Introduction
Multidrug resistance (MDR), a cross-resistance of
cancer cells to seemingly unrelated drugs, such as anthracyclines
vinca alkaloids (doxorubicin and daunorubicin),
epipodophyllotoxins, (vincristine and vinblastine) and taxanes
(taxol and taxotere), is a major clinical concern in the treatment
of human cancers with conventional chemotherapeutic drugs (1).
Topoisomerase II (Topo II) poisons widely used in
clinical practice, such as etoposide, adriamycin (ADM) and their
analogues often induce dose-limiting toxicity and MDR, resulting in
treatment failure after the initial effective therapy (2,3).
Therefore, increasing research has focused on the development of
novel Topo II-targeting drugs, with the aim to overcome current
hurdles (4–6).
Although reversal agents have been assessed for
their efficacy against cancers with MDR, the majority have shown
little or no therapeutic potential due to their high toxicity in
vivo at the doses required to reverse MDR, as observed with
verapamil (7). Over the past few
years, podophyllotoxin derivatives have been widely used as cancer
chemotherapeutic agents (8). For
instance, etoposide, a low toxicity semisynthetic podophyllotoxin
analogue, has been utilized for the treatment of a broad spectrum
of tumors, for its Topo II-targeting properties (9,10).
The suppression of Topo II activity by Topo II-targeting drugs,
such as etoposide and ADM, can lead to double-stranded DNA breaks
(11,12). In addition, other newly developed
derivatives (e.g., NPF and GL-331) (13–15) which have displayed better
pharmacology profiles are currently being evaluated in clinical
trials (16). However, compared
to many previously described drugs, they have not shown sufficient
potency in the treatment of cancers with MDR. Recently, a novel
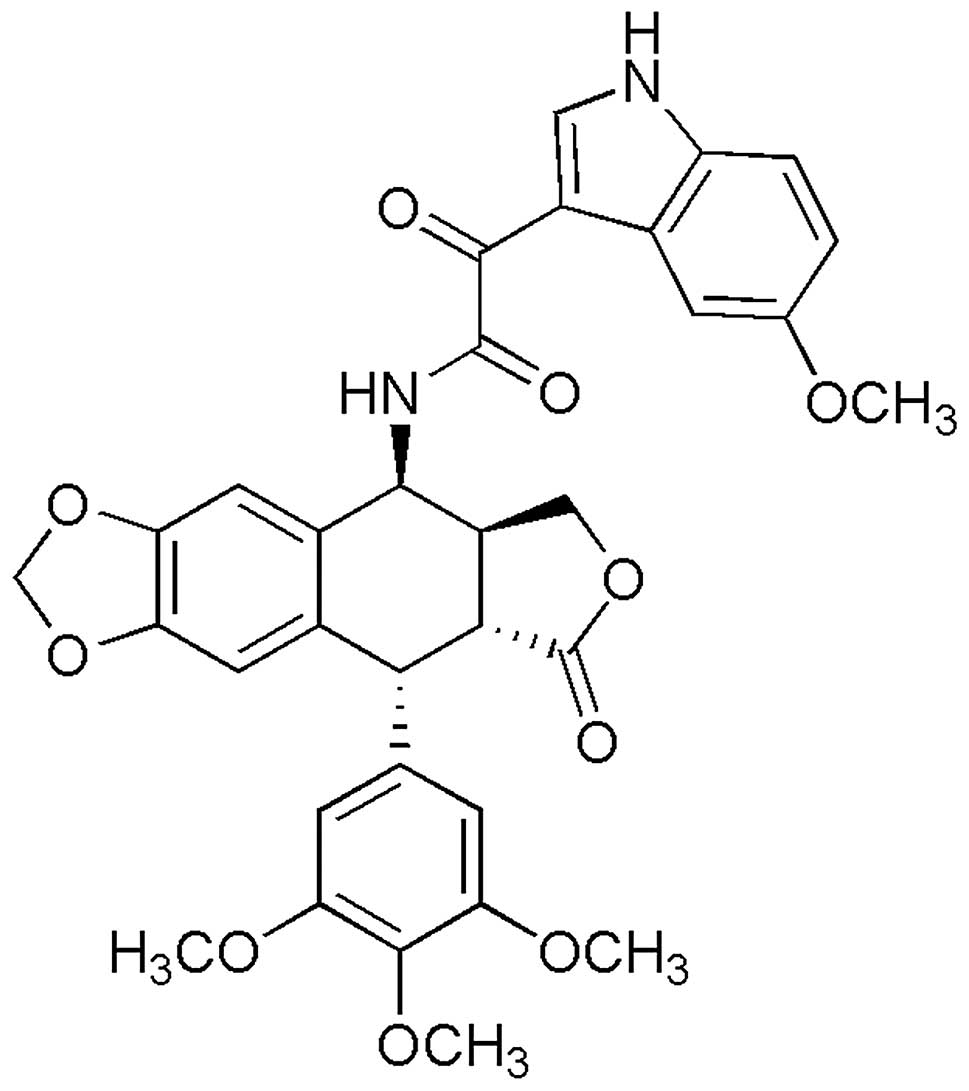
podophyllotoxin derivate, CIP-36 (Fig. 1), was synthesized in our
laboratory and presented the advantages of effectiveness, stability
and low toxicity. In this study, we aimed to investigate CIP-36 for
its effects on cancer cells with MDR. In vitro enzymatic
assay demonstrated the effectiveness of CIP-35 in inhibiting Topo
IIα activity. CIP-36 inhibited the proliferation of multiple cancer
cells, including multidrug-resistant K562/A02 cells, suggesting
that it has the potential for use as an optional chemical agent in
the treatment of cancers with MDR.
Materials and methods
Anticancer drugs
CIP-36 (purity >98%) was synthesized in the
laboratory of Professor Hong Chen by Dr Pengfei Yu. Its molecular
structure is illustrated in Fig.
1. ADM was purchased from Shenzhen Wanle Pharmaceutical Co.,
Ltd. (Shenzhen, China) and etoposide (VP-16) was from Jiangsu
Hengrui Medicine Co., Ltd. (Jiangsu, China).
Cells
The human leukemia cell line, K562, and the ADM
subline, K562/A02, were obtained from the Institute of Hematology
and Blood Disease Hospital, Chinese Academy of Medical Sciences and
Peking Union Medical College (Beijing, China). Other cell lines,
including human uterine cervical adenocarcinoma cells (HeLa), human
breast adenocarcinoma cells (MCF-7), human oral squamous carcinoma
cells (KB) and their multidrug resistant counterpart (KBv200
cells), human osteosarcoma cells (HOS), human colon carcinoma cells
(LoVo), human hypertrophic scar fibroblasts (FBs) and human
vascular endothelial cells (VECs) were supplied by the Institute of
Materia Medica, Chinese Academy of Medical Sciences and Peking
Union Medical College (Beijing, China). These cell lines were
cultured in RPMI-1640 (Sigma-Aldrich, St. Louis, MO, USA)
containing 10% heat-inactivated fetal bovine serum, penicillin (100
U/ml) and streptomycin (100 μg/ml) in a humidified
environment with 5% CO2 at 37°C. The K562/A02 cells were
stable and cultured in medium containing 1 μg/ml ADM in
order to obtain the stability of drug resistance. ADM (10 and 0.5
μg/ml) was used to measured the cell growth curve. The
KBv200 cells were stable and cultured in medium containing 200
nmol/l vincristine (Shenzhen Main Luck Pharmaceuticals Inc.,
Wanleyaoye, Shenzhen, China) in order to obtain the stability of
drug resistance. The KBv200 cells were used to measured the
cytotoxic effects of CIP-36. The drugs were removed 2 weeks prior
to the experiment.
Cytotoxicity assays
Cytotoxicity was assessed by sulforhodamine B (SRB)
or 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT) (Sigma-Aldrich) cytotoxicity assay in 96-well microtiter
plates as previously described (17,18). Briefly, the medium was replaced
with fresh medium containing 0.5 mg/ml of MTT. After 4 h of
incubation at 37°C, the cellular formazan product was dissolved in
dimethylsulfoxide (DMSO) and the absorbance was measured at a
wavelength of 570 nm using a spectrophotometer (PerkinElmer Inc.,
Boston, MA, USA).
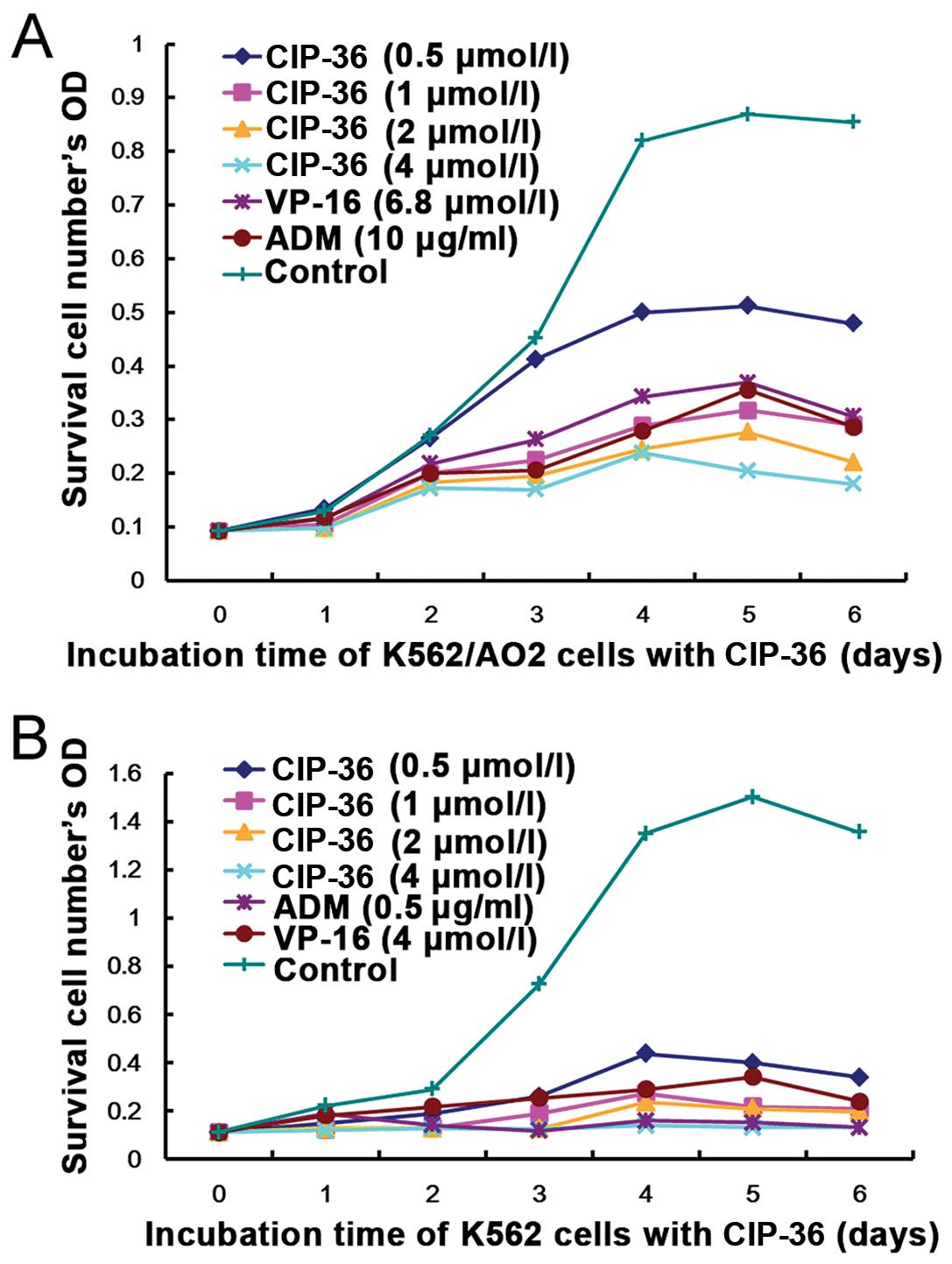
Cell growth curve following treatment
with CIP-36
The K562 and K562/A02 cells in the log phase were
seeded in 96-well plates at a density of 8,000 cells/ml. The cells
were then treated with various concentrations CIP-36 in RPMI-1640.
The number of viable cells was quantified by SRB assay every 24 h
for 6 consecutive days in order to establish the growth curve in
vitro.
Assessment of apoptosis by Hoechst 33342
and propidium iodide (PI) staining
The cells were exposed to CIP-36 at various
concentrations for 24 h, washed twice with phoshate-buffered saline
(PBS) and fixed with 4% formaldehyde for 10 min. The fixed cells
were then washed and stained with 10 μg/ml of Hoechst 33342
and PI for 10 min. The cells were examined under a fluorescence
microscope (XSZ-D2; Olympus, Tokyo, Japan).
Cell cycle analysis by flow
cytometry
The K562/A02 cells (1×106) were treated
with various concentrations of CIP-36 for 6, 12 and 24 h at 37°C,
harvested, washed with PBS and fixed with 70% ethanol. The fixed
cells were kept overnight at −20°C and washed with PBS prior to
treatment with 50 μg/ml PI solution in PBS, containing RNase
(50 μg/ml). Cell cycle analysis was carried out on an Epics
XL flow cytometer (Beckman Coulter, Miami, FL, USA).
Topo IIα DNA cleavage assay
Recombinant DNA Topo IIα was cloned and purified as
previously described (19). DNA
cleavage assays were carried according to the procedure described
in the study by Lemke et al (20) with minor modifications. The total
volume reaction mixture of 20 μl contained 20 mM Tris/HCl,
pH 7.5, 7.5 mM MgCl2, 0.5 mM dithiothreitol, 150 mM KCl,
1 mM ATP and 200 ng of pBR322 DNA (Toyobo Co. Ltd., Japan). The
reaction was inititated by the addition of 5 units of DNA Topo IIα
followed by incubation at 30°C for 10 min. One unit of enzyme
activity was defined as the amount of enzyme decatenating 0.2
μg of kinetoplast DNA in 30 min at 37°C, according to the
manufacturer’s instructions (T8944; Sigma-Aldrich). The reactions
were terminated by the addition of sodium dodecyl sulfate and
proteinase K at final concentrations of 0.35% and 0.3 mg/ml,
respectively. After an additional incubation for 60 min at 37°C, 5
μl of gel loading buffer were added to each reaction
mixture. The samples were loaded on 1% agarose gels containing 0.5
μg/ml ethidium bromide and separated for 18 h in TBE buffer
at 0.5 V/cm. The gels were then destained in distilled water and
photographed using a gel-imaging system (Bio-Rad, Hercules, CA,
USA). The percentage of supercoiled DNA in each sample was
determined using Quantity One Image software (Bio-Rad), and the
relative activity of Topo IIα in the drug-treated cells, as
previously described (21).
Statistical analysis
The statistical package SPSS 17.0 (SPSS, Chicago,
IL, USA) was used for all analyses. Data are presented as the means
± standard deviation (SD) and all the experiments were repeated at
least 3 times. Statistical significance between 2 groups was
determined by the Student’s t-test. For 3 groups or more, one-way
analysis of variance (ANOVA) was used and post hoc analysis by
least significant difference. A value of P<0.05 was considered
to indicate a statistically significant difference.
Results
Effects of CIP-36 on the proliferation of
human cancer and normal cells
First, CIP-36 was compared to ADM for its efficacy.
As shown in Table I, the K562
cells were sensitive to all drugs tested. However, the K562/A02
cells were resistant to ADM, whereas no cross-resistance to CIP-36
was observed. Indeed, a resistance index (RI) of 3.27 was obtained
for CIP-36, markedly lower than the RI values obtained for ADM (RI
of 68) and VP-16 (RI of 33.85). Subsequently, the
anti-proliferative activity of CIP-36 was further assessed in 8
human cancer cell lines. CIP-36 showed a broad-spectrum
anti-proliferative activity, with rather similar inhibitory
properties against various human cancer cells: the concentration
for 50% of maximal inhibition of cell proliferation
(GI50) or half maximal inhibitory concentration
(IC50) values ranged from 0.14–3.34 μmol/l for
CIP-36, generally lower than those of etoposide (VP-16; 0.45–34.76
μmol/l). Of note, as observed for the K562/A02 cells, the
KBv200 cells were resistant to etoposide, but not CIP-36 (Table II). Importantly, CIP-36 displayed
less cytotoxicity towards normal human cell lines (fibroblasts,
VECs), with significantly higher IC50 values recorded
for the normal cells in comparison with the cancer cells.
Furthermore, we demonstrated that the effects of CIP-36 on the K562
and K562/A02 cells occurred in a concentration- and time-dependent
manner, confirming the above-mentioned results (Fig. 2).
 | Table ICytotoxic activity of ADM, CIP-36 and
VP-16 against K562 and K562/A02 cells by SRB assay. |
Table I
Cytotoxic activity of ADM, CIP-36 and
VP-16 against K562 and K562/A02 cells by SRB assay.
| Cancer cell
lines | GI50
|
|---|
| ADM
(μg/ml) | VP-16
(μmol/l) | CIP-36
(μmol/l) |
|---|
| K562 | 0.21±0.17 | 1.08±0.42 | 1.02±0.58 |
| K562/A02 | 14.28±1.21 | 36.56±2.31 | 3.34±1.12 |
| RI | 68 | 33.85 | 3.27a |
 | Table IICytotoxic effects of CIP-36 on
different cell lines. |
Table II
Cytotoxic effects of CIP-36 on
different cell lines.
| Cancer cells | VP-16 | CIP-36 |
|---|
| KB | 1.71±0.04 | 1.41±0.06 |
| KBv200 |
12.1±1.23 |
2.06±0.38a |
| HeLa | 2.56±0.53 | 1.96±0.46 |
| MCF-7 | 8.61±0.88 | 3.13±0.22 |
| LoVo | 2.38± 0.76 | 2.01±0.36 |
| HOS | 2.32±0.25 | 3.39±0.85 |
| VECs | 55.57±1.78 | 15.11±0.77 |
| FBs | 57.87±3.45 | 26.08±2.29 |
Effects of CIP-36 on the apoptosis of
K562/A02 cells
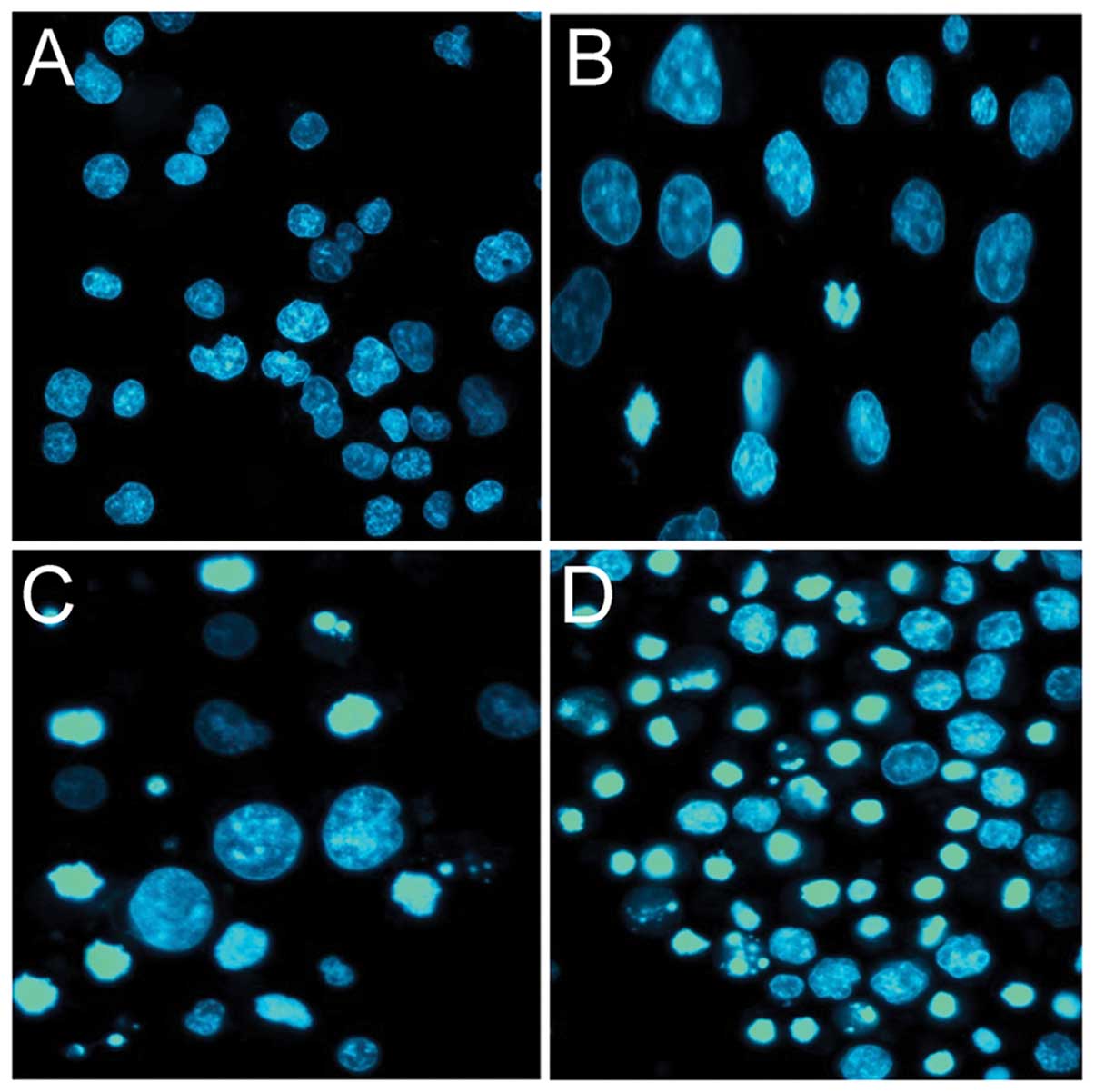
To determine the mechanisms of the CIP-36-induced
cytotoxic effects, we evaluated the ability of the compound to
induce apoptosis, using Hoechst 33342 staining and flow cytometry.
We found that CIP-36 induced morphological changes, characteristic
of apoptosis in the K562/A02 cells, such as chromosome condensation
(Fig. 3). Flow cytometric
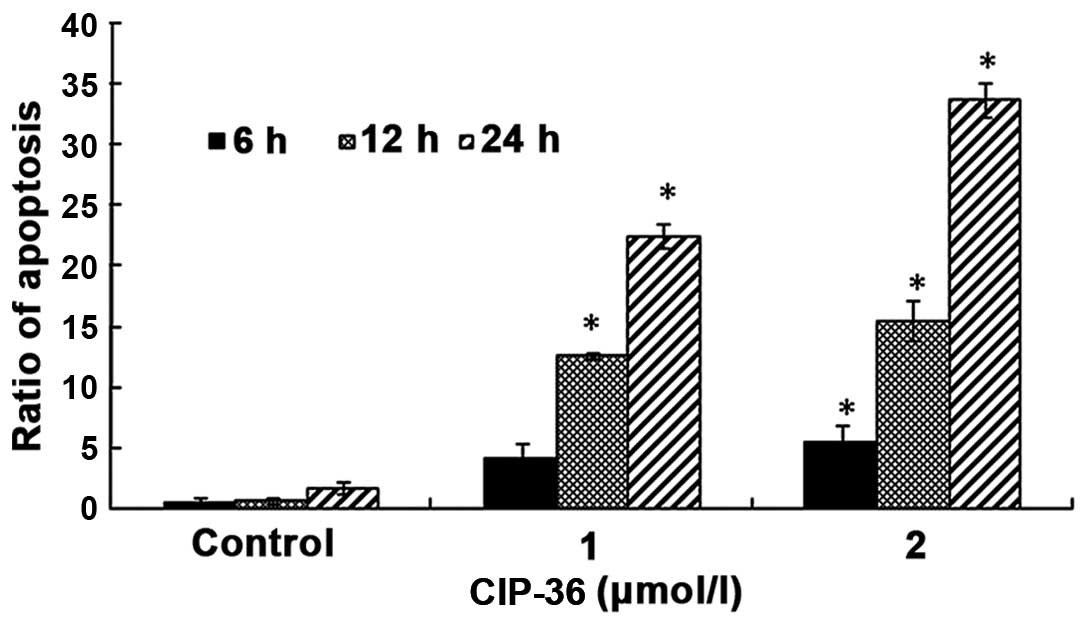
analysis of the K562/A02 cells treated with CIP-36 confirmed the
morphological observations mentioned above. At a low concentration
(1 μmol/l) CIP-36 induced the apoptosis of 4.14, 8.82 and
22.25% of K562/A02 cells after 6, 12 and 24 h, respectively
(Fig. 4). The proportion of
apoptotic cells increased at a high CIP-36 concentration (4
μmol/l), with 5.4, 15.5 and 35.2% of K562/A02 cells
undergoing apoptosis after 6, 12 and 24 h, respectively. These data
indicated that the apoptotic effects of CIP-36 occurred in a time-
and dose-dependent manner.
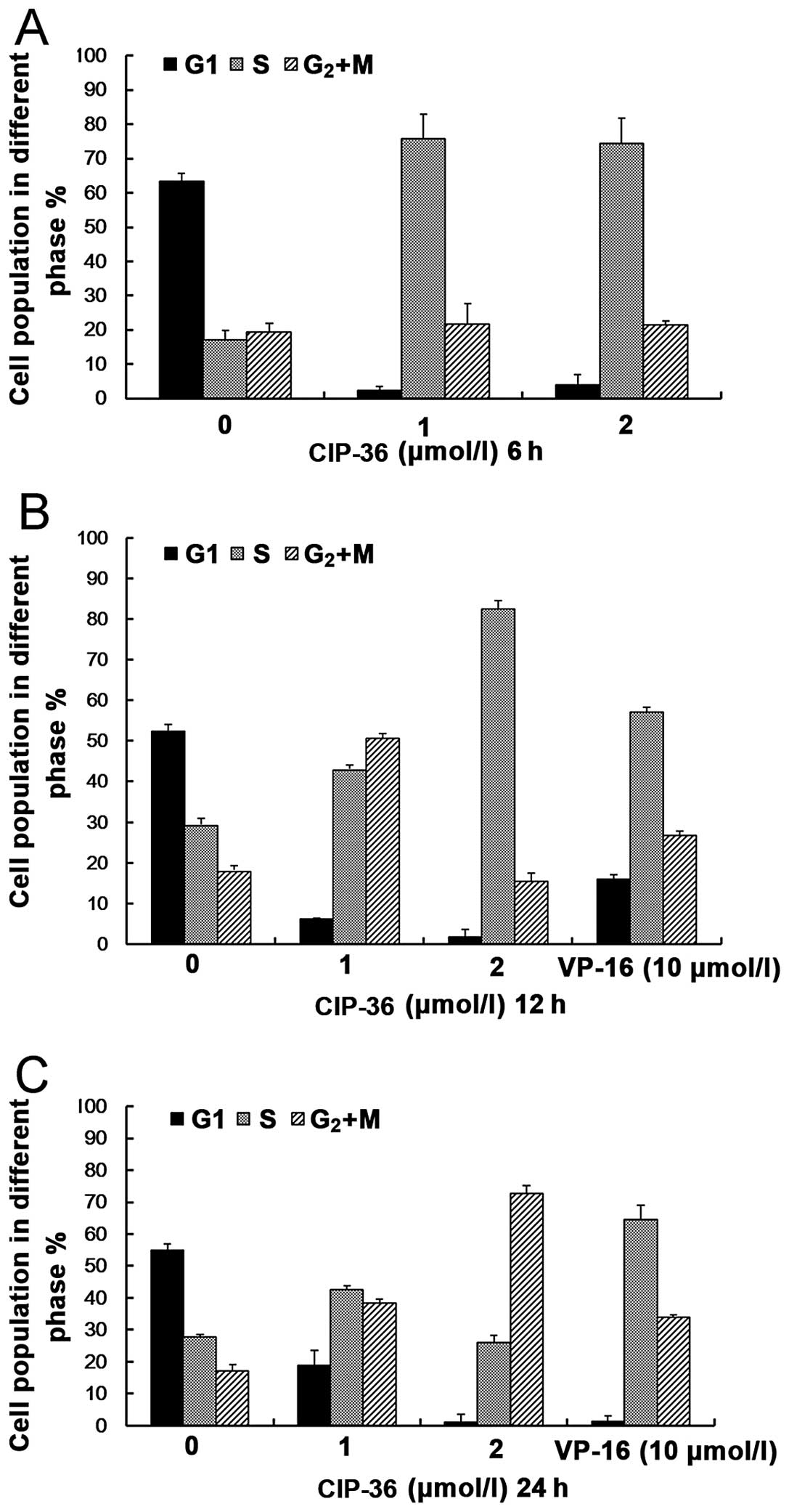
Effects of CIP-36 on cell cycle
progression
The cells were treated with CIP-36 at the indicated
concentrations (1 and 4 μmol/l) for 6, 12 and 24 h, and
distinct changes in the cell cycle distribution were observed
(Fig. 5). At 6 and 12 h, flow
cytometric analysis revealed higher DNA contents (S phase) in the
CIP-36-treated cells compared with the controls (treated with
DMSO). However, the cells had mainly accumulated in the
S/G2 + M phase after 24 h. These results suggest that
CIP-36 blocks K562/A02 cells in the S/G2 + M phase, in
contrast to VP-16, which blocks the K562/A02 cells in the S phase.
These findings demonstrate the differences in the mechanisms
underlying the antitumor activities of CIP-36 and VP-16.
CIP-36 inhibits Topo IIα activity
The novel podophyllotoxin derivative, CIP-36, was
examined for its effects on DNA cleavage mediated by human DNA Topo
IIα. We found that CIP-36 increased Topo II-DNA cleavage complex
(nicked DNA) levels. Indeed, the DNA bands corresponding to nicked
DNA were more intense with 2 or 4 μmol/l CIP-36 (Fig. 6A, lanes 4 and 5). The effects of
CIP-36 on DNA cleavage were more prominent than those of the
reference compound, etoposide (Fig.
6A, lane 6). The quantification of DNA bands by gel
densitometry confirmed these results. CIP-36 increased the amounts
of nicked DNA while reducing the quantities of linear DNA, in a
dose-dependent manner (Fig.
6B).
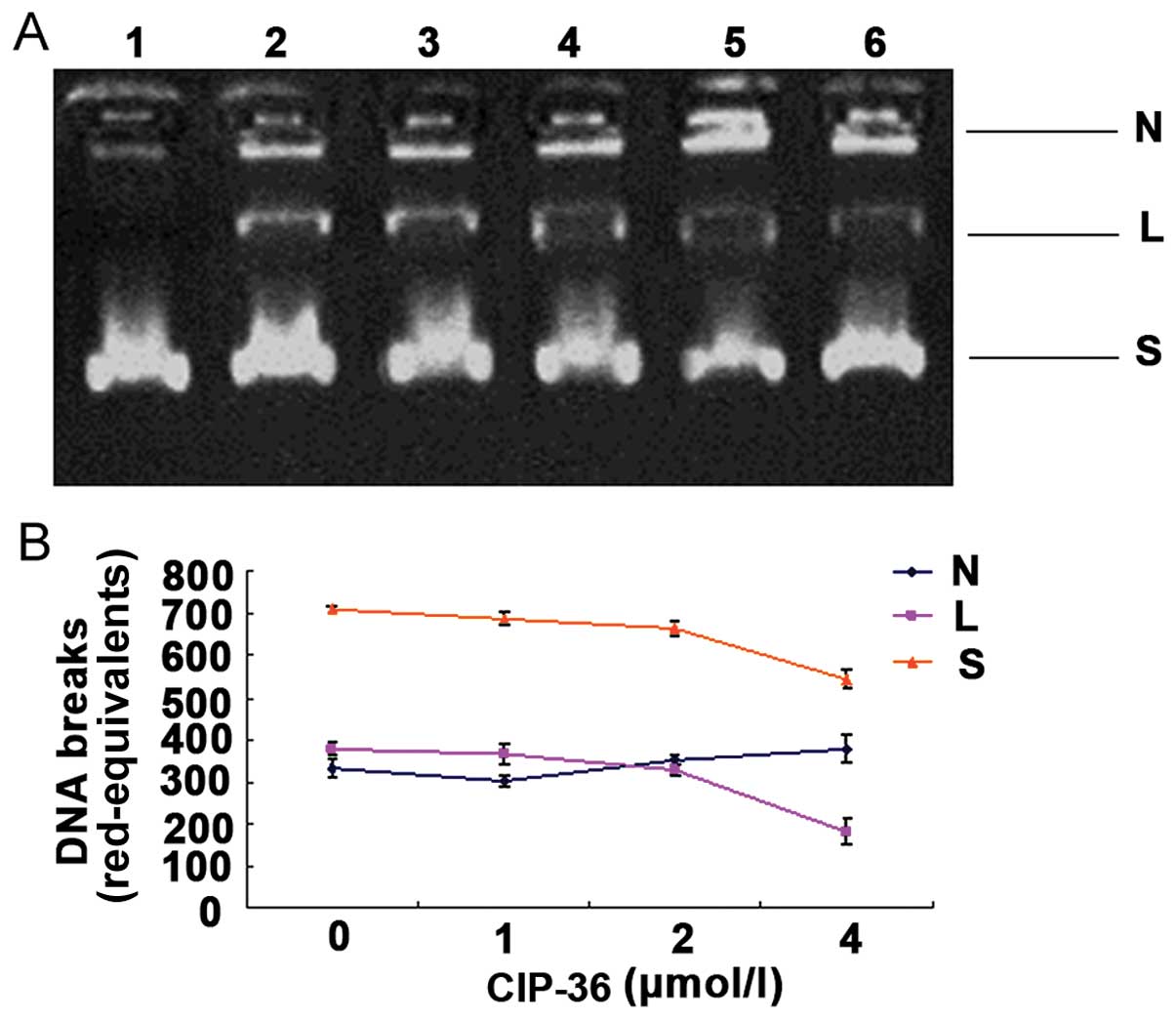 | Figure 6Effects of CIP-36 on DNA cleavage by
topoisomerase IIα (Topo IIα). (A) Representative images of agarose
gels are shown. Supercoiled pBR322 DNA was incubated with 4 units
of Topo IIα in the absence or presence of drugs. Lanes 1, DNA
substrate; lane 2, reaction mixture containing enzyme but no drugs;
lanes 3–5, reaction in the presence of 1, 2 and 4 μΜ CIP-36;
lane 6, 10 μΜ etoposide. (B) The rad-equivalents of the
supercoiled form, nicked form and linear form in each lane were
quantified, and the relative activity of Topo IIα was calculated.
The data presented were obtained from triplicate experiments. N,
nicked DNA; L, linear DNA; S, supercoiled DNA. |
Discussion
It is now clear that chemotherapy is indispensable
for cancer treatment. However, the occurrence of MDR constitutes
one of the main obstacles facing the field of oncology. In this
study, we demonstrated that CIP-36 effectively killed not only
parental K562 and KB cell lines, but also MDR sublines, such as
K562/A02, KBv200 to an equivalent degree. To date, 3 different
forms of MDR have been described in more detail: classical MDR,
non-Pgp MDR and atypical MDR (22). Atypical MDR has been shown to be
associated with quantitative and qualitative alterations in Topo
IIα, a nuclear enzyme that actively participates in the lethal
action of cytotoxic drugs. Topo II is an essential enzyme involved
in DNA replication and cell division through the cleavage and
religation of double-stranded DNA (23). It is known that the expression of
this enzyme begins to increase in the late G1 phase, peaks in the
G2/M phase and markedly decreases in the G1/G0 phase of the cell
cycle (23,24). Topo II exists in 2 forms, namely
Topo IIα (170 kDa) and Topo IIβ (180 kDa). The α form is highly
expressed in proliferating cells, whereas the β form is
preferentially expressed in cells in the stationary phase (25,26). Several studies characterizing Topo
II expression and activity in mammalian cells have demonstrated
that the enzyme is more abundant and active in neoplastic cells
compared to normal cells (27–29). Therefore, mammalian Topo II has
been used as a primary cellular target in the development of
several antitumor drugs, such as anthracyclines, acridines,
epipodophyllotoxins and amonafide (30). However, the majority of drugs
targeting Topo IIα, including retigeric acid B (31), 19-tert-butyldiphenylsilyl-8,
17-epoxy andrographolide (32)
and others (33) have been mainly
characterized for conventional cancer cells and those tested in
cancer cells with MDR are usually effective only at toxic doses
(34), indicating their limited
potential in the treatment of MDR cancer types.
In the present study, the novel epipodophyllotoxin
derivative, CIP-36, displayed a broad-spectrum activity and exerted
significant antitumor activity against the K562 and K562/A02 cells
in vitro. Furthermore, the novel drug selectivity inhibited
cancer cells, with an IC50 value significantly lower
compared with the values obtained for normal cells, including human
VECs and fibroblasts (FBs). Of note, we demonstrated that CIP-36
inhibited Topo IIα activity, which may explain these findings.
The induction of apoptosis is a strategy used widely
in the treatment of cancer (35).
Our data demonstrated that CIP-36 induced the apoptosis of the
K562/A02 cells in time- and concentration-dependent manner, as
demonstrated by Hoechst 33342 staining and flow cytometry. Of note,
it has been demonstrated that 5k, a novel
β-O-demethyl-epipodophyllotoxin analogue, is effective
against cells with MDR both in vitro and in vivo,
albeit inducing apoptotic signaling pathways only at high
concentrations of 1.25–5.00 μmol/l (36).
In conclusion, in the present study, we demonstrated
that CIP-36 inhibited Topo IIα activity and induced apoptosis, thus
inhibiting the growth of multiple cancer cells, including K562/A02
cells with MDR. These findings suggest that CIP-36 has the
potential to be used in the treatment of patients with cancers with
MDR. Ongoing studies are being carried out in our laboratory for
further characterization of this important molecule.
Acknowledgments
The authors are gratefully to the Great Program of
Science Foundation of Tianjin (06YFJZJCO2700) and the Program of
Science Foundation of Tianjin (08JCYBJC070000) for financially
supporting this study. This study was also supported by a grant
from the National Natural Science Foundation of China (no.
30873363).
References
|
1
|
Huff LM, Lee JS, Robey RW and Fojo T:
Characterization of gene rearrangements leading to activation of
MDR-1. J Biol Chem. 281:36501–36509. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sinha BK, Kumar A, Bhattacharjee S, Espey
MG and Mason RP: Effect of nitric oxide on the anticancer activity
of the topoisomerase-active drugs etoposide and adriamycin in human
melanoma cells. J Pharmacol Exp Ther. 347:607–614. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhu CY, Lv YP, Yan DF and Gao FL:
Knockdown of MDR1 increases the sensitivity to adriamycin in drug
resistant gastric cancer cells. Asian Pac J Cancer Prev.
14:6757–6760. 2013. View Article : Google Scholar
|
|
4
|
Deng S, Yan T, Jendrny C, Nemecek A,
Vincetic M, Gödtel-Armbrust U and Wojnowski L: Dexrazoxane may
prevent doxorubicin-induced DNA damage via depleting both
topoisomerase II isoforms. BMC Cancer. 14:8422014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Miura JT, Johnston FM, Thomas J, George B,
Eastwood D, Tsai S, Christians KK, Turaga KK and Gamblin TC:
Molecular profiling in gastric cancer: examining potential targets
for chemotherapy. J Surg Oncol. 110:302–306. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Smith NA, Byl JA, Mercer SL, Deweese JE
and Osheroff N: Etoposide quinone is a covalent poison of human
topoisomerase IIβ. Biochemistry. 53:3229–3236. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pennock GD, Dalton WS, Roeske WR, et al:
Systemic toxic effects associated with high-dose verapamil infusion
and chemotherapy administration. J Natl Cancer Inst. 83:105–110.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hartmann JT and Lipp HP: Camptothecin and
podophyllotoxin derivatives: inhibitors of topoisomerase I and II -
mechanisms of action, pharmacokinetics and toxicity profile. Drug
Saf. 29:209–230. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bender RP, Jablonksy MJ, Shadid M, et al:
Substituents on etoposide that interact with human topoisomerase
IIalpha in the binary enzyme-drug complex: contributions to
etoposide binding and activity. Biochemistry. 47:4501–4509. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Holden JA: DNA topoisomerases as
anticancer drug targets: from the laboratory to the clinic. Curr
Med Chem Anticancer Agents. 1:1–25. 2001. View Article : Google Scholar
|
|
11
|
Osheroff N: Effect of antineoplastic
agents on the DNA cleavage/religation reaction of eukaryotic
topoisomerase II: inhibition of DNA religation by etoposide.
Biochemistry. 28:6157–6160. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Robinson MJ and Osheroff N: Effects of
antineoplastic drugs on the post-strand-passage DNA
cleavage/religation equilibrium of topoisomerase II. Biochemistry.
30:1807–1813. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen Y, Lin TY, Chen JC, Yang HZ and Tseng
SH: GL331, a topoisomerase II inhibitor, induces radiosensitization
of human glioma cells. Anticancer Res. 26:2149–2156.
2006.PubMed/NCBI
|
|
14
|
Whang PJ and Huang TS: New trials of
GL331, a novel topoisomerase II inhibitor, in treatment of solid
tumors. J Intern Med Taiwan. 8:6–11. 1997.
|
|
15
|
Zhang YL, Tropsha A, McPhail AT and Lee
KH: Antitumor agents. 152. In vitro inhibitory activity of
etoposide derivative NPF against human tumor cell lines and a study
of its conformation by X-ray crystallography, molecular modeling,
and NMR spectroscopy. J Med Chem. 37:1460–1464. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu YQ, Tian J, Qian K, Zhao XB,
Morris-Natschke SL, Yang L, Nan X, Tian X and Lee KH: Recent
progress on C-4-modified podophyllotoxin analogs as potent
antitumor agents. Med Res Rev. 35:1–62. 2015. View Article : Google Scholar
|
|
17
|
Chen H, Bi W, Cao B, et al: A novel
podophyllotoxin derivative (YB-1EPN) induces apoptosis and
down-regulates express of P-glycoprotein in multidrug resistance
cell line KBV200. Eur J Pharmacol. 627:69–74. 2010. View Article : Google Scholar
|
|
18
|
Skehan P, Storeng R, Scudiero D, et al:
New colorimetric cytotoxicity assay for anticancer-drug screening.
J Natl Cancer Inst. 82:1107–1112. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sullivan DM, Glisson BS, Hodges PK,
Smallwood-Kentro S and Ross WE: Proliferation dependence of
topoisomerase II mediated drug action. Biochemistry. 25:2248–2256.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lemke K, Poindessous V, Skladanowski A and
Larsen AK: The antitumor triazoloacridone C-1305 is a topoisomerase
II poison with unusual properties. Mol Pharmacol. 66:1035–1042.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li CH, Chen PY, Chang UM, et al: Ganoderic
acid X, a lanostanoid triterpene, inhibits topoisomerases and
induces apoptosis of cancer cells. Life Sci. 77:252–265. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nooter K and Stoter G: Molecular
mechanisms of multidrug resistance in cancer chemotherapy. Pathol
Res Pract. 192:768–780. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kellner U, Sehested M, Jensen PB, Gieseler
F and Rudolph P: Culprit and victim - DNA topoisomerase II. Lancet
Oncol. 3:235–243. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kimura K, Saijo M, Ui M and Enomoto T:
Growth state- and cell cycle-dependent fluctuation in the
expression of two forms of DNA topoisomerase II and possible
specific modification of the higher molecular weight form in the M
phase. J Biol Chem. 269:1173–1176. 1994.PubMed/NCBI
|
|
25
|
Chen W, Qiu J and Shen YM: Topoisomerase
IIα, rather than IIβ, is a promising target in development of
anti-cancer drugs. Drug Discov Ther. 6:230–237. 2012.PubMed/NCBI
|
|
26
|
Dingemans AM, Pinedo HM and Giaccone G:
Clinical resistance to topoisomerase-targeted drugs. Biochim
Biophys Acta. 1400:275–288. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Priel E, Aboud M, Feigelman H and Segal S:
Topoisomerase-II activity in human leukemic and lymphoblastoid
cells. Biochem Biophys Res Commun. 130:325–332. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Engstrøm MJ, Ytterhus B, Vatten LJ, Opdahl
S and Bofin AM: TOP2A gene copy number change in breast cancer. J
Clin Pathol. 67:420–425. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pendleton M, Lindsey RH Jr, Felix CA,
Grimwade D and Osheroff N: Topoisomerase II and leukemia. Ann NY
Acad Sci. 1310:98–110. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pourpak A, Landowski TH and Dorr RT:
Ethonafide-induced cytotoxicity is mediated by topoisomerase II
inhibition in prostate cancer cells. J Pharmacol Exp Ther.
321:1109–1117. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu Y, Gao F, Jiang H, et al: Induction of
DNA damage and ATF3 by retigeric acid B, a novel topoisomerase II
inhibitor, promotes apoptosis in prostate cancer cells. Cancer
Lett. 337:66–76. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nateewattana J, Dutta S, Reabroi S, et al:
Induction of apoptosis in cholangiocarcinoma by an andrographolide
analogue is mediated through topoisomerase II alpha inhibition. Eur
J Pharmacol. 723:148–155. 2014. View Article : Google Scholar
|
|
33
|
Zhang X, Bao B, Yu X, et al: The discovery
and optimization of novel dual inhibitors of topoisomerase II and
histone deacetylase. Bioorg Med Chem. 21:6981–6995. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bau JT, Kang Z, Austin CA and Kurz EU:
Salicylate, a catalytic inhibitor of topoisomerase II, inhibits DNA
cleavage and is selective for the α isoform. Mol Pharmacol.
85:198–207. 2014. View Article : Google Scholar
|
|
35
|
Chen H, Wang J, Zhang J, et al: L1EPO, a
novel podophyllotoxin derivative overcomes P-glycoprotein-mediated
multidrug resistance in K562/A02 cell line. Biol Pharm Bull.
32:609–613. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xu D, Cao J, Qian S, et al: 5k, a novel
β-O-demethyl-epipodophyllotoxin analogue, inhibits the
proliferation of cancer cells in vitro and in vivo via the
induction of G2 arrest and apoptosis. Invest New Drugs. 29:786–799.
2011. View Article : Google Scholar
|