promotes NPC
proliferation, and the inhibition of BMP signaling contributes to
miRNA-17-mediated increase of NPC proliferation (49). Therefore, miRNA-17 plays an
important role during cortex development by modulating the BMP
signaling pathway.
miRNA-22
BMPs such as BMP2, 3 and 4 are expressed at the
external germinal layer during postnatal cerebellum development and
function as powerful inhibitors of sonic hedgehog (Shh)-mediated
proliferation of cerebellar granular neuronal precursors (CGNPs)
(50). To address whether the BMP
signals that antagonize Shh-dependent proliferation are, at least
in part, mediated by miRNAs, miRNA expression profiles in CGNPs in
response to Shh were compared with those treated with Shh and BMP2
(51). miRNA-22 levels increased
significantly following treatment with BMP2. miRNA-22 acts
downstream of BMPs to modulate the activity of N-myc in CGNPs
during the development of the cerebellum. The overexpression of
miRNA-22 had a potent anti-proliferative effect, significantly
increasing the cell cycle duration in CGNPs. Moreover, in P7 rat
cerebellum, miRNA-22 distribution largely recapitulated the
combination of BMP2 and BMP4 expression patterns (51). Therefore, BMP-mediated regulation
of miRNA-22 is critical for neurogenesis by reducing the cell
proliferation rate.
miRNA-134
The expression of miRNA-134 has been shown to be
increased during embryonic neuronal differentiation and modulates
dendritic maturation in response to exogenous BMP4 by targeting the
BMP antagonist, Chordin-like 1 (Chrdl-1) (52). The reduction in Chrdl-1 levels
induced by miRNA-134 in dividing NPCs leads to the sensitization of
cortical progenitors to autocrine BMP7 signaling, affecting NPC
proliferation, neuron migration and neuronal maturation (52). Consistently, the sensitivity to
exogenous BMP signals is reduced in miRNA-134-knockdown cells.
Therefore, miRNA-134 is an essential mediator of BMP
signaling-associated cortical development.
5. Conclusions
The BMP signaling pathway is involved in many
cellular processes, including cell growth and differentiation. BMP
signals either upregulate or downregulate a subset of miRNAs, and
these coordinately regulated miRNAs cooperate for BMP
signaling-mediated cellular functions. Therefore, it is important
to understand how cells integrate the complicated regulation of
miRNAs whose expression levels are fine-tuned by BMP signaling
pathways and transmit a precise signal to control normal
development and maintain homeostasis.
In the present review, we summarized the regulation
of miRNA expression during BMP signaling pathway-mediated cellular
differentiation, in particular VSMC differentiation, osteogenesis
and neurogenesis. In most reported cases, miRNA levels are
regulated by BMP signals, but BMP signaling is able to be regulated
by miRNAs through targeting of BMP signal transducers or inhibitory
molecules. For example, miRNA-30 and miRNA-133/135 target Smad1 and
Smad5, respectively (27). This
information provides insight into the mechansisms throug which
miRNAs are integrated into the BMP signaling pathway and may help
in the development of miRNA-based novel approaches to modulate the
BMP signaling pathway for therapeutic applications.
Although the differential expression of a subset of
miRNAs during BMP signaling-mediated differentiation has been
elucidated in various contexts, many factors regulating the
cellular miRNA levels are still unknown. Some miRNAs, such as
miRNA-302, are transcriptionally regulated by direct binding of
Smads to the promoter of the miRNA gene (20). Alternatively, other miRNAs are
post-transcriptionally modulated by the association of Smads with
pri-miRNA, such as miRNA-21 (15). Therefore, elucidating the
mechanisms responsible for the regulation of miRNA remains a future
challenge.
Acknowledgments
The present study was supported by the Incheon
National University International Cooperative Research Grant in
2012 to H.K.
References
|
1
|
Massague J: TGFβ signalling in context.
Nat Rev Mol Cell Biol. 13:616–630. 2012. View Article : Google Scholar
|
|
2
|
Massague J, Seoane J and Wotton D: Smad
transcription factors. Genes Dev. 19:2783–2810. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Davis-Dusenbery BN and Hata A:
Smad-mediated miRNA processing: a critical role for a conserved RNA
sequence. RNA Biol. 8:71–76. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rodriguez A, Griffiths-Jones S, Ashurst JL
and Bradley A: Identification of mammalian microRNA host genes and
transcription units. Genome Res. 14:1902–1910. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Borchert GM, Lanier W and Davidson BL: RNA
polymerase III transcribes human microRNAs. Nat Struct Mol Biol.
13:1097–1101. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Han J, Lee Y, Yeom KH, et al: Molecular
basis for the recognition of primary microRNAs by the Drosha-DGCR8
complex. Cell. 125:887–901. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bernstein E, Caudy AA, Hammond SM and
Hannon GJ: Role for a bidentate ribonuclease in the initiation step
of RNA interference. Nature. 409:363–366. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hutvagner G, McLachlan J, Pasquinelli AE,
Balint E, Tuschl T and Zamore PD: A cellular function for the
RNA-interference enzyme Dicer in the maturation of the let-7 small
temporal RNA. Science. 293:834–838. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Carrington JC and Ambros V: Role of
microRNAs in plant and animal development. Science. 301:336–338.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
ten Dijke P and Arthur HM: Extracellular
control of TGFβ signalling in vascular development and disease. Nat
Rev Mol Cell Biol. 8:857–869. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Drake KM, Dunmore BJ, McNelly LN, Morrell
NW and Aldred MA: Correction of nonsense BMPR2 and SMAD9 mutations
by ataluren in pulmonary arterial hypertension. Am J Respir Cell
Mol Biol. 49:403–409. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lagna G, Ku MM, Nguyen PH, Neuman NA,
Davis BN and Hata A: Control of phenotypic plasticity of smooth
muscle cells by bone morphogenetic protein signaling through the
myocardin-related transcription factors. J Biol Chem.
282:37244–37255. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Owens GK, Kumar MS and Wamhoff BR:
Molecular regulation of vascular smooth muscle cell differentiation
in development and disease. Physiol Rev. 84:767–801. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Davis BN, Hilyard AC, Lagna G and Hata A:
SMAD proteins control DROSHA-mediated microRNA maturation. Nature.
454:56–61. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kang H, Davis-Dusenbery BN, Nguyen PH, et
al: Bone morpho-genetic protein 4 promotes vascular smooth muscle
contractility by activating microRNA-21 (miR-21), which
down-regulates expression of family of dedicator of cytokinesis
(DOCK) proteins. J Biol Chem. 287:3976–3986. 2012. View Article : Google Scholar :
|
|
17
|
Davis BN, Hilyard AC, Nguyen PH, Lagna G
and Hata A: Smad proteins bind a conserved RNA sequence to promote
microRNA maturation by Drosha. Mol Cell. 39:373–384. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim S, Hata A and Kang H: Down-regulation
of miR-96 by bone morphogenetic protein signaling is critical for
vascular smooth muscle cell phenotype modulation. J Cell Biochem.
115:889–895. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chan MC, Hilyard AC, Wu C, et al:
Molecular basis for antagonism between PDGF and the TGFβ family of
signalling pathways by control of miR-24 expression. EMBO J.
29:559–573. 2010. View Article : Google Scholar :
|
|
20
|
Kang H, Louie J, Weisman A, et al:
Inhibition of microRNA-302 (miR-302) by bone morphogenetic protein
4 (BMP4) facilitates the BMP signaling pathway. J Biol Chem.
287:38656–38664. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xin M, Small EM, Sutherland LB, et al:
MicroRNAs miR-143 and miR-145 modulate cytoskeletal dynamics and
responsiveness of smooth muscle cells to injury. Genes Dev.
23:2166–2178. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Davis-Dusenbery BN, Chan MC, Reno KE, et
al: down-regulation of Kruppel-like factor-4 (KLF4) by
microRNA-143/145 is critical for modulation of vascular smooth
muscle cell phenotype by transforming growth factor-beta and bone
morphogenetic protein 4. J Biol Chem. 286:28097–28110. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Balderman JA, Lee HY, Mahoney CE, et al:
Bone morphogenetic protein-2 decreases microRNA-30b and
microRNA-30c to promote vascular smooth muscle cell calcification.
J Am Heart Assoc. 1:e0039052012. View Article : Google Scholar
|
|
24
|
Lin GL and Hankenson KD: Integration of
BMP, Wnt, and notch signaling pathways in osteoblast
differentiation. J Cell Biochem. 112:3491–3501. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bandyopadhyay A, Tsuji K, Cox K, Harfe BD,
Rosen V and Tabin CJ: Genetic analysis of the roles of BMP2, BMP4,
and BMP7 in limb patterning and skeletogenesis. PLoS Genet.
2:e2162006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vimalraj S and Selvamurugan N: MicroRNAs:
synthesis, gene regulation and osteoblast differentiation. Curr
Issues Mol Biol. 15:7–18. 2012.PubMed/NCBI
|
|
27
|
Li Z, Hassan MQ, Volinia S, et al: A
microRNA signature for a BMP2-induced osteoblast lineage commitment
program. Proc Natl Acad Sci USA. 105:13906–13911. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee KS, Kim HJ, Li QL, et al: Runx2 is a
common target of transforming growth factor β1 and bone
morphogenetic protein 2, and cooperation between Runx2 and Smad5
induces osteoblast-specific gene expression in the pluripotent
mesenchymal precursor cell line C2C12. Mol Cell Biol. 20:8783–8792.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Itoh T, Nozawa Y and Akao Y: MicroRNA-141
and -200a are involved in bone morphogenetic protein-2-induced
mouse pre-osteoblast differentiation by targeting distal-less
homeobox 5. J Biol Chem. 284:19272–19279. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ulsamer A, Ortuno MJ, Ruiz S, et al: BMP-2
induces Osterix expression through up-regulation of Dlx5 and its
phosphorylation by p38. J Biol Chem. 283:3816–3826. 2008.
View Article : Google Scholar
|
|
31
|
Itoh T, Takeda S and Akao Y: MicroRNA-208
modulates BMP-2-stimulated mouse preosteoblast differentiation by
directly targeting V-ets erythroblastosis virus E26 oncogene
homolog 1. J Biol Chem. 285:27745–27752. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Itoh T, Ando M, Tsukamasa Y and Akao Y:
Expression of BMP-2 and Ets1 in BMP-2-stimulated mouse
pre-osteoblast differentiation is regulated by microRNA-370. FEBS
Lett. 586:1693–1701. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Raouf A and Seth A: Ets transcription
factors and targets in osteogenesis. Oncogene. 19:6455–6463. 2000.
View Article : Google Scholar
|
|
34
|
Zhang JF, Fu WM, He ML, et al: MiRNA-20a
promotes osteogenic differentiation of human mesenchymal stem cells
by co-regulating BMP signaling. RNA Biol. 8:829–838. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gazzerro E and Canalis E: Bone
morphogenetic proteins and their antagonists. Rev Endocr Metab
Disord. 7:51–65. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Goda S, Inoue H, Kaneshita Y, et al:
Emdogain stimulates matrix degradation by osteoblasts. J Dent Res.
87:782–787. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wu T, Zhou H, Hong Y, Li J, Jiang X and
Huang H: miR-30 family members negatively regulate osteoblast
differentiation. J Biol Chem. 287:7503–7511. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dobreva G, Chahrour M, Dautzenberg M, et
al: SATB2 is a multifunctional determinant of craniofacial
patterning and osteoblast differentiation. Cell. 125:971–986. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gong Y, Xu F, Zhang L, et al: MicroRNA
expression signature for Satb2-induced osteogenic differentiation
in bone marrow stromal cells. Mol Cell Biochem. 387:227–239. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gamez B, Rodriguez-Carballo E, Bartrons R,
Rosa JL and Ventura F: MicroRNA-322 (miR-322) and its target
protein Tob2 modulate Osterix (Osx) mRNA stability. J Biol Chem.
288:14264–14275. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yoshida Y, Tanaka S, Umemori H, et al:
Negative regulation of BMP/Smad signaling by Tob in osteoblasts.
Cell. 103:1085–1097. 2000. View Article : Google Scholar
|
|
42
|
Mizuno Y, Tokuzawa Y, Ninomiya Y, et al:
miR-210 promotes osteoblastic differentiation through inhibition of
AcvR1b. FEBS Lett. 583:2263–2268. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Miyazono K, Maeda S and Imamura T: BMP
receptor signaling: transcriptional targets, regulation of signals,
and signaling crosstalk. Cytokine Growth Factor Rev. 16:251–263.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Maeda S, Hayashi M, Komiya S, Imamura T
and Miyazono K: Endogenous TGF-β signaling suppresses maturation of
osteo-blastic mesenchymal cells. EMBO J. 23:552–563. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hegarty SV, O’Keeffe GW and Sullivan AM:
BMP-Smad 1/5/8 signalling in the development of the nervous system.
Prog Neurobiol. 109:28–41. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
De Pietri Tonelli D, Pulvers JN, Haffner
C, Murchison EP, Hannon GJ and Huttner WB: miRNAs are essential for
survival and differentiation of newborn neurons but not for
expansion of neural progenitors during early neurogenesis in the
mouse embryonic neocortex. Development. 135:3911–3921. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Smirnova L, Grafe A, Seiler A, Schumacher
S, Nitsch R and Wulczyn FG: Regulation of miRNA expression during
neural cell specification. Eur J Neurosci. 21:1469–1477. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Mao S, Li H, Sun Q, Zen K, Zhang CY and Li
L: miR-17 regulates the proliferation and differentiation of the
neural precursor cells during mouse corticogenesis. FEBS J. Dec
9–2013.(Epub ahead of print). PubMed/NCBI
|
|
49
|
Sun Q, Mao S, Li H, Zen K, Zhang CY and Li
L: Role of miR-17 family in the negative feedback loop of bone
morphogenetic protein signaling in neuron. PLoS One. 8:e830672013.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Rios I, Alvarez-Rodriguez R, Marti E and
Pons S: Bmp2 antagonizes sonic hedgehog-mediated proliferation of
cerebellar granule neurones through Smad5 signalling. Development.
131:3159–3168. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Berenguer J, Herrera A, Vuolo L, et al:
MicroRNA 22 regulates cell cycle length in cerebellar granular
neuron precursors. Mol Cell Biol. 33:2706–2717. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Gaughwin P, Ciesla M, Yang H, Lim B and
Brundin P: Stage-specific modulation of cortical neuronal
development by Mmu-miR-134. Cereb Cortex. 21:1857–1869. 2011.
View Article : Google Scholar : PubMed/NCBI
|