Introduction
Inflammation is a complex protective response caused
by endo- and exogenous stimuli such as bacterial lipopolysaccharide
(LPS) (1). LPS, the major
component in the outer membrane of gram-negative bacteria cell
walls, induces the production of pro-inflammatory cytokines, such
as tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, and IL-6,
as well as inflammatory mediators, such as nitric oxide (NO) and
prostaglandin E2 (PGE2), which are
synthesized by inducible nitric oxide synthase (iNOS) and
cyclooxygenase-2 (COX-2), respectively (2). These pro-inflammatory cytokines and
inflammatory mediators assist with the innate immune response.
However, their overproduction results in acute phase endotoxemia
that causes tissue injury, organ failure, shock and even death
(3).
Nuclear factor-κB (NF-κB), which is a key player in
the regulation of immune and inflammatory responses, has an
important role in modulating the transcription of several
inflammatory factors and cytokines such as TNF-α, IL-1β and IL-6
(4). In unstimulated cells, Rel
protein dimers, which are composed mainly of p50 and p65 subunits,
are normally sequestered in the cytosol as an inactive complex by
binding to inhibitor κB-α (IκB-α) (5). The activation of NF-κB mostly occurs
through the phosphorylation and subsequent degradation of IκB via
the activation of inhibitor κB kinase (IKK). When IκB is
phosphorylated, it is targeted for ubiquitination and subsequent
degradation by the 26S proteosome (6). The resulting free NF-κB then
translocates to the nucleus, where it binds to κB-binding sites in
the promoter regions of target genes and induces the transcription
of pro-inflammatory mediators (7).
The mitogen-activated protein kinases (MAPKs) are
serine/threonine-specific protein kinases that play a critical role
in the regulation and differentiation of cell survival/apoptosis,
as well as in controlling the cell response to cytokines, growth
factors and environmental stresses (8). These classical MAPKs, extracellular
signal-regulated kinase 1/2 (ERK 1/2), p38 MAPK and c-jun
NH2-terminal kinase (JNK) have been involved in the transcriptional
regulation of inflammatory genes (9). Specifically, p38 MAPK signaling
pathways constitute an additional level of gene regulation by
NF-κB, especially of the p65 subunit (10,11). Synergistically, MAPKs and NF-κB
can collaborate to induce pro-inflammatory cytokine gene products
and excretion (12). Accordingly,
therapy aimed at the inhibition of NF-κB and MAPKs have potential
therapeutic advantages in curing inflammatory diseases (13).
Canarium lyi C.D. Dai & Yakovlev (CL) is
a member of the Anacardiaceae family. To the best of our knowledge,
no studies on its anti-inflammatory effects have yet been
reported.
In the present study, we elucidated the assumptive
anti-inflammatory mechanism of CL on LPS-stimulated RAW 264.7
macrophages by measuring the levels of cytokines, NF-κB and MAPK
activation. In addition, we assessed the anti-inflammatory effects
of CL in a murine model of LPS-induced acute lung injury by
measuring the brochoalveolar lavage fluid (BALF) analysis,
pro-inflammatory protein expression and histological alteration of
lung tissue.
Materials and methods
In vitro experiment
Preparation of plant material
Canarium lyi C.D. Dai & Yakovlev of the
Burseraceae family was collected from the area of Gia Lai, K Bang,
So Pai, Vietnam in 2011. Plant samples were identified by Dr Tran
The Bach of the Institute of Ecology and Biological Resources. A
voucher specimen (KRIB 0036679) was deposited in the herbarium
(KRIB) of the Korea Research Institute of Bioscience and
Biotechnology. Canarium lyi (147 g) was treated with MeOH
and sonicated several times at room temperature for 3 days to
produce an extract (20.05 g).
Cell culture
RAW 264.7 cells were maintained at 1×105
cells/ml in Dulbecco’s modified Eagle’s medium (DMEM; Sigma, St.
Louis, MO, USA) supplemented with 10% heat-inactivated fetal bovine
serum (FBS; Invitrogen, Burlington, ON, Canada), and 1% (w/v) of an
antibiotic-antimycotic solution (Invitrogen, Grand Island, NY, USA)
in 95% air and 5% CO2 humidified atmosphere at 37°C.
Cytotoxicity assay
Cell viability was determined by assessing the
mitochondrion-dependent reduction of MTT (Amresco LLC, Solon, OH,
USA) to formazan. Briefly, 5 μl of a 5 mg/ml MTT solution
was added to the cell supernatant, and then incubation for 4 h at
37°C. DMSO was added following removal of the medium. The optical
density of formazan was measured using a microplate reader
(VersaMax; Molecular Devices, San Diego, CA, USA) at 570 nm. The
level of formazan generated by untreated cells was chosen as the
100% value.
Measurement of nitric oxide
Nitrite levels in the cultured media and serum,
which reflect intracellular nitric oxide (NO) synthase activity,
were determined by Griess reaction. The cells were incubated with
samples in the presence of LPS (0.5 μg/ml, Sigma-Aldrich, La
Jolla, CA, USA) at 37°C for 24 h. The cell supernatant was
dispensed into new 96-well plates, and 100 μl of each
supernatant was mixed with the same volume of the Griess reagent
[1% sulfanilamide, 0.1% N-(1-naphathyl)-ethylenediamine
dihydrochloride and 5% phosphoric acid] and incubated at room
temperature for 10 min. Sodium nitrite was used to generate a
standard curve, and the concentration of nitrite was measured for
absorbance at 540 nm.
Enzyme-linked immunosorbent assay
(ELISA) of IL-6
The levels of IL-6 in supernatant were determined
using a commercially available ELISA kit (R&D Systems Inc.,
Minneapolis, MN, USA) according to the manufacturer’s instructions.
IL-6 levels were determined from a standard curve. The
concentrations were expressed as pg/ml.
Enzyme immune assay of prostaglandin
E2
PGE2 levels in supernatants were
determined using a PGE2 EIA kit (Cayman Chemical Co.,
Inc., Ann Arbor, MI, USA) according to the manufacturer’s
instructions. Briefly, 50 μl diluted standards/samples were
pipetted into the wells of a 96-well plate precoated with goat
polyclonal anti-mouse IgG. Aliquots of a PGE2 monoclonal
antibody solution and a PGE2 acetylcholine esterase
conjugate solution were added to each well, and incubated at room
temperature for 18 h. The wells were washed six times with a wash
buffer containing 0.05% (v/v) Tween-20, followed by the addition of
200 μl Ellman’s reagent containing acetylthiocholine and
5,5′-dithio-bis-(2-nitrobenzoic acid). PGE2
concentrations were measured by absorbance at 405 nm.
Reverse transcriptase-polymerase chain
reaction analysis (RT-PCR)
Total RNA was isolated using TRIzol™ reagent (Life
Technologies Corp., Carlsbad, CA, USA). For RT-PCR, a single-strand
cDNA was synthesized from 2 μg total RNA. The primer
sequences used were: iNOS: sense, 5′-CAA GAG TTT GAC CAG AGG ACC-3′
and antisense, 5′-TGG AAC CAC TCG TAC TTG GGA-3′; COX-2: sense,
5′-GAA GTC TTT GGT CTG GTC TCC TG-3′ and antisense, 5′-GTC TGC TGG
TTT GGA ATA GTT GC-3′; TNF-α: sense and 5′-CAT CTT GCA AAA TTC GAG
TGA CAA-3′ and antisense, 5′-TGG GAG TAG ACA AGG TAC AAC CC-3′;
IL-6, sense, 5′-GAG GAT ACC ACT CCC AAC AGA CC-3′ and antisense,
5′-AAG TGC ATC ATC GTT GTT CAT ACA-3′, β-actin: sense, 5′-CGC TCA
TTG CCG ATA GTG AT-3′ and antisense 5′-TGT TTG AGA CCT TCA ACA
CC-3′. PCR products were fractionated on 1.5% agarose gel
electrophoresis and stained with 5 μg/ml ethidium bromide.
Images were captured by an Olympus C-4000 Zoom camera system
(Olympus America Inc., Center Valley, PA, USA).
Immunoblot analysis
Western blot analyses were performed as previously
described (14). Immunoblotting
was performed with the primary antibodies at 4°C overnight. A
horseradish peroxidase-labeled secondary antibody (Santa Cruz
Biotechnology Inc., Santa Cruz, CA, USA) was then used for 1 h. The
membranes were washed three times with TBST, and then developed
using an enhanced chemiluminescence (ECL) kit (Thermo Fisher
Scientific, San Jose, CA, USA). For quantitative analysis,
densitometric band values were determined using a bio-imaging
analyzer (LAS 4000 mini; Fujifilm, Tokyo, Japan).
In vivo experiment
Animals
Male C57BL/6 mice (6–8 weeks) were obtained from the
Koatech Co. (Pyeongtaek, Korea). The mice were fed with food and
water ad libitum in an animal facility with temperature
ranging from 22 to 24°C and a 12-h light/dark cycle under a
specific pathogen-free conditions. Prior to the initiation of the
experiment, the mice were housed for a minimum of one week in order
that they adapt to the environment. The animal experimental
procedures were approved by the Korea Research Institute of
Bioscience and Biotechnology and performed in compliance with the
National Institute of Health Guidelines for the care and use of
laboratory animals and the Korean national animal welfare law.
Experimental protocols
Mice were randomly allocated into 4 groups: Control,
LPS, LPS + dexamethasone (LPS + DEX) and LPS + CL. Dexamethasone
served as a positive control drug. The mice of the LPS + DEX and
LPS + CL groups received dexamethasone (3 mg/kg) and CL (30 mg/kg)
by oral gavage for 3 days, respectively. The mice from the control
and LPS groups received oral gavage at an equal volume of PBS. The
mice including the LPS, LPS + DEX and LPS + CL groups were
instilled with 10 μg of LPS dissolved in 50 μl PBS
intranasally to induce acute lung injury 1 h after final drug
treatment. Mice in the control group were intranasally given 50
μl PBS without LPS for 18 h. To obtain BALF, ice-cold PBS
(0.7 ml) was infused into the lung and withdrawn via tracheal
cannulation twice (total volume 1.4 ml).
Inflammatory cell counts in
bronchoalveolar lavage fluid
Total inflammatory cell numbers were assessed by
counting cells in at least five squares of a hemocytometer after
excluding dead cells by Trypan blue staining. To determine the
differential cell counts, 100 μl of BALF was centrifuged
onto slides using a Cytospin (Hanil Science Industrial Co., Ltd.,
Seoul, Korea) (200 × g for 5 min). The slides were dried, and the
cells were fixed and stained using a Diff-Quik® staining
reagent (B4132-1A; IMEB Inc., Deerfield, IL, USA) following the
manufacturer’s instructions. The supernatant obtained from BALF was
stored at −70°C for the biochemical analysis.
Western blot analysis of the lung
tissue
Lung tissue was homogenized (1/10 w/v) using a
homogenizer with a tissue lysis/extraction reagent (Sigma-Aldrich)
containing a protease inhibitor cocktail (Roche Diagnostics,
Indianapolis, IN, USA). Each protein concentration was determined
using a Bradford reagent (Bio-Rad Laboratories, Hercules, CA, USA).
Western blotting was performed as described above, and the levels
of COX-2, IκB-α and NF-κB were determined.
Measurement of the levels of
pro-inflammatory cytokines in the BALF
The levels of IL-6 (R&D Systems), TNF-α and
IL-1β (BD Biosciences, San Jose, CA, USA) in BALF were measured
using ELISA kits according to the manufacturer’s instructions.
Histological analysis
After BALF samples were obtained, lung tissues were
fixed in 4% (v/v) paraformaldehyde. Tissues were embedded in
paraffin, sectioned at 4 μm thickness, and stained with
H&E solution (Sigma-Aldrich) to estimate inflammation.
Statistical analysis
Data are expressed as the means ± the standard error
of the mean (SEM). Statistical significance was determined using
analyses of variance (ANOVAs) followed by multiple comparison tests
with Dunnet’s adjustment. P<0.05 was considered significant.
Results
In vitro study
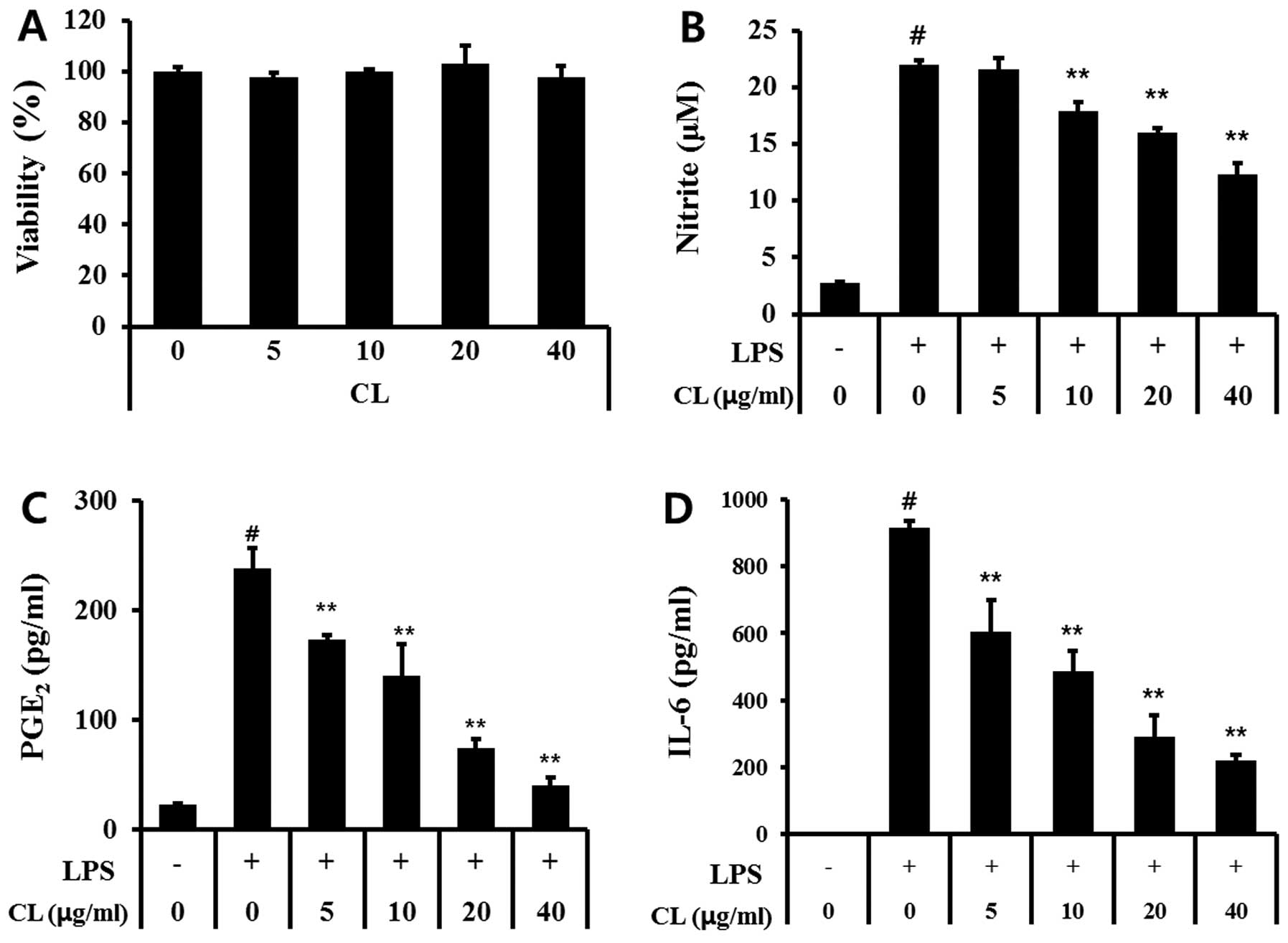
Effect of CL on cell viability
We determined the effect of CL on cell viability by
MTT assay after incubating cells for 24 h. The cytotoxicity of CL
was preliminarily evaluated to establish the appropriate
concentration ranges of CL for the analysis of ongoing experiments.
Results showed that the cells’ viabilities were not affected by CL
at the concentrations (5, 10, 20 and 40 μg/ml) used
(Fig. 1A). Therefore, we used
non-toxic concentrations (5–40 μg/ml) for the entire
experiment.
Effect of CL on the production of
nitrite, PGE2 and pro-inflammatory cytokines on
LPS-stimulated RAW 264.7 cells
LPS-stimulated cells markedly increased in NO,
whereas CL treatment significantly decreased NO production in a
concentration-dependent manner (Fig.
1B). We also examined the effects of CL on PGE2
production following LPS stimulation in RAW 264.7 cells. The amount
of PGE2 was increased by the LPS stimulation in the
culture supernatant, and this increase was effectively reduced by
treatment with CL (Fig. 1C).
Similarly, treatment of the RAW 264.7 cells with LPS
alone resulted in a significant increase in cytokine production
compared with the control group (Fig.
1D). However, CL treatment considerably inhibited the LPS
induction of IL-6 in a dose-dependent manner (Fig. 1D).
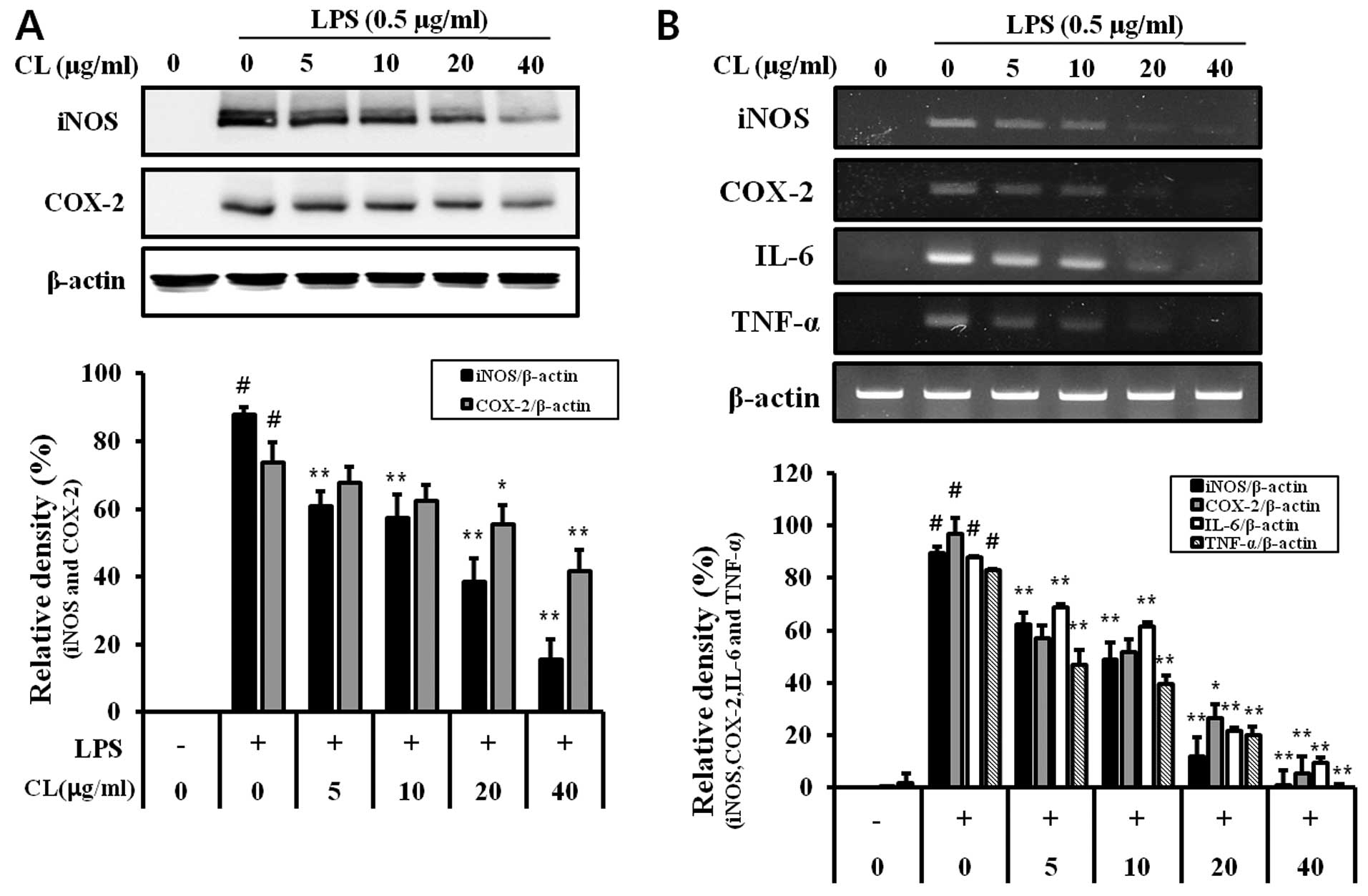
Effect of CL on mRNA and protein
expression levels of inflammatory mediators
The production of mRNA and protein of iNOS, COX-2
and pro-inflammatory cytokines, including IL-6 and TNF-α, increased
in LPS-stimulated RAW 264.7 cells (Fig. 2). However, treatment of the cells
with CL significantly decreased iNOS, COX-2 and pro-inflammatory
cytokine production compared to the LPS-stimulated cells in a
concentration-dependent manner (Fig.
2).
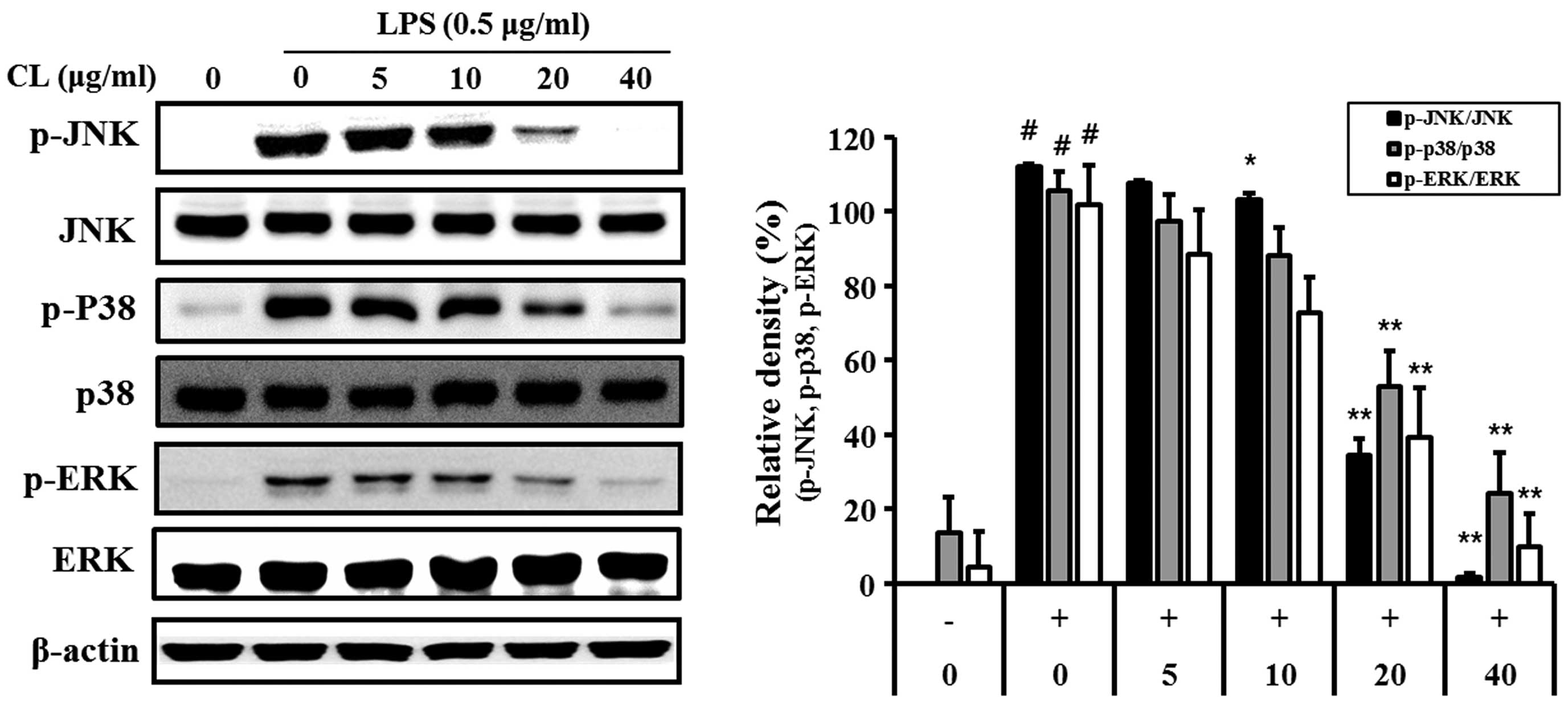
Effect of CL on LPS-induced MAPK
activation
LPS-stimulated cells showed an increase in the
phosphorylation levels of p38, ERK1/2 and JNK. By contrast,
treatment of the cells with CL significantly reduced the
phosphorylation of p38, ERK and JNK expression compared with the
LPS-stimulated cells in a concentration-dependent manner (Fig. 3).
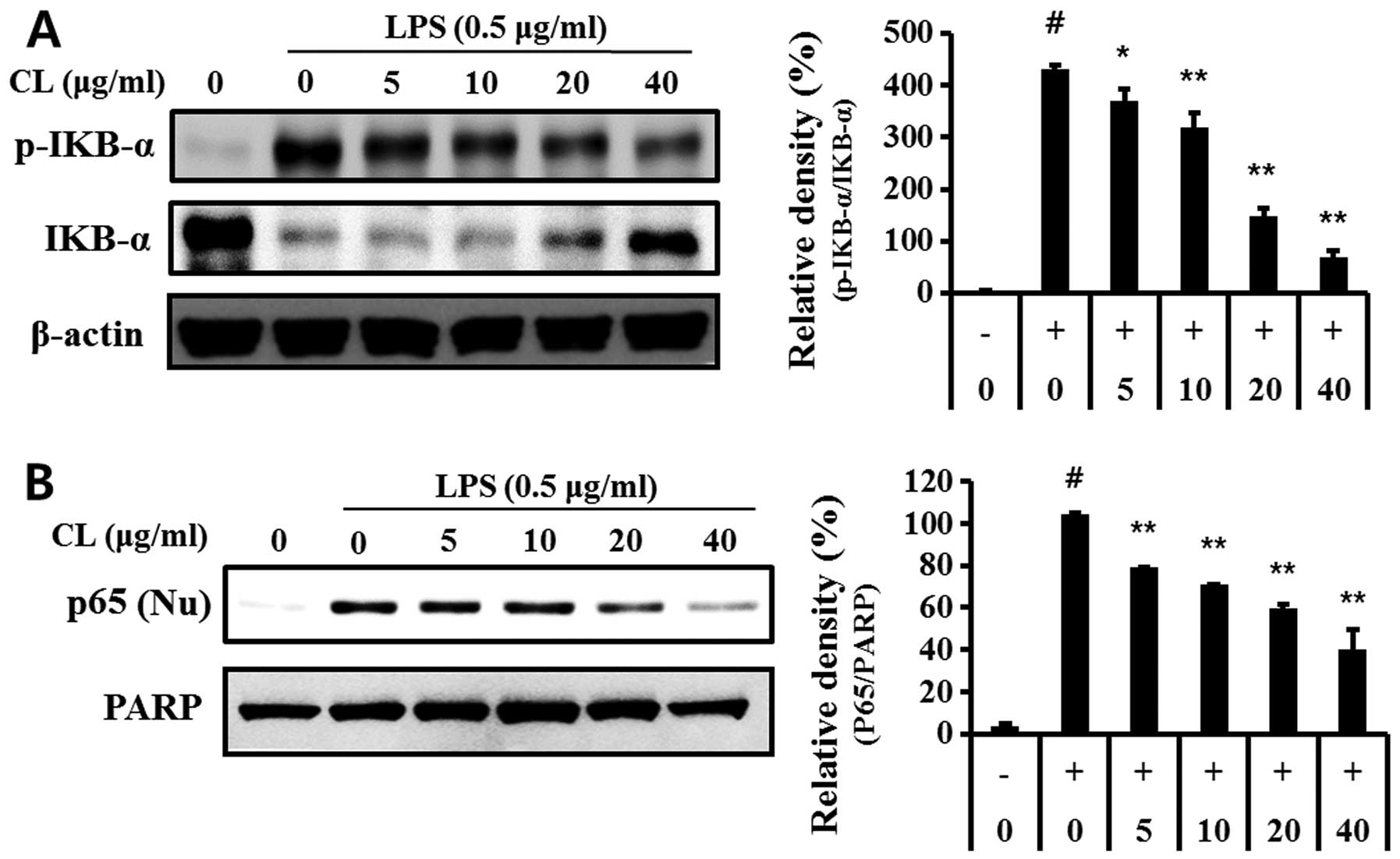
Effect of CL on LPS-induced NF-κB
activity
We examined the effect of CL on LPS-stimulated IκB-α
degradation. One hour of pre-treatment with CL followed by
treatment with LPS for 30 min markedly suppressed the
LPS-stimulated phosphorylation and degradation of IκB-α in a
dose-dependent manner (Fig. 4A).
We also investigated the translocation of the NF-κB subunit p65
from the cytosol to the nucleus using western blot analysis. LPS
stimulation caused p65 translocation from the cytosol to the
nucleus, while CL inhibited this translocation (Fig. 4B).
In vivo experiment
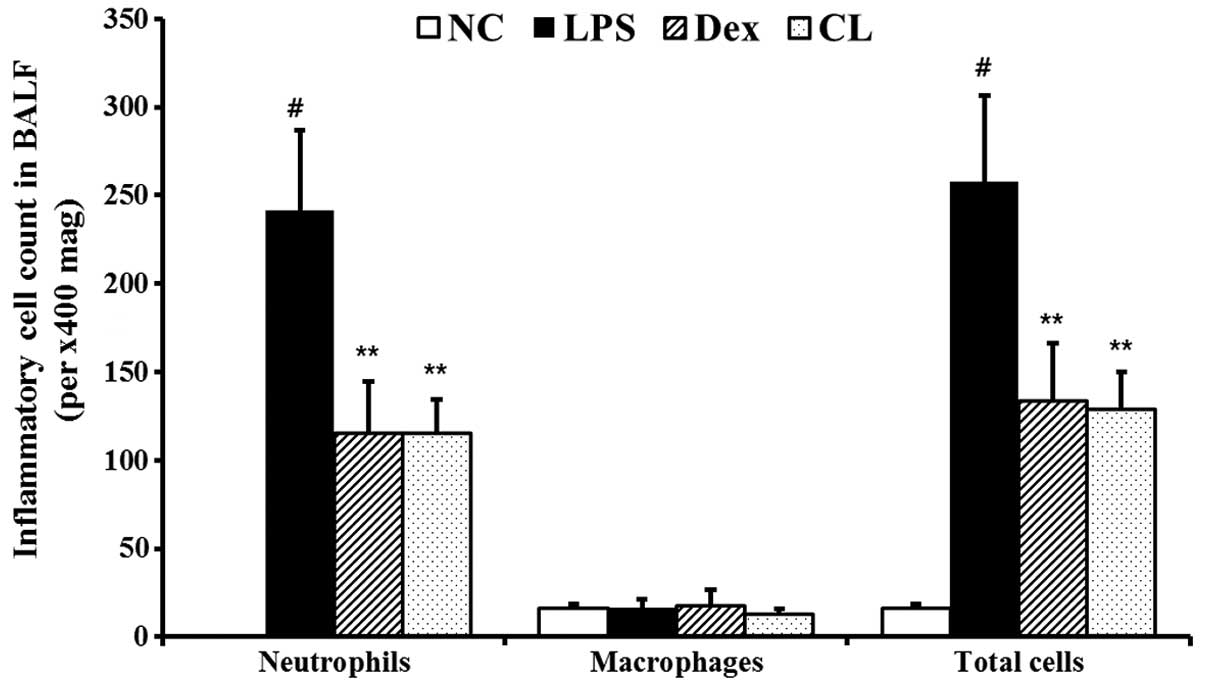
Effect of CL on inflammatory cell
count in the BALF of LPS-induced ALI mice
The LPS group showed a significant increase in the
number of total inflammatory cells and neutrophils in BALF compared
with the negative control group. However, pre-treatment with CL
significantly decreased the number of total inflammatory cells and
neutrophils compared with the LPS group (Fig. 5).
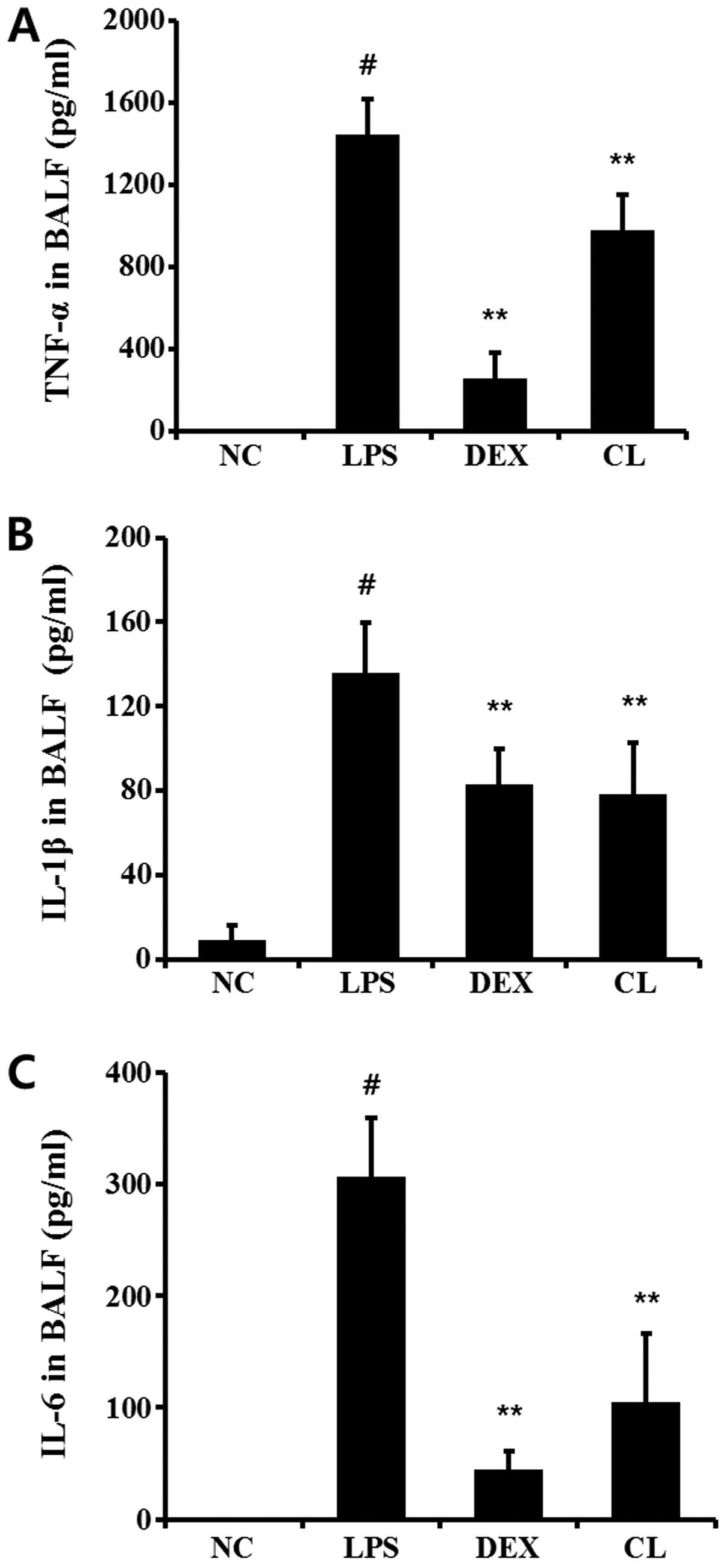
Effect of CL on pro-inflammatory
cytokines in the BALF of LPS-induced ALI mice
The levels of IL-6, IL-1β and TNF-α in BALF were
significantly higher in LPS-induced mice when compared with the
negative control group. By contrast, the CL-treated mice had
considerably lower levels of all these pro-inflammatory cytokines
compared to the LPS-treated mice (Fig. 6).
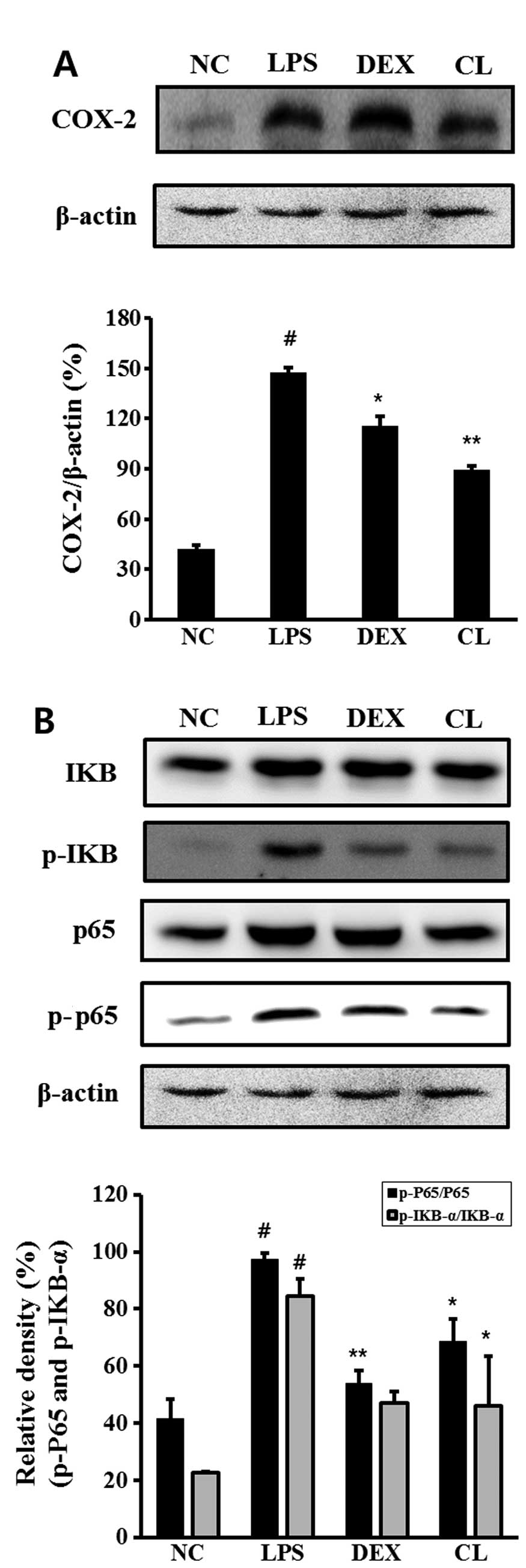
Effect of CL on COX-2 and NF-κB in the
lung tissue of LPS-induced ALI mice
The LPS-induced mice exhibited an increased
expression of COX-2, p-IκB and p-p65 in the lung tissue compared to
the negative control group. However, treatment of the mice with CL
significantly inhibited the expression of COX-2, IκB degradation
and the phosphorylation of p65 in the lung tissue compared to the
LPS-treated mice (Fig. 7).
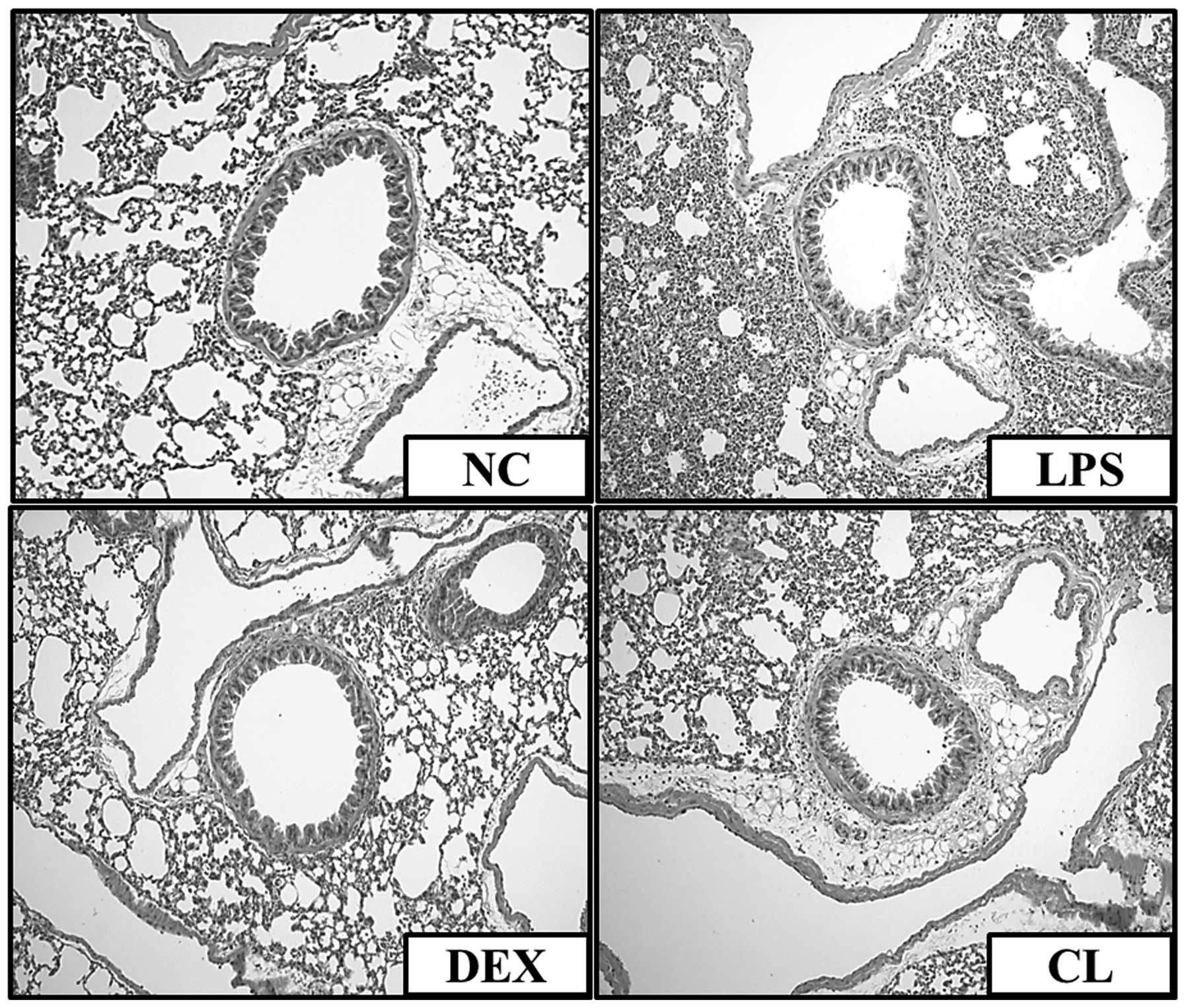
Effect of CL on histopathological
changes in the lung tissue of LPS-induced ALI mice
We confirmed an intact structure and clear pulmonary
alveoli in the lung sections of mice in the negative control group.
By contrast, lung sections obtained from mice in the LPS group
showed evidence of histological changes, including areas of
inflammatory cell infiltration, thickening of the alveolar wall,
edema and pulmonary congestion. By contrast, treatment of the mice
with CL attenuated the pathological changes that were observed in
the LPS-treated mice (Fig.
8).
Discussion
Macrophages are major inflammatory cells and immune
effector cells (15). The
activity of macrophages plays an important role in the inflammatory
responses when infected with pathogens such as LPS.
Macrophages can kill pathogens directly by
phagocytosis and indirectly through the secretion of various
pro-inflammatory mediators such as reactive oxygen and
pro-inflammatory cytokines such as TNF-α, IL-1β and IL-6 (16). The overproduction of the
inflammatory mediators by activated macrophages is involved in the
pathophysiology of many inflammatory diseases, including arthritis,
acute lung injury, chronic obstructive pulmonary disease (COPD),
asthma and inflammatory bowel disease (IBD) (17). LPS can directly activate
macrophages and trigger the production of inflammatory mediators,
such as pro-inflammatory cytokines, NO and PGE2, which
are the main cytotoxic and pro-apoptotic mechanisms participating
in the innate response of many mammals. Thus, LPS-stimulated RAW
264.7 macrophages can be effectively used as a model to study
inflammation and potential anti-inflammatory mediators with their
action mechanisms (18). The LPS
stimulation of murine macrophages has been known to induce the
phosphorylation and activation of MAPKs such as ERK 1/2, JNK, and
p38 (19,20). Previous studies have demonstrated
that LPS stimulation accelerates the phosphorylation of MAPKs in an
inflammatory response. Moreover, it has been reported that the
activation of NF-κB is triggered by MAPK (21). In the present study, we found that
CL inhibited the expression of iNOS, COX-2, IL-6 and TNF-α mRNA
simultaneously and concentration-dependently, suggesting that the
inhibition of NO and PGE2 release may be caused by the
suppression of iNOS and COX-2 expression at the mRNA level. In
addition, we investigated the effects of CL on the LPS-stimulated
phosphorylation of MAPKs in RAW 264.7 cells. The results showed
that CL significantly reduced the phosphorylation of MAPKs in the
LPS-stimulated RAW 264.7 cells compared with LPS-stimulated RAW
264.7 cells not treated with CL. Therefore, these results indicate
that CL may attenuate the inflammatory responses induced by LPS
stimulation.
NF-κB plays a central role in immunity since it
activates the pro-inflammatory genes that encode iNOS, COX-2 and
TNF-α (22). NF-κB is a
transcription factor and binds to the κB motifs in the promoters of
target genes, and thus, induces the transcriptions of iNOS, COX-2
and TNF-α. In unstimulated cells, Rel protein dimers, which are
composed mainly of p50 and p65 subunits, are sequestered in the
cytoplasm as complexes with a family of inhibitors known as IκB
(23). When the IκBs become
phosphorylated, NF-κB is released from its inhibition by IκB and
translocated to the nucleus where inflammation-associated genes are
then activated. NF-κB activation mediates the transactivation of
pro-inflammatory genes, including TNF-α and IL-6 (24,25). To elucidate the effect of CL on
NF-κB activation, we evaluated the expression of phosphorylated
IκB-α in vitro and in vivo. The results of the
present study demonstrate that CL blocked LPS-induced IκB-α
phosphorylation and, as a result, inhibited NF-κB activation. Since
LPS induces changes in the NF-κB and MAPK signaling pathways, MAPKs
may be another factor affected by CL exposure. These collective
results provide convincing evidence that CL had an
anti-inflammatory ability by inhibiting LPS-stimulated NF-κB and
MAPK activation and subsequent cytokine production in RAW 264.7
cells.
To investigate the potential anti-inflammatory
effects of CL in vivo, we evaluated the protective effects
of CL on a murine model of LPS-induced ALI. ALI is characterized by
interstitial edema, neutrophil accumulation, epithelial integrity
disruption, and protein leakage into the alveolar space, severely
altering gas exchange (26). Many
sequela associated with ALI result from the excessive production of
cytokine mediators such as TNF-α, IL-1β and IL-6 (27). Previous studies have shown that
increased levels of TNF-α, IL-1β and IL-6 in BALF may be noted in
the persistent elevation of pro-inflammatory cytokines in patients
with ALI (28). TNF-α, IL-1β and
IL-6 increase the inflammatory cascade, cause inflammatory injury
and recruit neutrophils into the lung (29). In our results, CL markedly
decreased the production of TNF-α, IL-1β and IL-6, as well as the
infiltration of inflammation cells, including macrophages and
neutrophils compared with LPS-induced ALI. These findings were
consistent with the histopathology of lung tissue. These results
indicate that the protective effects of CL on ALI induced by LPS
may result from the inhibition of inflammatory mediators and the
limitation of an inflammatory response in the lung.
In summary, the results of the present study have
demonstrated the anti-inflammatory effects of CL in in vitro
and in vivo experiments. CL inhibited the expression of
pro-inflammatory mediators in LPS-stimulated RAW 264.7 cells and
LPS-induced ALI mice, and it blocked the activation of MAPKs and
NF-κB. These results suggest that CL may have therapeutic potential
for effectively treating inflammatory diseases such as
pneumonia.
Acknowledgments
The present study was supported by a grant from the
Ministry of Science, ICT and Future planning (FGC 1011332) and the
KRIBB Research Initiative Program (KGM1221413) of the Republic of
Korea.
References
|
1
|
Kim JJ, Jiang J, Shim DW, et al:
Anti-inflammatory and anti-allergic effects of Agrimonia pilosa
Ledeb extract on murine cell lines and OVA-induced airway
inflammation. J Ethnopharmacol. 140:213–221. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huang MH, Wang BS, Chiu CS, et al:
Antioxidant, antinociceptive, and anti-inflammatory activities of
Xanthii Fructus extract. J Ethnopharmacol. 135:545–552. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hong YH, Chao WW, Chen ML, et al: Ethyl
acetate extracts of alfalfa (Medicago sativa L.) sprouts inhibit
lipopolysaccharide-induced inflammation in vitro and in vivo. J
Biomed Sci 14. 16:642009. View Article : Google Scholar
|
|
4
|
Su YW, Chiou WF, Chao SH, et al:
Ligustilide prevents LPS-induced iNOS expression in RAW 264.7
macrophages by preventing ROS production and down-regulating the
MAPK, NF-κB and AP-1 signaling pathways. Int Immunopharmacol.
11:1166–1172. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Oh JH, Kang LL, Ban JO, et al:
Anti-inflammatory effect of 4-O-methylhonokiol, compound isolated
from Magnolia officinalis through inhibition of NF-kappaB. Chem
Biol Interact. 180:506–514. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen ZJ: Ubiquitin signalling in the
NF-kappaB pathway. Nat Cell Biol. 7:758–765. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park HH, Kim MJ, Li Y, et al: Britanin
suppresses LPS-induced nitric oxide, PGE2 and cytokine production
via NF-κB and MAPK inactivation in RAW 264.7 cells. Int
Immunopharmacol. 15:296–302. 2013. View Article : Google Scholar
|
|
8
|
Gao Y, Jiang W, Dong C, et al:
Anti-inflammatory effects of sophocarpine in LPS-induced RAW 264.7
cells via NF-κB and MAPKs signaling pathways. Toxicol In Vitro.
26:1–6. 2012. View Article : Google Scholar
|
|
9
|
Dong C, Davis RJ and Flavell RA: MAP
kinases in the immune response. Annu Rev Immunol. 20:55–72. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim JY, Shin JS, Ryu JH, et al:
Anti-inflammatory effect of anemarsaponin B isolated from the
rhizomes of Anemarrhena asphodeloides in LPS-induced RAW 264.7
macrophages is mediated by negative regulation of the nuclear
factor-κB and p38 pathways. Food Chem Toxicol. 47:1610–1617. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Saccani S, Pantano S and Natoli G:
p38-dependent marking of inflammatory genes for increased NF-kappaB
recruitment. Nat Immunol. 3:69–75. 2002. View Article : Google Scholar
|
|
12
|
Craig R, Larkin A, Mingo AM, et al: p38
MAPK and NF-kappa B collaborate to induce interleukin-6 gene
expression and release. Evidence for a cytoprotective autocrine
signaling pathway in a cardiac myocyte model system. J Biol Chem.
275:23814–23824. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huo M, Cui X, Xue J, et al:
Anti-inflammatory effects of linalool in RAW 264.7 macrophages and
lipopolysaccharide-induced lung injury model. J Surg Res.
180:e47–e54. 2013. View Article : Google Scholar
|
|
14
|
Su YW, Chao SH, Lee MH, et al: Inhibitory
effects of citronellol and geraniol on nitric oxide and
prostaglandin E2 production in macrophages. Planta Med.
76:1666–1671. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim AR, Lee MS, Shin TS, et al:
Phlorofucofuroeckol A inhibits the LPS-stimulated iNOS and COX-2
expressions in macrophages via inhibition of NF-κB, Akt, and p38
MAPK. Toxicol In Vitro. 25:1789–1795. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Himaya SW, Ryu B, Qian ZJ, et al: Sea
cucumber, Stichopus japonicus ethyl acetate fraction modulates the
lipopolysaccharide induced iNOS and COX-2 via MAPK signaling
pathway in murine macrophages. Environ Toxicol Pharmacol. 30:68–75.
2010. View Article : Google Scholar
|
|
17
|
Vodovotz Y, Chow CC, Bartels J, et al: In
silico models of acute inflammation in animals. Shock. 26:235–244.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang SS, Chiu CS, Lin TH, et al:
Antioxidant and anti-inflammatory activities of aqueous extract of
Centipeda minima. J Ethnopharmacol. 147:395–405. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fengyang L, Yunhe F, Bo L, et al:
Stevioside suppressed inflammatory cytokine secretion by
downregulation of NF-κB and MAPK signaling pathways in
LPS-stimulated RAW264.7 cells. Inflammation. 35:1669–1675. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tuntipopipat S, Muangnoi C, Chingsuwanrote
P, et al: Anti-inflammatory activities of red curry paste extract
on lipopolysaccharide-activated murine macrophage cell line.
Nutrition. 27:479–487. 2011. View Article : Google Scholar
|
|
21
|
Cho EJ, An HJ, Shin JS, et al: Roxatidine
suppresses inflammatory responses via inhibition of NF-κB and p38
MAPK activation in LPS-induced RAW 264.7 macrophages. J Cell
Biochem. 112:3648–3659. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yun KJ, Shin JS, Choi JH, et al:
Quaternary alkaloid, pseudocoptisine isolated from tubers of
Corydalis turtschaninovi inhibits LPS-induced nitric oxide,
PGE2, and pro-inflammatory cytokines production via the
down-regulation of NF-kappaB in RAW 264.7 murine macrophage cells.
Int Immunopharmacol. 9:1323–1331. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
An HJ, Kim IT, Park HJ, et al: Tormentic
acid, a triterpenoid saponin, isolated from Rosa rugosa, inhibited
LPS-induced iNOS, COX-2, and TNF-α expression through inactivation
of the nuclear factor-κb pathway in RAW 264.7 macrophages. Int
Immunopharmacol. 11:504–510. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu H, Sidiropoulos P, Song G, et al:
TNF-alpha gene expression in macrophages: regulation by NF-kappa B
is independent of c-Jun or C/EBP beta. J Immunol. 164:4277–4285.
2000. View Article : Google Scholar
|
|
25
|
Yoshimura A: Signal transduction of
inflammatory cytokines and tumor development. Cancer Sci.
97:439–447. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang Y, Du Z, Zhou Q, et al: Remifentanil
attenuates lipopolysaccharide-induced acute lung injury by
downregulating the NF-κB signaling pathway. Inflammation. Apr
20–2014.Epub ahead of print. View Article : Google Scholar
|
|
27
|
Ward PA: Role of complement, chemokines,
and regulatory cytokines in acute lung injury. Ann NY Acad Sci.
796:104–112. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Minamino T and Komuro I: Regeneration of
the endothelium as a novel therapeutic strategy for acute lung
injury. J Clin Invest. 116:2316–2319. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Matthay MA and Zimmerman GA: Acute lung
injury and the acute respiratory distress syndrome: four decades of
inquiry into pathogenesis and rational management. Am J Respir Cell
Mol Biol. 33:319–327. 2005. View Article : Google Scholar : PubMed/NCBI
|