Introduction
The increasing prevalence of obesity is a major
health concern in industrialized countries. Obesity is a primary
causative factor in the development of metabolic disorders, such as
hypertention, insulin resistence and type II diabetes, which is a
complex, multi-factorial and chronic disease (1). Obesity is characterized by the
accumulation of inordinate fat in the body which involves the
pathological growth of adipocytes caused by an imbalance in energy
intake and expenditure (2).
The maintenance of lipid homeostasis and energy
balance is connected to adipocytes that regulate the storage of
triglycerides (TGs) or the release of free fatty acids (FFAs)
through changes in energy states (3,4).
Adipocytes not only control lipid metabolism, but also glucose
metabolism and endocrine function through insulin-dependent glucose
uptake and the secretion of hormones and cytokines (5). Adipogenesis is the process through
which an undifferentiated preadipocyte is reorganized into a fully
differentiated adipocyte (6). The
differentiation process through which preadipocytes are converted
into adipocytes is associated with the stimulation of
transcriptional factors, including CCAAT/enhancer-binding protein α
(C/EBPα) and peroxisome proliferator activated receptor γ (PPARγ).
When the cascade of transcription factors is initiated, the
induction of differentiation begins and the expression of C/EBPα
and PPARγ increases from undetectable levels in preadipocytes to
detectable levels within 2 days; C/EBPα and PPARγ are fully
expressed following the initiation of the differentiation process
within approximately 5 days (7).
This activation of transcription factors leads to terminal
differentiation and regulates the expression of genes involved in
the induction of the adipocyte phenotype (7).
Adipogenesis is characterized by the accumulation of
TGs accompanied by an increase in the expression of various enzymes
that are involved in lipogenesis or lipolysis. Sterol regulatory
element binding proteins (SREBPs) are transcription factors that
control both cholesterol and fatty acid biosynthetic processes,
termed lipogenesis. SREBP-1c which is one of the SREBP isoforms,
has been shown to play a role in fatty acid synthesis and
insulin-induced glucose metabolism (8). Fatty acid synthase (FAS), which is
key enzyme of lipogenesis that produces long-chain fatty acids from
acetyl-coA and malonyl-coA, is associated with the activation of
transcription factors, such as SREBP-1 and PPARγ at the
transcriptional level through signaling mechanisms (9,10).
Lipoprotein lipase (LPL), which plays an important role in TG
accumulation, hydrolyzes lipoproteins and provides substrates for
fatty acid uptake into adipose tissue (11). Hormone sensitive lipase (HSL) and
adipose triglyceride lipase (ATGL) are enzymes that play a role in
lipolysis and catabolize stored TGs in lipid droplets (12). ATGL decomposes TGs into
diglycerides (DGs) that are hydrolyzed by HSL (13,14).
Quercetin (3,30,40,5,7-pentahydroxyflavone) is one
of the most common and bioactive flavonoids found in a variety of
vegetables, fruits and botanicals, such as red onions, apples and
tea (15). It has attracted much
attention as a potential antioxidant of dietary origin. Previous
studies have indicated that quercetin has multiple pharmacological
effects, such as the scavenging of oxygen radicals, the prevention
of lipid peroxidation, as well as anti-adipogenic, anti-apoptotic
and anti-inflammatory effects (16–19). However, to the best of our
knowledge, there is no published study to date on adipogenesis and
lipolysis in OP9 cells. The association between quercetin and
lipolyis remains undetermined. Thus, the aim of this study was to
determine the effects of quercetin on adipogenesis and lipolysis.
Furthermore, we investigated the molecular mechanisms of action of
quercetin in OP9 mouse stromal cells, a new model of
adipocytes.
Materials and methods
Materials
Quercetin used in this study was purchased from
Sigma-Aldrich (St. Louis, MΟ, USA). The Oil Red O (ORO) staining
dye was also purchased from Sigma-Aldrich. The
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
(MTS) solution was purchased from Promega (Madison, WI, USA).
Anti-β-actin (sc-47778), anti-C/EBPα (sc-61), anti-PPARγ (sc-7273),
anti-SREBP-1c (sc-366), anti-mouse (sc-2005) and anti-rabbit
(sc-2004) immunoglobulin antibodies were obtained from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA).
Cell culture
OP9 cells, bone marrow-derived mouse stromal cells,
were obtained from the American Type Culture Collection (ATCC,
Manassas, VA, USA) and grown in α-minimum essential medium (α-MEM)
supplemented with 20% fetal bovine serum (FBS), 100 U/ml
penicillin, 100 μg/ml streptomycin and 2 mM L-glutamine
(HyClone®, Thermo Scientific, Logan, UT, USA). The cells
were cultured at 37°C in a humidified atmosphere of 95% air to 5%
CO2.
Cell differentiation into adipocytes
Adipocyte differentiation was induced as previously
described (20) with minor
modifications. OP9 preadipocytes were seeded at 60,000
cells/cm2. The cells allowed to reach confluence for 2
days. At this time point (day 0), the medium were switched to MDI
differentiation medium [α-MEM, 10% FBS, 0.25 μM
dexamethasone, 0.25 mM isobutylmethylxanthine (IBMX) and 10
μg/ml insulin] for 2 days. At this time point, the cells
were treated with differentiation medium in the presence of various
concentrations of quercetin to examine the effects of quercetin on
adipogenesis. On day 2, the dexamethasone and IBMX were removed,
leaving insulin in the cell medium with or without quercetin for an
additional 2 days. Thereafter, the cells were maintained in the
original propagation α-MEM with changes in medium every 2 days. On
day 6, when the differentiation process was completed, the cells
were harvested.
Cytotoxicity assay
Cell viability was examined by MTS assay. Briefly,
the OP9 cells were seeded at a density of 1×106 cells/ml
in 96-well plates. In order to determine the concentration of
quercetin which is non-toxic to the cells, quercetin (5, 10, 25, 50
and 100 μM) was then added to each well. The plates were
then incubated for 24 h at 37°C under 5% CO2. MTS
solution (5 mg/ml) was added to each well and the cells were then
cultured for a further 2 h, after which the optical density was
read at 490 nm. Cytotoxicity was then calculated using the
following formula: 1 - (mean absorbance value of treated cells/mean
absorbance value of untreated cells).
ORO staining
For the examination of fat accumulation in the OP9
cells, the cells were treated as described above in ‘Cell
differentiation into adipocytes’. The cells were rinsed with cold
phosphate-buffered saline (PBS) twice and fixed in 10%
paraformaldehyde for 30 min in room temperature. After the cells
were washed with 60% isopropanol, the cells were stained for at
least 1 h in a freshly diluted 0.3% ORO solution (6 parts 0.5% ORO
stock solution in isopropanol and 4 parts H2O). After
the stain was removed and the cells were washed with 60%
isopropanol, an image of each group was acquired using an Olympus
IX71 Research Inverted Phase microscope (Olympus Co., Tokyo,
Japan). The stained lipid droplets were then extracted with
isopropanol for quantification by measuring its absorbance at 490
nm.
Reverse transcriptase-polymerase chain
reaction (RT-PCR)
Total RNA was isolated using an easy-BLUE total RNA
extraction kit (iNtRon, Seongnam, Korea) according to the
instructions provided by the manufacturer. Single-strand cDNA
synthesis was performed using the QuantiTect reverse transcription
kit (Qiagen, Hilden, Germany) according to the manufacturer’s
instructions. PCR reactions were performed in a total volume of 20
μl comprising 2 μl of cDNA product, 0.2 mM of each
dNTP, 20 pmol of each primer and 0.8 units of Taq polymerase. The
primer sequences for C/EBPα, PPARγ, SREBP1, FAS, adipocyte fatty
acid-binding protein (aP2), ATGL, HSL, LPL and β-actin are
presented in Table I. PCR
reactions were conducted under the following conditions: 95°C for 3
min (1 cycle), 95°C for 30 sec, 50–62°C for 30 sec, 72°C for (40
cycles). The PCR products increased as the concentration of the RNA
increased. Finally, the products were electrophoresed on a 2.0%
agarose gel using the Dyne Gel Safe Red kit (II) (DyneBio,
Seongnam, Korea) visualized under UV light.
 | Table IGene-specific primers used for
RT-PCR. |
Table I
Gene-specific primers used for
RT-PCR.
| cDNA | Primer sequences |
|---|
| C/EBPα | F:
5′-CGCAAGAGCCGAGATAAAGC-3′ |
| R:
5′-AGAGGTCCACAGAGCTGATTCC-3′ |
| PPARγ | F
5′-CGCTGATGCACTGCCTATGA-3′ |
| R:
5′-AGAGGTCCACAGAGCTGATTCC-3′ |
| SREBP-1 | F:
5′-GGCACTAAGTGCCCTCAACCT-3′ |
| R:
5′-GCCACATAGATCTCTGCCAGTGT-3′ |
| FAS | F:
5′-CCTGGATAGCATTCCGAACCT-3′ |
| R:
5′-AGCACATCTCGAAGGCTACACA-3′ |
| aP2 | F:
5′-CATGGCCAAGCCCAACAT-3′ |
| R:
5′-CGCCCAGTTTGAAGGAAATC-3′ |
| ATGL | F:
5′-ATTTATCCCGGTGTACTGTG-3′ |
| R:
5′-GGGACACTGTGATGGTATTC-3′ |
| HSL | F:
5′-ACTCAGACCAGAAGGCACTA-3′ |
| R:
5′-TAGTTCCAGGAAGGAGTTGA-3′ |
| LPL | F:
5′-ACTTGTCATCTCATTCCTGG-3′ |
| R:
5′-TCTCATACATTCCCGTTACC-3′ |
| β-actin | F:
5′-TGTCCACCTTCCAGCAGATGT-3′ |
| R:
5′-AGCTCAGTAACAGTCCGCCTAGA-3′ |
Western blot analysis
Protein expression was assessed by western blot
analysis according to standard procedures. The OP9 cells which had
completed the differentiation process were washed twice in PBS. The
cell pellets were resuspended in lysis buffer on ice for 20 min,
and the supernatant was collected by centrifugation (13,000 rpm, 10
min, 4°C). The protein concentrations in the supernatant were
determined using the Bio-Rad protein assay reagent (Bio-Rad
Laboratories, Hercules, CA, USA) according to the manufacturer’s
instructions. Equal amounts of protein (20 μg) were
subjected to sodium dodecyl sulphate-polyacrylamide gel
electrophoresis (SDS-PAGE) and then transferred onto a
polyvinylidene membrane (Millipore, Bedford, MA, USA). The membrane
was blocked for >1 h with 5% skim milk in Tris-buffered saline
(150 mM NaCl and 20 mM Tris-HCl, pH 7.4) with 0.05% Tween-20. After
blocking, the membrane was incubated with primary antibodies for 18
h. The membrane was then washed with Tris-buffered saline with
Tween-20 and incubated with anti-mouse or anti-rabbit
immunoglobulin G horseradish peroxidase-conjugated secondary
antibodies. The proteins were then supplemented with ECL prime
western blot detection reagents and the ImageQuant LAS 4000 Mini
Biomolecular Imager (both from GE Healthcare, Buckinghamshire, UK)
was used for evaluating the bands. The bands were quantified using
ImageJ software.
Statistical analysis
Statistical analysis was performed followed by
one-way analysis of variance (ANOVA) using IBM SPSS statistical
software version 19. The data from the experiments are presented as
the means ± SD.
Results
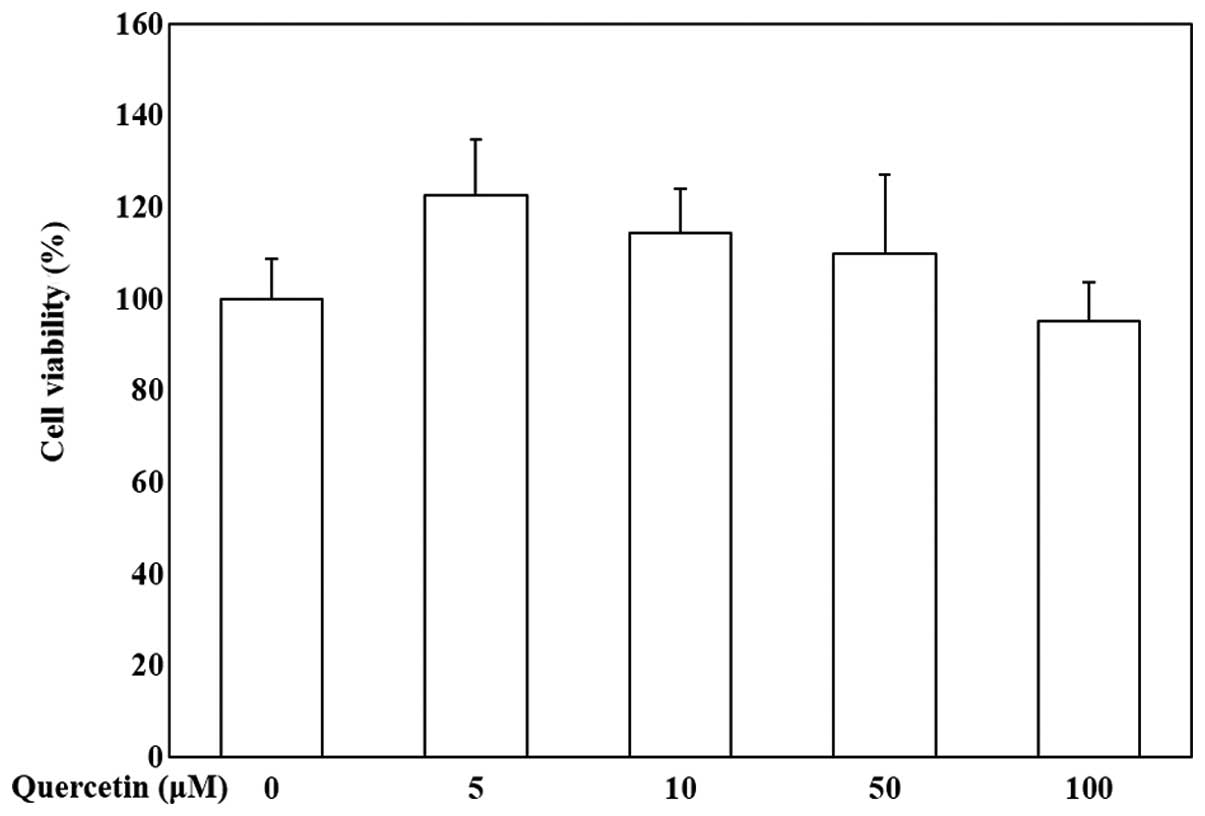
Cytotoxicity of quercetin in OP9
cells
To evaluate the effects of quercetin on the
viability of OP9 cells, the cells were treated with various
concentrations of quercetin (0, 5, 10, 50 and 100 μM) and
MTS assay was then conducted. Our data indicated that quercetin was
not cytotoxic to the OP9 cells (Fig.
1). Quercetin at the concentration of 5, 10 and 50 μM
was used to evaluate its anti-adipogenic activity.
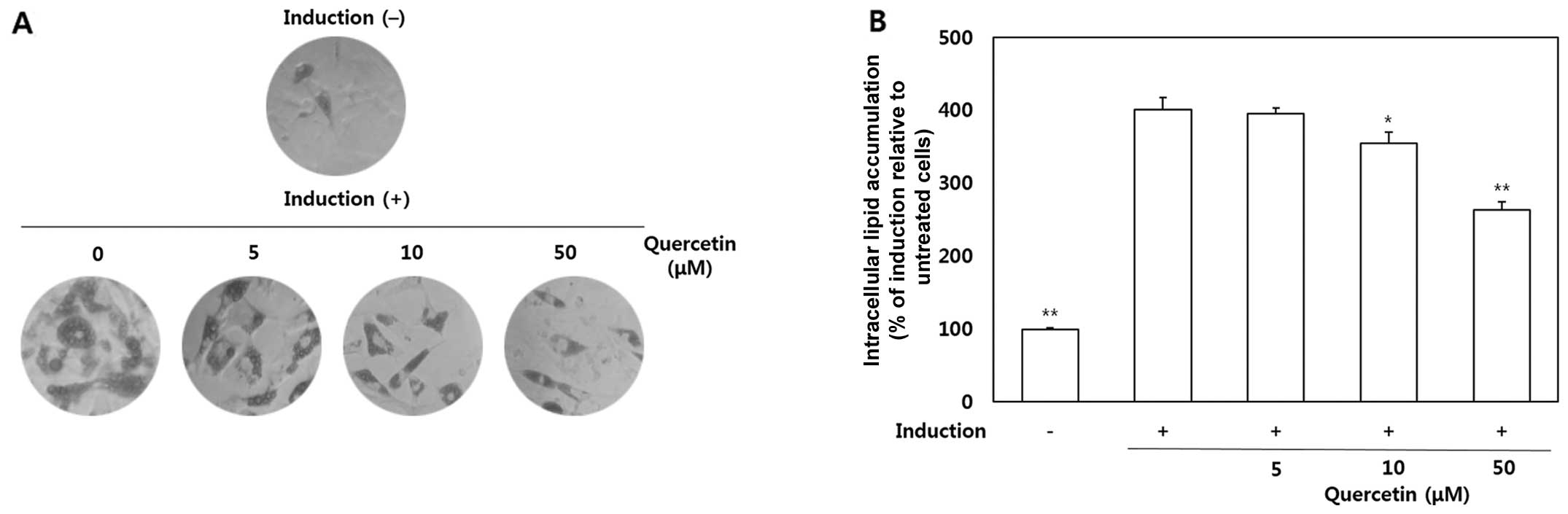
Inhibitory effects of quercetin on the
differentiation of OP9 cells into adipocytes
The OP9 cells were treated with quercetin (5, 10 and
50 μM) to determine its effects on the accumulation of lipid
droplets in the cytoplasm. After the preadipocytes had
differentiated into adipocytes, morphological alterations were
observed due to the accumulation of intracellular lipids (Fig. 2A). As shown by our results, lipid
accumulation in the quercetin-treated cells was significantly
decreased compared with the untreated cells. The quantitative data
of ORO staining indicated that treatment with quercetin at 10 and
50 μM led to a 11.5 and 34.4% decrease in lipid
accumulation, respectively (Fig.
2B).
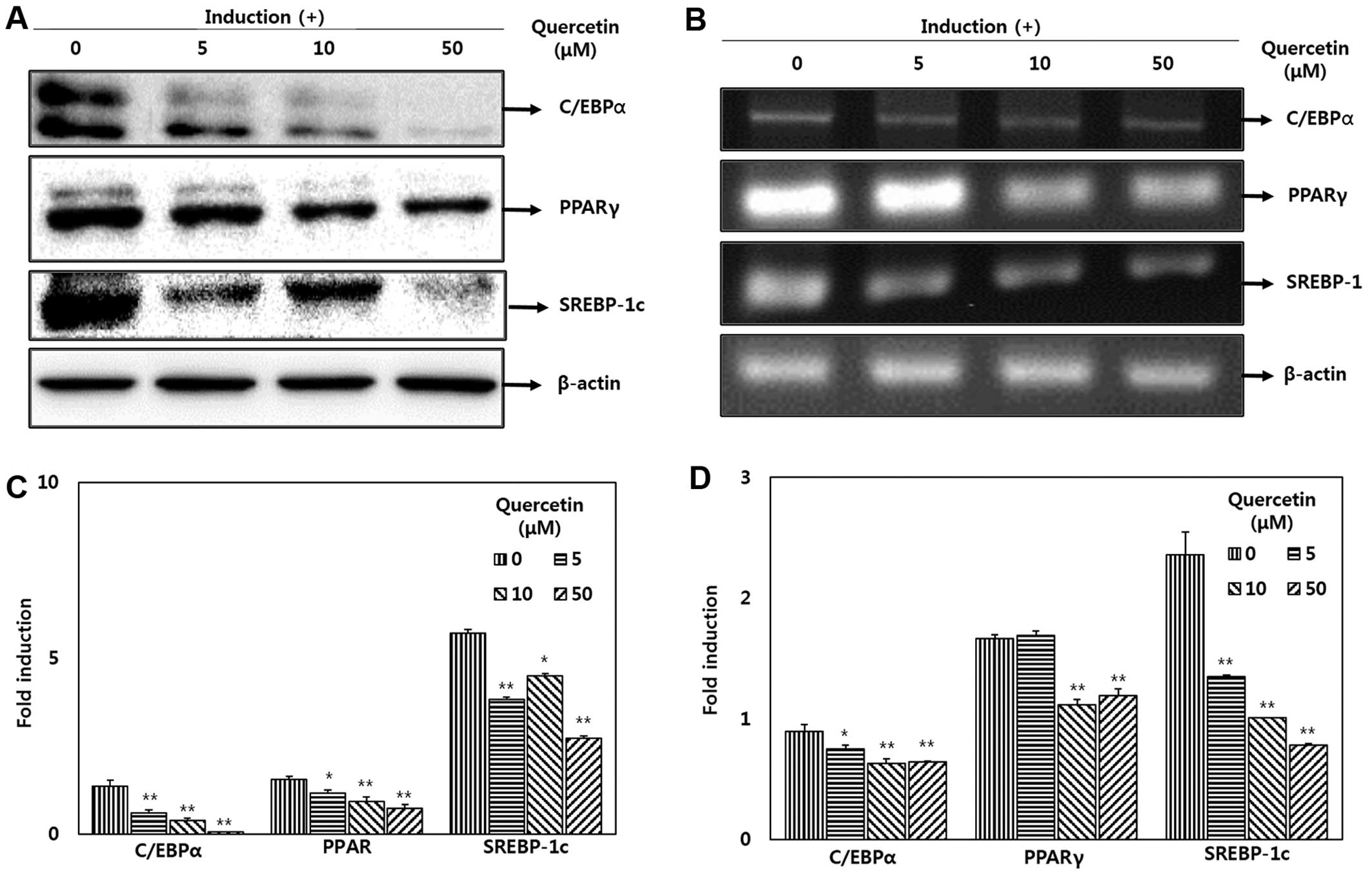
Effect of quercetin on the expression of
transcriptional regulators
To determine the effects of quercetin on adipocyte
differentiation at the protein level, western blot analysis was
performed to measure the expression of factors and enzymes
associated with adipogenis. The adipocytes undergoing MDI-induced
differentiation were treated with various doses of quercetin (0, 5,
10 and 50 μM). The increase in C/EBPα, PPARγ and SREBP-1
protein expression was significantly suppressed by treatment with
quercetin (Fig. 3A). The
expression levels of C/EBPα and PPARγ were decreased following
treatment with quercetin in a dose-dependent manner. The mRNA
expression levels were also significantly decreased in the cells
treated with quercetin following adipocyte differentiation compared
with the untreated cells (Fig.
3B). Following treatment with 5, 10 and 50 μM quercetin,
the expression of SREBP-1 was reduced to 57.2, 42.7 and 33%,
respectively in a dose-dependent manner compared with the untreated
cells. Following treatment with 50 μM quercetin, the
expression level of C/EBPα and PPARγ also decreased to 71.6 and
71.7%, respectively (Fig.
3D).
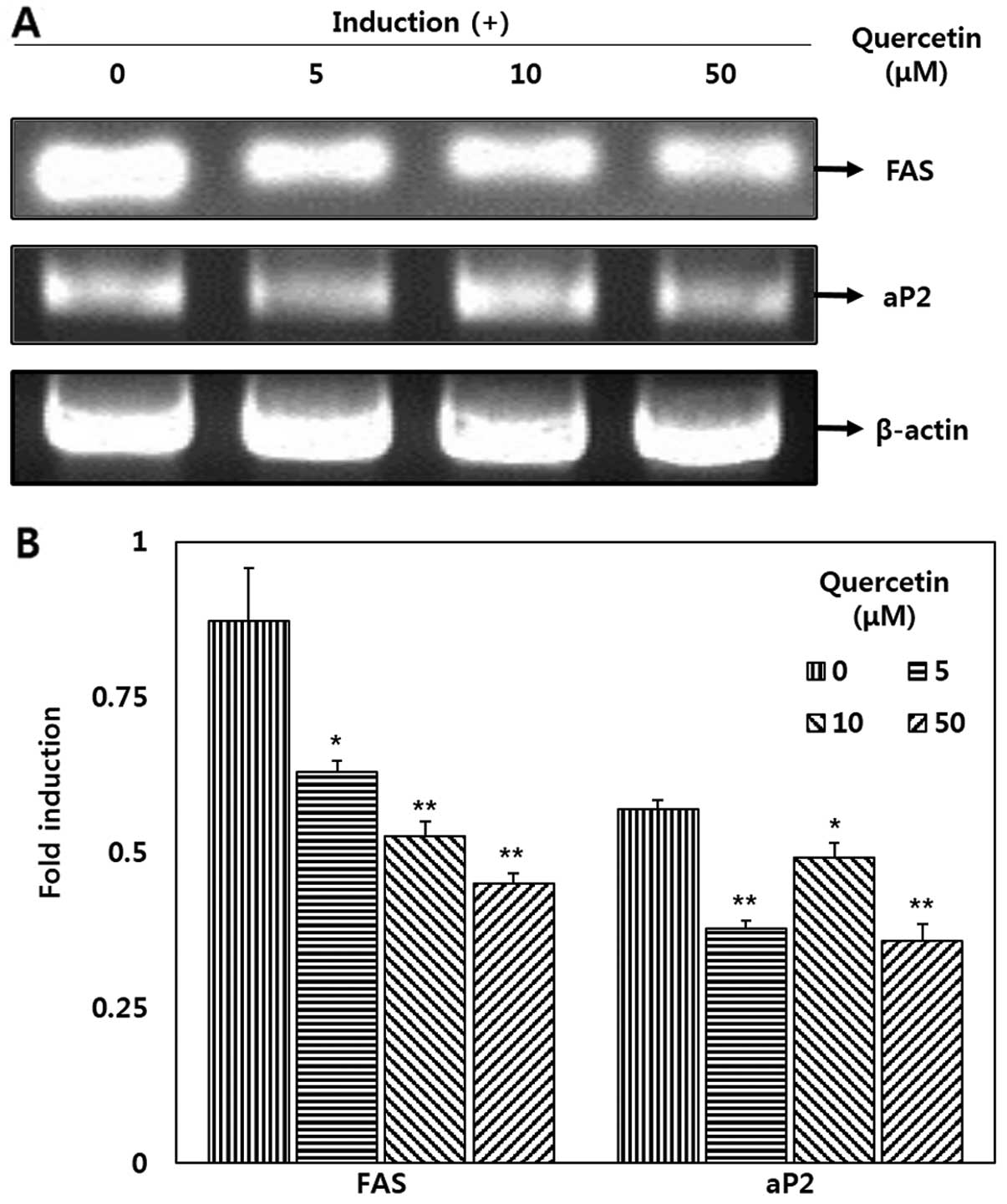
Downregulatory effects of quercetin on
FAS and aP2 expression
Quercetin significantly decreased the mRNA
expression levels of FAS and aP2 in the OP9 adipocytes (Fig. 4). Our results revealed that
treatment with quercetin (50 μM) significantly decreased FAS
and aP2 mRNA expression by 48.3 and 37.1%, respectively when
compared with the untreated cells (Fig. 4B). Additionally, a decrease in FAS
mRNA expression was observed following treatment with various
concentrations of quercetin in a dose-dependent manner.
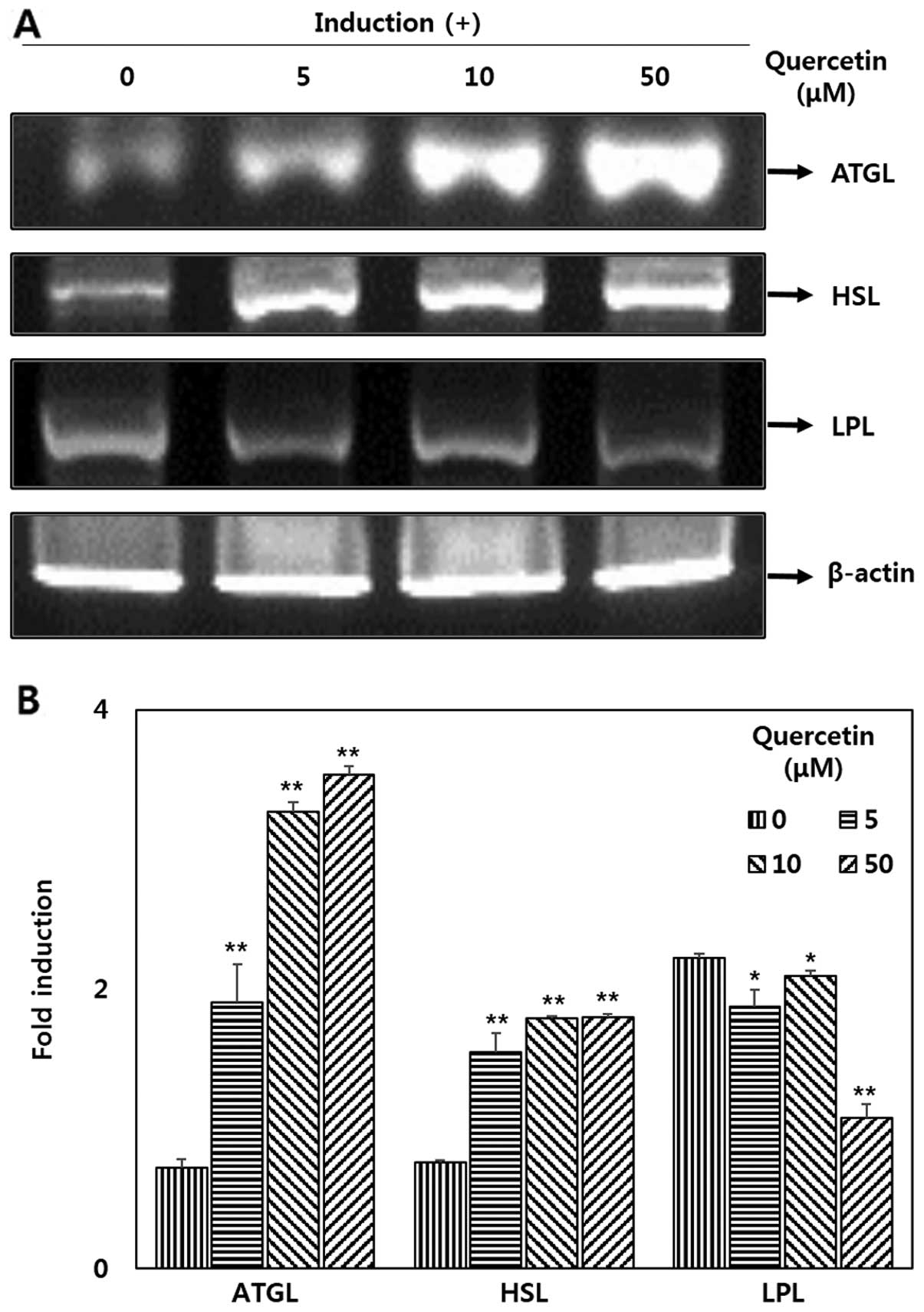
Effect quercetin on the mRNA expression
of ATGL, HSL and LPL in OP9 adipocytes
To determine the effects of quercetin on lipases,
RT-PCR was performed. The results revealed that there was a
significant increase in the expression of ATGL and HSL (Fig. 5A) following treatment with
quercetin. The mRNA expression levels of of ATGL increased by
>4-fold in a dose-dependent manner compared with the untreated
cells. In addition, the expression of HSL increased by >2-fold
compared with the untreated cells (Fig. 5B). It can be observed from the
results presented in Fig. 5 that
LPL expression was deceased in the OP9 adipocytes treated with
quercetin.
Discussion
The present study demonstrated that quercetin
inhibited the differentiation of OP9 preadipocytes induced by
hormone cocktail treatment. In fact, treatment with quercetin
decreased adipogenesis in the differentiated OP9 cells, as
indicated by measurements of total lipid accumulation; quercetin
also downregulated the expression of key adipogenesis-related
transcription factors and their target genes. Moreover, we
demonstrated that the treatment of adipocytes with quercetin
enhanced lipolytic activity by increasing the expression levels of
lipases, thereby diminishing lipid storage in adipocytes.
OP9 mouse stromal cells have received attention as a
new useful model of rapid adipogenesis for the study of adipocyte
biology (20). Adipocyte
differentiation in response to various stimuli is a complex process
involving coordinated changes in hormone sensitivity and gene
expression. Adipogenesis is characterized by the accumulation of
intracellular lipid droplets fully filled with TGs that are
synthesized followed by lipogenesis with glycerol and fatty acids.
Our findings indicated that treatment with quercetin markedly
decreased the accumulation of intracellular TGs (Fig. 2). The number of lipid droplets in
the adipocytes was significantly decreased and the amount of lipids
in the cytoplasm also decreased following treatment with
quercetin.
Adipogenesis requires the sequential activation of
numerous transcription factors, including PPARγ, C/EBPs and SREBPs
(7,21–23). The expression C/EBPα and PPARγ
genes is sequentially activated by early-phase transcription
factors, such as C/EBPβ and C/EBPδ, key transcription factors in
the adipogenesis (24). The
expression of C/EBPα and PPARγ increases the expression of
downstream target genes that are involved in TG metabolism and
finally leads to fully differentiated adipocytes (25). As shown by our data, the mRNA and
protein expression levels of C/EBPα and PPARγ (Fig. 3) were significantly decreased by
treatment with quercetin compared with the untreated cells. It has
also been previously demonstrated that SREBP-1c plays an important
role in the regulation of the mRNA expression of genes involved in
adipocyte differentiation and fatty acid synthesis (26). In this study, the expression of
SREBP-1c was decreased in the adipocytes treated with quercetin
compared with the untreated cells. The fact that C/EBPα and PPARγ
are essential for the intitiation of the cascade of transcription
factors that lead to adipogenesis and that SREBP-1c is associated
with lipogenesis and transcription, suggests that quercetin has
ability to prevent adipogenesis, as shown by our results.
Adipogenesis can be induced through changes in the
expression of programmed specific genes, such as FAS and aP2. These
genes are regulated by transcription factors, such as PPARγ, C/EBPα
and SREBP-1c, which are known to be critical activators of
adipogenesis (5). The aP2 and FAS
genes are known as terminal differentiation markers of adipocytes.
The aP2 gene plays a central role in the pathway which links
obesity to insulin resistance and fatty acid metabolism. The
expression of the FAS enzyme is involved in lipoginesis and leads
to the activation of PPARγ and SREBP-1c as a metabolic cascade.
This activation is also clearly able to cross-activate the FAS
promoter (27). In the present
study, treatment with quercetin induced the downregulation of the
FAS gene, as well as the aP2 gene (Fig. 4). Thus, quercetin may suppress the
downstream adipocyte-specific gene promoters, including aP2 and
FAS, which are associated with adipocyte differentiation and
lipogenesis.
Since quercetin suppressed intracellular lipid
accumulation, we examined the effects of quercetion not only on
transcription factors involved in adipocyte differentiation, but
also on lipases, such as ATGL, HSL and LPL associated with
lipolysis. Previous studies have demonstrated that ATGL is capable
of hydrolyzing TGs and that the level of ATGL determines the rate
of lipolysis (14). ATGL
selectively performs the first step in TG hydrolysis resulting in
the formation of DGs and FFAs (12). HSL has been shown to exhibit broad
substrate specificity and is capable of hydrolyzing
cholesterylester, TGs, DGs and monoacylglycerol. The enzyme is most
active against DGs which are hydrolyzed ~10-fold nore rapidly than
TGs (28). In this study,
quercetin significantly increased the adipocyte mRNA levels of the
major TG lipases, ATGL and HSL. Treatment with quercetin stimulated
lipolysis, indicated by the increased expression of ATGL and HSL,
leading to TG hydrolysis. LPL is a main lipoprotein enzyme in fat
cells and is secreted into capillary vessels and cell envelopes and
participates in lipoprotein metabolism (29). LPL is an early marker of adipocyte
differentiation that dissolves lipids in lipoproteins, such as very
low density lipoprotein (VLDL) and low density lipoprotein (LDL)
into one monoacylglycerol and two FFAs to allow fatty acid entry
into fat tissue; thus, the overexpression of LPL indicates the
initiation of lipid accumulation (30).
In our study, the mRNA expression level of LPL in
adipocytes treated with quercetin was markedly decreased by up to
48.4%. This result indicated that quercetin suppressed the
accumulation of intracellular TGs by downregulating LPL and
upregulating ATGL and HSL (Fig.
5).
In the present study, we examined the effects of
quercetin on the differentiation of preadipocytes into adipocytes
and the mechanisms responsible for the prevention of adipogenesis.
Our data indicated quercetin suppressed the expression of
transcription factors, such as PPARγ, C/EBPα and SREBP-1c and their
downstream target genes, including FAS and aP2. In addition,
quercetin regulated the expression of enzymes associate with
lipolysis, such as ATGL, HSL and LPL. These results suggest that
quercetin has potential for use in the prevention of adipogenesis.
However, further studies are required to explore the full potential
of quercetin in the management of obesity.
Acknowledgments
This study was financially supported by a grant from
the Ministry of Knowledge Economy (MKE), the Korea Institute for
Advancement of Technology (KIAT) through the Inter-ER Cooperation
Projects (R0002019), the National Research Foundation of Korea
(NRF) grant funded by the Korean government (MSIP) (2008-0062484)
and NRF-2013R1A1A2064673.
References
|
1
|
Kopelman PG: Obesity as a medical problem.
Nature. 404:635–643. 2000.PubMed/NCBI
|
|
2
|
Isganaitis E and Lustig RH: Fast food,
central nervous system insulin resistance, and obesity.
Arterioscler Thromb Vasc Biol. 25:2451–2462. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Frühbeck G1, Gómez-Ambrosi J, Muruzábal FJ
and Burrell MA: The adipocyte: a model for integration of endocrine
and metabolic signaling in energy metabolism regulation. Am J
Physiol Endocrinol Metab. 280:E827–E847. 2001.PubMed/NCBI
|
|
4
|
Tang QQ, Jiang MS and Lane MD: Repressive
effect of Sp1 on the C/EBP alpha gene promoter: role in adipocyte
differentiation. Mol Cell Biol. 19:4855–4865. 1999.PubMed/NCBI
|
|
5
|
Gregoire FM, Smas CM and Sul HS:
Understanding adipocyte differentiation. Physiol Rev. 78:783–809.
1998.PubMed/NCBI
|
|
6
|
Otto TC and Lane MD: Adipose development:
from stem cell to adipocyte. Crit Rev Biochem Mol Biol. 40:229–242.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
White UA and Stephens JM: Transcriptional
factors that promote formation of white adipose tissue. Mol Cell
Endocrinol. 318:10–14. 2010. View Article : Google Scholar
|
|
8
|
Eberlé D1, Hegarty B, Bossard P, Ferré P
and Foufelle F: SREBP transcription factors: master regulators of
lipid homeostasis. Biochimie. 86:839–848. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Griffin MJ and Sul HS: Insulin regulation
of fatty acid synthase gene transcription: roles of USF and
SREBP-1c. IUBMB Life. 56:595–600. 2004. View Article : Google Scholar
|
|
10
|
Lodhi IJ, Yin L, Jensen-Urstad AP, et al:
Inhibiting adipose tissue lipogenesis reprograms thermogenesis and
PPARγ activation to decrease diet-induced obesity. Cell Metab.
16:189–201. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Benkalfat NB, Merzouk H, Bouanane S, et
al: Altered adipose tissue metabolism in offspring of dietary obese
rat dams. Clin Sci (Lond). 121:19–28. 2011. View Article : Google Scholar
|
|
12
|
Zimmermann R, Lass A, Haemmerle G and
Zechner R: Fate of fat: the role of adipose triglyceride lipase in
lipolysis. Biochim Biophys Acta. 1791:494–500. 2009. View Article : Google Scholar
|
|
13
|
Bezaire V, Mairal A, Ribet C, et al:
Contribution of adipose triglyceride lipase and hormone-sensitive
lipase to lipolysis in hMADS adipocytes. J Biol Chem.
284:18282–18291. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zimmermann R, Strauss JG, Haemmerle G, et
al: Fat mobilization in adipose tissue is promoted by adipose
triglyceride lipase. Science. 306:1383–1386. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bischoff SC: Quercetin: potentials in the
prevention and therapy of disease. Curr Opin Clin Nutr Metab Care.
11:733–740. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vicentini FT, Fonseca YM, Pitol DL,
Iyomasa MM, Bentley MV and Fonseca MJ: Evaluation of protective
effect of a water-in-oil microemulsion incorporating quercetin
against UVB-induced damage in hairless mice skin. J Pharm Pharm
Sci. 13:pp. 274–285. 2010, PubMed/NCBI
|
|
17
|
Wagner C, Vargas AP, Roos DH, et al:
Comparative study of quercetin and its two glycoside derivatives
quercetin and rutin against methylmercury (MgHg)-induced ROS
production in rat brain slices. Arch Toxicol. 84:pp. 89–97. 2010,
View Article : Google Scholar
|
|
18
|
Chen-yu G, Chun-fen Y, Qi-lu L, Qi T,
Yan-wei X, Wei-na L and Guang-xi Z: Development of a
quercetin-loaded nanostructured lipid carrier formulation for
topical delivery. Int J Pharm. 430:292–298. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ahn J, Lee H, Kim S, Park J and Ha T: The
anti-obesity effect of quercetin is mediated by the AMPK and MAPK
signaling pathways. Biochem Biophys Res Commun. 373:545–549. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wolins NE, Quaynor BK, Skinner JR, Tzekov
A, Park C, Choi K and Bickel PE: OP9 mouse stromal cells rapidly
differentiate into adipocytes: characterization of a useful new
model of adipogenesis. J Lipid Res. 47:450–460. 2006. View Article : Google Scholar
|
|
21
|
Rosen E, Eguchi J and Xu Z:
Transcriptional targets in adipocyte biology. Expert Opin Ther
Targets. 13:975–986. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rosen ED and Spiegelman BM: Molecular
regulation of adipogenesis. Annu Rev Cell Dev Biol. 16:145–171.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rosen ED, Walkey CJ, Puigserver P and
Spiegelman BM: Transcriptional regulation of adipogenesis. Genes
Dev. 14:1293–1307. 2000.PubMed/NCBI
|
|
24
|
Darlington GJ, Ross SE and MacDougald OA:
The role of C/EBP genes in adipocyte differentiation. J Biol Chem.
273:30057–30060. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Farmer SR: Transcriptional control of
adipocyte formation. Cell Metab. 4:263–273. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shimano H: Sterol regulatory
element-binding protein family as global regulators of lipid
synthetic genes in energy metabolism. Vitam Horm. 65:167–194. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Palmer DG, Rutter GA and Tavare JM:
Insulin-stimulated fatty acid synthase gene expression does not
require increased sterol response element binding protein 1
transcription in primary adipocytes. Biochem Biophys Res Commun.
291:439–443. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yeaman SJ, Smith GM, Jepson CA, Wood SL
and Emmison N: The multifunctional role of hormone-sensitive lipase
in lipid metabolism. Adv Enzyme Regul. 34:355–370. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim MS, Wang Y and Rodrigues B:
Lipoprotein lipase mediated fatty acid delivery and its impact in
diabetic cardiomyopathy. Biochim Biophys Acta. 1821:800–808. 2012.
View Article : Google Scholar
|
|
30
|
Gonzales AM and Orlando RA: Role of
adipocyte-derived lipoprotein lipase in adipocyte hypertrophy. Nutr
Metab (Lond). 4:222007. View Article : Google Scholar
|