Introduction
Mesenchymal stem cells (MSCs) are of stromal origin
and can be isolated from various human tissues, including bone
marrow, adipose tissue, skeletal muscle, synovium, gingiva,
amniotic fluid, cord blood, and the umbilical cord. MSCs are an
extremely promising source of adult stem cells that may be used for
cell-based therapeutics, due in part to their substantial ability
to differentiate into multilineages and broad secretory activities
(1–4). Preclinical studies have suggested
the potential for using MSCs in the settings of tissue repair in
type 1 diabetes (5), acute lung
injury (6,7), radiation protection (8) and nephropathy (9).
However, it was suggested that the immunomodulatory
activity of MSCs is an important consideration in cell therapy
(10). Additionally, in
vitro evidence suggests that MSCs directly modulate T-cell
function. MSCs inhibit the maturation and migration of various
antigen-presenting cells, suppress B-cell activation, induce
suppressor T-cell formation, and alter the expression of several
receptors necessary for antigen capture and processing (11,12). This immunosuppressive activity of
MSCs may also play an important role in the therapy of autoimmune
diseases (13), and the
prevention of acute graft-versus-host disease (aGVHD) (14).
Previous findings have shown that the recommended
infusion of adoptively transferred MSCs ranges from
105/kg to 107/kg body weight, and that an
association exists between the anticipated therapeutic effect and
the increasing quantity of MSCs (15–19). However, infused MSCs are, not only
found in the bone marrow and injured tissues, but are also located
in the lungs (20,21). The dangers of pulmonary embolism
and cardiac dysfunction increase with the number of adoptively
transferred input cells (22).
Therefore, this study aimed to obtain a new population of MSCs
exhibiting more potent immunosuppressive functions with subsequent
improvement in their clinical application without the need for an
increasing cell dose.
Numerous molecules participate in the
immunomodulation process of MSCs, including prostaglandin E2 (PGE2)
(23), indoleamine
2,3-dioxygenase (IDO) (24),
nitric oxide (25), transforming
growth factor-β (TGF-β) (26),
and interleukin-6 (IL-6) (27).
Of these molecules, IDO-1 and cyclooxygenase-2 (COX-2) are key
factors in tryptophan catabolism and PGE2 synthesis, respectively.
When PGE2 production is inhibited, immunomodulation is weakened
(23,24,28). Tryptophan is an essential amino
acid in T-cell proliferation, and its depletion or accumulation of
its degradation product kynurenine, adversely affect T-cell
amplification (24).
In view of the critical roles of IDO-1 and COX-2 in
immunomodulation, we attempted to upregulate the expression levels
of IDO-1 and COX-2 to enhance the synthesis of PGE2 and kynurenine,
and to determine whether the immunosuppressive ability of MSCs can
be improved.
Materials and methods
Culture of MSCs
Umbilical cords were obtained from clinically normal
pregnancies after participants provided informed consent. The study
was approved by the local ethics committee of Qilu Hospital.
Umbilical cords were excised and washed in 0.1 mol/l phosphate
buffer (pH 7.4) to remove residual blood. The cords were dissected,
and the blood vessels were removed. The remaining tissues were cut
into small sections (1–2 mm3) and placed in tissue
culture plates with low-glucose Dulbecco’s modified Eagle’s medium
(DMEM), which was supplemented with 10% fetal bovine serum (FBS)
(Gibco-BRL, Grand Island, NY, USA), 2 ng/ml vascular endothelial
growth factor (VEGF), 2 ng/ml epidermal growth factor (EGF), 2
ng/ml fibroblast growth factor (all from R&D Systems,
Minneapolis, MN, USA), 100 U/ml penicillin, and 100 μg/ml
streptomycin (Gibco-BRL). Cultures were maintained at 37°C in a
fully-humidified atmosphere with 5% CO2 in air. The
media were changed every 3–4 days. Adherent cells proliferated from
individual explanted tissues 7–12 days after the culture was
initiated. Subsequently, small tissue specimens were removed from
the culture, and the adherent fibroblast-like cells were cultured
to confluence for 2–3 weeks. The cells were trypsinized using 0.25%
trypsin (Gibco-BRL) and then passaged at a density of
1×104 cells/ml in the culture medium exactly as
described above. The cells were used after 5 or more passages.
Cell surface antigen phenotyping
Fifth- to seventh-passage cells were collected and
treated with 0.25% trypsin. The cells were then stained with either
fluorescein isothiocyanate (FITC)-conjugated or phycoerythrin
(PE)-conjugated monoclonal antibodies in 100 μl phosphate
buffer for 15 min at room temperature, as per the manufacturer’s
instructions. The antibodies used in this assay were targeted
against human cell-surface expressed antigens including CD29, CD34,
CD31, CD44, CD45, CD73, CD90 and CD105 (SeroTec, Raleigh, NC, USA).
The cells were analyzed by flow cytometry (Guava easyCyte 6HT-2L;
Merck Millipore, Billerica, MA, USA). Positive cells were counted
and compared with the signal of the corresponding immunoglobulin
isotypic controls.
Recombinant plasmid construction and
transfection
For construction of the expression plasmid, the
IDO-1 and the COX-2 inserts were isolated by polymerase chain
reaction (PCR) amplification from a complementary DNA (cDNA)
library (GeneChem, Shanghai, China) and digested with the
restriction endonucleases EcoRI and BglII (MBI
Fermentas, Burlington, ON, Canada). The inserts were subsequently
linked to the pEGFP-N3 expression plasmids (Bio-Asia Co., Shanghai,
China) with T4 DNA ligase (TransGen, Beijing, China). The ligation
products were transformed into competent Escherichia coli
DH5α (TransGen) and then selected using the kanamycin resistance
method. Recombinant plasmids were sequenced (ABI Prism 3100 DNA
Sequencer; Applied Biosystems, Foster City, CA, USA) and confirmed
to contain the entire coding sequence of IDO-1 or COX-2. Clones
with the correct sequence were amplified for further transfection.
The new recombinant plasmids were designated as pEGFP-IDO-1 and
pEGFP-COX-2.
The recombinant plasmids were transfected into MSCs
with a gene transfection instrument (SCIENTZ-2C; Scientz Co.,
Ningbo, China) and electroporation cuvettes (Bio-Rad, Hercules, CA,
USA). A successful transduction was confirmed by visualizing the
expression of enhanced green fluorescent protein (EGFP; included in
the pEGFP-N3 vector) after 4 days of culture. The cells were
maintained and allowed to grow for another 3–5 days, and the
expression level of IDO-1 and COX-2 was confirmed by western
immunoblotting as described below.
The experiments were divided into four groups: i)
untreated MSCs were the control group, ii) cells transfected with
only the IDO-1 gene were the MSCs (IDO-1) group, iii) cells
transfected with only the COX-2 gene were the MSCs (COX-2)
group, and iv) cells described as the MSCs (IDO-1/COX-2) group,
were simultaneously transfected with the IDO-1 and COX-2 plasmids.
The expression levels of the transferred plasmids were the same in
each group.
Protein extraction and western
immunoblotting
To verify the expression levels of IDO-1 and COX-2,
the transfected cells were collected and resuspended in protein
lysis buffer (RIPA: PMSF at a ratio of 100:1; Solarbio, Beijing,
China) according to the manufacturer’s instructions. Lysates were
incubated on ice for 30 min and then centrifuged at 12,000 rpm and
4°C for 20 min. Equal amounts of proteins (15 μg for each
sample) were separated by sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) and then transferred to polyvinylidene
difluoride (PVDF) membranes. The membranes were incubated with
antibodies against IDO-1, COX-2 or β-actin (Abcam Inc., Cambridge,
MA, USA) at 4°C overnight. Antibody binding was assessed by
incubation with horseradish peroxidase-conjugated secondary
antibodies (Beyotime, Shanghai, China). Chemiluminescence was
detected using an ECL Plus immunoblotting detection system
(Beyotime).
Reverse transcription-quantitative PCR
(RT-qPCR) assay of immune-regulated gene mRNA expression
In order to detect changes in immune-related genes
in transfected MSCs, total RNA was extracted using TRIzol reagent
(Invitrogen, Carlsbad, CA, USA) following the manufacturer’s
instructions. Total RNA was reverse transcribed to cDNA with the
Omniscript cDNA Synthesis kit (Qiagen, Hamburg, Germany) according
to the manufacturer’s instructions. In a typical procedure, 0.2
μg of total RNA was reverse transcribed in a final volume of
20 μl containing the following: 1X RT buffer,
deoxynucleotide triphosphate mix (5 mM each), RNase inhibitor (10
U/μl RNase out; Invitrogen), oligo(dT) primers and 4 units
Omniscript RT. The samples were incubated at 37°C for 60 min, and
the resulting cDNA was stored at −80°C until RT-qPCR analysis was
initiated using an ABI 7500 PCR system and SYBR-Green I dye
(Toyobo, Osaka, Japan). The primers used are provided in Table I. The reagents and primers were
obtained from Bioasi Co., Ltd., Shanghai, China. β-actin was used
as an internal control. The expression of each gene was determined
using the 2−ΔΔCT method. The RT-qPCR conditions used
were: 1 cycle at 95°C for 4 min followed by 94°C for 15 sec at 60°C
for 1 min, and for a total of 40 cycles. Data were analyzed using
Sequence Detection software version 1.4 (Applied Biosystems). Data
were reported as mean ± standard deviation (SD) of at least three
independent experiments. mRNA expression was presented as
fold-change compared with the untreated control groups. Control
group values were set at a fold-change equal to unity.
 | Table IPrimer sequences used in PCR. |
Table I
Primer sequences used in PCR.
| Genes | Primer
sequences | Size (bp) |
|---|
|
IDO-1a |
5′-GGAAGATCTTCCATGGCACACGCTATGGAAAAC | 1,209 |
|
5′-CCGGAATTCCGGACCTTCCTTCAAAAGGGATTTC |
|
COX-2a |
5′-GGAAGATCTTCCATGCTCGCCCGCGCCCTGCTGC | 1,812 |
|
5′-CCGGAATTCCGGCAGTTCAGTCGAACGTTCTTTTAG |
| IDO-1 |
5′-GCCCTTCAAGTGTTTCACCAA | 90 |
|
5′-GCCTTTCCAGCCAGACAAATAT |
| COX-2 |
5′-GGTCTGGTGCCTGGTCTGAT | 80 |
|
5′-TCCTGTTTAAGCACATCGCATACT |
| HMOX-1 |
5′-AGGGAAGCCCCCACTCAAC | 81 |
|
5′-ACTGTCGCCACCAGAAAGCT |
| IL-2 |
5′-CCAGGATGCTCACATTTAAGTTTTAC | 87 |
|
5′-GAGGTTTGAGTTCTTCTTCTAGACACTGA |
| IL-10 |
5′-GCCTTGTCTGAGATGATCCAGTT | 85 |
|
5′-TCACATGCGCCTTGATGTCT |
| iNOS |
5′-GGTGGAAGCGGTAACAAAGG | 81 |
|
5′-TGCTTGGTGGCGAAGATGA |
| TGF-β1 |
5′-GGGAAATTGAGGGCTTTCG | 83 |
|
5′-GAACCCGTTGATGTCCACTTG |
| IL-1α |
5′-GACGCCCTCAATCAAAGTATAATTC | 89 |
|
5′-TCAAATTTCACTGCTTCATCCAGAT |
| IL-1β |
5′-GCGGCATCCAGCTACGAAT | 80 |
|
5′-GTCCATGGCCACAACAACTG |
| IFN-γ |
5′-CCAACGCAAAGCAATACATGA | 72 |
|
5′-TTTTCGCTTCCCTGTTTTAGCT |
| β-actin |
5′-GGACATCCGCAAAGACCTGTA | 80 |
|
5′-GCATCCTGTCGGCAATGC |
Inhibition of peripheral blood
mononuclear cell (PBMC) proliferation by IDO-1- and COX-2-modified
MSCs
Allogeneic PBMCs were isolated by Ficoll/Hypaque
gradient centrifugation of peripheral venous blood (20-30 ml, that
was collected from healthy volunteers). The preliminary tests
showed that phytohemagglutinin (PHA; Sigma-Aldrich, St. Louis, MO,
USA) stimulated lymphocyte proliferation at a final concentration
of 10 μg/ml. Additionally, the inhibitory effect of MSCs on
lymphocyte proliferation was notable at a ratio of MSCs to
lymphocytes of 1:10 to 1:20 (data not shown).
MSCs of the four groups were adapted to the
co-culture medium (RPMI-1640 medium without phenol red, and
supplemented with 10% FBS, 2 mM L-glutamine, 100 U/ml penicillin,
and 100 μg/ml streptomycin) by gradient reduction of DMEM
and plated in triplicate in 96-well microtiter plates. When MSCs
achieved a 100% confluence, the cells were co-cultured with
PHA-activated PBMCs. After three days, the suspended cells (mainly
lymphocytes) from each well were transferred to a new 96-well
plate, and then incubated with 10 μl of Cell Counting kit-8
(CCK-8; Dojindo, Kumamoto, Japan). Absorbance at 450 nm was
measured with a model 450 microplate reader (Bio-Rad Laboratories,
Richmond, CA, USA). Experiments were performed in triplicate and
were repeated at least twice.
Cytokine measurement in culture
supernatant
After three days of co-culture of lymphocytes and
MSCs, culture supernatants were collected to measure the levels of
interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α) by
enzyme-linked immunosorbent assay (ELISA) according to the
manufacturer’s instructions (R&D Systems).
Cytotoxicity analysis
HeLa cells at a density of 3×104, were
resuspended in RPMI-1640 medium (without phenol red), supplemented
with 10% FBS, and seeded in 96-well plates and used as target cells
for cytotoxicity analysis. PHA-activated PBMCs were respectively
co-cultured with MSCs from the four groups at a 1:10 ratio of MSCs
to lymphocytes, respectively. After three days, suspended cells
(mainly lymphocytes) from each group were transferred to HeLa cell
pre-cultured 96-well plates with lymphocytes seeded at a density of
3×105 lymphocytes per well. The cells were incubated at
37°C in 5% CO2 in air for 6 h. After this time period,
10 μl of CCK-8 was added to the cultures and incubations
were continued for an additional 1.5 h. Relative absorbance values
were measured at 450 nm as described above. Cytotoxic activity was
calculated as: [OD(PBMC+HeLa) −
OD(PBMC)]/OD(HeLa).
Statistical analysis
Data are presented as the means ± standard
deviation. Data sets were compared via analysis of variance (ANOVA)
followed by the Student’s t-test for paired analysis of data sets
between and within groups. Data were analyzed using SPSS software
version 14.0 (SPSS Inc., Chicago, IL, USA). Differences between
values were considered statistically significant at an α-value of
P<0.05.
Results
Biological characteristics of UC-derived
MSCs
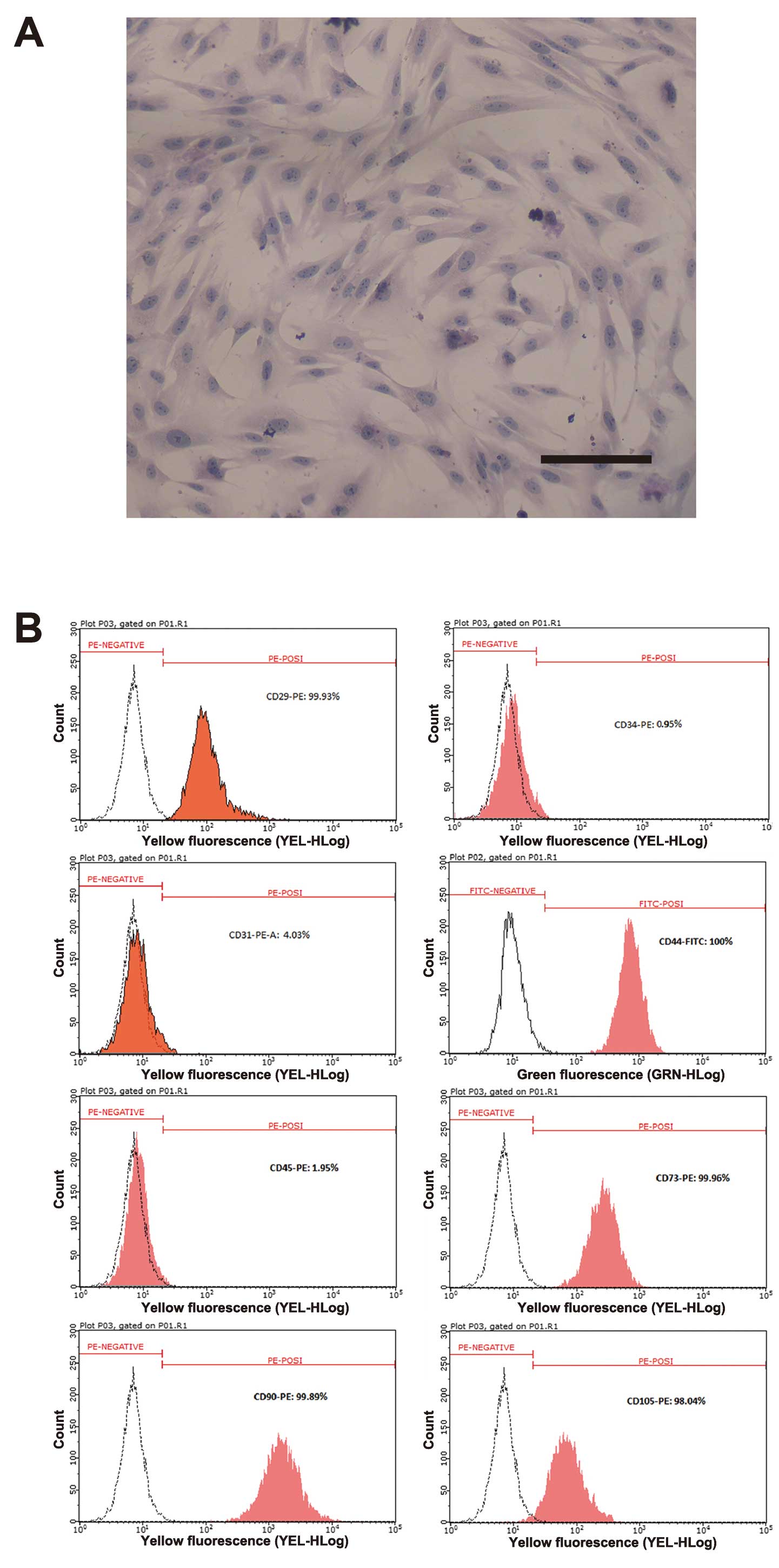
After several passages, adherent cells from UC
formed a monolayer of typical fibroblastic cells (Fig. 1A). Flow cytometric results showed
that UC-derived cells shared most of their immunophenotype with
MSCs, including a positive expression for stromal markers (CD29,
CD44, CD73, CD90 and CD105), but a negative expression for the
endothelial marker CD31, and the hematopoietic markers (CD34 and
CD45) (Fig. 1B).
Construction of recombinant eukaryotic
expression plasmids and electro-transfection
The amplification products of the IDO-1 and
COX-2 genes (with relative size of 1,209 and 1,812 bp,
respectively) were purified and digested with the restriction
endonucleases BglII and EcoRI to construct a
recombinant plasmid by insertion into the pEGFP-N3 (4729 bp)
vector. The result of rDNA sequence analysis confirmed the correct
sequence and reading frame of IDO-1 (GenBank, NM_002164.5) and
COX-2 (GenBank, NM_000963). MSCs were transfected with recombinant
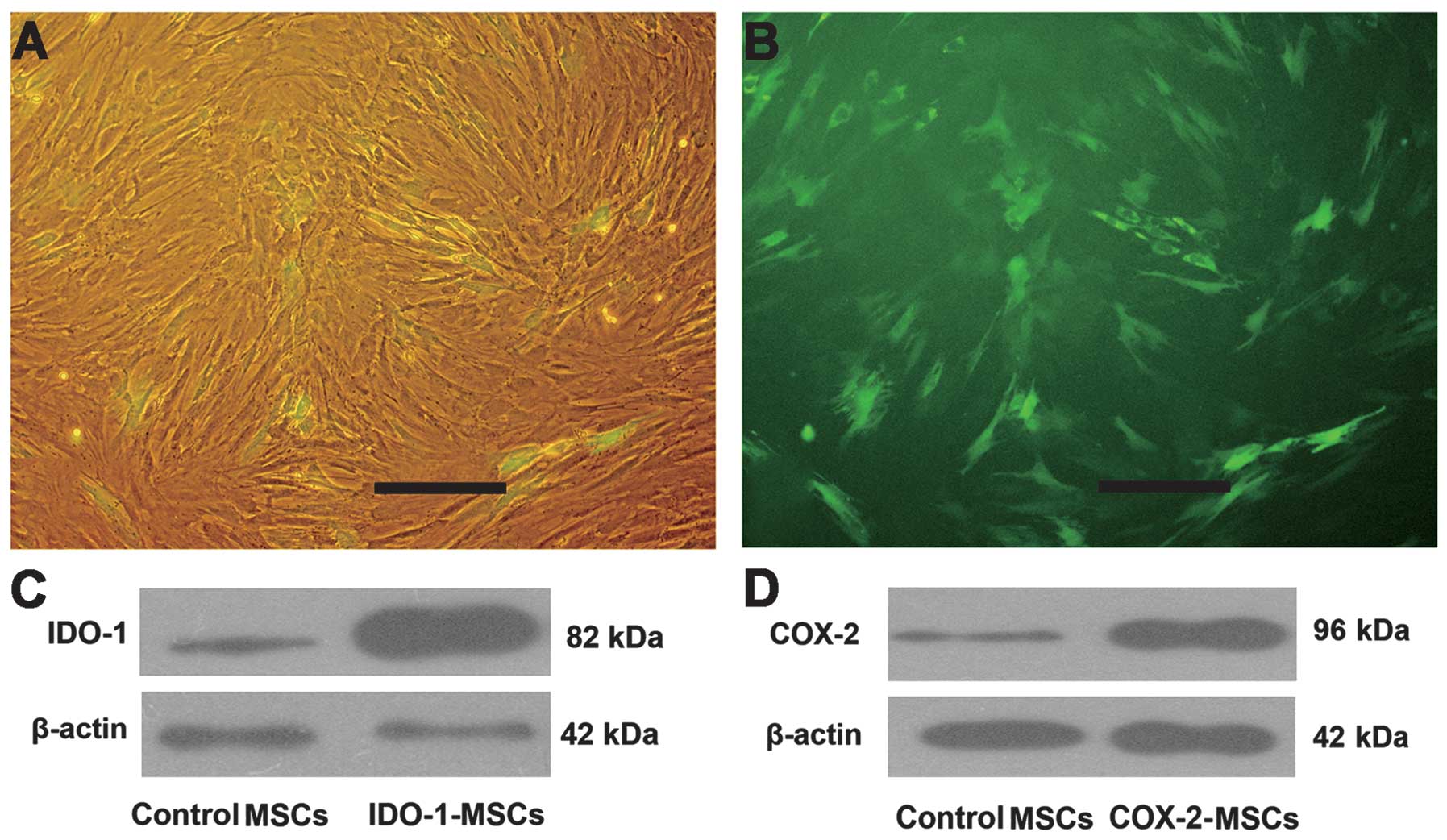
vectors with a transfection efficiency of ~30% (Fig. 2A and B, as evaluated by
observation of GFP fluorescence). The results of the western
immunoblot analysis confirmed the existence of a thicker band for
the IDO-1 and COX-2 proteins in the transfected MSCs as compared to
MSCs without transfection, which demonstrated that the IDO-1 and
COX-2 cDNA was expressed in MSCs (Fig. 2C).
Expression of immune-related genes in
IDO-1- and COX-2-modified MSCs
The results showed that the MSCs transfected with
recombinant plasmids markedly upregulated the expression of the
inserted genes (Fig. 3). Compared
with the control group, the MSCs (IDO-1) group expressed very high
levels of IDO-1, and downregulated other immunosupressive factors,
such as COX-2, heme-oxygenase-1 (HO-1), inducible nitric-oxide
synthase (iNOS), TGF-β, IL-10, TNF-α-stimulated gene/protein-6
(TSG-6) and human leukocyte antigen molecule 5 (HLA-G5). For the
MSCs (COX-2) group, the expression levels of COX-2 and other immune
regulatory genes were all elevated. However, apart from the
expression of IDO-1 and COX-2, almost all the detected genes were
dampened in the MSCs (IDO-1) and (IDO-1/COX-2) groups.
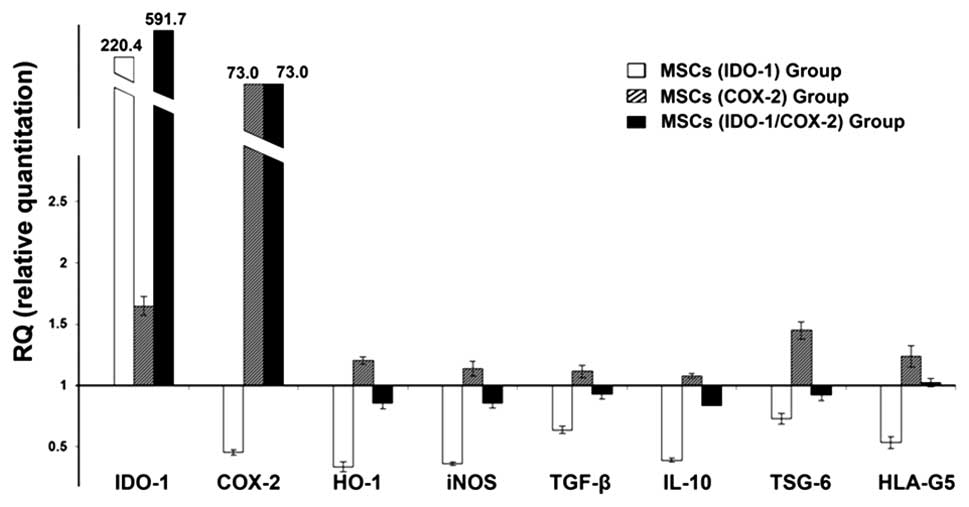 | Figure 3Immune-related gene expression in
indoleamine 2,3-dioxygenase-1 (IDO-1)- and cyclooxygenase-2
(COX-2)-modified mesenchymal stem cells (MSCs).
Immunomodulation-related gene expression was quantified by the
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) assay (the control group values were set at fold-change,
1). Compared with the untreated control group, the expression of
IDO-1, COX-2, heme-oxygenase-1 (HO-1), inducible nitric-oxide
synthase (iNOS), tumor necrosis factor-α (TNF-α) stimulated
gene/protein-6 (TSG-6), transforming growth factor-β (TGF-β), human
leukocyte antigen molecule 5 (HLA-G5) and interleukin-10 (IL-10)
were enhanced in the COX-2 MSC group. However, apart from IDO-1 and
COX-2, almost all the detected genes were dampened in the IDO-1 MSC
group and the IDO-1/COX-2 MSC group. |
Potent immunosuppressive ability of
COX-2-modified MSCs
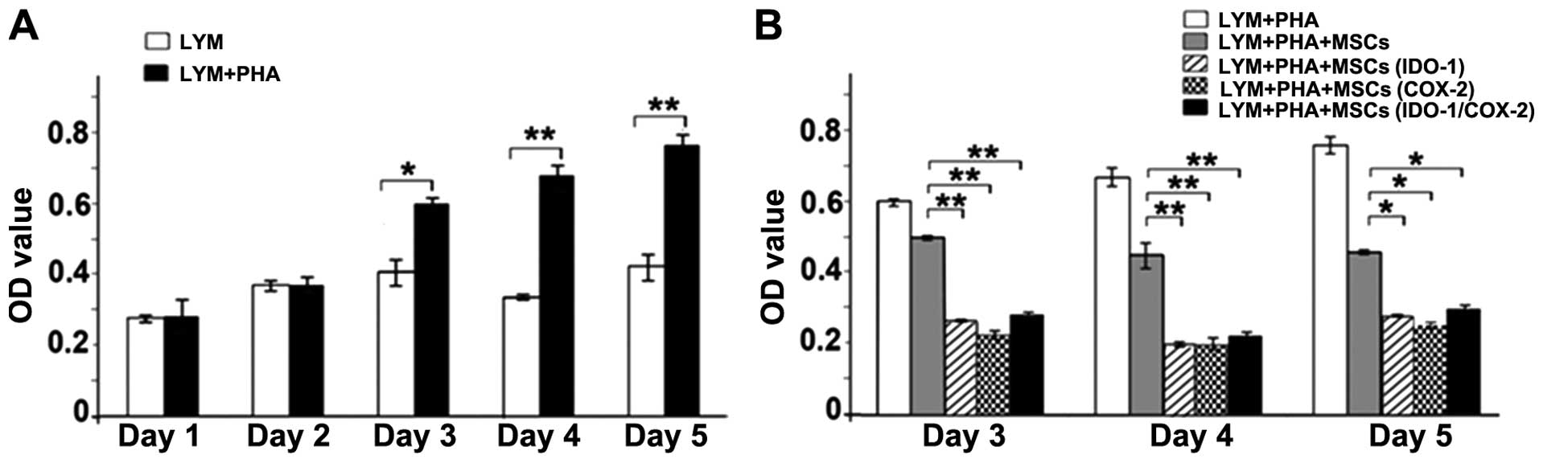
In the preliminary experiments, PHA-stimulated
lymphocytes showed significant proliferation from day 3 onwards
compared to the untreated group (Fig.
4A). Thus, we collected the data from day 3 in subsequent
co-culture assays. The results (Fig.
4B) showed that the inhibitory effect of MSCs on lymphocyte
proliferation was statistically significant when transfected with
IDO-1 or COX-2 compared with the control MSCs. However, the
inhibitory effect of MSCs (IDO-1/COX-2) group was lower than that
of the single-transfected MSCs (IDO-1) group or MSCs (COX-2)
group.
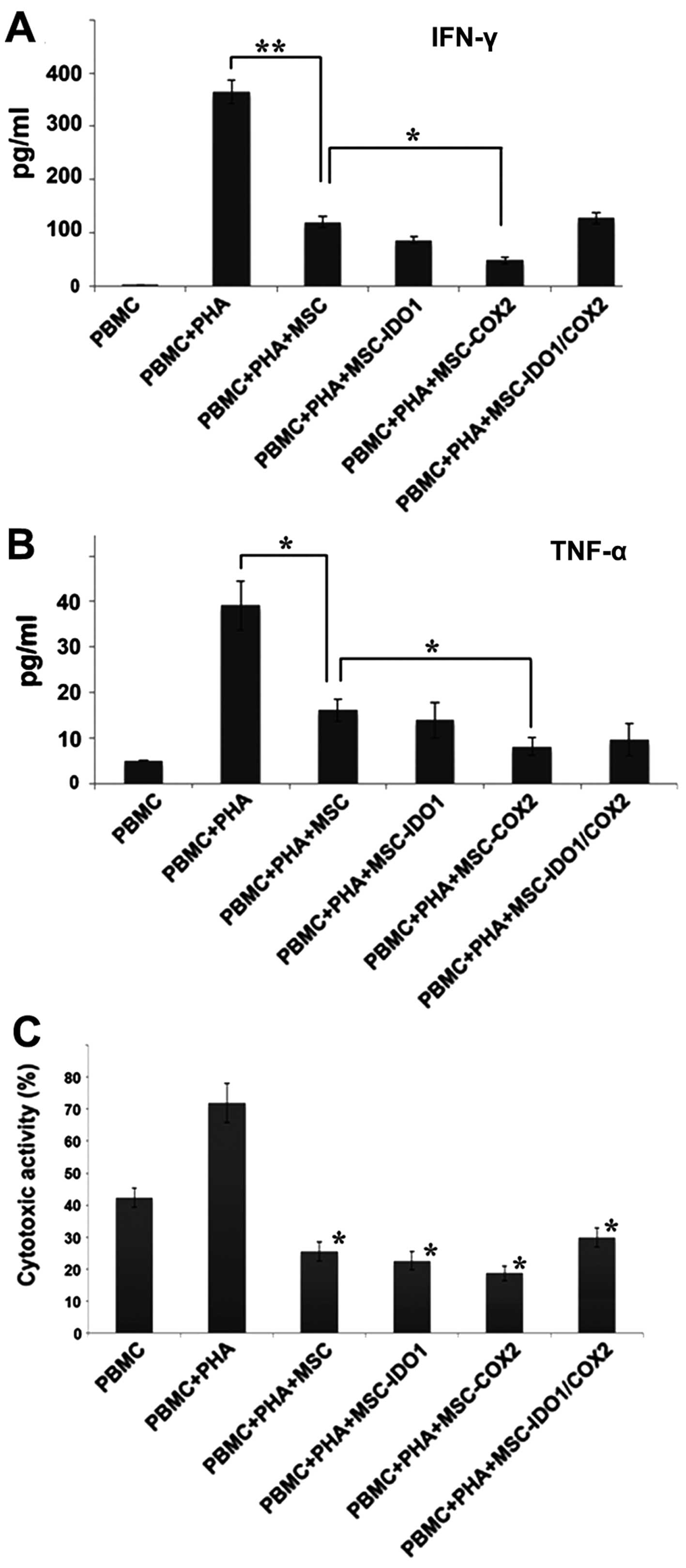
Inflammatory cytokines expressed by PBMC, such as
IFN-γ and TNF-α, were activated by PHA stimulation. However,
following co-culture with MSCs, the expression levels of IFN-γ and
TNF-α were significantly reduced in PBMC. By contrast, the MSCs
(COX-2) group more effectively inhibited the protein expression
levels of IFN-γ and TNF-α than their control group counterparts
(P<0.05) (Fig. 5A and B).
In the cytotoxicity analysis, the results showed
that the 10:1 ratio of lymphocytes relative to HeLa cells exerted a
highly acceptable killing effect. By contrast, PHA-activated PBMC
exhibited markedly increased cytotoxicity against HeLa cells. After
co-culture with MSCs, the cytotoxic activity of PBMC was
significantly lower than the non-transfected MSCs group
(P<0.05). In addition, PBMC incubated with the MSCs (COX-2)
group, had the lowest killing activity as compared with the
non-transfected control group. However, the observations were not
statistically different from each other (Fig. 5C).
Discussion
The immunosuppressive properties of MSCs potentially
endow them with properties ideally suited as cellular products in
the treatment of autoimmune and other immune-mediated disorders
(29,30). Improving the immune regulatory
efficiency of MSCs inevitably leads to improved clinical outcomes.
As previously mentioned, IDO-1 and COX-2 play critical roles in the
immunomodulation of MSCs (23,24). In tryptophan catabolism, IDO-1 is
a rate-limiting enzyme that inhibits antigen-specific T-cell
proliferation and suppresses T-cell responses. In addition, COX-2
plays a key role in prostaglandin biosynthesis, which is helpful in
PGE2-mediated immunosuppression (31–34). Therefore, in the current study, we
attempted to improve the expression levels of IDO-1 or COX-2 to
expand the active functional expression of key immune regulatory
molecules that are needed in adoptive cellular immune therapy,
thereby increasing the potential immune regulatory efficiency of
MSCs.
Considering the clinical safety of the cell product,
viral vectors are not the optimal choice. The short lifespan of
allogeneic MSCs in recipient subjects also does not require a
stable transfection (35–37). Thus, we reconstructed two
recombinant plasmids to enhance the expression levels of IDO-1 and
COX-2. The results of RT-qPCR and western immunoblotting confirmed
the expression of IDO-1 and COX-2 in transfected MSCs. Further
functional tests in co-culture assays, including lymphocyte
proliferation and cytotoxicity assays, showed that
COX-2-transfected MSCs exhibit more potent immunomodulatory
properties than other groups. As shown by ELISA, the synthesis of
IFN-γ and TNF-α was significantly inhibited in PBMC after
co-culture with COX-2-transfected MSCs. IFN-γ is a cytokine that is
produced primarily by T-lymphocytes and natural killer cells. IFN-γ
is critical for innate and adaptive immunity against viral and
intracellular bacterial infections and for tumor control (38). It also alters the transcription of
up to 30 genes that produce a variety of physiological and cell
responses, including driving NK cell activity, increasing antigen
presentation and lysosomal activity of macrophages. TNF-α was
produced by macrophages, lymphoid cells, and fibroblasts, among
other cells. TNF-α was originally characterized by its ability to
induce tumor cell apoptosis and cachexia, and is now considered a
central mediator of inflammation (39). Reduction in the levels of IFN-γ
and TNF-α dampens the occurrence of inflammation and immune
rejection.
To elucidate the changes caused by transfection, we
examined the expression of several immune regulatory genes in MSCs.
In addition to IDO-1 and COX-2, the expression of
HO-1, iNOS, TSG-6, TGF-β, HLA-G5
and IL-10 were all increased after COX-2 transfection in
MSCs. Previous findings have demonstrated that secretion of TGF-β
and induction of IL-10 contribute to the immunological dampening
functions of MSC (40).
MSC-released TSG-6 was also identified and shown to improve
immunosuppression by interacting with CD44 on resident macrophages,
which decreased TLR2/NFκ-B signaling and thereby decreased the
secretion of proinflammatory mediators (41,42).
Another important molecule involved in MSC immune
regulation involved the expression and secretion of HLA-G5. The
soluble isoform of HLA-G5 that is secreted by MSCs is responsible
for the suppression of T-cell prolife ration, NK cell-mediated
cytolysis, and IFN-γ secretion (43). It was shown that iNOS is important
in MSC immune suppression by limiting T-cell proliferation
(44). The stress-inducible
enzyme HO-1, which catalyzes the rate-limiting step of the
degradation of heme to biliverdin, also has a suppressive effect on
T-cell proliferation in human and rat MSCs (45,46). Increased expression of these genes
indicated that COX-2-enhanced MSCs were the most effective at
enhancing immunosuppression, which is consistent with the results
obtained from the functional tests.
The immunosuppressive effect of the MSCs (IDO-1)
group was less than that of the MSCs (COX-2) group, suggesting the
importance of PGE2 as compared to tryptophan/kynurenine among
several immunomodulatory mechanisms. Notably, the immunosuppressive
effect of MSCs with the two recombinant plasmids was weaker than
MSCs transfected with only one recombinant plasmid. We hypothesize
that the two plasmids may have competitive properties in the cell,
resulting in inadequate translation. However, COX-2 transfection
promoted positive outcomes, despite the instability of
electrotransfection among different batches and cell membrane
damage caused by the electrical pulses, which may have affected the
efficacy of cell therapy. Additionally, the influence of COX-2
overexpression on cell viability remains to be investigated.
In conclusion, we obtained a novel population of
MSCs that was derived following the overexpression of the
immune-related COX-2 gene. The modified MSCs more potently
inhibited the activation and proliferation of PBMCs. Thus, this
gene-modified population of MSCs overexpressing COX-2, are expected
to reduce the requirement of the number of infused cells, and
improve the efficacy of treating autoimmune diseases and aGVHD.
Acknowledgments
The Major State Basic Research Development Program
(2012CB966504), Shandong Province Natural Science Foundation
(ZR2011HM007 and 2013GSF11812), and the Innovation Fund Project of
Shandong University (grant no. 2012ZD023) all supported this
study.
References
|
1
|
Johnson TV, Bull ND, Hunt DP, Marina N,
Tomarev SI and Martin KR: Neuroprotective effects of intravitreal
mesenchymal stem cell transplantation in experimental glaucoma.
Invest Ophthalmol Vis Sci. 51:2051–2059. 2010. View Article : Google Scholar :
|
|
2
|
Matsushita K, Morello F, Wu Y, et al:
Mesenchymal stem cells differentiate into renin-producing
juxtaglomerular (JG)-like cells under the control of liver X
receptor-alpha. J Biol Chem. 285:11974–11982. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Williams AR and Hare JM: Mesenchymal stem
cells: biology, pathophysiology, translational findings, and
therapeutic implications for cardiac disease. Circ Res.
109:923–940. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Musumeci G, Lo Furno D, Loreto C, et al:
Mesenchymal stem cells from adipose tissue which have been
differentiated into chondrocytes in three-dimensional culture
express lubricin. Exp Biol Med (Maywood). 236:1333–1341. 2011.
View Article : Google Scholar
|
|
5
|
Jurewicz M, Yang S, Augello A, et al:
Congenic mesenchymal stem cell therapy reverses hyperglycemia in
experimental type 1 diabetes. Diabetes. 59:3139–3147. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Curley GF, Hayes M, Ansari B, et al:
Mesenchymal stem cells enhance recovery and repair following
ventilator-induced lung injury in the rat. Thorax. 67:496–501.
2012. View Article : Google Scholar
|
|
7
|
Li J, Li D, Liu X, Tang S and Wei F: Human
umbilical cord mesenchymal stem cells reduce systemic inflammation
and attenuate LPS-induced acute lung injury in rats. J Inflamm
(Lond). 9:332012. View Article : Google Scholar
|
|
8
|
Hu KX, Sun QY, Guo M and Ai HS: The
radiation protection and therapy effects of mesenchymal stem cells
in mice with acute radiation injury. Br J Radiol. 83:52–58. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zoja C, Garcia PB, Rota C, et al:
Mesenchymal stem cell therapy promotes renal repair by limiting
glomerular podocyte and progenitor cell dysfunction in
adriamycin-induced nephropathy. Am J Physiol Renal Physiol.
303:F1370–F1381. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nauta AJ and Fibbe WE: Immunomodulatory
properties of mesenchymal stromal cells. Blood. 110:3499–3506.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Aggarwal S and Pittenger MF: Human
mesenchymal stem cells modulate allogeneic immune cell responses.
Blood. 105:1815–1822. 2005. View Article : Google Scholar
|
|
12
|
Atoui R, Shum-Tim D and Chiu RC:
Myocardial regenerative therapy: immunologic basis for the
potential ‘universal donor cells’. Ann Thorac Surg. 86:327–334.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rice CM, Kemp K, Wilkins A and Scolding
NJ: Cell therapy for multiple sclerosis: an evolving concept with
implications for other neurodegenerative diseases. Lancet.
382:1204–1213. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim N, Im KI, Lim JY, et al: Mesenchymal
stem cells for the treatment and prevention of graft-versus-host
disease: experiments and practice. Ann Hematol. 92:1295–1308. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ning H, Yang F, Jiang M, et al: The
correlation between cotransplantation of mesenchymal stem cells and
higher recurrence rate in hematologic malignancy patients: outcome
of a pilot clinical study. Leukemia. 22:593–599. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ball LM, Bernardo ME, Roelofs H, et al:
Cotransplantation of ex vivo expanded mesenchymal stem cells
accelerates lymphocyte recovery and may reduce the risk of graft
failure in haploidentical hematopoietic stem-cell transplantation.
Blood. 110:2764–2767. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lazarus HM, Koc ON, Devine SM, et al:
Cotransplantation of HLA-identical sibling culture-expanded
mesenchymal stem cells and hematopoietic stem cells in hematologic
malignancy patients. Biol Blood Marrow Transplant. 11:389–398.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Koç ON, Gerson SL, Cooper BW, et al: Rapid
hematopoietic recovery after coinfusion of autologous-blood stem
cells and culture-expanded marrow mesenchymal stem cells in
advanced breast cancer patients receiving high-dose chemotherapy. J
Clin Oncol. 18:307–316. 2000.PubMed/NCBI
|
|
19
|
Le Blanc K, Samuelsson H, Gustafsson B, et
al: Transplantation of mesenchymal stem cells to enhance
engraftment of hematopoietic stem cells. Leukemia. 21:1733–1738.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ji JF, He BP, Dheen ST and Tay SS:
Interactions of chemokines and chemokine receptors mediate the
migration of mesenchymal stem cells to the impaired site in the
brain after hypoglossal nerve injury. Stem Cells. 22:415–427. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Devine SM, Cobbs C, Jennings M,
Bartholomew A and Hoffman R: Mesenchymal stem cells distribute to a
wide range of tissues following systemic infusion into nonhuman
primates. Blood. 101:2999–3001. 2003. View Article : Google Scholar
|
|
22
|
Jung JW, Kwon M, Choi JC, et al: Familial
occurrence of pulmonary embolism after intravenous, adipose
tissue-derived stem cell therapy. Yonsei Med J. 54:1293–1296. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Spaggiari GM, Abdelrazik H, Becchetti F
and Moretta L: MSCs inhibit monocyte-derived DC maturation and
function by selectively interfering with the generation of immature
DCs: central role of MSC-derived prostaglandin E2. Blood.
113:6576–6583. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Opitz CA, Litzenburger UM, Lutz C, et al:
Toll-like receptor engagement enhances the immunosuppressive
properties of human bone marrow-derived mesenchymal stem cells by
inducing indoleamine-2,3-dioxygenase-1 via interferon-beta and
protein kinase R. Stem Cells. 27:909–919. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sato K, Ozaki K, Oh I, et al: Nitric oxide
plays a critical role in suppression of T-cell proliferation by
mesenchymal stem cells. Blood. 109:228–234. 2007. View Article : Google Scholar
|
|
26
|
Yoo SW, Chang DY, Lee HS, et al: Immune
following suppression mesenchymal stem cell transplantation in the
ischemic brain is mediated by TGF-β. Neurobiol Dis. 58:249–257.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Djouad F, Charbonnier LM, Bouffi C, et al:
Mesenchymal stem cells inhibit the differentiation of dendritic
cells through an interleukin-6-dependent mechanism. Stem Cells.
25:2025–2032. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Németh K, Leelahavanichkul A, Yuen PS, et
al: Bone marrow stromal cells attenuate sepsis via prostaglandin
E(2)-dependent reprogramming of host macrophages to increase their
interleukin-10 production. Nat Med. 15:42–49. 2009. View Article : Google Scholar
|
|
29
|
Sui W, Hou X, Che W, et al: Hematopoietic
and mesenchymal stem cell transplantation for severe and refractory
systemic lupus erythematosus. Clin Immunol. 148:186–197. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Luz-Crawford P, Kurte M, Bravo-Alegria J,
et al: Mesenchymal stem cells generate a
CD4+CD25+Foxp3+ regulatory T cell
population during the differentiation process of Th1 and Th17
cells. Stem Cell Res Ther. 4:652013. View
Article : Google Scholar
|
|
31
|
Spaggiari GM, Capobianco A, Abdelrazik H,
Becchetti F, Mingari MC and Moretta L: Mesenchymal stem cells
inhibit natural killer-cell proliferation, cytotoxicity, and
cytokine production: role of indoleamine 2,3-dioxygenase and
prostaglandin E2. Blood. 111:1327–1333. 2008. View Article : Google Scholar
|
|
32
|
English K, Ryan JM, Tobin L, Murphy MJ,
Barry FP and Mahon BP: Cell contact, prostaglandin E(2) and
transforming growth factor beta 1 play non-redundant roles in human
mesenchymal stem cell induction of CD4+CD25(High)
forkhead box P3+ regulatory T cells. Clin Exp Immunol.
156:149–160. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Duffy MM, Pindjakova J, Hanley SA, et al:
Mesenchymal stem cell inhibition of T-helper 17
cell-differentiation is triggered by cell-cell contact and mediated
by prostaglandin E2 via the EP4 receptor. Eur J Immunol.
41:2840–2851. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yañez R, Oviedo A, Aldea M, Bueren JA and
Lamana ML: Prostaglandin E2 plays a key role in the
immunosuppressive properties of adipose and bone marrow
tissue-derived mesenchymal stromal cells. Exp Cell Res.
316:3109–3123. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pittenger MF and Martin BJ: Mesenchymal
stem cells and their potential as cardiac therapeutics. Circ Res.
95:9–20. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tamama K, Kawasaki H and Wells A:
Epidermal growth factor (EGF) treatment on multipotential stromal
cells (MSCs). Possible enhancement of therapeutic potential of MSC.
J Biomed Biotechnol. 2010:7953852010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Freyman T, Polin G, Osman H, et al: A
quantitative, randomized study evaluating three methods of
mesenchymal stem cell delivery following myocardial infarction. Eur
Heart J. 27:1114–1122. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schroder K, Hertzog PJ, Ravasi T and Hume
DA: Interferon-gamma: an overview of signals, mechanisms and
functions. J Leukoc Biol. 75:163–189. 2004. View Article : Google Scholar
|
|
39
|
Pfeffer K: Biological functions of tumor
necrosis factor cytokines and their receptors. Cytokine Growth
Factor Rev. 14:185–191. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu H, Lu K, MacAry PA, et al: Soluble
molecules are key in maintaining the immunomodulatory activity of
murine mesenchymal stromal cells. J Cell Sci. 125:200–208. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Qi Y, Jiang D, Sindrilaru A, et al: TSG-6
released from intradermally injected mesenchymal stem cells
accelerates wound healing and reduces tissue fibrosis in murine
full-thickness skin wounds. J Invest Dermatol. 134:526–537. 2014.
View Article : Google Scholar
|
|
42
|
Prockop DJ: Concise review: two negative
feedback loops place mesenchymal stem/stromal cells at the center
of early regulators of inflammation. Stem Cells. 31:2042–2046.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Selmani Z, Naji A, Zidi I, et al: Human
leukocyte antigen-G5 secretion by human mesenchymal stem cells is
required to suppress T lymphocyte and natural killer function and
to induce CD4+CD25highFOXP3+
regulatory T cells. Stem Cells. 26:212–222. 2008. View Article : Google Scholar
|
|
44
|
Ren G, Zhang L, Zhao X, et al: Mesenchymal
stem cell-mediated immunosuppression occurs via concerted action of
chemokines and nitric oxide. Cell Stem Cell. 2:141–150. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chabannes D, Hill M, Merieau E, et al: A
role for heme oxygenase-1 in the immunosuppressive effect of adult
rat and human mesenchymal stem cells. Blood. 110:3691–3694. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Brusko TM, Wasserfall CH, Agarwal A,
Kapturczak MH and Atkinson MA: An integral role for heme
oxygenase-1 and carbon monoxide in maintaining peripheral tolerance
by CD4+CD25+ regulatory T cells. J Immunol.
174:5181–5186. 2005. View Article : Google Scholar : PubMed/NCBI
|