Introduction
In China, the incidence of bladder cancer is the
most prominent type of malignancy of the urinary system (1). With the exception of surgical
treatment, chemotherapy is important for the therapy of bladder
cancer. Cisplatin-based chemotherapy is widely used in bladder
cancer treatment, with clear effects (2). However, a number of patients exhibit
poor sensitivity to cisplatin, and this drug resistance is a
problem that requires investigation. The low sensitivity to
cisplatin and resulting drug resistance affect the therapeutic
efficacy of bladder cancer treatment (3,4).
Therefore, it is necessary to investigate the resistance mechanism
of bladder cancer to cisplatin and attempt to improve the
sensitivity of bladder cancer cells to it.
The specific binding of insulin-like growth factor
binding protein (IGFBP) with insulin-like growth factor-1 (IGF-1),
through regulating the activity of IGF-1, is important in the
process of cell growth, development and proliferation (5,6).
In the family of IGFBPs, IGFBP-2 has been investigated more
extensively. Several studies have suggested that IGFBP-2 is
associated with the occurrence and development of tumors, and a
consensus of these reports is that IGFBP-2 overexpression is
observed in tumor patients. For example, studies have suggested
that the expression of IGFBP-2 is high in ovarian cancer, lung
cancer and prostate cancer (7–9).
These results suggest that excessive expression of IGFBP-2 sustains
the growth of tumor cells and inhibits apoptosis. These effects of
IGFBP-2 have been suggested as being not only IGF-1-dependent
(10,11). It is not difficult to hypothesize
that IGFBP-2 may be associated with the chemotherapy drug
resistance of tumor cells, and changing the expression of IGFBP-2
expression may change the sensitivity of tumor cells to
chemotherapeutic drugs. In terms of tumor drug resistance, previous
investigation has revealed that increased expression of IGFBP-2 is
associated with chemotherapy tolerance of prostate cancer induced
by hyperglycemia (12).
Trastuzumab-resistance in breast cancer is also associated with the
overexpression of IGFBP-2, which overactivates ErbB2 (13). However, whether IGFBP-2 is
associated with the drug resistance of bladder cancer cells to
cisplatin, and how IGFBP-2 may be regulated to alter the
sensitivity of bladder cancer cells to cisplatin remain to be fully
elucidated.
Maspin can inhibit serine protease, and several
studies have demonstrated that maspin can inhibit tumor growth by
inducing apoptosis (14,15). In certain types of tumor, maspin
is often expressed at low levels (16). Therefore, the present study
hypothesized that maspin is associated with the sensitivity of
bladder cancer cells to cisplatin. To confirm this, the present
study examined the expression of IGFBP-2 in bladder cancer tissues,
BIU87 cells and cisplatin-resistant BIU87 (BIU87-CisR) cells.
Furthermore, small interfering (si)RNA technology was used to
inhibit the expression of IGFBP-2 in bladder cancer BIU87-CisR
cells, to determine changes of the sensitivity of BIU87-CisR cells
to cisplatin and to examine changes in the expression of maspin
following IGFBP-2 inhibition.
Materials and methods
Patients and preparation of tissue
specimens
The present study recruited 32 patients with bladder
cancer (18 male and 14 female, median age 65.9, range 51–76), who
had undergone surgery and cisplatin-based combination chemotherapy
between March 2012 and February 2013. Of these patients, 20 were
sensitive to chemotherapy, while 12 were resistant to chemotherapy,
however the information obtained regarding demographic data,
including age, gender, stage of disease and treatment were not
statistically different. Tumor tissue specimens were obtained
during the surgical bladder cancer tissue resection and were stored
in liquid nitrogen until further analysis. The present study was
approved by the Ethics Committee and the informed consent was
obtained from all patients.
Cell culture
The BIU87 cell line was purchased from the Type
Culture Collection of the Chinese Academy of Sciences (Shanghai,
China). The cisplatin-resistant subline, termed the BIU87-CisR cell
line, was established as in our preliminary experiment, and its
resistance to cisplatin was confirmed. BIU87-CisR cells were
obtained from parental BIU87 cells through continuous exposure to
increasing concentrations of cisplatin over 12 months, with the
final concentration of cisplatin being 6 μM. In brief, BIU87
cells were planted in 6-well plate with a density of 100,000/well,
and treated with 0.25, 0.5, 1, 2, 4 and 6 μM cisplatin for 2
months. The BIU87 cell line and BIU87-CisR cell line were
maintained in RPMI-1640 medium (Thermo Fisher Scientific, Waltham,
MA, USA) supplemented with 10% fetal bovine serum at 37°C in 5%
CO2 conditions.
Transfection of IGFBP-2 siRNA
Downregulation of the expression of IGFBP-2 in
BIU87-CisR cells was induced using siRNA. siRNA targeted to human
IGFBP-2 was designed and synthesized (GenePharma, Shanghai, China)
with the target sequence: 5′-UGG CGA UGA CCA CUC AGA A-3′ and
transfected into the cells in 24-well plate (100,000 cells/well)
using Lipofectamine® 2000 reagent (Invitrogen Life
Technologies, Carlsbad, CA, USA). The concentration of siRNA was
100 nM and the BIU87-CisR cells were cultured for 48 h following
transfection. The silencing effect was then assessed at the mRNA
and protein levels.
RNA isolation and quantification of the
mRNA expression levels of IGFBP-2 and maspin in the tissues and
cell lines
Reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) was performed to quantify the mRNA expression
levels of IGFBP-2. The total RNA of the tumor tissue specimens
(n=32), BIU87 cells, BIU87-CisR cells and IGFBP-2-silenced
BIU87-CisR cells were extracted using TRIzol reagent (Thermo Fisher
Scientific). The purity and concentration of the total RNA were
verified using spectrophotometry (NanoDrop 3300; Thermo Fisher
Scientific). The qualified RNA was reverse-transcribed into cDNA
using PrimeScript® RT reagent (Takara Biotechnology Co.,
Ltd., Dalian, China), according to manufacturer’s instructions, The
cDNA was then amplified using a SYBR® Premix Ex Taq™ II
kit (Takara Biotechnology Co., Ltd.) on a 7500 Real-Time PCR system
(Applied Biosystems, Foster City, CA, USA). The cycling conditions
were set (20 μl reaction volumes), according to the
manufacturer’s instructions. The cells were incubated for 30 sec at
95°C and then amplified for 40 cycles; in each cycle reaction
volume the cells were incubated at 95°C for 5 sec and 60°C for 30
sec. The primers (all designed by Takara Biotechnology Co., Ltd.)
of the targeted genes and glyceraldehyde 3-phosphate dehydrogenase
(GAPDH) were as follows: IGFBP-2, forward 5′-CAA GCA TGG CCT GTA
CAA CCTC-3′ and reverse 5′-GGG TTC ACA CAC CAG CAC TC-3′; Maspin,
forward 5′-AAC TGA AGA TGG TGG GGA TT-3′ and reverse 5′-TGG GAA GAA
GAG CTT CCA AA-3′; and GAPDH, forward 5′-CGG AGT CAA CGG ATT TGG
TCG TAT-3′ and reverse 5′-AGC CTT CTC CAT GGT GGT GAA GAC-3′. The
fold changes in the target genes were calculated using the
comparative cycle threshold (2−ΔΔCt) method (17), and the result was normalized by
GAPDH.
Western blot analysis for the protein
expression levels of IGFBP-2 and maspin
The proteins in the tissue samples and cells were
extracted using the using radioimmunoprecipitation assay reagent
combined with 1% protease inhibitor cocktail (Applygen
Technologies, Inc., Beijing, China). The protein samples (30
μg) were separated by 12% sodium dodecyl
sulfate-polyacrylamide gel electrophoreseis (SDS-PAGE; 100 V, 1.5
h; Applygen Technologies, Inc.) and then transferred onto
polyvinylidene difluoride membranes (150 mA, 1 h; Applygen
Technologies, Inc.). The membranes were blocked with 5% skim milk
in phosphate-buffered saline (PBS) for 1 h and incubated at 4°C for
12 h with the primary antibodies mouse anti-human IGFBP-2 (Cat. no.
sc-130070; 1:1,000), mouse anti-human β-actin (Cat. no. sc-130301;
1:5,000) or mouse anti-human maspin (Cat. no. sc-166260; 1:500).
All primary antibodies were monoclonal and purchased from Santa
Cruz Biotechnology Co., Ltd. (Shanghai, China). The membranes were
washed with PBST buffer, followed by incubation with
horseradish-peroxidase-conjugated secondary antibodies for 2 h at
room temperature. The bands were detected using an ECL Detection
Reagent kit (Applygen Technologies, Inc.) and analyzed using Image
J 1.48 software (National Institutes of Health, Bethesda, MD,
USA).
Cell inhibition analysis
The induction of cell inhibition by cisplatin was
detected using a Cell Counting Kit-8 (CCK-8) kits (Boster
Biological Engineering Co., Ltd., Wuhan, China). The BIU87 cells,
BIU87-CisR cells and IGFBP-2-silenced BIU87-CisR cells were plated
at a density of 1×104 cells per well in a 96-well plate
and treated with 3 μM cisplatin for 0, 6, 12, 18 and 24 h at
37°C. Following cisplatin exposure, 10 μl CCK-8 cell
counting reagent was added and incubated at 37°C for 4 h, the
optical density of the culture solution in each plate was measured
using a plate reader (ELX800; BioTec, Winooksi, VT, USA) at 450
nm.
Cell cycle and cell apoptosis
analysis
The BIU87-CisR cells and IGFBP-2-silenced BIU87-CisR
cells were treated with 3 μM cisplatin for 24 h, and cell
cycle and cell apoptosis analysis were performed using a flow
cytometer. For cell cycle analysis, the cells were collected and
fixed with pre-cold 70% ethanol. After 12 h, 500 μl
propidium iodide (Sigma-Aldrich, St. Louis, MO, USA) was added, and
the cells were incubated for 30 min. Cell cycle analysis was
performed using a BD FACSCalibur flow cytometer (Beckman Coulter,
Miami, FL, USA) within 15 min. For cell apoptosis analysis, an
Annexin V-FITC Assay kit was used, according to the manufacturer’s
protocol.
Overexpression of maspin and its effect
on sensitivity to cisplatin of BIU87-CisR cells
The pcDNA-maspin recombinant plasmids, constructed
in our preliminary experiment, were transfected into the BIU87-CisR
cells planted in 24-well plate (100,000 cells/well) using
Lipofectamine® 2000 reagent in order to increase the
expression of maspin. The proliferation inhibition of the
maspin-overexpressing BIU87-CisR cells treated with 3 μM
cisplatin for 24 h was analyzed using a CCK-8 kit, with the same
methods as mentioned above.
Statistical analysis
All data are expressed as the mean ± standard
deviation. All calculations were performed using SPSS 18.0
statistical software (SPSS, Chicago, IL, USA). A one-way analysis
of variance, followed by a least significant difference test, were
used to determine the statistical significance among four groups
and between each group. P<0.05 was considered to indicate a
statistically significant difference.
Results
Higher expression levels of IGFBP-2 are
detected in tumor tissue from chemoresistant patients and in
BIU87-CisR cells
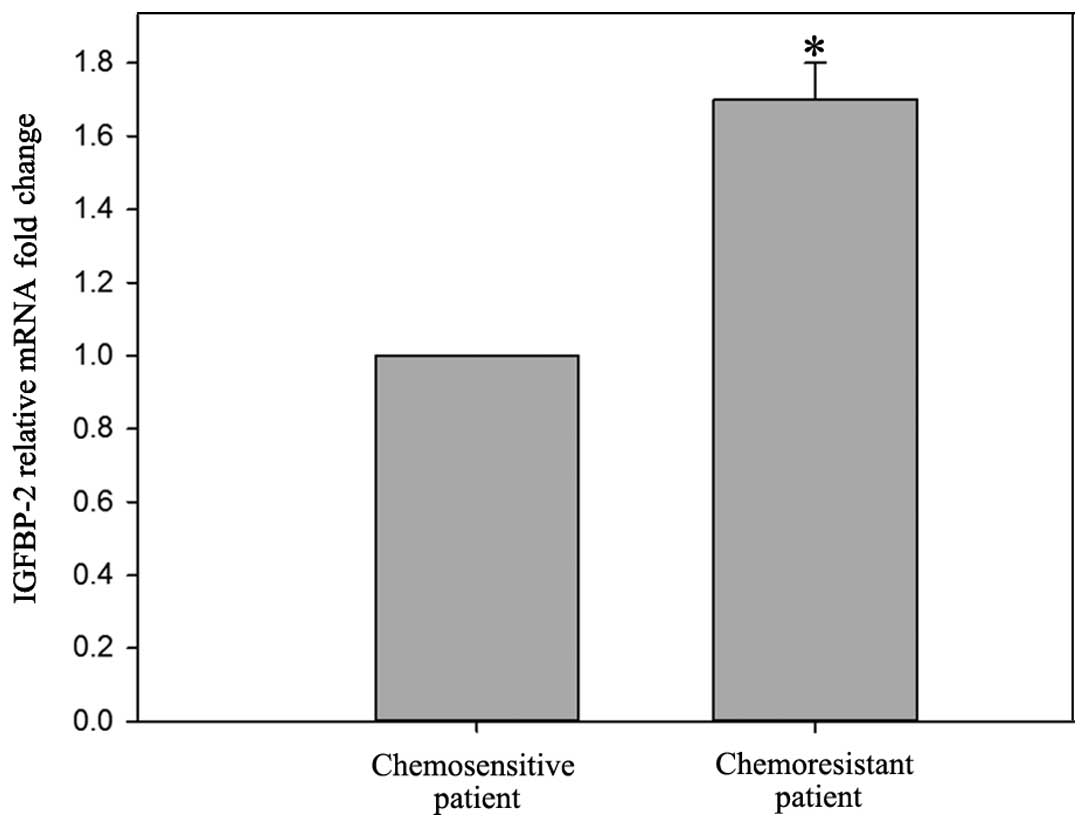
The mRNA levels of IGFBP-2 were higher
(1.70±0.08-fold) in the chemoresistant patients, compared with the
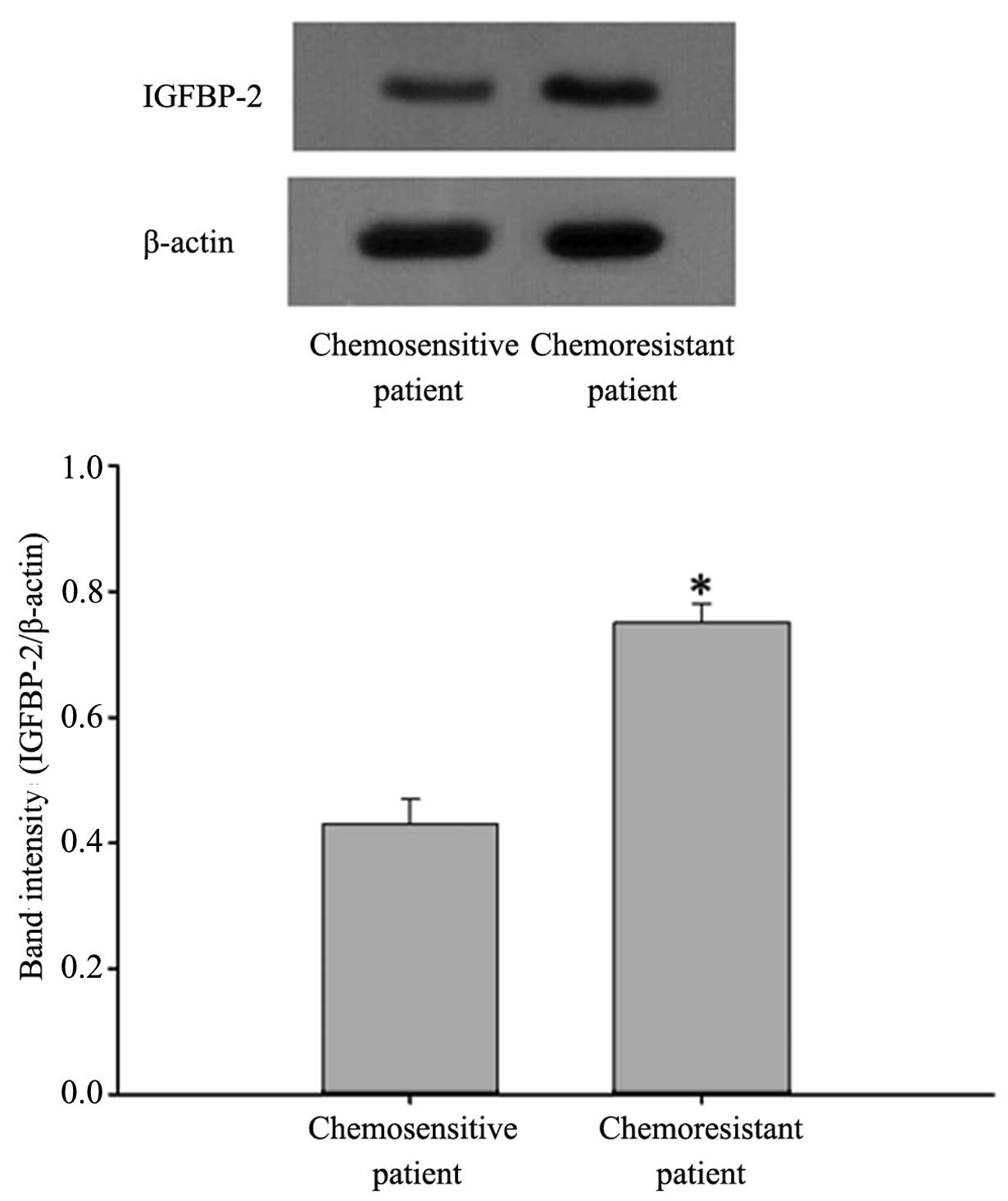
chemosensitive patients (P<0.05; Fig. 1). Western blot analysis confirmed
the protein expression of IGFBP-2 was also higher in the
chemo-resistant patients (Fig.
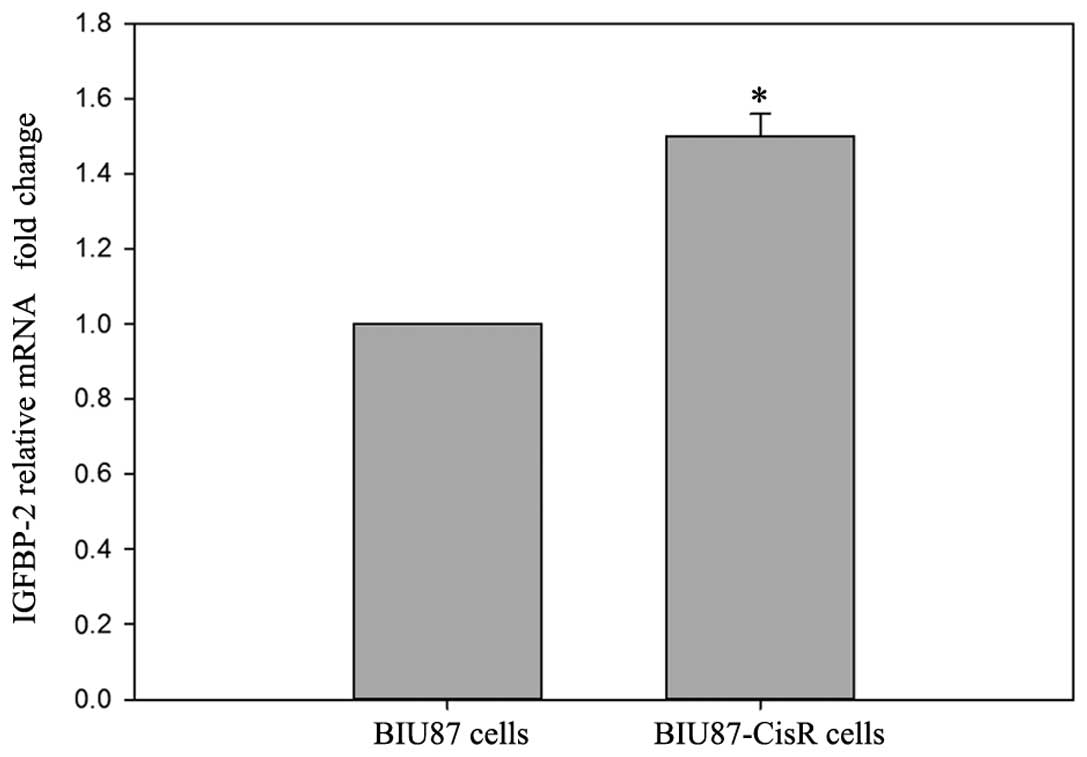
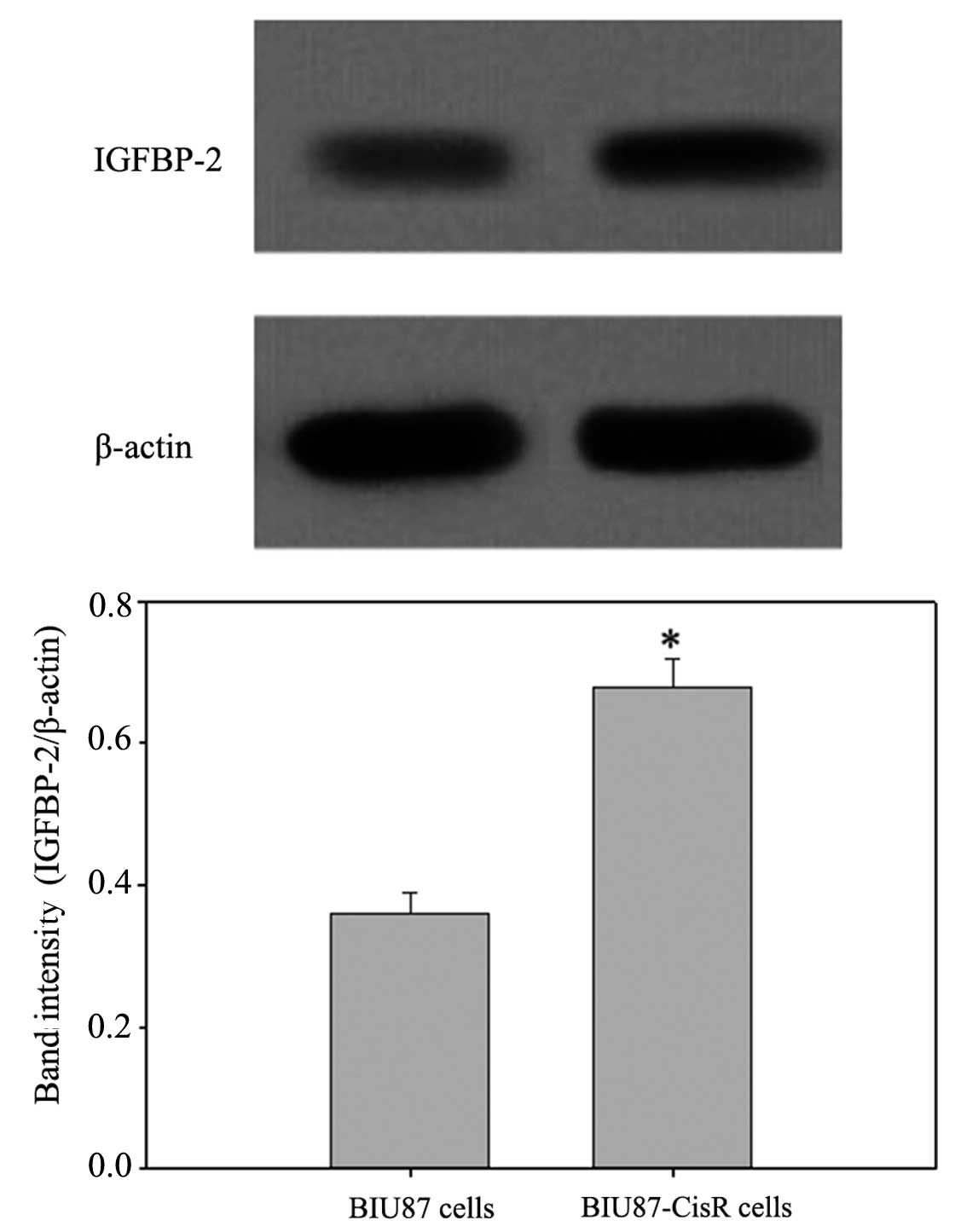
2). In the BIU87-CisR cells, higher expression levels of
IGFBP-2 were also observed, compared with the BIU87 cells
(1.55±0.06-fold; P<0.05), at the mRNA and protein levels
(Figs. 3 and 4).
Lower expression levels of maspin are
detected in tumor tissues of chemoresistant patients and in
BIU87-CisR cells
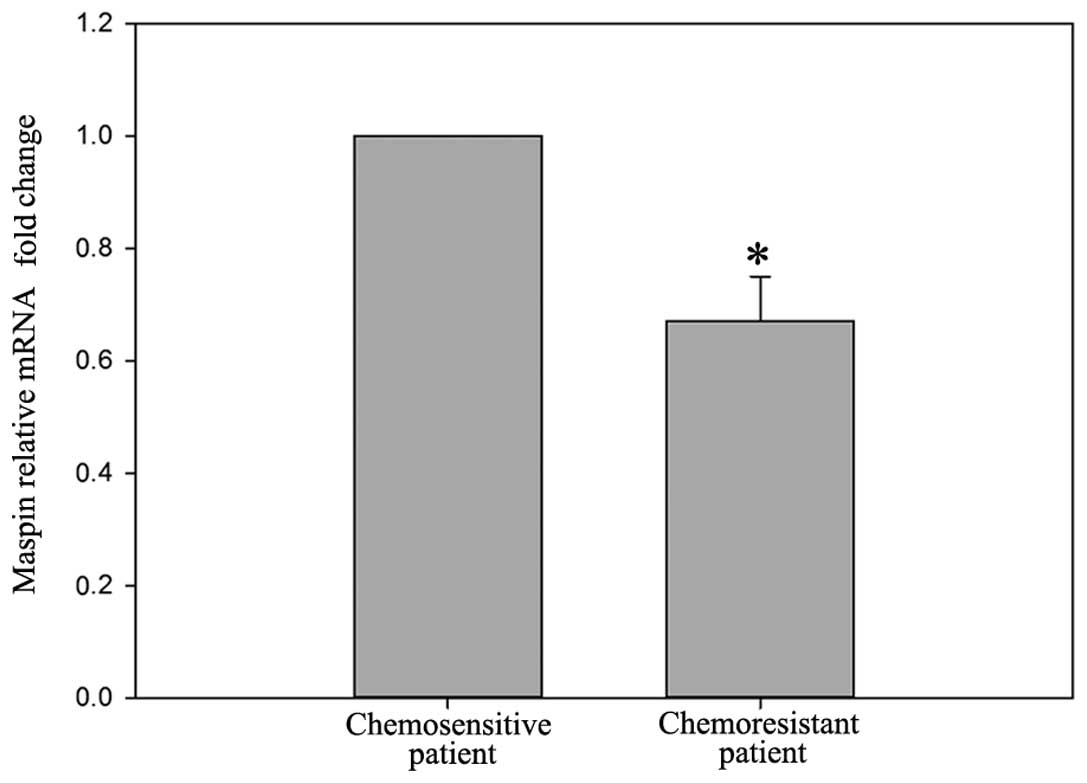
In contrast to the expression of IGFBP-2, the mRNA
level of maspin was lower (0.67±0.08-fold) in the chemoresistant
patients (Fig. 5), compared with
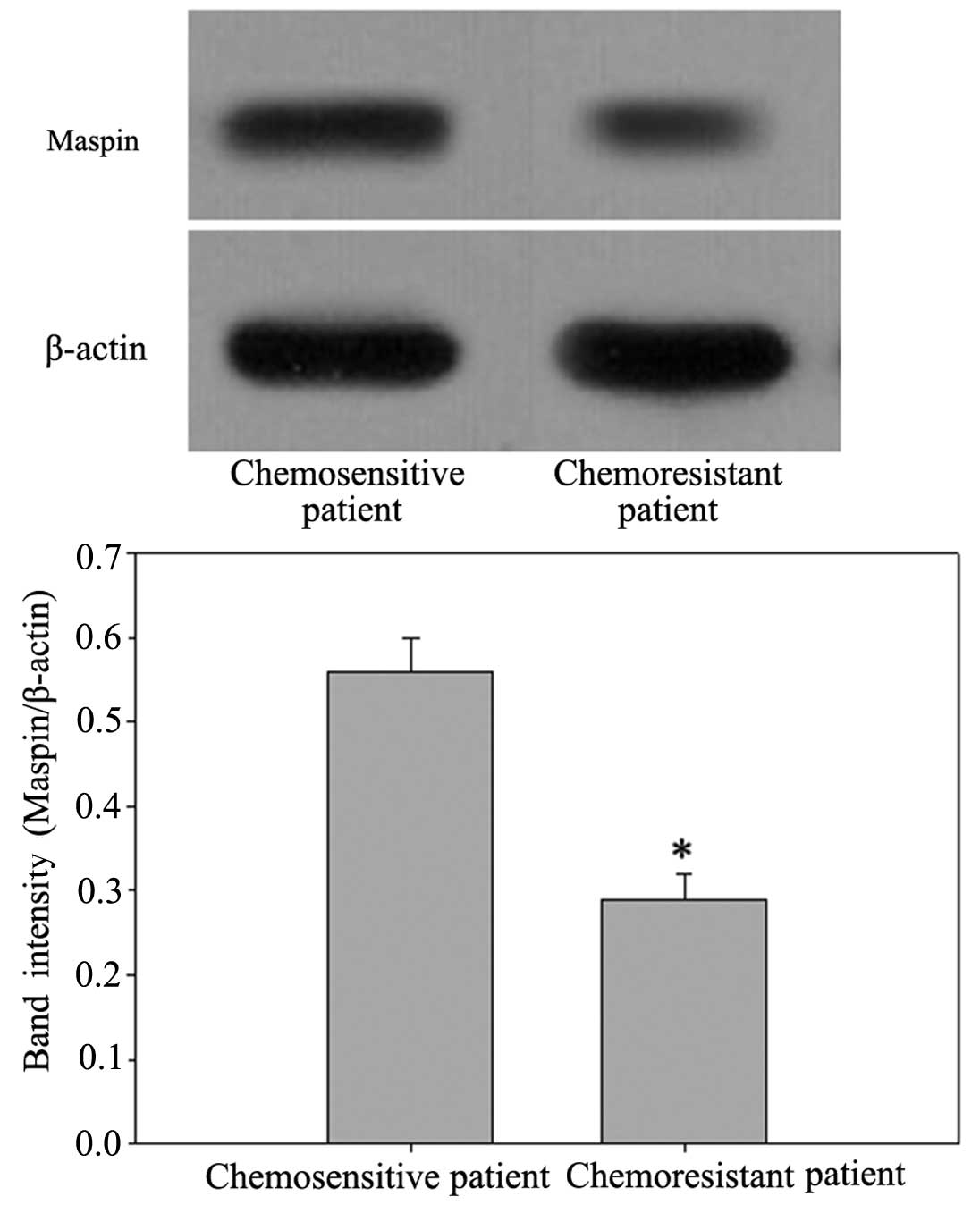
the chemosensitive patients (P<0.05). Western blot analysis also
confirmed the same observation in the protein levels of maspin
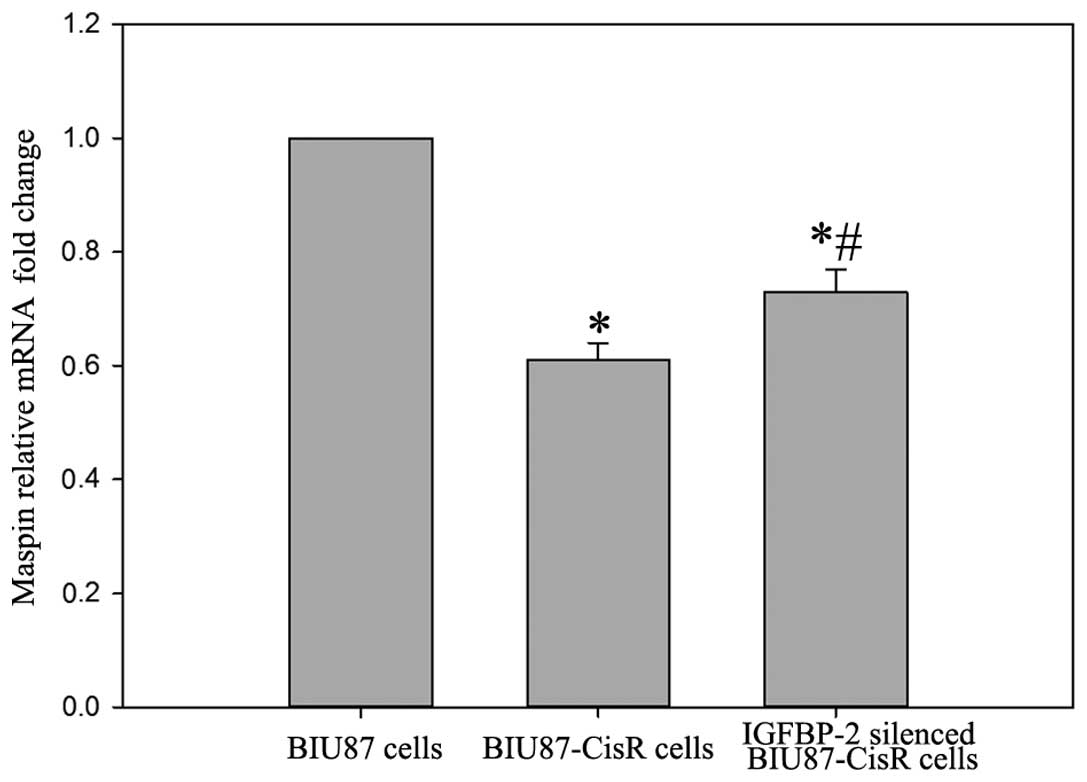
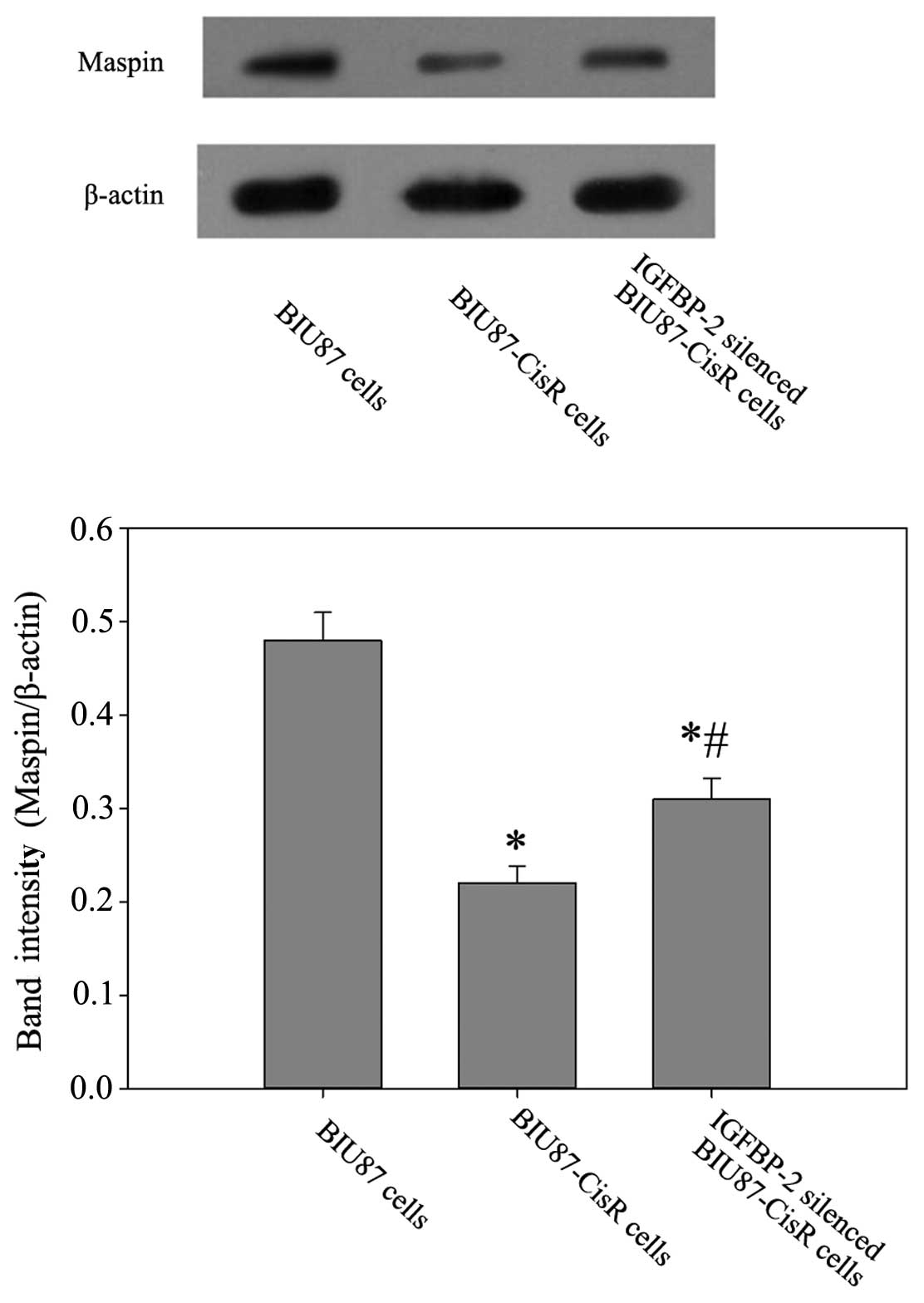
(Fig. 6). In the BIU87-CisR cell
line, lower expression levels of maspin were observed at the mrRNA
and protein levels, compared with the BIU87 cells (0.61±0.04-fold;
P<0.05). In addition, following IGFBP-2 inhibition, the mRNA and
protein expression levels of maspin in the BIU87-CisR cells
increased significantly (Figs. 7
and 8).
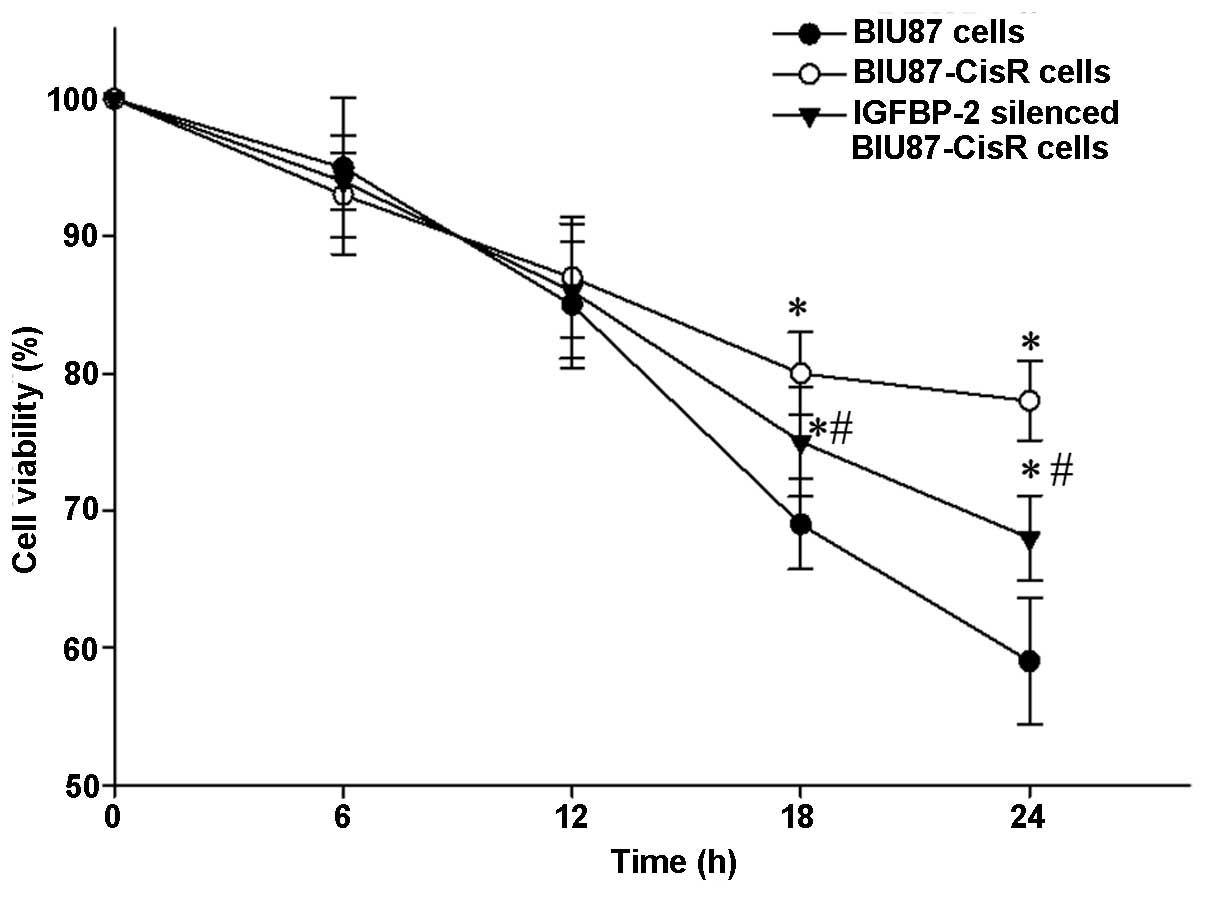
Knockdown of IGFBP-2 enhances the
inhibitory effect of cisplatin on proliferation
Cisplatin (3 μM) treatment was observed to
induce cell death in a time-dependent manner (Fig. 9). Following 24 h cisplatin
exposure, the cell viability was significantly lower in the BIU87
cells (P<0.05), compared with the BIU87-CisR cells and IGFBP-2
silenced BIU87-CisR cells. The normal BIU87 cells were the most
sensitive to cisplatin, and the BIU87-CisR cells exhibited
significant resistance to cisplatin, compared with the normal BIU87
cells. However, the IGFBP-2-silenced BIU87-CisR cells were more
sensitive to cisplatin, compared with the BIU87-CisR cells without
siRNA treatment.
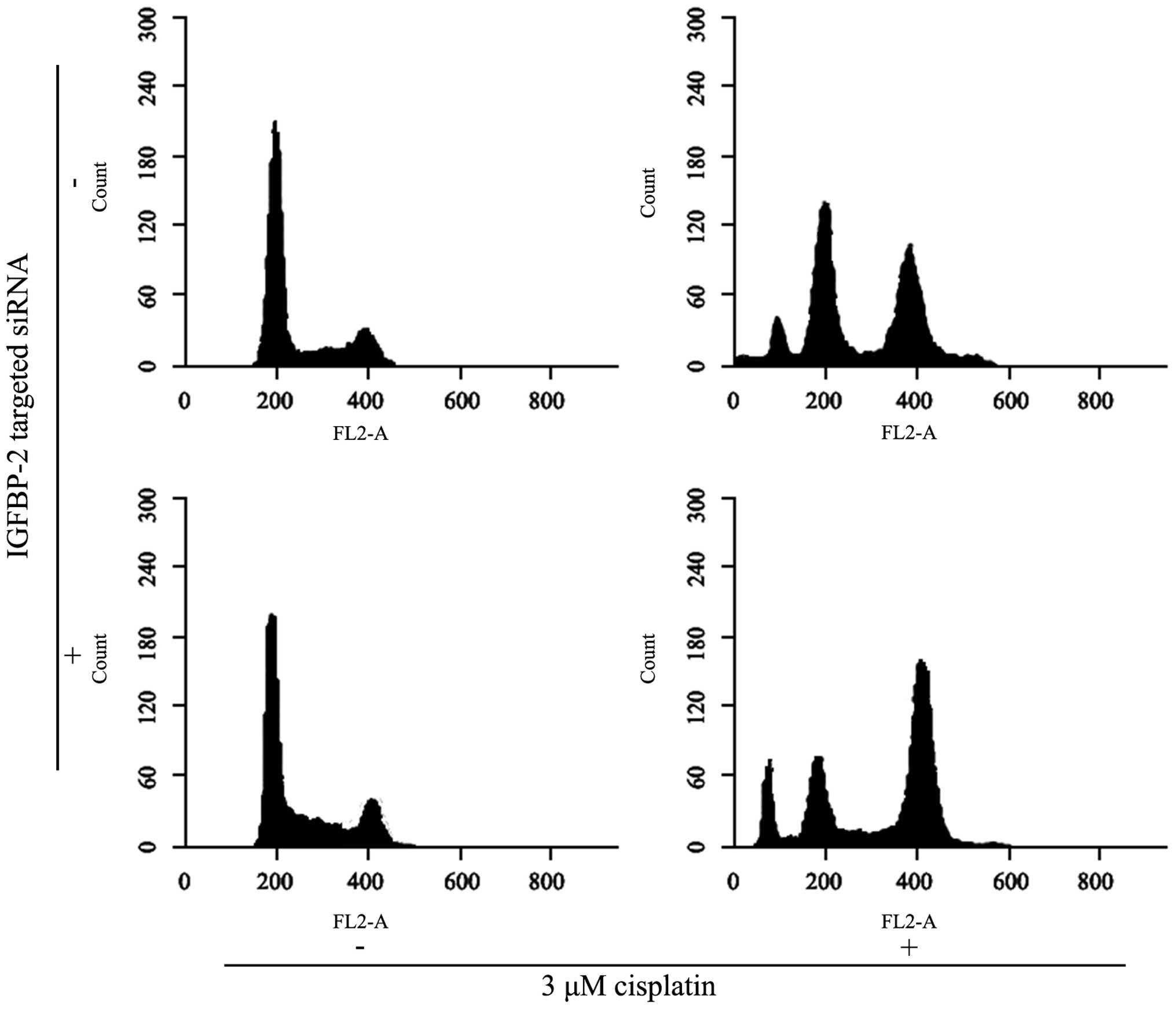
Knockdown of IGFBP-2 contributes to
G2/M phase arrest of BIU87-CisR cells induced by
cisplatin
Cisplatin was observed to arrest BIU87 cells at the
G2/M phase (Fig. 10).
In the BIU87-CisR group, the percentages of the cell population at
the G0/G1 phase was 70.34±4.45% and at the
G2/M phase was 8.5±0.9%. Following 3 μM cisplatin
treatment, the proportion of cells at the
G0/G1 phase deceased to 56.9±4.3%, Whereas
the proportion of cells at the G2/M phase increased to
38.3±4.1%. In addition, IGFBP-2 silencing marginally suppressed the
proportion of cells at the G0/G1 phase
(35.5±6.5%) and elevated the percentage of cells at the
G2/M phase (48.3±6.1%). The differences between each
group were statistically significant (P<0.05).
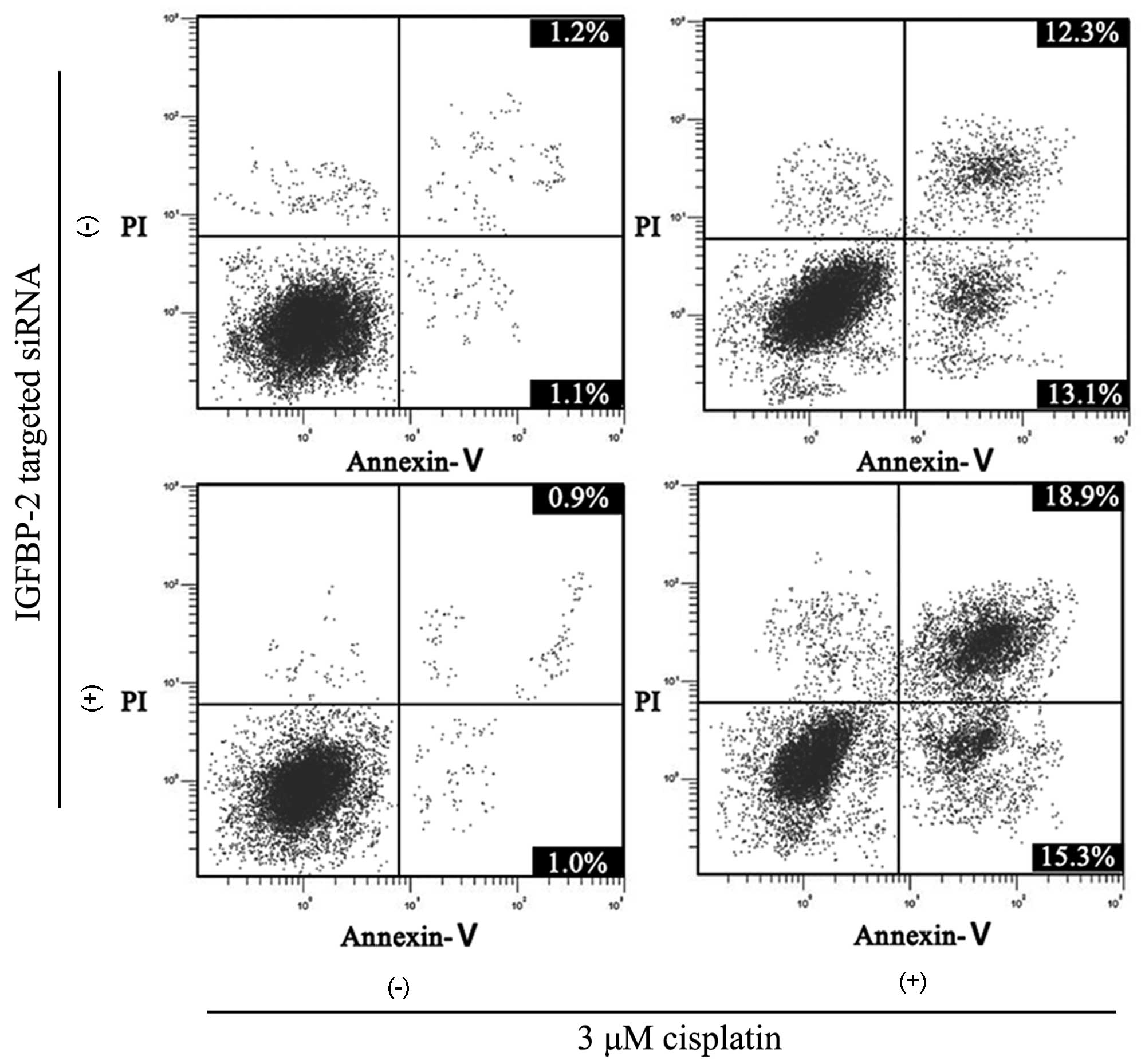
Apoptosis of BIU87 cells caused by
cisplatin increases following inhibition of IGFBP-2
Similar to the results of the cell cycle analysis,
the silencing of IGFBP-2 enhanced the apoptosis-promoting effect of
cisplatin. The apoptotic rate in the BIU87-CisR cells was 2.9±0.6%
and, following 3 μM cisplatin treatment, the apoptotic rate
increased to 22.6±2.4%. However, in the IGFBP-2-silenced BIU87-CisR
cells, the apoptotic rate induced by cisplatin was 35.9±3.2%
(Fig. 11). The differences
between each group were statistically significant (P<0.05).
Overexpression of maspin may improve the
sensitivity of BIU87-CisR cells to cisplatin (Fig. 12)
Following 3 μM cisplatin treatment for 24 h,
the maspin-overexpressing BIU87-CisR cells exhibited a lower cell
viability, compared with the normal BIU87-CisR cells (68.1±5.3, vs.
75.3±4.6%, respectively; P<0.05). This suggests that inhibition
of IGFBP-2 may improve the sensitivity of bladder cancer cells to
cisplatin via upregulating the expression of maspin.
Discussion
Carcinoma of the urinary bladder is a common and
life-threatening malignant tumor of the urinary system. The
preferred treatment option for this type of cancer is resection of
the bladder and pelvic lymphadenectomy. However, for patients with
advanced-stage disease, a high recurrence rate following surgery
has been reported, and certain elderly patients with cardiac or
pulmonic insufficiency cannot tolerate radical surgery (18). Therefore, chemotherapy has become
an important aspect of bladder cancer treatment, particularly for
patients with tumor metastasis or advanced-stage cancer (19). During the process of using
cisplatin as a treatment approach, the response of patients to the
drug is low and certain patients exhibit drug resistance, which
leads to ineffective chemotherapy, increased recurrence rate, poor
prognosis and increased economic burden (20). The present study was designed to
reveal the molecular mechanism underlying the sensitivity of
bladder cancer cells to cisplatin, and to provide novel methods to
improve the sensitivity of bladder cancer cells to cisplatin.
In the present study, the expression of IGFBP-2 in
patients with cisplatin-resistance was significantly higher at the
mRNA and protein levels. Previous studies have demonstrated that
IGFBP-2 overexpression reduces prostate cancer cell apoptosis
induced by docetaxel and reduces the sensitivity of non-small cell
lung cancer to dasatinib (12,21); it also increases chemotherapy
resistance in patients with adult acute myeloid leukemia (22). Therefore, the present study
hypothesized that IGFBP-2 overexpression may be associated with
reduced sensitivity of bladder cancer cells to cisplatin. To
investigate this, siRNA was used to downregulate the expression of
IGFBP-2 in the BIU87-CisR cells. The results revealed that,
following IGFBP-2 inhibition, the proliferation of cells exposed to
cisplatin was further inhibited, more cells were in G2/M
phase arrest, and the apoptotic rate also increased. These results
indicated that inhibiting the expression of IGFBP-2 improved the
sensitivity of the bladder cancer cells to cisplatin.
There are two mechanisms by which IGFBP-2 reduces
cancer cell apoptosis and increases its proliferation. The first is
the IGF-1-dependent pathway, which involves adjustment of the IGF-1
system through the binding of IGF-1 and IGFBP-2, which reduces the
interaction of IGF-1 with its receptor and inhibits signal
transmission (23). The other
mechanism is the IGF-1-independent pathway. Lu et al
(21) demonstrated that IGFBP-2
may activate the FAK pathway to inhibit the apoptosis of non-small
cell lung cancer, and used dasatinib combined with FAK inhibitor to
inhibit small-cell lung cancer with overexpressed IGFBP-2. In lung
adenocarcinoma, the overexpression of IGFBP-2 inhibits the activity
of IGF-1 signaling pathways and reduces the activity of caspase-3,
which limits cancer cell apoptosis (24). By using the recombination of
IGFBP-2 to process breast cancer cell Hs578T, Frommer et al
(25) found that a series of
factors associated with apoptosis resistance, including nuclear
factor-κ and B-cell lymphoma-2 (Bcl-2) were increased. The present
study demonstrated that, following the inhibition of IGFBP-2, the
expression of maspin was significantly increased. Maspin has tumor
suppressor functions as, in addition to inhibiting cancer cell
metastasis, maintaining cell adhesion and inhibiting tumor
angiogenesis (26), it can induce
cancer cell apoptosis through Bcl-2 and B-cell-associated X protein
(27). Therefore, IGFBP-2 may
increase the expression of maspin and further increase the
susceptibility of bladder cancer cells to cisplatin with the
assistance of the tumor suppressor function of maspin. It is
generally acknowledged that the expression of maspin is
predominantly regulated by methylation/demethylation, and the
methylation enhancement of its gene promoter may lead to reduced
expression of maspin (28),
however, the mechanism underlying the increased expression of
maspin by IGFBP-2 remains to be elucidated. Further in-depth
investigations are required to determine whether IGFBP-2 affects
the promoter methylation of maspin. Based on the findings of the
present study, inhibiting IGFBP-2 may improve the susceptibility of
bladder cancer cells to cisplatin, the mechanism of which is
associated with changes to the expression of maspin.
References
|
1
|
Xu W, Wang F, Ying L and Wang HH:
Association between glutathione S-transferase M1 null variant and
risk of bladder cancer in Chinese Han population. Tumour Biol.
35:773–777. 2014. View Article : Google Scholar
|
|
2
|
Costantini C and Millard F: Update on
chemotherapy in the treatment of urothelial carcinoma.
ScientificWorldJournal. 11:1981–1994. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shirato A, Kikugawa T, Miura N, et al:
Cisplatin resistance by induction of aldo-keto reductase family 1
member C2 in human bladder cancer cells. Oncol Lett. 7:674–678.
2014.PubMed/NCBI
|
|
4
|
Galluzzi L, Vitale I, Michels J, et al:
Systems biology of cisplatin resistance: past, present and future.
Cell Death Dis. 5:e12572014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hjortebjerg R and Frystyk J: Determination
of IGFs and their binding proteins. Best Pract Res Clin Endocrinol
Metab. 27:771–781. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Malaguarnera R and Belfiore A: The
emerging role of insulin and insulin-like growth factor signaling
in cancer stem cells. Front Endocrinol (Lausanne). 5:102014.
|
|
7
|
Lancaster JM, Sayer RA, Blanchette C, et
al: High expression of insulin-like growth factor binding protein-2
messenger RNA in epithelial ovarian cancers produces elevated
preoperative serum levels. Int J Gynecol Cancer. 16:1529–1535.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Y, Ying X, Han S, et al:
Autoantibodies against insulin-like growth factorbinding protein-2
as a serological biomarker in the diagnosis of lung cancer. Int J
Oncol. 42:93–100. 2013.
|
|
9
|
Uzoh CC, Holly JM, Biernacka KM, et al:
Insulin-like growth factor-binding protein-2 promotes prostate
cancer cell growth via IGF-dependent or -independent mechanisms and
reduces the efficacy of docetaxel. Br J Cancer. 104:1587–1593.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hoeflich A, Fettscher O, Preta G, et al:
Increased activity of catalase in tumor cells overexpressing
IGFBP-2. Horm Metab Res. 35:816–821. 2003. View Article : Google Scholar
|
|
11
|
Wheatcroft SB and Kearney MT:
IGF-dependent and IGF-independent actions of IGF-binding protein-1
and -2: implications for metabolic homeostasis. Trends Endocrinol
Metab. 20:153–162. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Biernacka KM, Uzoh CC, Zeng L, et al:
Hyperglycaemia-induced chemoresistance of prostate cancer cells due
to IGFBP2. Endocr Relat Cancer. 20:741–751. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dokmanovic M, Shen Y, Bonacci TM, et al:
Trastuzumab regulates IGFBP-2 and IGFBP-3 to mediate growth
inhibition: implications for the development of predictive
biomarkers for trastuzumab resistance. Mol Cancer Ther. 10:917–928.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Snoeren N, Emmink BL, Koerkamp MJ, et al:
Maspin is a marker for early recurrence in primary stage III and IV
colorectal cancer. Br J Cancer. 109:1636–1647. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hall DC, Johnson-Pais TL, Grubbs B, Bernal
R, Leach RJ and Padalecki SS: Maspin reduces prostate cancer
metastasis to bone. Urol Oncol. 26:652–658. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Machowska M, Wachowicz K, Sopel M and
Rzepecki R: Nuclear location of tumor suppressor protein maspin
inhibits proliferation of breast cancer cells without affecting
proliferation of normal epithelial cells. BMC Cancer. 14:1422014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
18
|
Shariat SF, Milowsky M and Droller MJ:
Bladder cancer in the elderly. Urol Oncol. 27:653–667. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Drayton RM, Dudziec E, Peter S, et al:
Reduced expression of miRNA-27a modulates cisplatin resistance in
bladder cancer by targeting the cystine/glutamate exchanger
SLC7A11. Clin Cancer Res. 20:1990–2000. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Svatek RS, Hollenbeck BK, Holmang S, et
al: The economics of bladder cancer: costs and considerations of
caring for this disease. Eur Urol. 66:253–262. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lu H, Wang L, Gao W, et al: IGFBP2/FAK
pathway is causally associated with dasatinib resistance in
non-small cell lung cancer cells. Mol Cancer Ther. 12:2864–2873.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kühnl A, Kaiser M, Neumann M, et al: High
expression of IGFBP2 is associated with chemoresistance in adult
acute myeloid leukemia. Leuk Res. 35:1585–1590. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fukushima T and Kataoka H: Roles of
insulin-like growth factor binding protein-2 (IGFBP-2) in
glioblastoma. Anticancer Res. 27:3685–3692. 2007.PubMed/NCBI
|
|
24
|
Migita T, Narita T, Asaka R, et al: Role
of insulin-like growth factor binding protein 2 in lung
adenocarcinoma: IGF-independent antiapoptotic effect via caspase-3.
Am J Pathol. 176:1756–1766. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Frommer KW, Reichenmiller K, Schutt BS, et
al: IGF-independent effects of IGFBP-2 on the human breast cancer
cell line Hs578T. J Mol Endocrinol. 37:13–23. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Taskiran C, Erdem O, Onan A, et al: Maspin
expression in endometrial hyperplasia and carcinoma, and its
relation with angiogenesis. Eur J Gynaecol Oncol. 35:134–139.
2014.PubMed/NCBI
|
|
27
|
Romani AA, Soliani P, Desenzani S, et al:
The associated expression of maspin and Bax proteins as a potential
prognostic factor in intrahepatic cholangiocarcinoma. BMC Cancer.
6:2552006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rivenbark AG, Stolzenburg S, Beltran AS,
et al: Epigenetic reprogramming of cancer cells via targeted DNA
methylation. Epigenetics. 7:350–360. 2012. View Article : Google Scholar : PubMed/NCBI
|