Introduction
Diabetes mellitus (DM) is a complex metabolic
syndrome, the prevalence of which is rapidly increasing worldwide.
Among the pathophysiological mechanisms of diabetes, vascular
complications are a main cause of morbidity and mortality in
diabetic patients (1). Oxidative
stress is the main pathophysiological mechanism of macrovascular
injury and contributes to endothelial cell injury. The aggregation
of reactive oxygen species (ROS) frequently damages the cytoplasm,
lipids and proteins, thus resulting in vascular endothelial cell
apoptosis (2–5). Cell apoptosis is the initial step in
macrovascular injury and is critical to the development and
progression of cardiovascular diseases (6). NADPH oxidase 4 (Nox4), a subunit of
NADPH oxidase, is abundantly expressed in several types of tissue
and generates free radicals in vascular endothelial cells (7,8).
The inhibition of Nox4 activity has been shown to prevent
adipose-derived stem cell apoptosis (9). Nevertheless, the molecular
mechanisms responsible for Nox4-induced endothelial cell apoptosis
remain unclear.
Transforming growth factor-β1 (TGF-β1) plays a role
in the apoptosis and proliferation of a variety of cells (10–12). Smad2, a downstream cytokine of
TGF-β1, is activated following the activation of TGF-β1. Activated
Smad2 then translocates to the nucleus and modulates the
transcription of TGF-β1 target genes (13,14). Previous studies have demonstrated
that TGF-β1/Smad2 possesses potent proliferative activity in
various cell types (15–17), whereas others have demonstrated
that it induces apoptosis in a number of cells (18–20). Nevertheless, to the best of our
knowledge, few studies have investigated whether the TGF-β1/Smad2
pathway is involved in vascular endothelial cell apoptosis in
DM.
Astragaloside IV (AST IV), which is used in
traditional Chinese medicine, is a monomer located in an extract of
astragaloside (Fig. 1). Our
previous studies confirmed that AST IV has pharmacological effects,
including anti-inflammatory and antioxidant effects in some
diseases (21–23). However, to the best of our
knowledge, the protective effects of AST IV against vascular injury
in DM in vitro have not been investigated to date.
In the present study, we aimed to investigate the
role of Nox4-dependent ROS production and whether the TGF-β1/Smad2
signaling pathway plays a critical role in endothelial cell
apoptosis in vitro induced by oxidative stress, which causes
vascular injury in DM, and whether AST IV inhibits hydrogen
peroxide (H2O2)-induced HUVEC apoptosis by
suppressing Nox4 expression through the TGF-β1/Smad2 pathway.
Materials and methods
Cell culture and administration
Human umbilical vein endothelial cells (HUVECs;
China Center for Type Culture Collection, Wuhan, China) were
incubated in Dulbecco’s modified Eagle’s medium (DMEM) with 4.5 g/l
D-glucose supplemented with 10% fetal bovine serum (FBS) (both from
HyClone, Logan, UT, USA), 100 U/ml penicillin and 100 μg/ml
streptomycin in a humidified atmosphere of 5% CO2 at
37°C. The HUVECs were subcultured at a 1:2 ratio interval of 2
days. Vascular injury associated with DM in vitro was
mimicked by incubation with H2O2 100
μmol/l for 18 h to induce damage to the HUVECs, as
previously described (24). The
cells were treated with each agent in the medium for 1 h prior to
exposure to H2O2.
The cells were divided into 3 groups as follows: i)
the control group: cells were left untreated; ii) the model group:
cells were treated with H2O2 100
μmol/l for 18 h; and iii) the AST IV group: cells were
treated with AST IV for 1 h and then treated with
H2O2. In addition, some cells were treated
with diphenyliodonium (DPI, a specific inhibitor of Nox4; from
Sigma, St. Louis, MO, USA) or LY2109761 (a selective inhibitor of
TGF-β1/Smad2; from MedChem Express, LLC, Princeton, NJ, USA).
MTT assay
The half maximal effective concentration
(EC50) of AST IV in preventing the
H2O2-induced damage to HUVECs was determined
by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT) assay (Sigma). In brief, the cells were seeded at a density
of 1×104 cells/well in 96-well plates. They were then
exposed to serial dilutions of AST IV in DMEM-high glucose medium
and allowed to grow for the indicated periods of time. Following
treatment, the cells were incubated with 100 μl DMEM-high
glucose medium containing 5 mg/ml MTT. Following incubation for 4 h
at 37°C, the supernatants were discarded, MTT crystals were
dissolved in 100 μl dimethyl sulfoxide (DMSO) and the
optical density (OD) was measured at 570 nm using a Bio-Rad
microplate reader (Bio-Rad, Hercules, CA, USA).
ROS assay
Intracellular ROS production was detected using a
probe, the redox-sensitive fluorophore
carboxy-2′,7′-dichlorodihydrofluorescein diacetate
(H2DCFDA; Sigma) as the following steps. After delivery,
the cells were washed with phosphate-buffered saline (PBS) and
incubated with 20 μmol/l H2DCFDA in the dark for
30 min. The cells were then briefly exposed to 0.5 g/l trypsin. The
deactivation of trypsin was accomplished by the addition of PBS
supplemented with 3% FBS. All cells were examined using a FACScan
flow cytometer (Beckman Coulter, Miami, FL, USA) and the data were
processed using FlowJo 7.6 software (Tree Star, Inc., Ashland, OR,
USA) (25).
Annexin-V and propidium iodide (PI)
staining to detect apoptosis
Following treatment, apoptosis was determined
according to the following steps: the cells were collected by 0.25%
ethylenediaminetetraacetic acid (EDTA)-free trypsin to digest the
cells followed by centrifugation (at 1,500 rpm) to collect the
cells; the cells then were suspended with 500 μl binding
buffer, and the concentration was then adjusted to 1×106
followed by the addition of 5 μl Annexin V-FITC staining
fluid, gentle blending and incubation at 4°C in the dark for 15
min; the cells were then treated with PI dyeing liquid at 4°C in
the dark for 5 min. Finally the cells were immediately examined
using a FACScan flow cytometer (Beckman Coulter). Annexin V-FITC
detection was carried out using the FL-1 and PI detection using the
FL-2 channel.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol reagent
(Invitrogen, Carlsbad, CA, USA) and reverse transcribed using the
First-Strand cDNA Synthesis kit (Life Technology, Carlsbad, CA,
USA) according to the manufacturer’s instructions. The cDNA were
used as the template for the qPCR amplification using oligo
primers, and the internal control for the qPCR reaction was
glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The primer
sequences of the Nox4, TGF-β1, Smad2, Bax, Bcl-2 and caspase-3
genes (Shanghai Sangon Biotech, Shanghai, China) are presented in
Table I. qPCR was performed using
an ABI 7500 Real-Time PCR system (Applied Biosystems, Foster City,
CA, USA). qPCR was performed with a total volume of 12 μl in
each well, containing 5 μl of SYBR-Green® PCR
Master Mix (Applied Biosystems), 5 μl of cDNA, and 1
μmol forward primers and 1 μmol reverse primers. Each
sample was run in triplicate in a separate tube. The qPCR
conditions were conducted as follows: 40 cycles of denaturation at
95°C for 15 sec, annealing at 60°C for 1 min, and extension at 72°C
for 1 min. Initial heating at 95°C for 10 min and a final extension
at 72°C for 7 min was performed for all qPCR reactions. The cycle
threshold (CT) values from all the qPCR experiments were calculated
using the 2−ΔΔCT method.
 | Table ISequence of primers used for
RT-qPCR. |
Table I
Sequence of primers used for
RT-qPCR.
| Gene | Sequence forward
primer 5′→3′ | Sequence reverse
primer 5′→3′ | Length (bp) |
|---|
| Nox4 |
TGGACCTTTGTGCCTGTACTGT |
TGAGGATGACTTATGACCGAAA | 89 |
| Smad2 |
CTTTTGTTGTGTAAGCTCTCACTG |
GACCTTCTACCACTTTCAGAGTTG | 243 |
| TGF-β1 |
TGGACACGCAGTACAGCAAG |
GCCCACGTAGTACACGATGG | 119 |
| Bax |
TGGCAGCTGACATGTTTTCTGAC |
TCACCCAACCACCCTGGTCTT | 195 |
| Bcl-2 |
TTTGAGTTCGGTGGGGTCATG |
TCACTTGTGGCTCAGATAGGC | 269 |
| Caspase-3 |
AGAGGGGATCGTTGTAGAAGTC |
ACAGTCCAGTTCTGTACCACG | 81 |
| GAPDH |
TCCCTGAGCTGAACGGGAAG |
GGAGGAGTGGGTGTCGCTGT | 217 |
Western blot analysis
Following treatment, the cells were lysed using RIPA
buffer (Beyotime, Shanghai, China) containing 1%
phenylmethanesulfonyl fluoride (PMSF; Sigma) on ice. The total
protein concentrations were measured using the BCA Protein Assay
kit (Beyotime). Proteins were separated by 8 or 10% SDS-PAGE and
then transferred onto PVDF membranes (Millipore Corp., Billerica,
MA, USA). Subsequently, the membranes were blocked with 5% non-fat
milk at room temperature (RT) for 1 h, and incubated with primary
antibodies as follows: anti-Nox4 (ab109225; Abcam, Cambridge, UK),
anti-TGF-β1 (3712S; Cell Signaling Technology Inc., Beverly, MA,
USA), anti-Smad2 (ab33875; Abcam), anti-Bax (sc-6236), anti-Bcl-2
(sc-783) (both from Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA), anti-caspase 3 (9665S; Cell Signaling Technology Inc.) and
anti-β-actin (A1978; Sigma) antibodies overnight at 4°C.
Subsequently, the membranes were incubated with horseradish
peroxidase-conjugated secondary anti-mouse, anti-rabbit antibodies
(1:2,000 dilution) for 1 h at RT. Moreover, the proteins were
visualized using an ECL advanced western blot detection kit
(Pierce, Thermo, Rockford, IL, USA). Densitometric measurements of
band intensity in the western blot analysis were performed using
ImageJ software (National Institutes of Health, Bethesda, MD,
USA).
Statistical analysis
All experiments were carried out 3 times. All
quantified results are expressed as the means ± SEM in graphical
representation. Data analysis of all results was carried out using
one-way analysis of variance (ANOVA) followed by Fisher’s LSD-based
post-hoc analysis. All P-values were two sided and considered
significant when P<0.05. Statistical analyses were performed
with SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA).
Results
EC50 of AST IV in HUVECs
treated with H2O2
MTT (Sigma) assay was performed to determine the
EC50 of AST IV in inhibiting
H2O2-induced damage to HUVECs. The cells were
treated with AST IV at 5, 10, 25, 50, 100, 200, 400 and 800
μmol/l for 1 h prior to treatment with
H2O2 and cell viability was then examined by
MTT assay. The results revealed that the EC50 of AST IV
in inhibiting H2O2-induced injury to HUVECs
was 100 μmol/l [calculated by regression equation (x, drug
concentration; y, cell survival rate)].
Protective effect of AST IV against
H2O2-induced HUVEC apoptosis
To assess the protective effects of AST IV against
H2O2-induced HUVEC apoptosis, the mRNA and
protein expression levels of Nox4, TGF-β1, Smad2, Bcl-2, Bax and
caspase-3 were determined; the intercellular ROS level and the
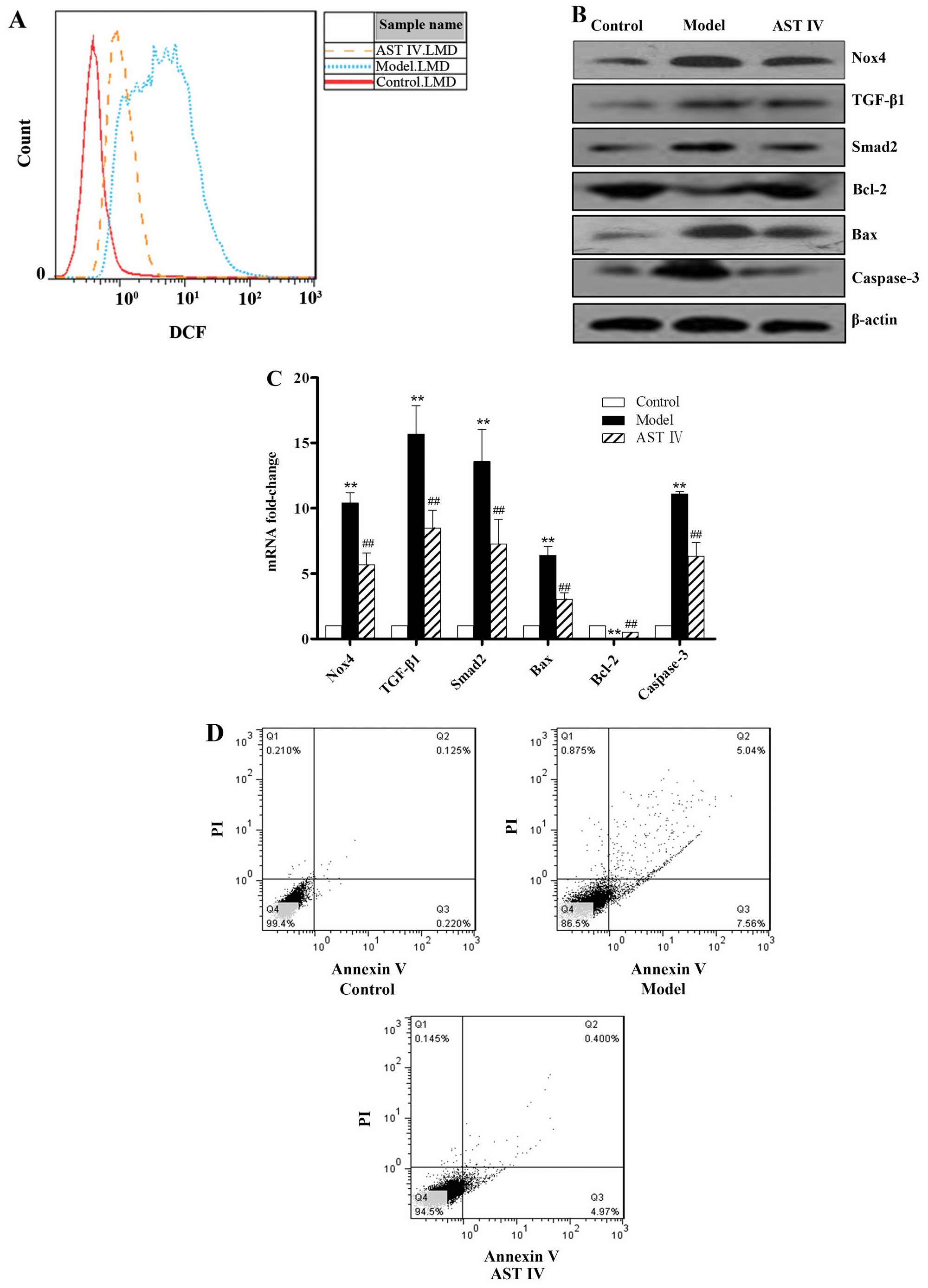
apoptotic rate were also determined. Nox4 expression in the model
group was significantly increased in comparison to the control
group (P<0.01; Fig. 2B and C).
The geometric mean fluorescence intensity indicating ROS production
in the control group was lower, whereas that in the model group was
markedly increased (P<0.01; Fig.
2A). In comparison with the control group, the expression of
TGF-β1/Smad2 was markedly elevated in the model group (P<0.01).
In addition, the expression of Bax (pro-apoptotic gene) was lower
and that of Bcl-2 (anti-apoptotic gene) was higher in the control
group compared to the model group (P<0.01; Fig. 2B and C). The expression of
caspase-3, a main terminal shear enzyme involved in apoptosis, was
barely detectable in the control group; by contrast, its expression
was upregulated in the model group (P<0.01; Fig. 2B and C). In comparison with the
control group, the apoptotic rate was significantly increased in
the model group (P<0.01; Fig.
2D). However, treatment with AST IV100 μmol/l reversed
these effects (P<0.01).
Expression of Nox4 is an important
promoting event in the onset of H2O2-induced
HUVEC apoptosis
To explore the molecular mechanisms responsible for
H2O2-induced HUVEC apoptosis, the HUVECs were
treated with diphenyliodonium (DPI; 10 μmol, a Nox4
inhibitor) prior to treatment with H2O2. We
found that treatment with DPI or AST IV decreased the expression of
Nox4 (Fig. 3B and C), as well as
the intercellular ROS levels in the HUVECs treated with
H2O2 (Fig.
3A). Our results revealed that DPI or AST decreased
TGF-β1/Smad2 expression in the HUVECs damaged by
H2O2 (Fig. 3B
and C). Our results also revealed that treatment with DPI or
AST IV decreased Bax and caspase-3 expression, and increased Bcl-2
expression (Fig. 3B and C). In
addition, treatment with DPI or AST IV decreased the HUVEC
apoptotic rate (Fig. 3D). The
exposure of the cells to AST IV at 100 μmol/l had a similar
effect to that of treament with DPI (Fig. 3).
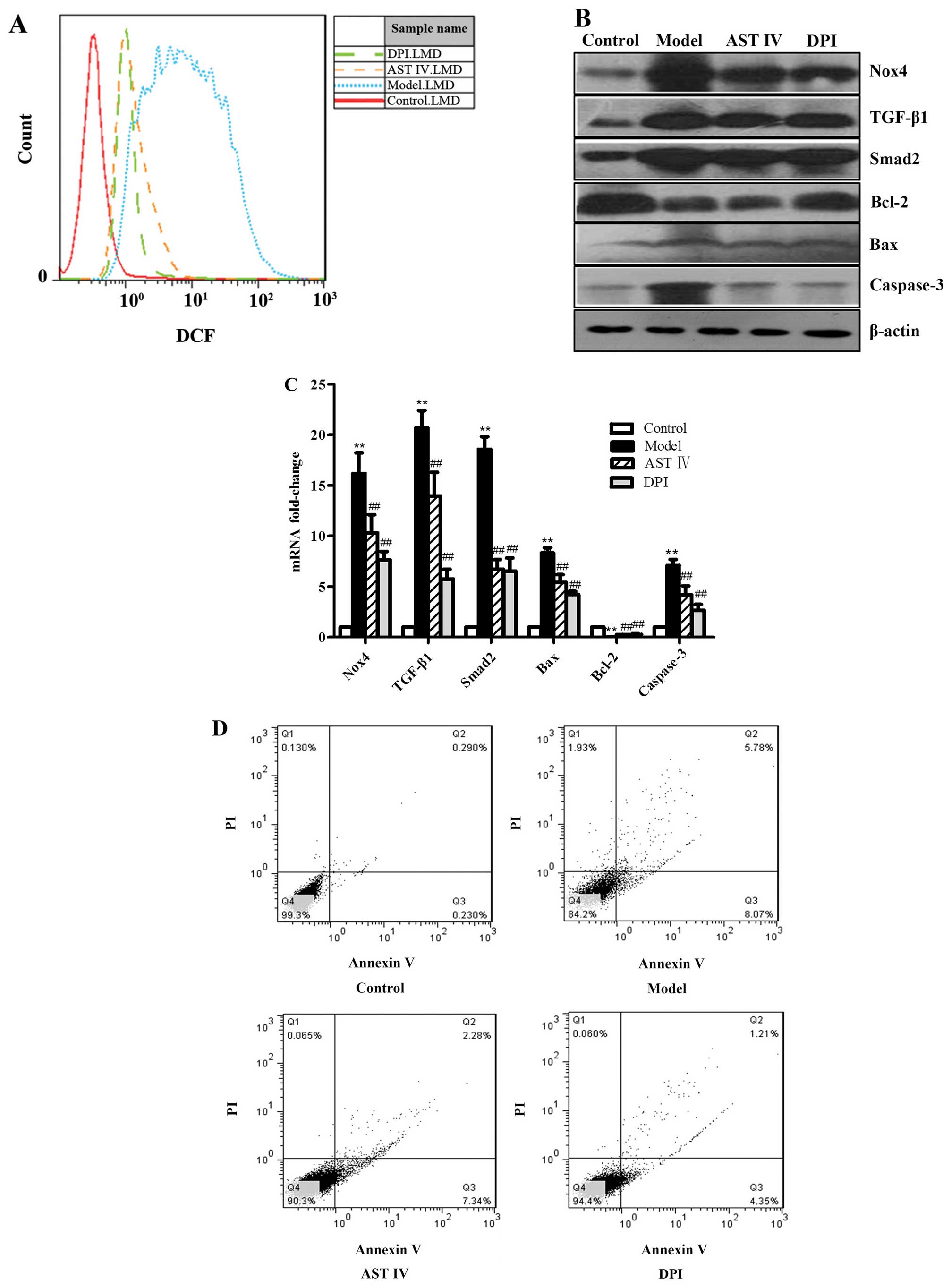 | Figure 3The overexpression of Nox4 is an
important promoting event in the onset of
H2O2-induced human umbilical vein endothelial
cell (HUVEC) apoptosis. (A) FACS analysis was performed to
determine intercellular reactive oxygen species (ROS) production in
the HUVECs in the control, model, astragaloside IV (AST IV), and
diphenyliodonium (DPI) groups. (B) Western blot analysis was
performed to determine protein expression in the HUVECs in the
control, model, AST IV and DPI groups. (C) RT-qPCR was performed to
determine mRNA expression in the HUVECs in the control, model, AST
IV and DPI groups. (D) Annexin V-FITC/PI staining was performed to
determine the apoptotic rate of the HUVECs in the control, model,
AST IV and DPI groups. **P<0.01 vs. control group;
##P<0.01 vs. model group. Nox4, NADPH oxidase 4;
TGF-β1, transforming growth factor-β1. |
Inhibition of the TGF-β1/Smad2 signaling
pathway decreases H2O2-induced HUVEC
apoptosis, but not oxidative stress
To further investigate the role of the TGF-β1/Smad2
signaling pathway in H2O2-induced HUVEC
apoptosis, LY2109761 (0.1 μmol/l), a selective inhibitor of
TGF-β1/Smad2, was used to suppress the activation of the
TGF-β1/Smad2 pathway. The results revealed that TGF-β1 and Smad2
mRNA and protein expression was detected at extremely low levels in
the control group, whereas the overexpression of TGF-β1 and Smad2
was observed in the model group; there was a statistically
significant difference between the control group and the model
group (P<0.01; Fig. 4B and C).
However, treatment with LY2109761 decreased TGF-β1 and Smad2
expression, as well as Bax and caspase-3 expression, and increased
Bcl-2 expression (P<0.01; Fig. 4B
and C). In addition, treatment with LY2109761 decreased HUVEC
apoptosis (P<0.01; Fig. 4D),
but had no effect on Nox4 expression and the ROS levels (P>0.05;
Fig. 4A–C). Treatment with AST IV
at 100 μmol/l significantly ameliorated these risk factors;
it downregulated Nox4 expression (Fig. 4B and C), decreased ROS levels
(Fig. 4A), decreased TGF-β1,
Smad2, Bax and caspase-3 expression (Fig. 4B and C) and upregulated Bcl-2
expression (Fig. 4B and C)
(P<0.05 and P<0.01 compared to model group), further
decreasing HUVEC apoptosis (Fig.
4D).
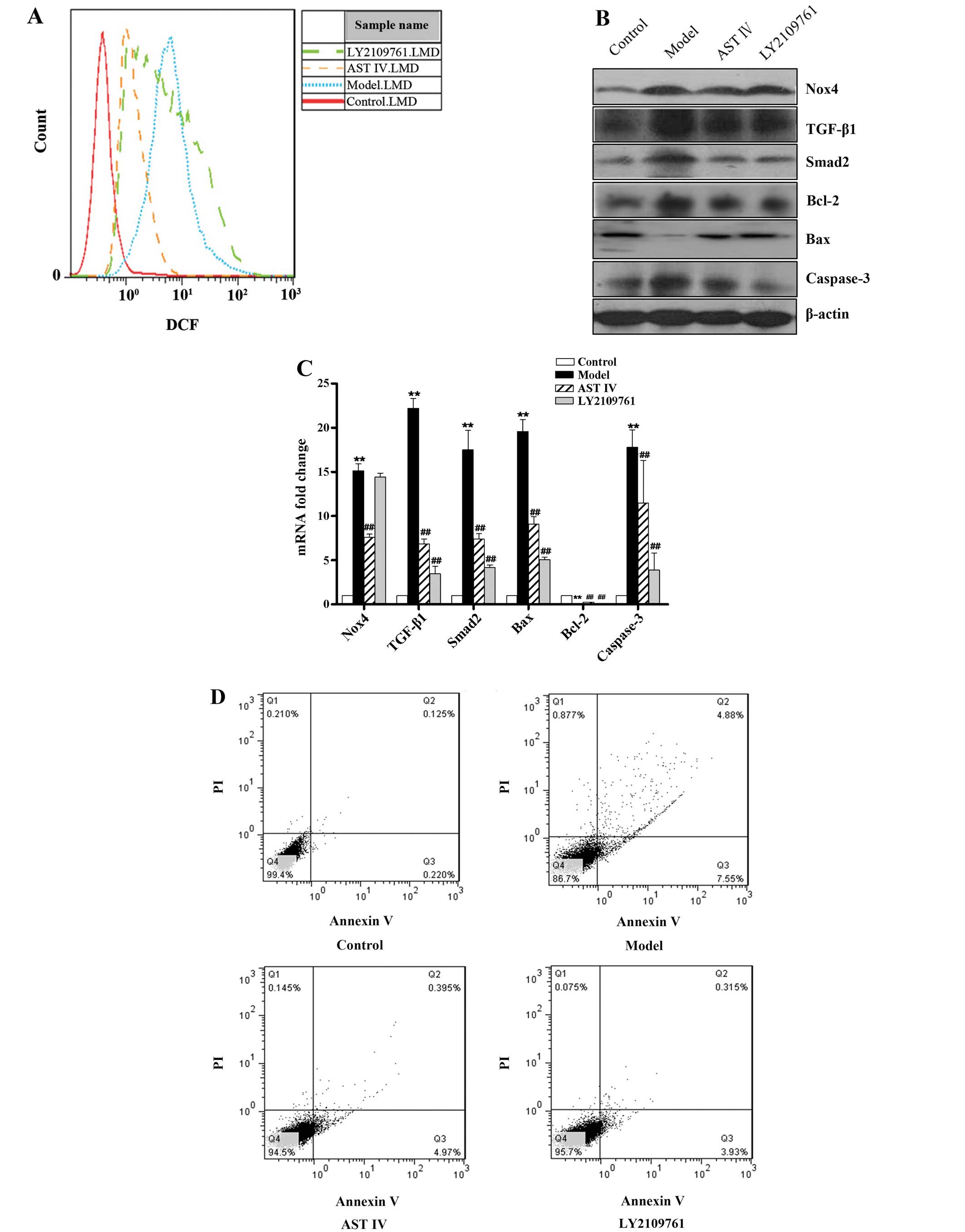 | Figure 4Inhibition of the TGF-β1/Smad2
pathway decreases H2O2-induced human
umbilical vein endothelial cell (HUVEC) apoptosis, but not
oxidative stress.(A) FACS analysis was performed to determine
intercellular reactive oxygen species (ROS) production in the
HUVECs in the control, model, astragaloside IV (AST IV) and
LY2109761 groups. (B) Western blot analysis was performed to
determine protein expression in the HUVECs in the control, model,
AST IV and LY2109761 groups. (C) RT-qPCR was performed to determine
mRNA expression in the HUVECs in the control, model, AST IV and
LY2109761 groups. (D) Annexin V-FITC/PI staining was performed to
determine the apoptotic rate of the HUVECs in the control, model,
AST IV and LY2109761 groups. **P<0.01 vs. control
group; ##P<0.01 vs. model group. Nox4, NADPH oxidase
4; TGF-β1, transforming growth factor-β1. |
Discussion
As is known, Nox4, a subunit of NADPH oxidase,
mainly catalyzes and generates intracellular ROS in vascular
endothelial cells (26). The
elevation of intracellular ROS production results in
pathophysiological changes, including vascular inflammation in DM
(27,28). This study confirmed that
incubation with H2O2 at 100 μmol/l for
18 h induced Nox4 expression and ROS generation, and that the
aggregation of ROS in HUVECs led to the development of endothelial
cell disorders, finally resulting in vascular complications in
vitro, as those observed in DM (28,29). On the one hand, increased ROS
freely transmits the cell membrane and induces membrane lipid
peroxidation and DNA damage. On the other hand, ROS augments the
cell oxidative reaction system and induces cell apoptosis (30–33). In this study, following treatment
with DPI, a compound which inhibits Nox4 generation, or AST IV
markedly suppressed Nox4 expression, significantly decreased the
generation of intracellular ROS, markedly decreased the expression
of apoptosis-related genes and the apoptotic rate of the HUVECs, as
demonstrated in previous studies using other agents (34–42). Therefore, the expression of Nox4
is an important promoting event in the onset of
H2O2-induced HUVEC apoptosis. AST IV may thus
inhibit H2O2-induced HUVEC apoptosis by
suppressing Nox4 expression.
Moreover, apoptosis, or programmed cell death, is
associated with the activation of multiple genes and multiple
signaling pathways. The TGF-β signaling pathway is associated with
oxidative stress and the apoptotic process (43–45). In this study, our results also
revealed that the TGF-β1/Smad2 pathway was activated in the
H2O2-treated HUVECs. TGF-β1 stimulates cell
responses by signaling through the canonical Smad protein pathway,
as well as using alternative pathways involving Smads,
mitogen-activated protein kinases (MAPKs), protein kinase C (PKC)
and phosphoinositide 3-kinase (PI3K). Activated Smad2, a downstream
effector of TGF-β1 signaling, then promotes cell apoptosis
(46–48). In this study, treatment with
LY2109761, a selective TGF-β1/Smad2 pathway inhibitor, produced
results similar to those obtained with DPI; however, LY2109761 had
no effect on Nox4 expression and ROS levels. Nevertheless, AST IV
decreased Nox4 expression and ROS levels, decreased TGF-β1 and
Smad2 expression, decreased Bax and caspase-3 expression, and
increased Bcl-2 expression and decreased HUVEC apoptosis.
Taken together, these results suggest that AST IV
exerts an anti-inflammatory effect by decreasing the apoptosis of
HUVECs induced by H2O2 through the inhibition
of the activation of the TGF-β1/Smad2 signaling pathway. A previous
study also demonstrated that AST IV possessed strong antioxidant
capabilities by scavenging and neutralizing free radicals, as well
as anti-inflammatory properties by inhibiting ROS formation and
accumulation (23). Nevertheless,
the findings of this study indicate that AST IV exerts effects
similar to those of DPI, but not LY2109761. Thus, the protective
effects of AST IV against vascular injury in DM in vitro,
are mainly related to the decrease in Nox4 expression. Moreover,
our results also suggest that potential therapeutic strategies to
combat anti-vascular complications in DM may be developed through
the manipulation of the redox status in DM. Furthermore, the
inhibition of the activation of the TGF-β1/Smad2 signaling pathway
may be another potential therapeutic strategy in the treatment of
DM. However, the signaling pathways related to apoptosis are
complex, multiple, and a number of pathways interact with each
other. In this study, we only investigated the TGF-β/Smad signaling
pathway; further investigations are warranted to investigate the
other pathways involved. At the present time, the pharmacological
effects of AST IV on vascular injury in DM need to be explored and
further studies are required to determine the role of other
signaling pathways.
Acknowledgments
This study was supported by a grant form the
National Natural Science Foundation of China (no. 81173624).
Abbreviations:
|
DM
|
diabetes mellitus
|
|
ROS
|
reactive oxygen species
|
|
TGF-β1
|
transforming growth factor-β1
|
|
AST IV
|
astragaloside IV
|
|
HUVECs
|
human umbilical vein endothelial
cells
|
|
H2O2
|
hydrogen peroxide
|
|
EC50
|
half maximal effective
concentration
|
|
MTT
|
3-(4,5-
dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
|
OD
|
optical density
|
|
H2DCFDA
|
2′,7′-dichloro dihydrofluorescein
diacetate
|
|
PBS
|
phosphate-buffered saline
|
|
FBS
|
fetal bovine serum
|
|
EDTA
|
ethylenediaminetetraacetic acid
|
|
qPCR
|
quantitative polymerase chain
reaction
|
|
CT
|
cycle threshold
|
|
PMSF
|
phenylmethanesulfonyl fluoride
|
|
RT
|
room temperature
|
|
ANOVA
|
one-way analysis of variance
|
|
DPI
|
diphenyliodonium
|
References
|
1
|
Haring R, Wallaschofski H, Nauck M, Felix
SB, Schmidt CO, Dörr M, Sauer S, Wilmking G and Völzke H: Total and
cardiovascular disease mortality predicted by metabolic syndrome is
inferior relative to its components. Exp Clin Endocrinol Diabetes.
118:685–691. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Erdei N, Bagi Z, Edes I, Kaley G and
Koller A: H2O2 increases production of
constrictor prostaglandins in smooth muscle leading to enhanced
arteriolar tone in type 2 diabetic mice. Am J Physiol Heart Circ
Physiol. 292:H649–H656. 2007. View Article : Google Scholar
|
|
3
|
Farah R, Shurtz-Swirski R and Lapin O:
Intensification of oxidative stress and inflammation in type 2
diabetes despite antihyperglycemic treatment. Cardiovasc Diabetol.
7:202008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hamed S, Brenner B and Roguin A: Nitric
oxide: A key factor behind the dysfunctionality of endothelial
progenitor cells in diabetes mellitus type-2. Cardiovasc Res.
91:9–15. 2011. View Article : Google Scholar
|
|
5
|
Yiu KH and Tse HF: Specific role of
impaired glucose metabolism and diabetes mellitus in endothelial
progenitor cell characteristics and function. Arterioscler Thromb
Vasc Biol. 34:1136–1143. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zernecke A, Bidzhekov K, Noels H, et al:
Delivery of microRNA-126 by apoptotic bodies induces
CXCL12-dependent vascular protection. Sci Signal. 2:ra812009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lambeth JD: NOX enzymes and the biology of
reactive oxygen. Nat Rev Immunol. 4:181–189. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Spillmann F, Van Linthout S, Miteva K,
Lorenz M, Stangl V, Schultheiss HP and Tschöpe C: LXR agonism
improves TNF-α-induced endothelial dysfunction in the absence of
its cholesterol-modulating effects. Atherosclerosis. 232:1–9. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Scioli MG, Cervelli V, Arcuri G, Gentile
P, Doldo E, Bielli A, Bonanno E and Orlandi A: High insulin-induced
down-regulation of Erk-1/IGF-1R/FGFR-1 signaling is required for
oxidative stress-mediated apoptosis of adipose-derived stem cells.
J Cell Physiol. 229:2077–2087. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Camelo A, Dunmore R, Sleeman MA and Clarke
DL: The epithelium in idiopathic pulmonary fibrosis: Breaking the
barrier. Front Pharmacol. 4:1732014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Herman-Edelstein M, Weinstein T and Gafter
U: TGFβ1-dependent podocyte dysfunction. Curr Opin Nephrol
Hypertens. 22:93–99. 2013. View Article : Google Scholar
|
|
12
|
Kamato D, Burch ML, Piva TJ, Rezaei HB,
Rostam MA, Xu S, Zheng W, Little PJ and Osman N: Transforming
growth factor-β signalling: Role and consequences of Smad linker
region phosphorylation. Cell Signal. 25:2017–2024. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Blanco FF, Sanduja S, Deane NG, Blackshear
PJ and Dixon DA: Transforming growth factor β regulates P-body
formation through induction of the mRNA decay factor
tristetraprolin. Mol Cell Biol. 34:180–195. 2014. View Article : Google Scholar :
|
|
14
|
Bohanon FJ, Wang X, Ding C, Ding Y,
Radhakrishnan GL, Rastellini C, Zhou J and Radhakrishnan RS:
Oridonin inhibits hepatic stellate cell proliferation and
fibrogenesis. J Surg Res. 190:55–63. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li YC, An YS, Wang T and Zang HR: Analysis
of transforming growth factor β signaling in chronic
rhinosinusitis. Chin Med J (Engl). 126:3340–3343. 2013.
|
|
16
|
Wang X, Chu J, Wen CJ, Fu SB, Qian YL, Wo
Y, Wang C and Wang DR: Functional characterization of TRAP1-like
protein involved in modulating fibrotic processes mediated by
TGF-β/Smad signaling in hypertrophic scar fibroblasts. Exp Cell
Res. 332:202–211. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Z, Song Y, Tu W, He X, Lin J and Liu
F: β-2 spectrin is involved in hepatocyte proliferation through the
interaction of TGFβ/Smad and PI3K/AKT signalling. Liver Int.
32:1103–1111. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Janeesh PA and Abraham A: Robinin
modulates doxorubicin-induced cardiac apoptosis by TGF-β1 signaling
pathway in Sprague Dawley rats. Biomed Pharmacother. 68:989–998.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu T, Peng YF, Jia C, Yang BH, Tao X,
Fang X and Zhong W: Effect of HGF on the apoptosis of rat corpus
cavernosum smooth muscle cells induced by TGFβ1. Andrologia. Nov
11–2014.Epub ahead of print. View Article : Google Scholar
|
|
20
|
Merle P and Trepo C: Molecular mechanisms
underlying hepatocellular carcinoma. Viruses. 1:852–872. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li WZ, Li WP, Zhang W, Yin YY, Sun XX,
Zhou SS, et al: Protective effect of extract of Astragalus on
learning and memory impairments and neurons’ apoptosis induced by
glucocorticoids in 12-month-old male mice. Anat Rec (Hoboken).
294:1003–1014. 2011. View Article : Google Scholar
|
|
22
|
Yin YY, Li WP, Gong HL, Zhu FF, Li WZ and
Wu GC: Protective effect of astragaloside on focal cerebral
ischemia/reperfusion injury in rats. Am J Chin Med. 38:517–527.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun L, Li W, Li W, Xiong L, Li G and Ma R:
Astragaloside IV prevents damage to human mesangial cells through
the inhibition of the NADPH oxidase/ROS/Akt/NF-κB pathway under
high glucose conditions. Int J Mol Med. 34:167–176. 2014.PubMed/NCBI
|
|
24
|
Ruan Y, Wu S, Zhang L, Chen G and Lai W:
Retarding the senescence of human vascular endothelial cells
induced by hydrogen peroxide: Effects of 17beta-estradiol
(E2) mediated mitochondria protection. Biogerontology.
15:367–375. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wardman P: Fluorescent and luminescent
probes for measurement of oxidative and nitrosative species in
cells and tissues: Progress, pitfalls, and prospects. Free Radic
Biol Med. 43:995–1022. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yan F, Wang Y, Wu X, Peshavariya HM,
Dusting GJ, Zhang M and Jiang F: Nox4 and redox signaling mediate
TGF-β-induced endothelial cell apoptosis and phenotypic switch.
Cell Death Dis. 5:e10102014. View Article : Google Scholar
|
|
27
|
Anderson TJ: Assessment and treatment of
endothelial dysfunction in humans. J Am Coll Cardiol. 34:631–638.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hadi HA, Carr CS and Al Suwaidi J:
Endothelial dysfunction: Cardiovascular risk factors, therapy, and
outcome. Vasc Health Risk Manag. 1:183–198. 2005.
|
|
29
|
Tousoulis D, Briasoulis A, Papageorgiou N,
Tsioufis C, Tsiamis E, Toutouzas K and Stefanadis C: Oxidative
stress and endothelial function: Therapeutic interventions. Recent
Patents Cardiovasc Drug Discov. 6:103–114. 2011. View Article : Google Scholar
|
|
30
|
Bramlage CP, Müller GA, Tampe B, et al:
The role of bone morphogenetic protein-5 (BMP-5) in human
nephrosclerosis. J Nephrol. 24:647–655. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gray SP, Di Marco E, Okabe J, et al: NADPH
oxidase 1 plays a key role in diabetes mellitus-accelerated
atherosclerosis. Circulation. 127:1888–1902. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu XQ, Sheng R and Qin ZH: The
neuroprotective mechanism of brain ischemic preconditioning. Acta
Pharmacol Sin. 30:1071–1080. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Urbański K, Nowak M and Guzik TJ:
Oxidative stress and vascular function. Postepy Biochem.
59:424–431. 2013.In Polish.
|
|
34
|
Dao J, Zhu L, Luo R, Hu C, Wang Y, Li H,
Lu K, Liu J, Lin J and Cheng G: Molecular characterization of
SjBIRP, another apoptosis inhibitor, from Schistosoma japonicum.
Parasitol Res. 113:4065–4071. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Han HJ, Kwon HY, Sohn EJ, Ko H, Kim B,
Jung K, Lew JH and Kim SH: Suppression of E-cadherin mediates
gallotannin induced apoptosis in Hep G2 hepatocelluar carcinoma
cells. Int J Biol Sci. 10:490–499. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kvansakul M and Hinds MG: Structural
biology of the Bcl-2 family and its mimicry by viral proteins. Cell
Death Dis. 4:e9092013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Martel C, Wang Z and Brenner C: VDAC
phosphorylation, a lipid sensor influencing the cell fate.
Mitochondrion. 19:69–77. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Qiao D, Xu J, Le C, Huang E, Liu C, Qiu P,
Lin Z, Xie WB and Wang H: Insulin-like growth factor binding
protein 5 (IGFBP5) mediates methamphetamine-induced dopaminergic
neuron apoptosis. Toxicol Lett. 230:444–453. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang G, Jiang MY, Meng Y, Song HR and Shi
W: Cellular mechanisms of a new pyrazinone compound that induces
apoptosis in SKOV-3 cells. Asian Pac J Cancer Prev. 15:797–802.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Eachkoti R, Reddy MV, Lieu YK, Cosenza SC
and Reddy EP: Identification and characterisation of a novel heat
shock protein 90 inhibitor ONO4140. Eur J Cancer. 50:1982–92. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hou XQ, Wu DW, Zhang CX, et al: Bushen
Yizhi formula ameliorates cognition deficits and attenuates
oxidative stress related neuronal apoptosis in scopolamine induced
senescence in mice. Int J Mol Med. 34:429–439. 2014.PubMed/NCBI
|
|
42
|
Tharaheswari M, Jayachandra Reddy N, Kumar
R, Varshney KC, Kannan M and Sudha Rani S: Trigonelline and
diosgenin attenuate ER stress, oxidative stress-mediated damage in
pancreas and enhance adipose tissue PPARγ activity in type 2
diabetic rats. Mol Cell Biochem. 396:161–174. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Isfort RJ, Cody DB, Stuard SB, et al: The
combination of epidermal growth factor and transforming growth
factor-beta induces novel phenotypic changes in mouse liver stem
cell lines. J Cell Sci. 110:3117–3129. 1997.
|
|
44
|
Lee HS: Mechanisms and consequences of
TGF-β overexpression by podocytes in progressive podocyte disease.
Cell Tissue Res. 347:129–140. 2012. View Article : Google Scholar :
|
|
45
|
Wakui H, Dejima T, Tamura K, et al:
Activation of angiotensin II type 1 receptor-associated protein
exerts an inhibitory effect on vascular hypertrophy and oxidative
stress in angiotensin II-mediated hypertension. Cardiovasc Res.
100:511–519. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
He C, Zhu H, Zhang W, Okon I, Wang Q, Li
H, Le YZ and Xie Z: 7-Ketocholesterol induces autophagy in vascular
smooth muscle cells through Nox4 and Atg4B. Am J Pathol.
183:626–637. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li J, Li X, Xu W, Wang S, Hu Z, Zhang Q,
et al: Antifibrotic effects of luteolin on hepatic stellate cells
and liver fibrosis by targeting AKT/mTOR/p70S6K and TGFbeta/Smad
signalling pathways. Liver Inter. 5–Aug;2014.Epub ahead of
print.
|
|
48
|
Martínez-Palacián A, del Castillo G,
Suárez-Causado A, García-Álvaro M, de Morena-Frutos D, Fernández M,
Roncero C, Fabregat I, Herrera B and Sánchez A: Mouse hepatic oval
cells require Met-dependent PI3K to impair TGF-β-induced oxidative
stress and apoptosis. PLoS One. 8:e531082013. View Article : Google Scholar
|