Introduction
Acne vulgaris is the most common skin disease of the
pilosebaceous follicle that results in non-inflammatory and
inflammatory lesions. Acne induces inflammation at the skin surface
of the face, neck, chest or back (1). The pathogenic factors of acne
include increased sebum production, ductal cornification, bacterial
colonization of the pilosebaceous ducts and inflammation (2,3).
Propionibacterium acnes (P. acnes) is one of the
major factors contributing to the inflammatory reaction in acne
vulgaris (4). A P. acnes
challenge occurs and a cascade of inflammatory events then ensues.
P. acnes contributes to the inflammatory responses of acne
by activating inflammatory cells, keratinocytes and sebocytes to
secrete pro-inflammatory cytokines such as interleukin (IL)-1β,
IL-8 and tumor necrosis factor (TNF)-α (5). Keratinocytes are the first line of
defense in the skin immune system and, in conjunction with
sebocytes, produce a variety of cytokines and chemokines (6). Monocytes also activate P.
acnes in an inflammatory nature of acne to secrete
pro-inflammatory cytokines such as IL-1β, IL-8 and TNF-α (7). These cytokines, including IL-1β,
IL-8 and TNF-α, are produced by human keratinocytes and monocytes,
and activate neutrophils and macrophages (6,7).
In particular, IL-8 is a member of the CXC chemokine family
involved in recruitment of leukocytes to the site of inflammation
(8).
Various therapeutic agents involving antibiotics for
acne have been used to inhibit inflammation or kill bacteria
(9). However, antibiotics may
lead to the emergence of resistant pathogens and side effects
(10,11). Therefore, new therapeutic agents
have been developed for acne with a higher therapeutic activity,
but fewer side effects (12,13).
Bee venom is composed of several active peptides,
including melittin, apamin, adolapin, mast cell-degranulating
peptide and enzymes (14,15). Bee venom has been used in the
treatment of inflammatory diseases such as rheumatoid arthritis,
back pain and skin diseases (16–18). The anticancer properties of bee
venom have also been shown in lung cancer cells, breast cancer
cells, hepatocellular carcinoma cells and prostate cancer cells
(19–21). Previous studies identified that
bee venom induced IL-1β and IL-18 release via the activation of
cytosolic DNA receptor in cultured keratinocytes (22). However, there has not yet been a
robust trial to prove a therapeutic effect of bee venom in skin
inflammation. In the present study, the anti-inflammatory
properties of bee venom were investigated in skin inflammation
stimulated by heat-killed P. acnes using human keratinocyte
and monocyte cell lines.
Materials and methods
Bee venom collection
The colonies of natural honeybees (Apis
mellifera L.) used in the present study were maintained at the
National Academy of Agricultural Science (Suwon, Korea). Bee venom
was the collecting device (Chung Jin Biotech Co., Ansan, Korea)
used in a sterile manner under strict laboratory conditions. In
brief, the bee venom collector was placed on the hive, and the bees
were administered enough electric shocks to cause them to sting a
glass plate from which dried bee venom was later removed by
scraping. The collected venom was purified by the methods of Han
et al (23). Purified bee
venom was stored in a refrigerator for later use. Bee venom used in
the experiment was confirmed with size exclusion gel chromatography
(AKTA Explorer; GE Healthcare, Pittsburgh, PA, USA) by dissolving
in 0.02 M phosphate buffer with 0.25 M NaCl adjusted to pH 7.2
using a Superdex peptide colum (Amersham Biosciences, GE
Healthcare).
Preparation of bacteria
P. acnes (ATCC 6919) was obtained from the
Korean Culture Center of Microorganisms (Seoul, Korea) and cultured
on Reinforced Clostridium Medium (BD Diagnostics, Sparks Glencoe,
MD, USA) at 37°C under anaerobic conditions until it reached
OD600=1.0 (stationary phase). The cells were harvested
by centrifugation at 5,000 × g for 15 min at 4°C. The bacterial
pellet was washed three times in 100 ml of phosphate-buffered
saline (PBS; pH 7.4) and finally suspended in 10 ml of PBS. The
P. acnes suspension was incubated at 80°C for 30 min for the
heat-killing reaction. The heat-killed P. acnes suspension
was stored at 4°C until use.
Cell culture
HaCaT and THP-1 cells were maintained in Dulbecco’s
modified Eagle’s medium (DMEM) and RPMI-1640 medium, respectively,
supplemented with 10% fetal bovine serum and 100 units
penicillin-streptomycin antibiotics (Gibco, Gaithersburg, MD, USA).
Cells were cultured at 37°C in a humidified incubator under 5%
CO2 atmosphere.
HaCaT (5×105 cells/ml) and THP-1
(1×106 cells/ml) cells were seeded in complete medium
for 24 h. The cells were changed to fresh serum-free medium
containing the indicated concentration of bee venom (1, 10 and 100
ng/ml; Sigma, St. Louis, MO, USA). After 30 min, the cells were
treated with heat-killed P. acnes (1.0×107
colony-forming units/ml) for 8 h.
Cell viability assay
To determine the effects of bee venom on cell
viability, the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) and Cell Counting kit-8 (CCK-8; Dojindo Laboratories,
Kumamoto, Japan) assays were performed on the HaCaT and THP-1
cells. HaCaT cells (5.0×104 cells/well) were seeded in a
96-well plate and allowed to attach for 24 h. Cells were treated
with serum-free media containing bee venom (1, 10 and 100 ng/ml)
for 8, 12 and 24 h. Cells were washed with PBS. MTT was added to
each well to a final concentration of 0.5 mg/ml followed by
incubation for 4 h at 37°C in a humidified incubator containing 5%
CO2. Finally, MTT containing medium was removed by
aspiration and 100 μl of dimethyl sulfoxide was added to
each well. The absorbance value was measured at 540 nm using a
microplate reader (BMG Labtech, Ortenberg, Germany). THP-1 cells
(1.0×104 cells/well) were seeded in a 96-well plate and
incubated with different concentrations of bee venom for 8, 12 and
24 h. After experimental treatment, 10 μl of WST-8 solution
[2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium,
monosodium salt] was added to each well. Plates were incubated for
4 h at 37°C. The absorbance value was measured at 450 nm using a
microplate reader (BMG Labtech).
Enzyme-linked immunosorbent assay
(ELISA)
The concentrations of IFN-γ, IL-1β and TNF-α in the
supernatant of cultured cells were measured using a commercially
available ELISA kit (R&D Systems, Minneapolis, MN, USA),
according to the manufacturer’s instructions. Reading of the
absorbance at 450 nm was performed by an ELISA reader (BMG
Labtech).
Western blot analysis
Cells were lysed in a lysis buffer [50 mmol/l Tris
(pH 8.0), 150 mmol/l NaCl, 5 mmol/l EDTA, 0.5% NP-40, 100 mmol/l
phenylmethylsulfonyl fluoride, 1 mol/l dithiothreitol, 10 mg/ml
leupeptin and aprotinin]. After incubation for 30 min on ice, total
extract was centrifuged at 8,000 × g for 15 min at 4°C and the
supernatant was used as total protein extract. Protein samples were
separated on 8–12% SDS-polyacrylamide gels and transferred to
polyvinylidene fluoride membrane (Millipore, Billerica, MA, USA)
using standard SDS-PAGE gel electrophoresis procedure. Membranes
were incubated with primary antibodies for 4 h and horseradish
peroxidase (HRP)-conjugated secondary antibodies (sc-2004 and
sc-2005) were used for detection. Signals were detected using an
enhanced chemiluminescence detection system (Amersham Biosciences
Corp., Piscataway, NJ, USA) and film. Primary antibodies used in
the present study were anti-TNF-α (ab1793; Abcam, Cambridge, MA,
USA), anti-IL-1β (sc-7884), anti-TLR2 (sc-10739) and anti-GAPDH
(sc-32233; Santa Cruz Biotechnology, Inc., Dallas, TX, USA). Signal
intensity was quantified by an image analyzer (LAS-3000; Fuji,
Tokyo, Japan).
Immunofluorescence staining
Visual identification expression of IL-8 through
TLR2 was achieved by Hoechst 33342 staining of cells. For Hoechst
evaluation, heat-killed P. acnes-treated HaCaT cells were
fixed using 4% paraformaldehyde for 5 min, followed by 2
μg/ml Hoechst staining at 37°C for 30 min. Antibodies used
in the experiments were IL-8, TLR2 (Santa Cruz Biotechnology,
Inc.), and anti-goat- and anti-rabbit-biotinylated secondary
antibodies conjugated with fluorescein isothiocyanate (Invitrogen,
Carlsbad, CA, USA) or Texas Red (Invitrogen). Stained nuclei were
observed under fluorescence microscopy (Nikon, Tokyo, Japan).
Statistical analysis
The experimental results are expressed as mean ±
standard error. Analysis of variance and paired or unpaired t-tests
were performed for statistical analysis as appropriate. P<0.05
was considered to indicate a statistically significant
difference.
Results
Effects of bee venom on cell
viability
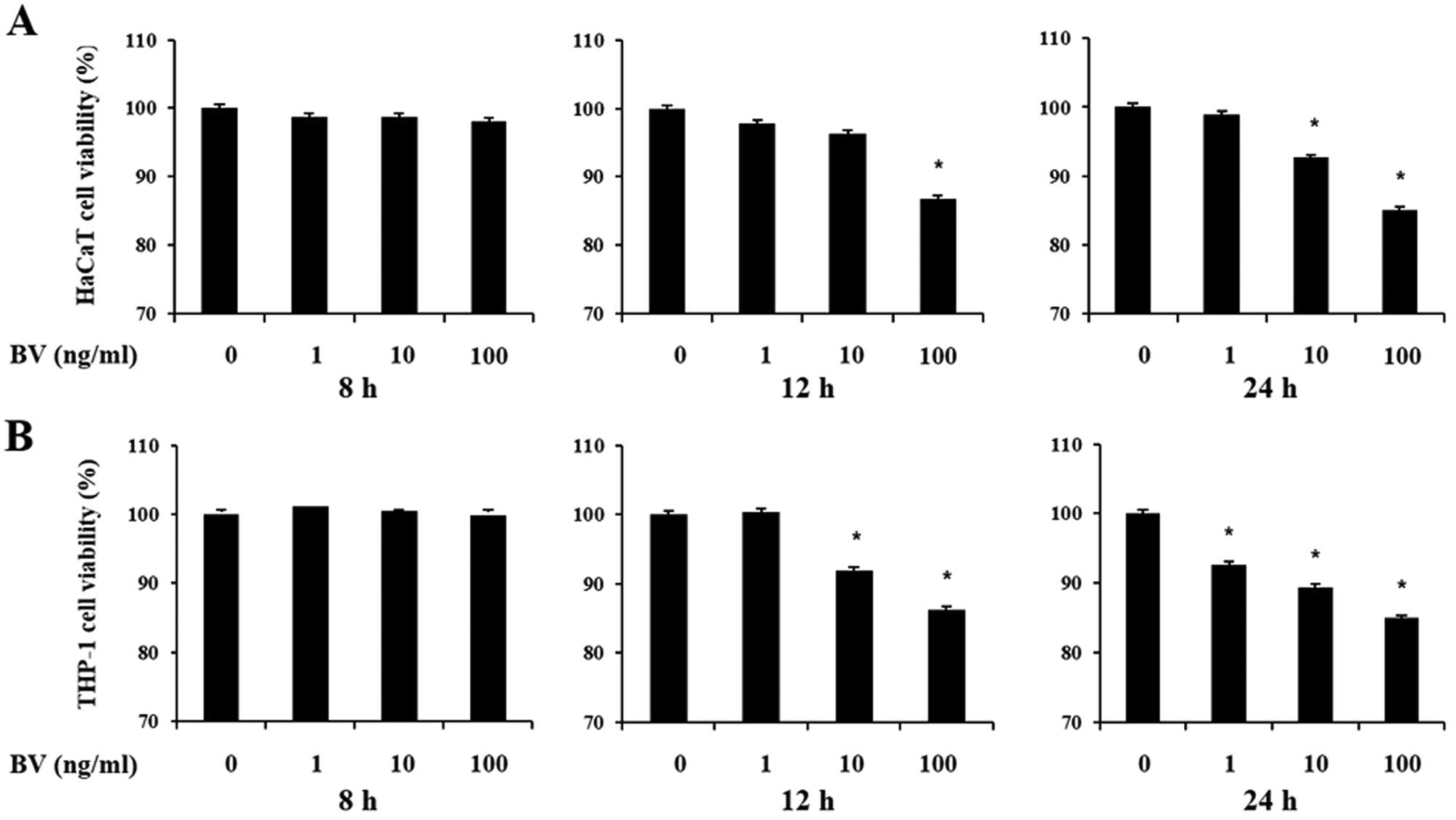
The cytotoxic effects of bee venom were first
assessed on the viability of cultured human keratinocytes and
monocytes using the MTT and CCK-8 assays, respectively. HaCaT and
THP-1 cells were treated with increasing doses of bee venom for 8,
12 and 24 h. Decreases in cell viability following treatment with
increasing doses of bee venom for 12 and 24 h were 10–20% compared
to normal untreated cell lines (Fig.
1). However, cells did not lose viability at 8 h in the
presence of bee venom. As a result, cells were treated with bee
venom for 8 h in subsequent experiments.
Bee venom inhibits the heat-killed P.
acnes-induced pro-inflammatory cytokines and chemokine in HaCaT and
THP-1 cells
To investigate the anti-inflammatory effects of bee
venom in heat-killed P. acnes-treated HaCaT and THP-1 cells,
ELISA analysis was performed to measure the pro-inflammatory
cytokines and chemokines. Cell lines were incubated with increasing
doses of bee venom for 8 h and heat-killed P. acnes
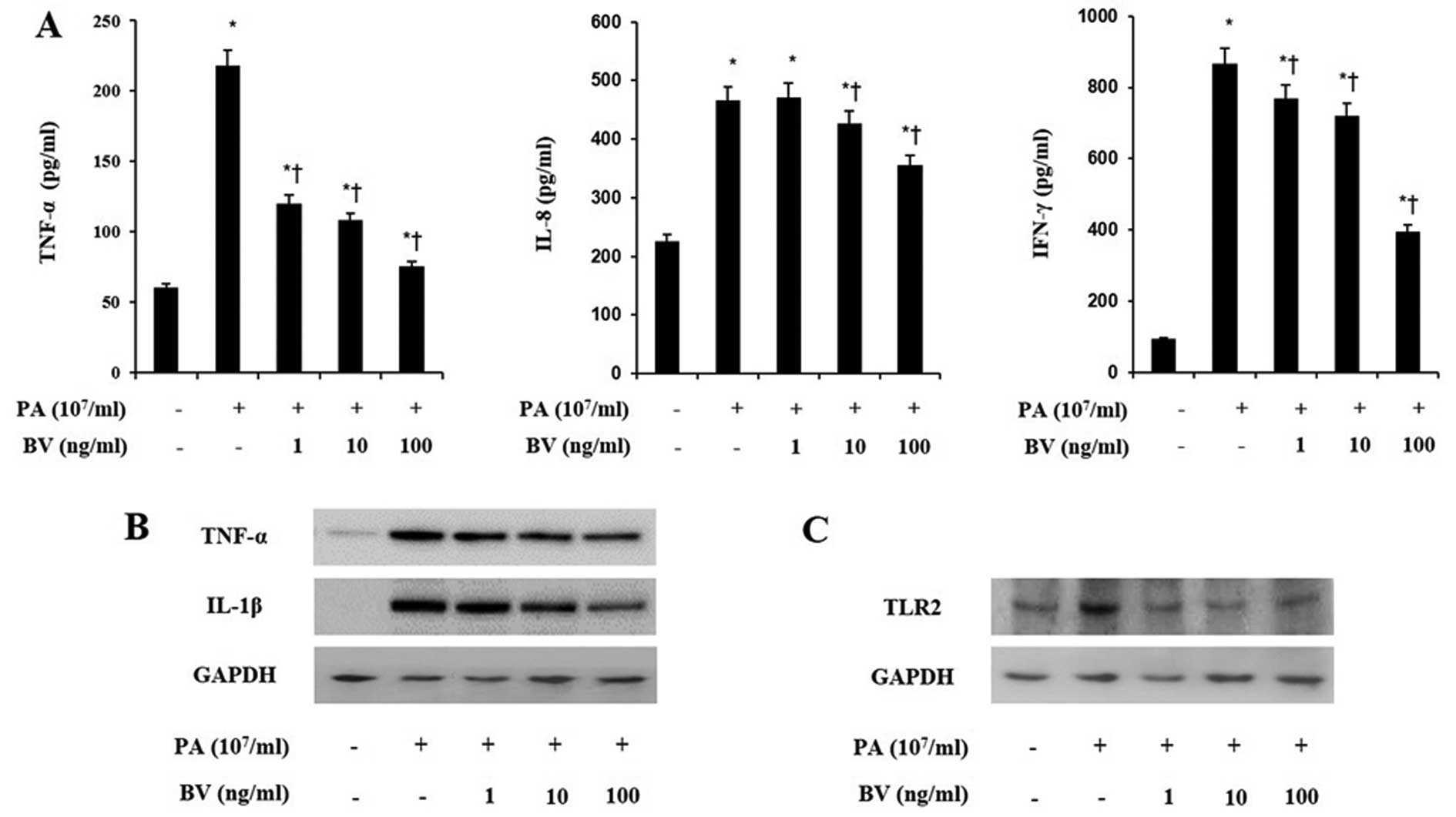
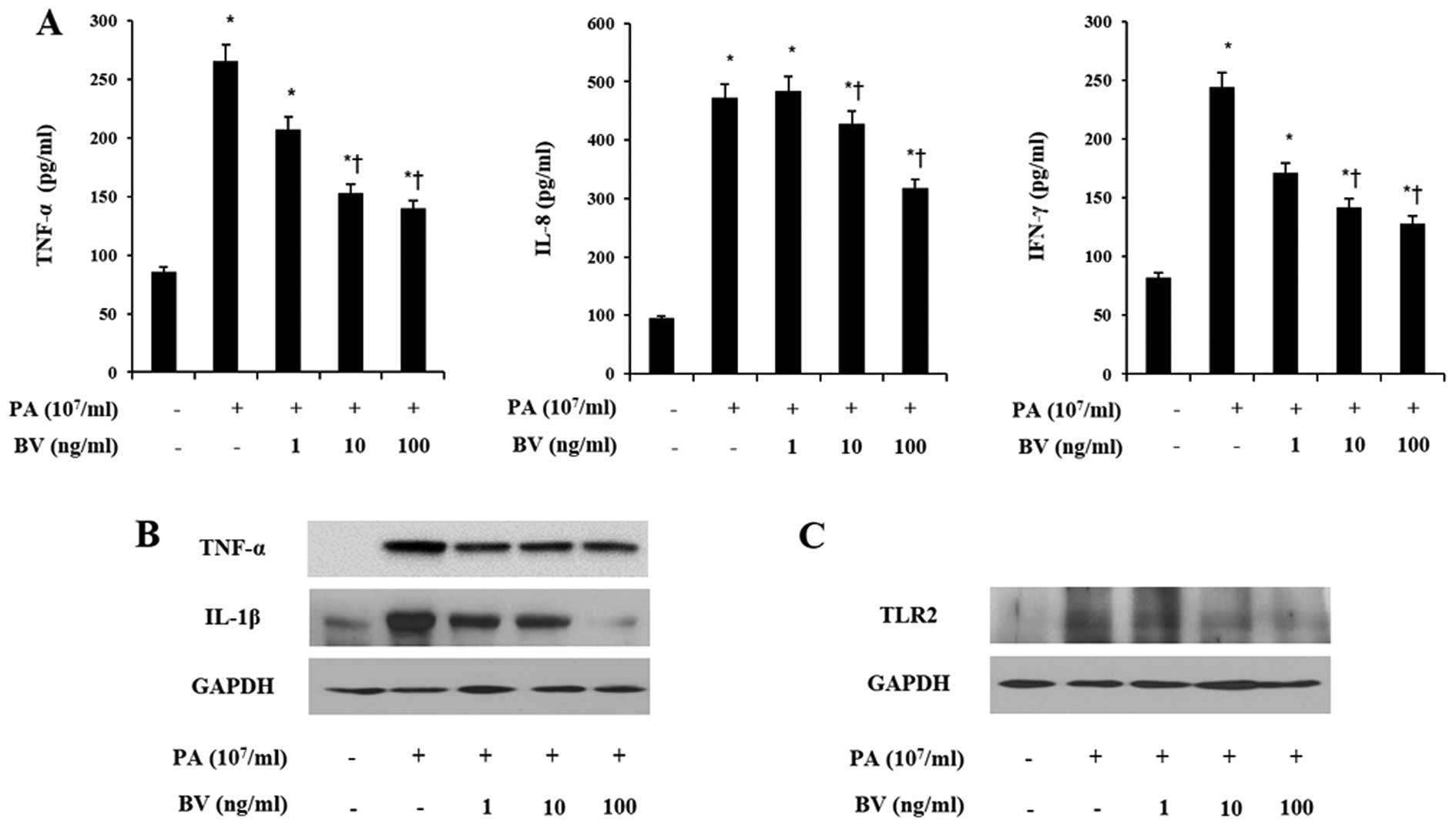
treatment followed. Heat-killed P. acnes markedly increased
the secretions of TNF-α, IL-8 and IFN-γ in HaCaT and THP-1 cells
(Figs. 2A and 3A). By contrast, bee venom treatment
decreased the secretions of those pro-inflammatory cytokines in
HaCaT and THP-1 cells induced with P. acnes. These results
indicate that heat-killed P. acnes effectively induced the
secretion of pro-inflammatory cytokines in HaCaT and THP-1 cells.
By contrast, bee venom specifically attenuated the secretion of
TNF-α, IL-8 and IFN-γ in HaCaT and THP-1 cells.
Bee venom suppresses the expression of
pro-inflammatory cytokines through TLRs in heat-killed P.
acnes-treated HaCaT and THP-1 cells
In order to assess the effects of bee venom on
inflammatory changes by P. acnes induction, western blot
analysis was utilized to analyze the expression of pro-inflammatory
cytokines. As shown in Figs. 2B
and 3B, heat-killed P.
acnes strongly increased the expression of TNF-α and IL-1β in
HaCaT and THP-1 cells. By contrast, bee venom significantly
suppressed the TNF-α and IL-1β expression in heat-killed P.
acnes-treated cells. Since the activation of TLRs leads to
production of inflammatory cytokines, further western blot analysis
was performed on HaCaT and THP-1 cells.
As observed in Figs.
2C and 3C, heat-killed P.
acnes caused a marked increase in the TLR2 expression of cell
lines. Bee venom dose-dependently inhibited heat-killed P.
acnes-induced TLR2 expression in HaCaT and THP-1 cells. These
data suggest that bee venom suppressed the protein levels of TNF-α,
IL-1β and TLR2 in heat-killed P. acnes-treated HaCaT and
THP-1 cells.
Bee venom inhibits the expression of IL-8
and TLR2 in P. acnes-treated HaCaT cells
Further investigation using immunofluorescence
labeling was performed to assess the effect of bee venom on the
expression of IL-8 and TLR2 in heat-killed P. acnes-treated
HaCaT cells (Fig. 4). The cell
surface expression of IL-8 and TLR2 on HaCaT cells was visualized.
Heat-killed P. acnes treatment induced the expression of
IL-8 and TLR2 in the cytoplasm and plasma membrane of HaCaT cells.
However, the concentration of 100 ng/ml bee venom treatment
suppressed the expression of IL-8 and TLR2 in heat-killed P.
acnes-treated HaCaT cells. These results showed that bee venom
effectively inhibited the secretion of IL-8 and expression of TLR2
in the cytoplasm and plasma membrane of HaCaT cells.
Discussion
As therapeutic agents for acne, antibiotics have
been used to suppress inflammation and action of P. acnes
(24). Currently, the available
topical therapeutic agents for the treatment of acne contain
tetracyclins, clindamycin and erythromycin (25). Several reports suggest that
topical therapeutic products have side effects such as occurrence
of resistant bacteria, organ damage and skin irritation (26). Therefore, safer and more
systematic agents are required.
Bee venom therapy has been used in oriental medicine
for the relief of pain and the treatment of inflammatory diseases
such as rheumatoid arthritis and multiple sclerosis (17,27). Previous studies have demonstrated
the anti-inflammatory effect of bee venom in rheumatoid arthritis,
allergic asthma and atherosclerosis (16,17). We have previously reported that
bee venom inhibits the development of atherosclerosis in mice
induced by injection of lipopolysaccharide (LPS) with the feeding
of an atherogenic diet (28).
However, a direct role of bee venom in skin inflammation has not
been well-established. Therefore, we examined the anti-inflammatory
properties of bee venom in skin inflammation induced by heat-killed
P. acnes using human keratinocytes and monocytes cell
lines.
While P. acnes induced inflammatory
reactions, epidermal and dermal cells contribute to immune and
inflammatory reactions by cellular interactions followed by the
release of cytokines that constitute the skin immune system
(7). Keratinocytes have an
important role in the initiation and progression of acne.
Keratinocytes are metabolically active cells that can secrete
pro-inflammatory cytokines such as IFN-γ, IL-1β and TNF-α (2,6).
Additionally, monocytes activate the induction of pro-inflammatory
cytokines by P. acnes (5).
Several studies demonstrated that keratinocytes and monocytes
induce pro-inflammatory cytokines in acne through a TLR2-dependent
pathway (29,30).
TLRs play a critical role in the innate
immunological response to a variety of microbial pathogens. TLRs
may include pattern recognition receptors of the innate immune
system (31). TLRs are expressed
by various cells of the innate immune system such as monocytes,
macrophages and granulocytes (32). Activation of TLRs promotes the
production of pro-inflammatory cytokines, prostaglandins,
leukotrienes and chemokines (33). Ten human TLRs with different
ligand specificities have been identified. TLR4 is associated with
CD14 and is mainly involved in mediating LPS-induced cellular
signaling of gram-negative bacteria (34). By contrast, TLR2 recognizes
lipopeptides from gram-positive bacteria and contributes to the
innate immune response of human epidermal keratinocytes (35). In particular, TLR2 is expressed on
the cell surface of macrophages surrounding pilosebaceous
follicles in acne lesions (30).
Several studies have suggested that P. acnes may trigger
inflammatory cytokine responses in acne via activation of TLR2
(32). During an inflammatory
response by P. acnes, keratinocytes and monocytes
synthe-sized pro-inflammatory cytokines such as IL-1, IL-8, IFN-γ
and TNF-α (5). Therefore, we
investigated whether bee venom suppresses the expression of TLR2
and pro-inflammatory cytokines in heat-killed P.
acnes-treated HaCaT and THP-1 cell lines. In the present study,
heat-killed P. acnes increased the secretion of
pro-inflammatory cytokines through the active expression of TLR2.
By contrast, bee venom treatment suppressed heat-killed P.
acnes-induced protein levels of TLR2, TNF-α and IL-1β, as well
as the secretion of IFN-γ, IL-1β, IL-8 and TNF-α.
TNF-P. acnes and IL-8 are well-described as
pro-inflammatory cytokines induced by P. acnes that may play
a role in the chemoattraction and maturation of inflammatory cells
(36). TNF-P. acnes is a
multifunctional cytokine that can induce a broad range of secondary
pro-inflammatory effects in response to microbial infections. It
also promotes keratinocyte proliferation and stimulates
angiogenesis (37). In addition,
IL-8 is one of the CXC chemokine with mitogenic activity on
keratinocytes and may play an important role in attracting
neutrophils to the pilosebaceous unit (38). Furthermore, it is well-known that
P. acnes induces keratinocyte IL-8 production through a
TLR2-dependent pathway (39). A
previous study demonstrated that the receptor blockage with TLR2
reduced the secretion of IL-8. It is, thus, suggested that
inhibition of TLR2 activation may be a novel and effective
therapeutic strategy for acne (30). The present results showed that
P. acnes induce the expression of IL-8 and TLR2 in the
cytoplasm and plasma membrane of HaCaT cells. However, bee venom
treatment effectively suppressed the expression of IL-8 and TLR2.
From these results, it can therefore be assumed that bee venom is
able to inhibit TLR2 expression, thereby it perhaps decreases
inflammation.
In conclusion, the present results demonstrate that
bee venom has effects on anti-inflammatory activity against P.
acnes in HaCaT and THP-1 cells. Bee venom blocked TLR2
expression and suppressed the production of IFN-γ, IL-1β, IL-8 and
TNF-α induced by P. acnes in HaCaT and THP-1 cells.
Therefore, we suggest that bee venom is an alternative treatment
for antibiotic therapy of acne. However, the anti-inflammatory
properties of the bee venom components were not determined. The
precise anti-inflammatory mechanism of the bee venom components
requires further investigation.
Acknowledgments
This study was carried out with the support of
‘Cooperative Research Program for Agriculture Science and
Technology Development (Project No. PJ01132501)’ Rural Development
Administration, Republic of Korea.
References
|
1
|
Leyden JJ: The evolving role of
Propionibacterium acnes in acne. Semin Cutan Med Surg. 20:139–143.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jappe U: Pathological mechanisms of acne
with special emphasis on Propionibacterium acnes and related
therapy. Acta Derm Venereol. 83:241–248. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Toyoda M and Morohashi M: Pathogenesis of
acne. Med Electron Microsc. 34:29–40. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Leyden JJ, McGinley KJ, Mills OH and
Kligman AM: Propionibacterium levels in patients with and without
acne vulgaris. J Invest Dermatol. 65:382–384. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vowels BR, Yang S and Leyden JJ: Induction
of proinflammatory cytokines by a soluble factor of
Propionibacterium acnes: implications for chronic inflammatory
acne. Infect Immun. 63:3158–3165. 1995.PubMed/NCBI
|
|
6
|
Raingeaud J and Pierre J: Interleukin-4
downregulates TNFalpha-induced IL-8 production in keratinocytes.
FEBS Lett. 579:3953–3959. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Feliciani C, Gupta AK and Sauder DN:
Keratinocytes and cytokine/growth factors. Crit Rev Oral Biol Med.
7:300–318. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Baggiolini M: Chemokines and leukocyte
traffic. Nature. 392:565–568. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ochsendorf F: Systemic antibiotic therapy
of acne vulgaris. J Dtsch Dermatol Ges. 4:828–841. 2006.In German.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Eady EA: Bacterial resistance in acne.
Dermatology. 196:59–66. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Eady EA, Cove JH, Holland KT and Cunliffe
WJ: Erythromycin resistant propionibacteria in antibiotic treated
acne patients: Association with therapeutic failure. Br J Dermatol.
121:51–57. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nam C, Kim S, Sim Y and Chang I: Anti-acne
effects of Oriental herb extracts: A novel screening method to
select anti-acne agents. Skin Pharmacol Appl Skin Physiol.
16:84–90. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tan HH: Antibacterial therapy for acne: A
guide to selection and use of systemic agents. Am J Clin Dermatol.
4:307–314. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hider RC: Honeybee venom: A rich source of
pharmacologically active peptides. Endeavour. 12:60–65. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Habermann E: Bee and wasp venoms. Science.
177:314–322. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kwon YB, Lee HJ, Han HJ, et al: The
water-soluble fraction of bee venom produces antinociceptive and
anti-inflammatory effects on rheumatoid arthritis in rats. Life
Sci. 71:191–204. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kwon YB, Lee JD, Lee HJ, et al: Bee venom
injection into an acupuncture point reduces arthritis associated
edema and nociceptive responses. Pain. 90:271–280. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stieger M, Wuthrich B, Wyss S and Kopper
E: Clinical picture and diagnosis of bee-venom allergy. A
comparison between skin tests and RAST determinations. Hautarzt.
29:632–637. 1978.In German. PubMed/NCBI
|
|
19
|
Ip SW, Liao SS, Lin SY, et al: The role of
mitochondria in bee venom-induced apoptosis in human breast cancer
MCF7 cells. In Vivo. 22:237–245. 2008.PubMed/NCBI
|
|
20
|
Orsolic N: Bee venom in cancer therapy.
Cancer Metastasis Rev. 31:173–194. 2012. View Article : Google Scholar
|
|
21
|
Park MH, Choi MS, Kwak DH, et al:
Anti-cancer effect of bee venom in prostate cancer cells through
activation of caspase pathway via inactivation of NF-kappaB.
Prostate. 71:801–812. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dombrowski Y, Peric M, Koglin S, et al:
Honey bee (Apis mellifera) venom induces AIM2 inflammasome
activation in human keratinocytes. Allergy. 67:1400–1407. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Han SM, Lee GG and Park KK: Acute dermal
toxicity study of bee venom (Apis mellifera L.) in rats. Toxicol
Res. 28:99–102. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guin JD, Huber DS and Gielerak PL:
Antibiotic sensitivity of comedonal Propionibacterium acnes. Acta
Derm Venereol. 59:552–554. 1979.PubMed/NCBI
|
|
25
|
Webster GF and Graber EM: Antibiotic
treatment for acne vulgaris. Semin Cutan Med Surg. 27:183–187.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Humphrey S: Antibiotic resistance in acne
treatment. Skin Therapy Lett. 17:1–3. 2012.PubMed/NCBI
|
|
27
|
Park HJ, Lee SH, Son DJ, et al:
Antiarthritic effect of bee venom: Inhibition of inflammation
mediator generation by suppression of NF-kappaB through interaction
with the p50 subunit. Arthritis Rheum. 50:3504–3515. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee WR, Kim SJ, Park JH, et al: Bee venom
reduces athero-sclerotic lesion formation via anti-inflammatory
mechanism. Am J Chin Med. 38:1077–1092. 2010. View Article : Google Scholar
|
|
29
|
Jugeau S, Tenaud I, Knol AC, et al:
Induction of toll-like receptors by Propionibacterium acnes. Br J
Dermatol. 153:1105–1113. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim J, Ochoa MT, Krutzik SR, et al:
Activation of toll-like receptor 2 in acne triggers inflammatory
cytokine responses. J Immunol. 169:1535–1541. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Koreck A, Pivarcsi A, Dobozy A and Kemeny
L: The role of innate immunity in the pathogenesis of acne.
Dermatology. 206:96–105. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim J: Review of the innate immune
response in acne vulgaris: Activation of Toll-like receptor 2 in
acne triggers inflammatory cytokine responses. Dermatology.
211:193–198. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hari A, Flach TL, Shi Y and Mydlarski PR:
Toll-like receptors: Role in dermatological disease. Mediators
Inflamm. 2010:4372462010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pivarcsi A, Bodai L, Rethi B, et al:
Expression and function of Toll-like receptors 2 and 4 in human
keratinocytes. Int Immunol. 15:721–730. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kollisch G, Kalali BN, Voelcker V, et al:
Various members of the Toll-like receptor family contribute to the
innate immune response of human epidermal keratinocytes.
Immunology. 114:531–541. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Schmidt N and Gans EH: Tretinoin: A review
of its anti-inflammatory properties in the treatment of acne. J
Clin Aesthet Dermatol. 4:22–29. 2011.PubMed/NCBI
|
|
37
|
Kock A, Schwarz T, Kirnbauer R, et al:
Human keratinocytes are a source for tumor necrosis factor alpha:
Evidence for synthesis and release upon stimulation with endotoxin
or ultraviolet light. J Exp Med. 172:1609–1614. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Beljaards RC, Van Beek P, Nieboer C, Stoof
TJ and Boorsma DM: The expression of interleukin-8 receptor in
untreated and treated psoriasis. Arch Dermatol Res. 289:440–443.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nagy I, Pivarcsi A, Koreck A, Szell M,
Urban E and Kemeny L: Distinct strains of Propionibacterium acnes
induce selective human beta-defensin-2 and interleukin-8 expression
in human keratinocytes through toll-like receptors. J Invest
Dermatol. 124:931–938. 2005. View Article : Google Scholar : PubMed/NCBI
|