Introduction
Late side-effects of anticancer treatment, such as
recurrence, secondary cancer and normal tissue injury from radio-
and chemotherapy, are clinical issues faced by cancer survivors.
Radiation-induced heart disease (RIHD) is a frequent and serious
adverse effect of cancer therapy, affecting the survival and
quality of life of patients (1).
RIHD may occur among patients with breast cancer and Hodgkin’s
lymphoma, as well as in pediatric cancers, as in these patients,
irradiation fields can encompass all or part of the heart (2). Some cancer patients treated with
ionizing radiation (IR) have been shown to exhibit increased
cardiovascular morbidity several years following the administration
of radiotherapy (1,2). The cardiovascular complications of
radiotherapy include atherosclerosis, myocardial degeneration and
valve dysfunction (3). Although
numerous studies performed over the years have expanded our
understanding of RIHD, the mechanisms responsible for RIHD remain
largely unknown. The only available strategies for preventing the
development of RIHD involve reducing the doses of radiotherapy to
the heart. Although the radiation doses to the heart have decreased
in recent years, they still remain high.
Exposure to IR results in the generation of reactive
oxygen species (ROS) that cause damage to DNA, proteins and lipids
(4). The formation of excessive
ROS has been implicated in the aging process and the pathogenesis
of cardiovascular diseases (5).
Cellular senescence is a physiological process of biological aging
through which cells lose their ability to divide and proliferate
and contributes to various physiological and pathological processes
of aging. Senescence is induced in response to various factors,
including IR, that encompasses irreversible growth arrest, normal
tissue repair and tumor suppression (6). Whereas replicative senescence has
been shown to be associated with telomere shortening after repeated
cell division, IR-induced premature senescence occurs in response
to DNA damage and occurs independently of telomere dysfunction
(7).
Natriuretic peptides are polypeptide hormones that
are secreted by the myocardium. These proteins act as powerful
regulators of blood pressure and body fluid homeostasis. In
addition to their endocrine functions, natriuretic peptides exert
autocrine activity that inhibits abnormal cardiac hypertrophy
(8). Corin has been identified as
a cardiac protease that targets natriuretic peptides,
proteolytically cleaving pro-atrial natriuretic peptide (ANP) and
pro-brain natriuretic peptide (BNP) to produce the corresponding
active hormone forms (9,10). The physiological significance of
the function of corin has been demonstrated in studies using
experimental animal models, where the disruption of the
corin gene has been shown to cause hypertension and cardiac
hypertrophy (11). Moreover, in
patients with heart failure, corin variants have been demonstrated
to be related to poor natriuretic peptide processing and overall
clinical outcomes (12). These
data suggest that a corin-deficient status may be a major
contributor to the onset of cardiovascular diseases and raises the
possibility that the pathophysiological function of corin is
relevant in the context of radiation-induced cardiac tissue
injury.
Although heart muscles have been reported to be
resistant to IR, analyses of RIHD have revealed an associated
dysfunction of cardiomyocytes (13). To the best of our knowledge, there
is no study available to date on the association between RIHD and
the IR-induced cellular senescence of cardiomyocytes. Thus, in the
current study, we focused on the involvement of cellular senescence
and possible alterations in corin expression levels in the
development of RIHD. We found that exposure to radiation induced
ROS-mediated cellular senescence in HL-1 and H9C2 myocardial cells.
Notably, corin expression was significantly decreased by the
IR-induced production of ROS in these cells through
post-translational regulation. Importantly, the knockdown of corin
by RNA interference enhanced the IR-induced senescence. On the
contrary, the overexpression of ANP and BNP reversed the IR-induced
senescence. Therefore, these data suggest that defects in corin
function and the reduction of the natriuretic peptide level in
response to IR may contribute to RIHD through the enhancement of
cellular senescence.
Materials and methods
Materials
N-acetylcysteine (NAC), cycloheximide and hydrogen
peroxide used in this study were obtained from Sigma-Aldrich (St.
Louis, MO, USA). The senescence staining kit (ab65351) and
antibodies against corin (ab125254) were purchased from Abcam
(Cambridge, MA, USA). Predesigned small inhibitory RNAs (siRNAs)
specific for corin (si-corin; 1341918) and for negative control
(si-control, SN-1001) were purchased from Bioneer (Seoul, Republic
of Korea). Antibodies against ANP (sc-18811), hemagglutinin (HA,
sc-805), furin (sc-20801), dipeptidyl peptidase IV (sc-9153), and
β-actin (sc-47778) were from Santa Cruz Biotechnology, Inc. (Santa
Cruz, CA, USA). Antibody against BNP (bs-2207R) was purchased from
Bioss (Kensington, Australia). V5-tagged expression plasmids of
natriuretic peptides (ANP-V5 and BNP-V5) were a gift from Dr Qingyu
Wu (Departments of Molecular Cardiology, Nephrology and
Hypertension, Lerner Research Institute, Cleveland Clinic,
Cleveland, OH, USA). The pcDNA3.1/V5-His-TOPO expression vector
(vector) and antibody against V5 (R961-25) were purchased from Life
Technologies (Carlsbad, CA, USA). Expression plasmids of Corin
(HA-corin, EX-U0424-M6) were from GeneCopoeia, Inc. (Rockville, MD,
USA).
Cell culture, radiation exposure, cell
counting and transfection
HL-1 cells, a gift from Dr William C. Claycomb
(Department of Biochemistry and Molecular Biology, LSU Health
Sciences Center, New Orleans, LA, USA), were grown in Claycomb
medium. H9C2 cells (ATCC® CRL-1446; ATCC, Manassas, VA,
USA) were grown in Dulbecco’s modified Eagle’s medium (DMEM),
supplemented with 10% heat-inactivated fetal bovine serum and
antibiotics. All cells were maintained at 37°C in a humidified 5%
CO2 atmosphere. For γ-irradiation, the cells were
exposed to a 137Cs γ-ray source (Atomic Energy of Canada
Ltd., Ontario, ON, Canada) at a dose rate of 3.2 Gy/min. Cells were
treated with cycloheximide (20 μg/ml) with or without
irradiation and were harvested at the indicated time points.
Hydrogen peroxide (H2O2) was added to the
cell culture medium at a final concentration of 100 μM. The
cells were counted using a trypan blue exclusion procedure to
identify live cells. The cells were transfected with plasmids or
siRNA using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA).
Propidium iodide (PI) staining
The trypsinized cells were washed twice with PBS
[137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4 and
1.4 mM KH2PO4 (pH 7.4)] and collected by
centrifugation at 200 × g for 10 min. The supernatant was discarded
and pelleted cells were carefully suspended in 500 μl PBS.
PI was then added to a final concentration of 40 μg/ml and
the cells were analyzed on a FACSCanto II flow cytometer (BD
Biosciences, San Jose, CA, USA).
Senescence-associated β-galactosidase
(SA-β-gal) staining
The cells were stained for SA-β-gal activity as
previously described (14).
Briefly, the cells were washed in PBS, fixed for 10 min (room
temperature) in 3% formaldehyde. The cells were then incubated at
37°C with fresh senescence-associated (SA-β-Gal) staining solution:
1 mg of 5-bromo-4-chloro-3-indolyl P3-D-galactoside (X-Gal) per ml
(stock = 20 mg of dimethylformamide per ml)/40 mM citric
acid/sodium phosphate, pH 6.0/5 mM potassium ferrocyanide/5 mM
potassium ferricyanide/150 mM NaCl/2 mM MgCl2. Staining
was evaluated after a 16-h incubation at oven temperature in a
CO2-free atmosphere. The percentage of blue cells (200
cells) observed under a light microscope was calculated.
Measurement of ROS generation
The cells were incubated with 10 μM
2′,7′-dichlorodihydrofluorescein diacetate (DCF-DA; Molecular
Probes, Eugene, OR, USA) dye for 30 min. The intensity of DCF-DA
fluorescence was determined using a FACSCanto II flow cytometer.
(BD Biosciences).
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was extracted and reverse transcribed
using Omniscript transcriptase (Qiagen, Hilden, Germany). PCR
amplifications were performed using the following primer pairs:
corin, 5′-ACC TCT CCT GCA GAG TCC CT-3′ (sense) and 5′-ATG TTC CCA
CAA AGG ACA GC-3′ (antisense); ANP, 5′-GGG GGT AGG ATT GAC AGG
ATT-3′ (sense) and 5′-CGT GAT AGA TGA AGG CAG GA-3′ (antisense);
BNP, 5′-GCC AGT CTC CAG AGC AAT TC-3′ (sense) and 5′-AAG AGA CCC
AGG CAG AGT CA-3′ (antisense); GAPDH, 5′-TGC TGA GTA TGT CGT GGA
GTC TA-3′ (sense) and 5′-AGT GGG AGT TGC TGT TGA AGT CG-3′
(antisense). The PCR thermocycling conditions were as follows: 95°C
for 5 min followed by 30 cycles of 94°C for 45 sec, 60°C for 2 min
and 72°C for 2 min.
Immunoblot analysis
Protein samples were prepared by extracting the
cells using lysis buffer (50 mM Tris-HCl pH 7.4, 1% NP-40, 0.25%
sodium deoxycholate, 150 mM NaCl, 1 mM EDTA, 1 mM PMSF, 1 mM sodium
orthovanadate, 1 mM NaF, 1 μg/ml aprotinin, 1 μg/ml
leupeptin and 1 μg/ml pepstatin). Proteins were resolved by
sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then
transferred onto a nitrocellulose membrane. The membrane was
incubated with the primary antibody at 4°C overnight and then with
the appropriate peroxidase-conjugated secondary antibody for 1 h at
room temperature. Immunoreactive proteins were visualized using
enhanced chemiluminescence reagents.
Statistical analysis
All data are expressed as the means ± standard
deviation (SD), and the mean values were obtained from at least 3
independent experiments. Statistical significance between 2 groups
was determined using a Wilcoxon signed-rank test; a value of
P<0.05 was considered to indicate a statistically significant
difference. All statistical analyses were performed using IBM SPSS
Statistical software version 20.0 (SPSS, Inc., Chicago, IL,
USA).
Results
IR induces cellular senescence in HL-1
and H9C2 myocardial cells
It is well accepted that cellular senescence occurs
in response to IR (6,7,15).
Thus, it is reasonable to investigate whether the exposure of
myocardial cells to radiation results in a senescent phenotype that
may be involved in myocardial dysfunction. In this study, before
examining the effects of IR on myocardial cell senescence, we
examined whether IR induces myocardial cell death through an
apoptotic mechanism. FACS analysis of PI-stained cells showed that
a 96-h exposure to IR at doses of 8 and 15 Gy induced <10 and
20% apoptosis, respectively (data not shown). Instead of inducing
cell death, IR significantly decreased the number of HL-1 and H9C2
cells compared with the untreated control cells, an effect that was
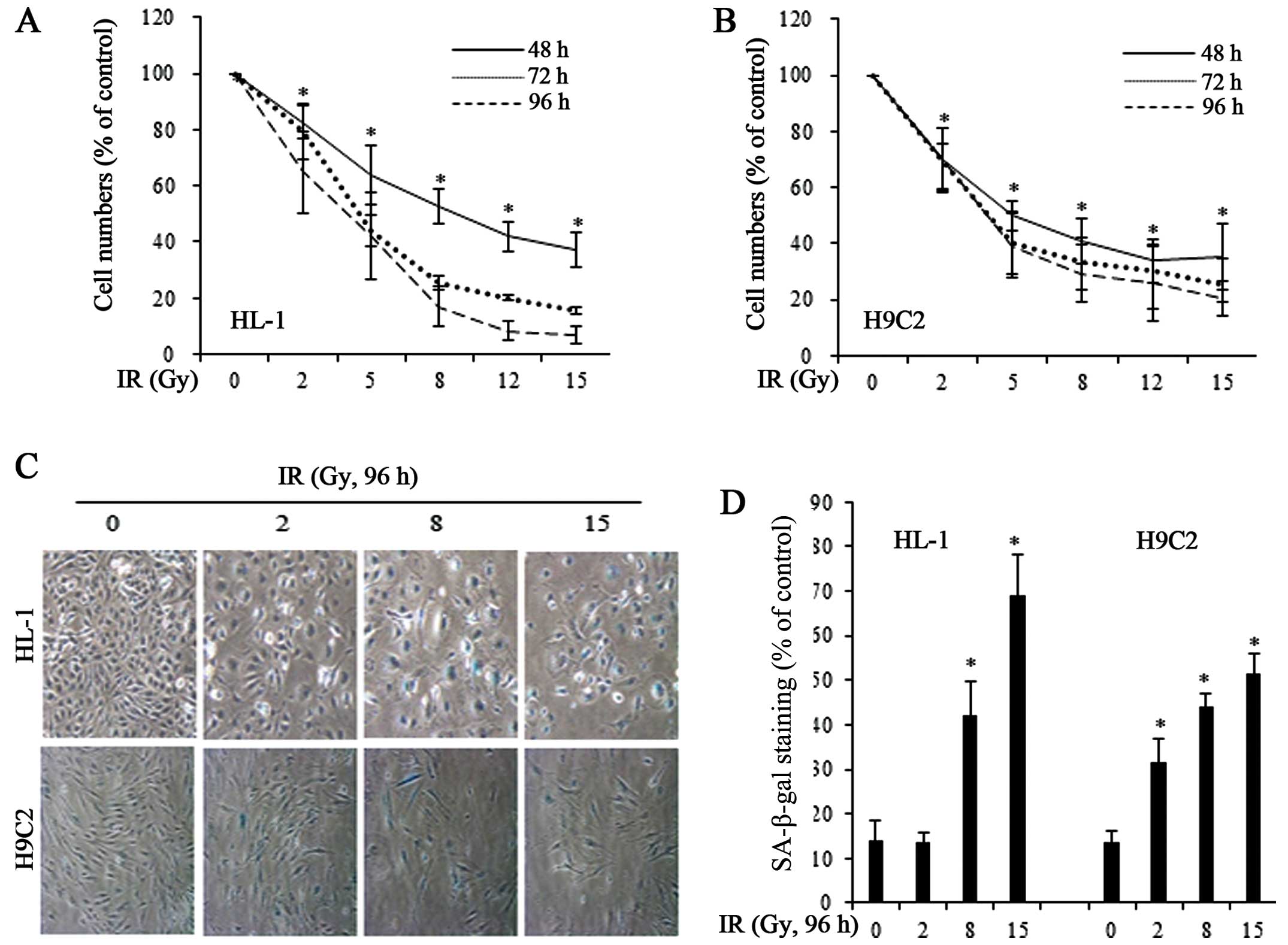
dose-dependent (Fig. 1A and B).
To determine whether the growth-inhibitory effects of IR were due
to cellular senescence, we exposed the HL-1 and H9C2 cells to IR
and stained for SA-β-gal, a marker of senescent cells (Fig. 1C). We found that 8 and 15 Gy of IR
significantly increased the number of SA-β-gal-positive, senescent
cells in both cell lines (Fig.
1D).
IR-induced ROS production causes
senescence of myocardial cells
To elucidate the mechanisms responsible for
IR-induced cellular senescence, we investigated the role of ROS
generation, which is generally considered a consequence of
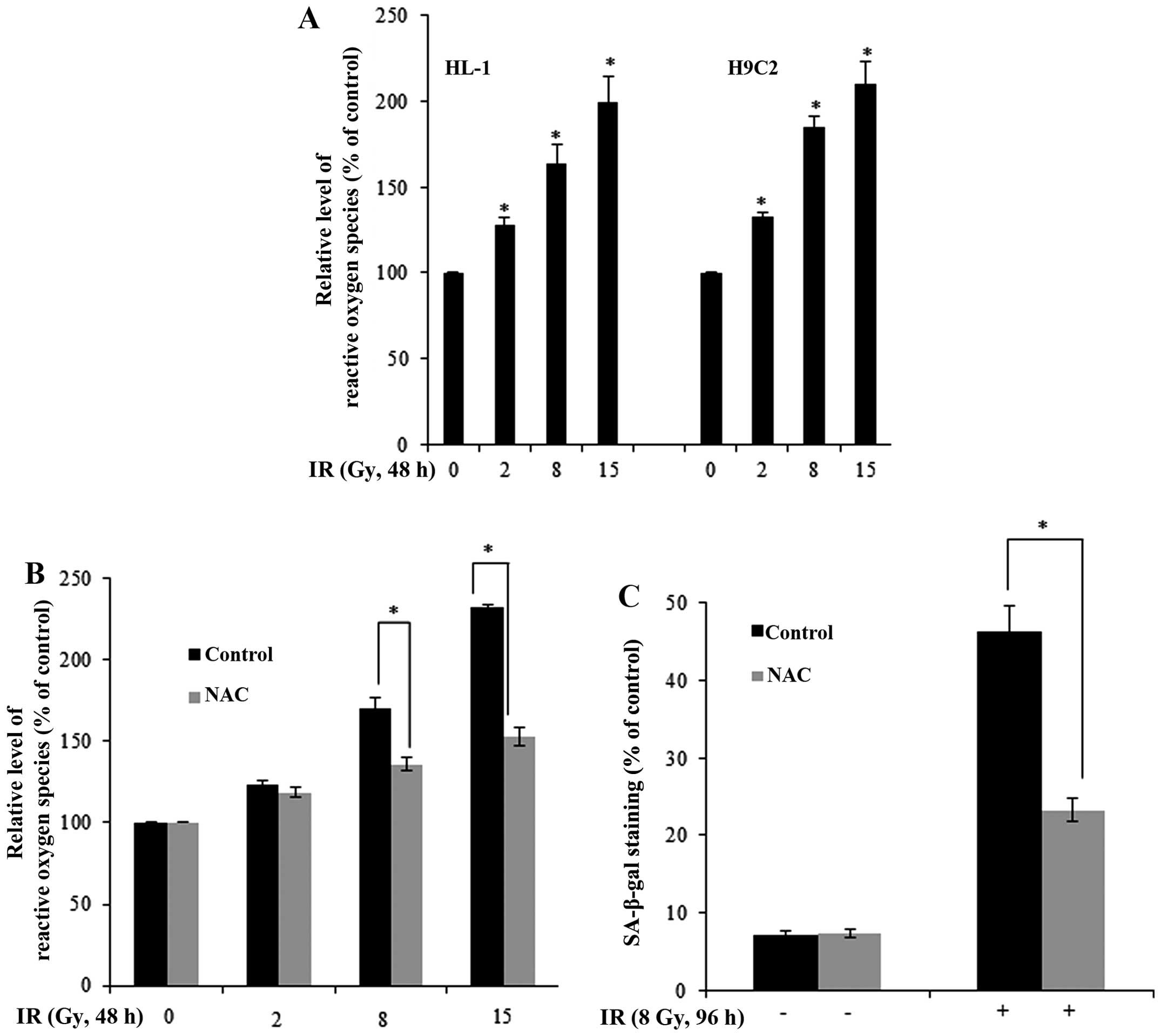
radiation exposure and a cause of cellular senescence (16,17). Using the fluorescent reactive
oxygen probe, DCF-DA, as an indicator, we found that IR increased
the generation of ROS in the HL-1 and H9C2 cells (Fig. 2A). Pre-treatment with NAC, a
scavenger of ROS, blocked the IR-induced generation of ROS in HL-1
cells (Fig. 2B). Notably, NAC
significantly inhibited the IR-induced cellular senescence in the
IR-exposed HL-1 cells (as shown by reduced SA-β-gal staining;
Fig. 2C), indicating that ROS is
a mediator of IR-induced cellular senescence in myocardial
cells.
Corin expression levels are decreased
following IR-induced ROS generation in myocardial cells
As previously demonstrated, a corin-deficient state
is closely associated with heart failure (11). However, the question of whether
the function of corin is critically involved in the process of RIHD
has not yet been answered. Therefore, in the present study, we
aimed to examine whether IR affects the activity of corin,
initially examining the protein levels of corin following the
exposure of HL-1 and H9C2 myocardial cells to IR. We found that the
corin expression levels were significantly decreased following
exposure of the HL-1 and H9C2 cells to IR (8 and 15 Gy) (Fig. 3A). In immunoblot analysis, the
protein levels of furin and dipeptidyl peptidase IV, which are also
proteases for natriuretic peptides, were not significantly altered
by exposure to radiation in the HL-1 cells, indicating that the
effect of IR was selective for corin (Fig. 3B). Likewise, irradiation for 48 h
resulted in a significant reduction in ANP and BNP in HL-1 cells
(Fig. 3B). The requirement of the
cyclin-dependent kinase inhibitor, p21, in cellular senescence was
confirmed by the upregulation of this protein following irradiation
(Fig. 3A). To investigate whether
the IR-induced decrease in corin protein expression is caused by
the inhibition of transcription, we determined corin mRNA levels by
RT-PCR. The corin mRNA levels were not altered in the cells exposed
to IR; the ANP and BNP mRNA levels were also unaltered in these
cells (Fig. 3C). Therefore, the
decrease in the protein levels of corin, ANP and BNP induced by IR
likely reflects post-translational regulation.
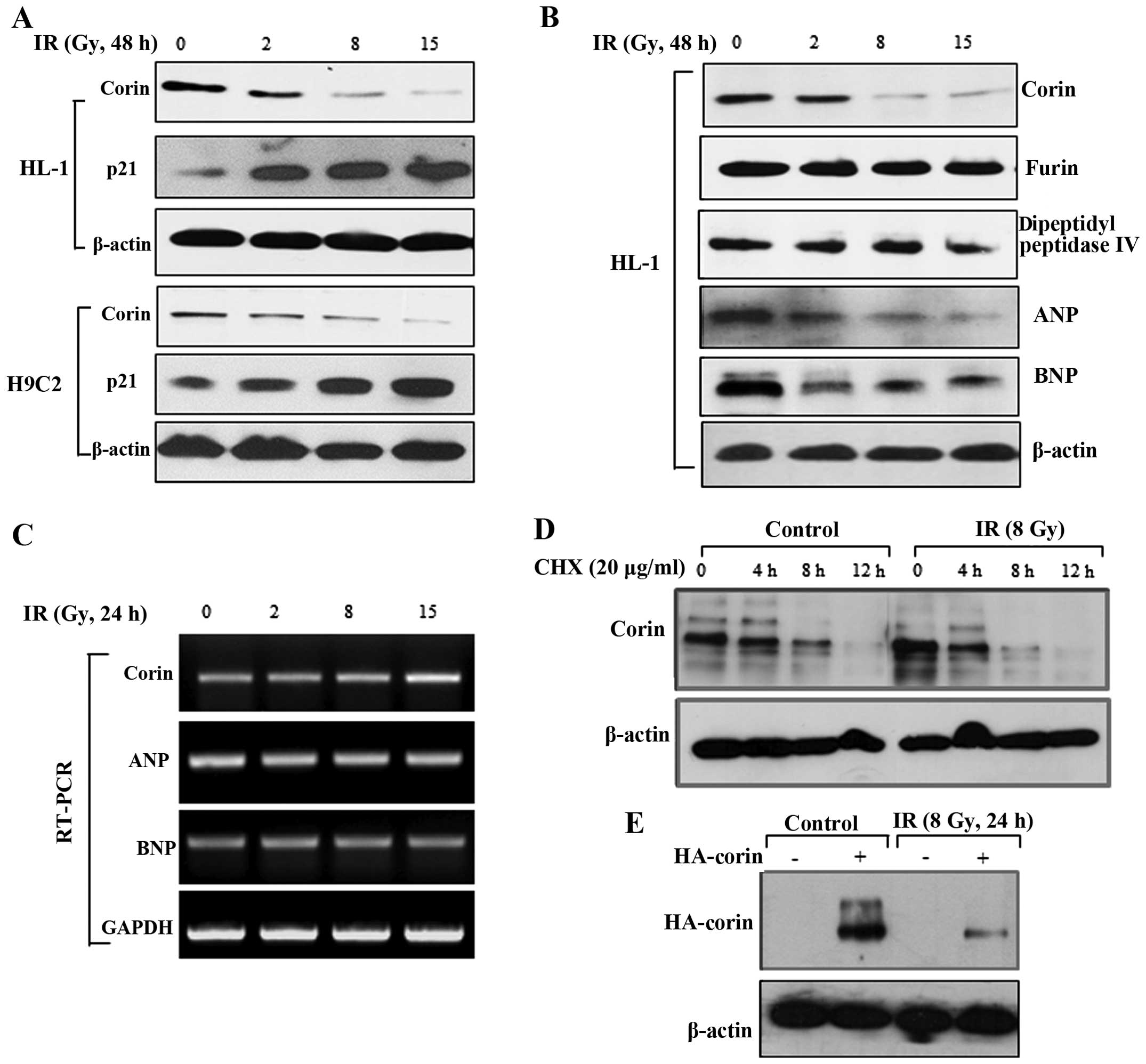 | Figure 3The levels of corin protein in
myocardial cells are decreased by exposure to ionizing radiation
(IR). (A) After 48 h of irradiation, the protein levels of corin
and p21 in the HL-1 and H9C2 cells were measured by immunoblot
analysis. (B) HL-1 cells were exposed to 2, 8 or 15 Gy of IR, and
then the protein levels of corin, furin, dipeptidyl peptidase,
atrial natriuretic peptide (ANP) and brain natriuretic peptide
(BNP) were analyzed by immunoblot analysis. (C) After 24 h of
irradiation, the mRNA levels of corin, ANP and BNP in the HL-1
cells were determined by RT-PCR. (D) HL-1 cells were treated with
cycloheximide (20 μg/ml) with or without irradiation. Cell
lysates were harvested at the indicated time points, and corin
expression levels were determined by immunoblot analysis. (E)
Following transient transfection with HA-tagged corin, the HL-1
cells were exposed to 8 Gy of IR, and the levels of HA-tagged corin
in cell lysates were determined by immunoblot analysis 24 h after
irradiation. |
To determine the effect of IR on corin protein
stability, we measured protein degradation in the HL-1 cells
treated with the protein synthesis inhibitor, cycloheximide.
Treatment with cycloheximide decreased corin stability in the
IR-exposed cells when compared with the control cells, suggesting
that corin stability was decreased by IR (Fig. 3D). We also transiently transfected
the HL-1 cells with an HA-tagged corin construct and then exposed
these cells to IR. The high of corin expression levels were
decreased following exposure to IR (Fig. 3E). These observations suggest that
IR affects corin protein stability in myocardial cells.
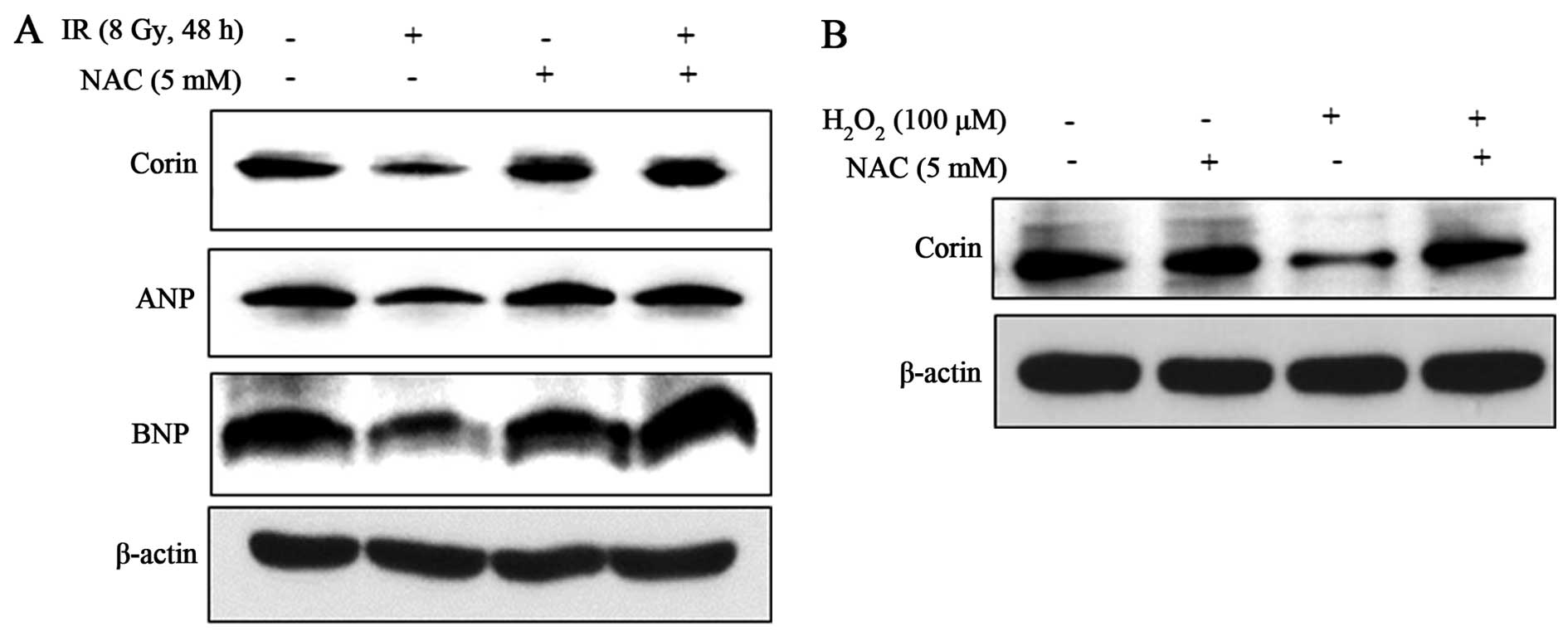
Importantly, the decrease in corin, ANP and BNP protein levels in
the IR (8 Gy)-exposed HL-1 cells was significantly attenuated by
NAC (Fig. 4A), indicating that IR
exerts its effects by promoting the generation of ROS to
downregulate these proteins. We confirmed the positive effects of
the IR-induced generation of ROS on the reduction of corin protein
expression by exposure of the cells to exogenous hydrogen peroxide
(100 μM), which resulted in a decrease in corin levels
(Fig. 4B). These results suggest
that IR-induced ROS is associated with the decrease in corin
expression levels in HL-1 cells.
A corin-deficient status enhances the
IR-induced senescence of myocardial cells
Given that corin may positively regulate cardiac
function through the activation of ANP and BNP, we examined whether
the IR-induced decrease in corin expression levels is critically
involved in the radiation-induced senescence of myocardial cells.
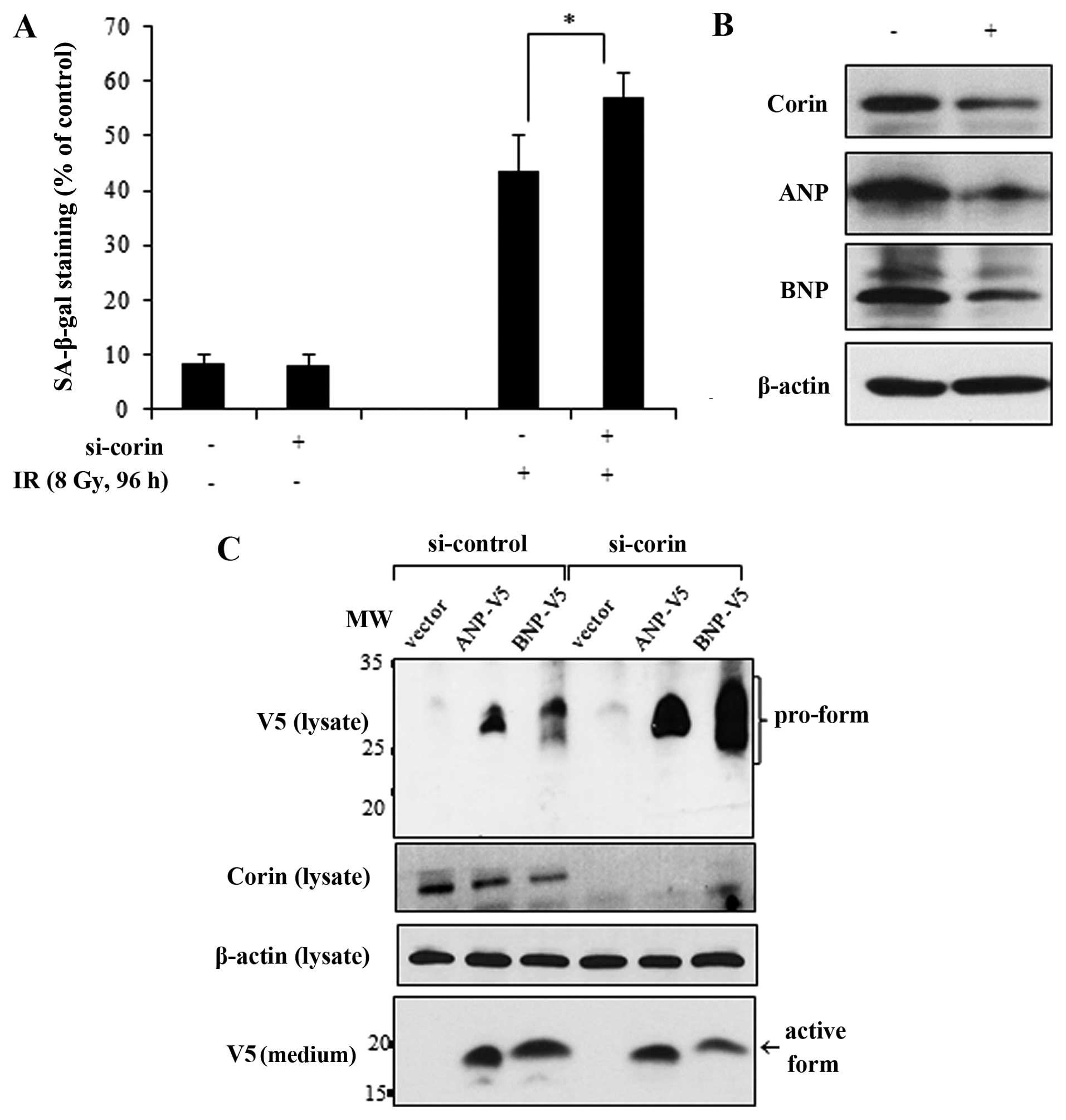
To accomplish this, we transfected the HL-1 cells with si-corin and
then exposed them to IR. Compared with the effects of IR in the
si-control-transfected cells, the IR-induced cellular senescence
was enhanced in the corin-deficient cells (Fig. 5A). The knockdown of corin and the
subsequent reduction of ANP and BNP expression levels were
confirmed by immunoblot analysis (Fig. 5B). ANP and BNP are synthesized in
cardiomyocytes as propeptides and must be cleaved by corin to be
secreted (9,10). If the pro-form of natriuretic
peptides is not cleaved by corin, it remains in an inactive state.
Therefore, to confirm the relevance of corin activity in the
proteolytic processing of natriuretic peptides in myocardial cells,
we overexpressed V5-tagged ANP and BNP in the si-corin-transfected
HL-1 cells and determined the cleavage and release of ANP and BNP
into the culture medium. The pro-form of each natriuretic peptide
with a C-terminal V5 tag was detected in the cell lysates by
immunoblot analysis, and the active forms of ANP and BNP were
detected in the medium as 25–35 and 12–17 kDa bands, respectively.
However, in the corin-deficient HL-1 cells, the protein levels of
pro-ANP and pro-BNP, which were not completely cleaved, were
increased in the cell lysates. Conversely, there was a decrease in
the amount of active ANP and BNP forms in the culture medium
(Fig. 5C). Collectively, these
results indicate that the IR-induced inhibition of corin and the
subsequent reduction in natriuretic peptides are critically
involved in the cellular senescence response to IR in myocardial
cells.
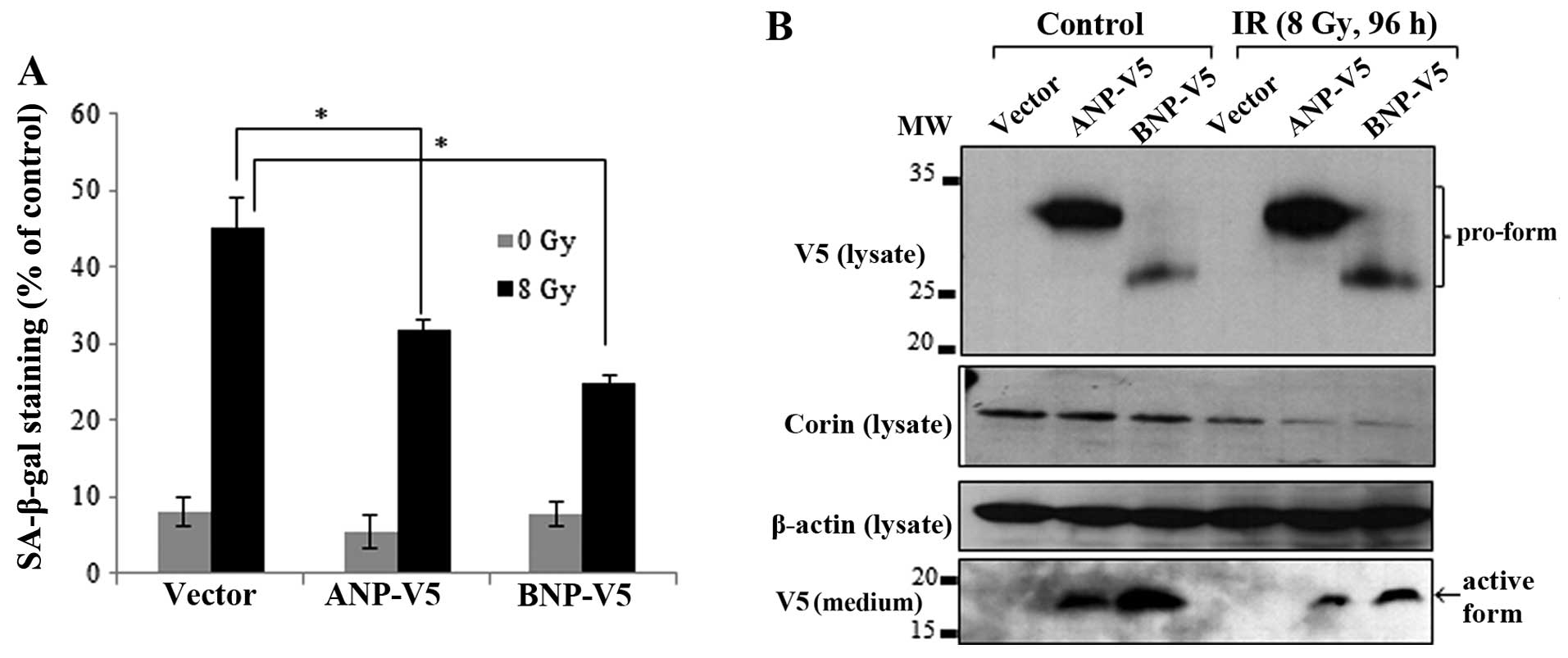
Overexpression of natriuretic peptides
reverses the IR-induced senescence of myocardial cells
If IR-induced senescence is indeed caused by the
inhibition of corin and a subsequent decrease in ANP and BNP
levels, the overexpression of these peptides should rescue cells
from IR-induced senescence. In order to confirm this hypothesis,
the HL-1 cells were transfected with V5-tagged ANP and BNP. The
overexpression of ANP and BNP partially reversed the IR-induced
senescence of HL-1 cells (Fig.
6A). The corin expression levels in the cell lysates and the
expression of V5-tagged ANP and BNP proteins in the cell lysates
and culture medium were confirmed by immunoblot analysis. Although
the levels of the pro-forms of ANP and BNP were increased in the
irradiated cell lysates, the active forms of ANP and BNP in the
culture medium were reduced (Fig.
6B).
Discussion
Despite advances in radiotherapy safety, RIHD may
occur among patients with breast cancer and Hodgkin’s lymphoma, as
well as in childhood cancers and in other types of cancer-following
treatment with radiation where the heart is also exposed (3). Although RIHD is becoming a great
concern for cancer patients and clinicians, there are only a few
methods available to prevent or reverse RIHD, and the underlying
biological mechanisms remain poorly understood. Importantly, in
vivo studies have suggested that myocardial degeneration and
fibrosis are involved in the pathogenesis of RIHD (13). Sridharan et al (18) demonstrated that a cardiac
inflammatory response is a major reaction of the heart to
irradiation. Another important finding is that IR causes
alterations in cardiac mitochondria, resulting in oxidative stress
that may ultimately lead to dysfunction of the myocardium (19). Given that oxidative stress has
been suggested to be a major contributor to IR-induced heart
damage, researchers have investigated the effects of antioxidants
on radiation injury as part of an effort to establish potential
therapeutic agents against RIHD (20). However, the underlying
mechanism(s) remain unknown, and therapeutic strategies for the
treatment or prevention of RIHD remain in the pre-clinical stage
(2). Thus, investigations into
the mechanisms of RIHD, as well as the identification of novel
therapeutic targets and the development of effective strategies for
the prevention of RIHD are essential.
To investigate the molecular mechanisms responsible
for the development of RIHD, we examined the effects of IR in
myocardial cells. We found that IR induced ROS-mediated cellular
senescence in myocardial cells (Figs.
1 and 2). This increase in
the senescence of IR-exposed myocardial cells was associated with a
decrease in corin expressino levels and the inactivation of
natriuretic peptides (Figs. 3 and
4). Importantly, the knockdown of
corin enhanced the IR-induced senescence of myocardial cells
(Fig. 5). By contrast, the
overexpression of ANP and BNP reversed this phenomenon (Fig. 6). Overall, these observations
suggest that the senescence of the myocardium and its progressive
degeneration, caused by a decrease in corin expression and its
substrates, may be involved in the pathogenesis of RIHD.
Corin is known as an essential protease for
processing pro-natriuretic peptides in cardiomyocytes and thereby
regulates multiple physiological functions in the cardiovascular
system (9). The fact that corin
has been placed center-stage in the study of the progression of
heart diseases highlights the importance of exploring the
association between corin expression and RIHD severity and/or
phenotype in cancer patients receiving radiotherapy (21). The two most intensively studied
substrates of corin, ANP and BNP, are closely linked to
compensatory mechanisms in failing hearts (11,21). An elevation in BNP levels has been
suggested to reflect an attempt to correct a serious dysfunction of
the heart (22). Significantly, a
synthetic natriuretic peptide analogue has been used clinically in
patients with heart failure (12,22). However, the potential use of
natriuretic peptide analogues in cancer patients receiving
radiotherapy is underappreciated, and may represent a promising
therapeutic strategy in RIHD.
Importantly, it has been shown that corin expression
is downregulated during heart failure (11,21). Soluble corin is detected in human
plasma, and its levels are decreased in patients with heart failure
(21). In addition, high levels
of the pro-form of plasma natriuretic peptides have also been found
in cardiovascular disease patients (23). In agreement with these studies,
our results demonstrated that corin and the active forms of
natriuretic peptides were decreased in response to IR in myocardial
cells. The IR-induced decrease in corin protein expression was
accompanied by a decrease in corin protein stability without a
change in mRNA levels, suggesting a post-translational mechanism
(Fig. 3). Similar to the decrease
observed in corin protein stability, both the levels of ANP and BNP
were also significantly reduced by IR (Fig. 3B and C). The mechanisms behind
this concomitant decrease in proteases and their substrates remain
unresolved, as it is questionable whether the loss of corin
function is the reason underlying the decrease in ANP and BNP
levels. Further studies are required to clarify the association
between the reduction in corin protein levels and the decrease in
the active forms of natriuretic peptides in irradiated myocardial
cells.
Since corin is generally considered the master
protease of natriuretic peptides, we overexpressed V5-tagged ANP
and BNP in HL-1 cells in conjunction with si-corin transfection or
IR exposure to evaluate the corin-mediated processing of ANP and
BNP. Importantly, the knockdown of corin or exposure to IR
increased the pro-form of natriuretic peptides and subsequently
decreased the active forms of ANP and BNP (Figs. 5C and 6B). These results suggest that a lesser
degree of active natriuretic peptides cleavage ensues as a
consequence of the IR-induced decrease in corin expression
levels.
However, the decrease in the active forms of
natriuretic peptides in the culture medium as a result of the
knockdown of corin or the IR-induced decrease in corin expression
levels was only partial (Figs. 5C
and 6B), suggesting that the
pro-forms of natriuretic peptides may be processed by other
proteases, such as furin and dipeptidyl peptidase IV, which are
expressed in HL-1 cells (11,24). Although the natriuretic peptide
processing induced by IR was incomplete (Fig. 6B), the overexpression of ANP and
BNP reversed the IR-induced senescence of HL-1 cells (Fig. 6A). It remains to be determined
whether IR affects the enzymatic activity of multiple proteases,
including furin and dipeptidyl peptidase IV in cardiomyocytes.
Future studies on senescence in the myocardium and
changes in the expression levels of corin and natriuretic peptides
in vivo using experimental animal models of RIHD are
required to provide fundamental information on the diagnosis and
prognosis of RIHD, as well as on the therapeutic applications of
natriuretic peptide analogues. In view of our findings, corin may
be required for appropriate myocardial function during or following
exposure to IR. Additionally, the modulation of corin activity and
treatment with natriuretic peptides analogues, which are valuable
for further understanding the myocardial responses to IR, may
become possible therapeutic strategies for RIHD.
Acknowledgments
We would like to thank Dr Willliam C. Claycomb
(Department of Biochemistry and Molecular Biology, LSU Health
Sciences Center, New Orleans, LA, United States of America) and Dr
Qingyu Wu (Departments of Molecular Cardiology, Nephrology and
Hypertension, Lerner Research Institute, Cleveland Clinic,
Cleveland, OH, USA) for providing the HL-1 cells and the
natriuretic peptide expression plasmids. This study was supported
by the Nuclear Research and Development Program through the
National Research Foundation of Korea (NRF) (grant no.
2012M2A2A7012483).
References
|
1
|
Jaworski C, Mariani JA, Wheeler G and Kaye
DM: Cardiac complications of thoracic irradiation. J Am Coll
Cardiol. 61:2319–2328. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Darby SC, Cutter DJ, Boerma M, et al:
Radiation-related heart disease: current knowledge and future
prospects. Int J Radiat Oncol Biol Phys. 76:656–665. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee PJ and Mallik R: Cardiovascular
effects of radiation therapy: practical approach to radiation
therapy-induced heart disease. Cardiol Rev. 13:80–86. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Azzam EI, Jay-Gerin JP and Pain D:
Ionizing radiation-induced metabolic oxidative stress and prolonged
cell injury. Cancer Lett. 327:48–60. 2012. View Article : Google Scholar
|
|
5
|
Tsutsui H, Kinugawa S and Matsushima S:
Oxidative stress and heart failure. Am J Physiol Heart Circ
Physiol. 301:H2181–H2190. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Suzuki M and Boothman DA: Stress-induced
premature senescence (SIPS) - influence of SIPS on radiotherapy. J
Radiat Res. 49:105–112. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Suzuki K, Mori I, Nakayama Y, Miyakoda M,
Kodama S and Watanabe M: Radiation-induced senescence-like growth
arrest requires TP53 function but not telomere shortening. Radiat
Res. 155:248–253. 2001. View Article : Google Scholar
|
|
8
|
Woods RL: Cardioprotective functions of
atrial natriuretic peptide and B-type natriuretic peptide: a brief
review. Clin Exp Pharmacol Physiol. 31:791–794. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ichiki T, Huntley BK, Heublein DM, et al:
Corin is present in the normal human heart, kidney, and blood, with
pro-B-type natriuretic peptide processing in the circulation. Clin
Chem. 57:40–47. 2011. View Article : Google Scholar
|
|
10
|
Yan W, Wu F, Morser J and Wu Q: Corin, a
transmembrane cardiac serine protease, acts as a pro-atrial
natriuretic peptide-converting enzyme. Proc Natl Acad Sci USA.
97:8525–8529. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ichiki T, Boerrigter G, Huntley BK, et al:
Differential expression of the pro-natriuretic peptide convertases
corin and furin in experimental heart failure and atrial fibrosis.
Am J Physiol Regul Integr Comp Physiol. 304:R102–R109. 2013.
View Article : Google Scholar :
|
|
12
|
Dong N, Chen S, Wang W, Zhou Y and Wu Q:
Corin in clinical laboratory diagnostics. Clin Chim Acta.
413:378–383. 2012. View Article : Google Scholar
|
|
13
|
Stewart FA: Mechanisms and dose-response
relationships for radiation-induced cardiovascular disease. Ann
ICRP. 41:72–79. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Debacq-Chainiaux F, Erusalimsky JD,
Campisi J and Toussaint O: Protocols to detect
senescence-associated beta-galactosidase (SA-betagal) activity, a
biomarker of senescent cells in culture and in vivo. Nat Protoc.
4:1798–1806. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tapio S: Ionizing radiation effects on
cells, organelles and tissues on proteome level. Adv Exp Med Biol.
990:37–48. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Leach JK, Van Tuyle G, Lin PS, et al:
Ionizing radiation-induced, mitochondria-dependent generation of
reactive oxygen/nitrogen. Cancer Res. 61:3894–3901. 2001.PubMed/NCBI
|
|
17
|
Hong EH, Lee SJ, Kim JS, et al: Ionizing
radiation induces cellular senescence of articular chondrocytes via
negative regulation of SIRT1 by p38 kinase. J Biol Chem.
285:1283–1295. 2010. View Article : Google Scholar :
|
|
18
|
Sridharan V, Tripathi P, Sharma SK, et al:
Cardiac inflammation after local irradiation is influenced by the
kallikrein-kinin system. Cancer Res. 72:4984–4992. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Barjaktarovic Z, Shyla A, Azimzadeh O, et
al: Ionising radiation induces persistent alterations in the
cardiac mitochondrial function of C57BL/6 mice 40 weeks after local
heart exposure. Radiother Oncol. 106:404–410. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Finnberg N, Wambi C, Kennedy AR and
El-Deiry WS: The effects of antioxidants on gene expression
following gamma-radiation (GR) and proton radiation (PR) in mice in
vivo. Cell Cycle. 12:2241–2247. 2013. View
Article : Google Scholar :
|
|
21
|
Chen S, Sen S, Young D, Wang W, Moravec CS
and Wu Q: Protease corin expression and activity in failing hearts.
Am J Physiol Heart Circ Physiol. 299:H1687–H1692. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Maisel AS, Krishnaswamy P, Nowak RM, et
al: Rapid measurement of B-type natriuretic peptide in the
emergency diagnosis of heart failure. N Engl J Med. 347:161–167.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
D’Errico MP, Grimaldi L, Petruzzelli MF,
et al: N-terminal pro-B-type natriuretic peptide plasma levels as a
potential biomarker for cardiac damage after radiotherapy in
patients with left-sided breast cancer. Int J Radiat Oncol Biol
Phys. 82:e239–e246. 2012. View Article : Google Scholar
|
|
24
|
Semenov AG, Tamm NN, Seferian KR, et al:
Processing of pro-B-type natriuretic peptide: furin and corin as
candidate convertases. Clin Chem. 56:1166–1176. 2010. View Article : Google Scholar : PubMed/NCBI
|