Introduction
Heat-shock proteins (HSPs) are induced by a variety
of physiological and environmental stresses, such as heat stress
(1). As molecular chaperones,
HSPs facilitate the refolding of non-native proteins, or assist in
their elimination via chaperone-mediated autophagy or via the
ubiquitin proteasome system. HSPs have recently been classified
into seven families; HSPH (HSP110), HSPC (HSP90), HSPA (HSP70),
HSPD/E (HSP60/HSP10), CCT (TRiC), DNAJ (HSP40) and HSPB (small HSP)
(1,2). HSP27 (HSPB1) belongs to the HSPB
family, with monomeric molecular masses ranging from 15 to 30 kDa.
The primary structure of HSP27 is highly homologous to other small
HSPs, including αB-crystallin and HSP20, which all contain amino
acid sequences known as ʽα-crystallin domains' (3). Although HSP27 is ubiquitously
expressed in human cells and tissues, its functions have mainly
been studied in skeletal, smooth and cardiac muscles, which have
higher expression levels (1,4).
The HSP27 expression level is significantly changed under various
cellular conditions (1). It is
generally recognized that the functions of HSP27 are regulated by
post-translational modifications, such as phosphorylation. HSP27,
which normally exists as an unphosphorylated oligomer, possesses
three phosphorylatable serine residues (Ser-15, Ser-78 and Ser-82)
(5,6). Once HSP27 is phosphorylated, a
conformational change occurs from the aggregated form to the dimer
(5–7). Phosphorylated HSP27 has been
previously shown to suppress the growth of hepatocellular carcinoma
via inhibition of extracellular signal-regulated kinase (8). However, the exact role of
phosphorylated HSP27 has not yet been clarified.
Bone metabolism is strictly regulated through
continuous bone remodeling in order to maintain the structural bone
integrity and mineral homeostasis (9). Bone remodeling is mainly comprised
of two functional events, osteoblastic bone formation and
osteoclastic bone resorption (10). It has been reported that the
outcome for osteosarcoma patients with overexpression of HSP27 is
poorer (11). With regard to the
functions of HSP27 in osteoblasts, it is reportedly involved in the
balance between differentiation and apoptosis (12,13). The HSP27 protein is barely
detectable in osteoblasts under unstimulated conditions (14). In our previous study (15), we demonstrated that the level of
HSP27 is low in unstimulated osteoblast-like MC3T3-E1 cells. In
addition, we reported that various physiological stimuli, including
sphingosine-1-phosphate, are able to induce the expression of HSP27
protein in these cells, and that the induced HSP27 is in the
unphosphorylated form (16,17). Furthermore, unphosphorylated HSP27
has a suppressive effect on the osteocalcin (OC) synthesis induced
by triiodothyronine (T3) in MC3T3-E1 cells (17). However, the mechanisms underlying
the inhibitory effects of HSP27 on osteoblasts remain to be
elucidated.
Evidence is accumulating that HSP27 (HSPB1)
interacts with clients regulating gene expression (18). The signal transducer and activator
of transcription 2 (STAT2) protein, which is a crucial
transcription factor, is reportedly degraded in cells following the
downregulation of HSP27, indicating that HSP27 acts as a regulator
of transcription (19). In
addition, it has been shown that the HSP27-eukaryotic translation
initiation factor 4E (eIF4E) interaction decreases eIF4E
ubiquitination and proteasomal degradation in advanced prostate
cancer cells, and HSP27 has been demonstrated to confer resistance
to androgen ablation and chemotherapy through eIF4E (20). It has been firmly established that
eIF4E is an mRNA cap-binding protein and has a crucial role in
translational control (21,22). eIF4E binds to eIF4G in response to
stimulation, and forms the eIF4F complex with eIF4A during the mRNA
translation process (23). eIF4E
assembles the mRNA into this complex and promotes the ribosome
recruitment. In addition, eIF4E is negatively regulated by
4E-binding protein 1 (4E-BP1), which competes with eIF4G, and under
unstimulated conditions, 4E-BP1 binds to eIF4E by occupying the
same binding site for eIF4G (24). In the present study, in order to
investigate the exact mechanism by which unphosphorylated HSP27
suppresses T3-stimulated OC synthesis in osteoblasts,
the molecular targets of HSP27 were explored using osteoblast-like
MC3T3-E1 cells with HSP27 overexpression. Unphosphorylated HSP27
was shown to associate with eIF4E in osteoblasts and suppress the
translation initiation process.
Materials and methods
Materials
T3 and sphingosine-1-phosphate were
purchased from Sigma-Aldrich (St. Louis, MO, USA). HSP27,
glycer-aldehyde-3-phosphate dehydrogenase (GAPDH) and rabbit
immunoglobulin G (IgG) antibodies were purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). The rabbit monoclonal eIF4E
(#2067), rabbit monoclonal eIF4G (#5169) and rabbit 4E-BP1 (#9452)
antibodies were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA). Wild-type (WT) HSP27 and mutant human HSP27s
subcloned into the pcDNA3.1(+) mammalian expression vector were
kindly provided by Dr C. Schafer (Klinikum Grosshadern,
Ludwig-Maximilians University, Munich, Germany). For mutant HSP27
vectors, the HSP27 cDNAs were mutated from serine residues (Ser-15,
Ser-78 and Ser-82) to alanine (3A) to prevent the phosphorylation
of HSP27, or were mutated to aspartic acid (3D) to imitate the
phosphorylated HSP27 form, as described previously (25). The eukaryotic expression vector,
pcDNA3.1(+), Dynabeads Protein A and TRIzol reagent were purchased
from Life Technologies (Carlsbad, CA, USA). The Omniscript Reverse
Transcriptase kit was purchased from Qiagen (Hilden, Germany).
FastStart DNA Master SYBR-Green I was obtained from Roche
Diagnostics K.K. (Basel, Switzerland). The Bicinchoninic Acid
Protein Assay kit was purchased from Thermo Fisher Scientific, Inc.
(Waltham, MA, USA). An ECL Western Blotting Detection System was
purchased from GE Healthcare UK, Ltd. (Buckinghamshire, UK). Other
materials and chemicals were obtained from commercial sources.
Sphingosine-1-phosphate was dissolved in dimethyl sulfoxide. The
maximum concentration of dimethyl sulfoxide was 0.1%, which did not
affect the assay for OC or the detection of protein expression by a
western blot analysis.
Cell culture
Cloned osteoblast-like MC3T3-E1 cells derived from
newborn mouse calvaria (26) were
maintained as described previously (27). Briefly, the cells were cultured in
α-minimum essential medium (α-MEM) containing 10% fetal bovine
serum (FBS) at 37°C in a humidified atmosphere of 5%
CO2/95% air. The cells were seeded in 35-mm dishes
(5×104 cells/dish) in α-MEM containing 10% FBS. After 5
days, the medium was exchanged for α-MEM containing 0.3% FBS. The
cells were used for experiments after 48 h.
Transient transfections
For transient transfections, the MC3T3-E1 cells were
seeded in 35-mm dishes (1.5×104 cells/dish) in α-MEM
containing 10% FBS. After 4 days, the cultured cells were
transfected with 1 µg of the WT HSP27 plasmid or control
(empty) pcDNA3.1(+) vector using the UniFECTOR transfection reagent
(B-Bridge International, Mountain View, CA, USA) in 1 ml of α-MEM
medium without FBS. Five hours after transfection, 1 ml of medium
with α-MEM containing 0.6% FBS was added. At 3 days after
transfection, the medium was removed, cells were washed with 2 ml
of α-MEM medium without FBS, and were incubated in α-MEM with 0.3%
FBS for co-immunoprecipitation or reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
experiments. The cells were subsequently cultured for another 6
h.
Establishment of stable HSP27-transfected
cells
The stable HSP27-transfected cells were established
as described previously (17).
Briefly, the MC3T3-E1 cells (5×105 cells) were
transfected with 2 µg of the WT, mutant 3A or 3D HSP27
plasmids expressing geneticin (G418; EMD Chemicals, Inc., San
Diego, CA, USA) resistance using UniFECTOR transfection reagent in
α-MEM without FBS, and were incubated in the presence of 400
µg/ml of G418. The transfected cells were seeded in 35-mm
dishes (5×104 cells/dish) in α-MEM containing 10% FBS
and 200 µg/ml of G418. After 5 days, the medium was
exchanged for α-MEM containing 0.3% FBS and 200 µg/ml of
G418. The cells were used for experiments after 48 h.
Protein preparation
For co-immunoprecipitation studies, the cultured
MC3T3-E1 cells or transfected cells were lysed in ice-cold TNE
lysis buffer [10 mM Tris/HCl (pH 7.8), 1% Nonidet P-40, 150 mM
NaCl, 1 mM EDTA, 1 mM dithiothreitol, 1 mM sodium fluoride, 1 mM
sodium vanadate and protease inhibitor cocktail (Roche Diagnostics
K.K.)]. The lysates were subsequently centrifuged at 10,000 × g at
4°C for 30 min, and the supernatant was collected as TNE soluble
protein. For the western blot analysis of HSP27, eIF4E, eIF4G,
4E-BP1 or IgG, the cultured MC3T3-E1 cells were pre-treated with 30
µM of sphingosine-1-phosphate or vehicle for 6 h to induce
HSP27, and were stimulated by 3 nM of T3 or vehicle for
24 h. The HSP27 cDNA-transfected cells were stimulated by 3 nM of
T3 or vehicle for 24 h. Following stimulation, the cells
were lysed, homogenized and sonicated in lysis buffer containing
62.5 mM Tris/HCl (pH 6.8), 2% sodium dodecyl sulfate, 50 mM
dithiothreitol and 10% glycerol.
Co-immunoprecipitation
Co-immunoprecipitation was performed as described
previously (28). Briefly, the
indicated antibodies were added to the TNE-solubilized proteins,
and the mixture was agitated gently overnight at 4°C, followed by
the addition of Dynabeads Protein A and incubation for a further 1
h with continuous mixing. Protein immunocomplexes were isolated
using a magnetic particle concentrator. The immunoprecipitated
proteins and the TNE-soluble proteins (for analysis of the total
protein) were analyzed by western blot analysis.
RT-qPCR
The cultured MC3T3-E1 cells were pre-treated with 30
µM of sphingosine-1-phosphate or vehicle for 6 h to induce
HSP27, and were stimulated by 3 nM of T3 or vehicle in
α-MEM containing 0.3% FBS for 3 h. Transiently HSP27-overexpressing
cells were stimulated by 3 nM of T3 or vehicle in α-MEM
containing 0.3% FBS for 6 h. Total RNA was isolated and transcribed
into complementary DNA using the TRIzol reagent and an Omniscript
Reverse Transcriptase kit, respectively. RT-qPCR was performed
using a LightCycler system in capillaries and the FastStart DNA
Master SYBR-Green I provided with the kit. Sense and antisense
primers were synthesized based on the report by Zhang et al
(29) for mouse OC mRNA and
Simpson et al (30) for
mouse GAPDH mRNA. The amplified products were determined using a
melting curve analysis and agarose electrophoresis. The OC mRNA
levels were normalized to those of GAPDH mRNA.
Western blot analysis
A western blot analysis was performed as described
previously (17). SDS-PAGE of the
prepared cell lysates was performed by the method described by
Laemmli (31) in 10%
polyacrylamide gels. The protein was fractionated and transferred
onto an Immun-Blot polyvinylidene difluoride (PVDF) membrane
(Bio-Rad, Hercules, CA, USA), and a western blot analysis was
performed using the indicated primary antibodies with
peroxidase-labeled antibodies as secondary antibodies. The primary
and secondary antibodies were diluted at 1:1,000 with 5% skimmed,
dried milk in TBST. The peroxidase activity on the PVDF membranes
was visualized on X-ray film by means of the ECL Western Blotting
Detection System.
Statistical analysis
The data were analyzed by analysis of variance
followed by the Bonferroni method for multiple comparisons between
pairs, and P<0.05 was considered to indicate a statistically
significant difference. All the data are presented as the means ±
standard error of the mean of triplicate determinations from three
independent cell preparations.
Results
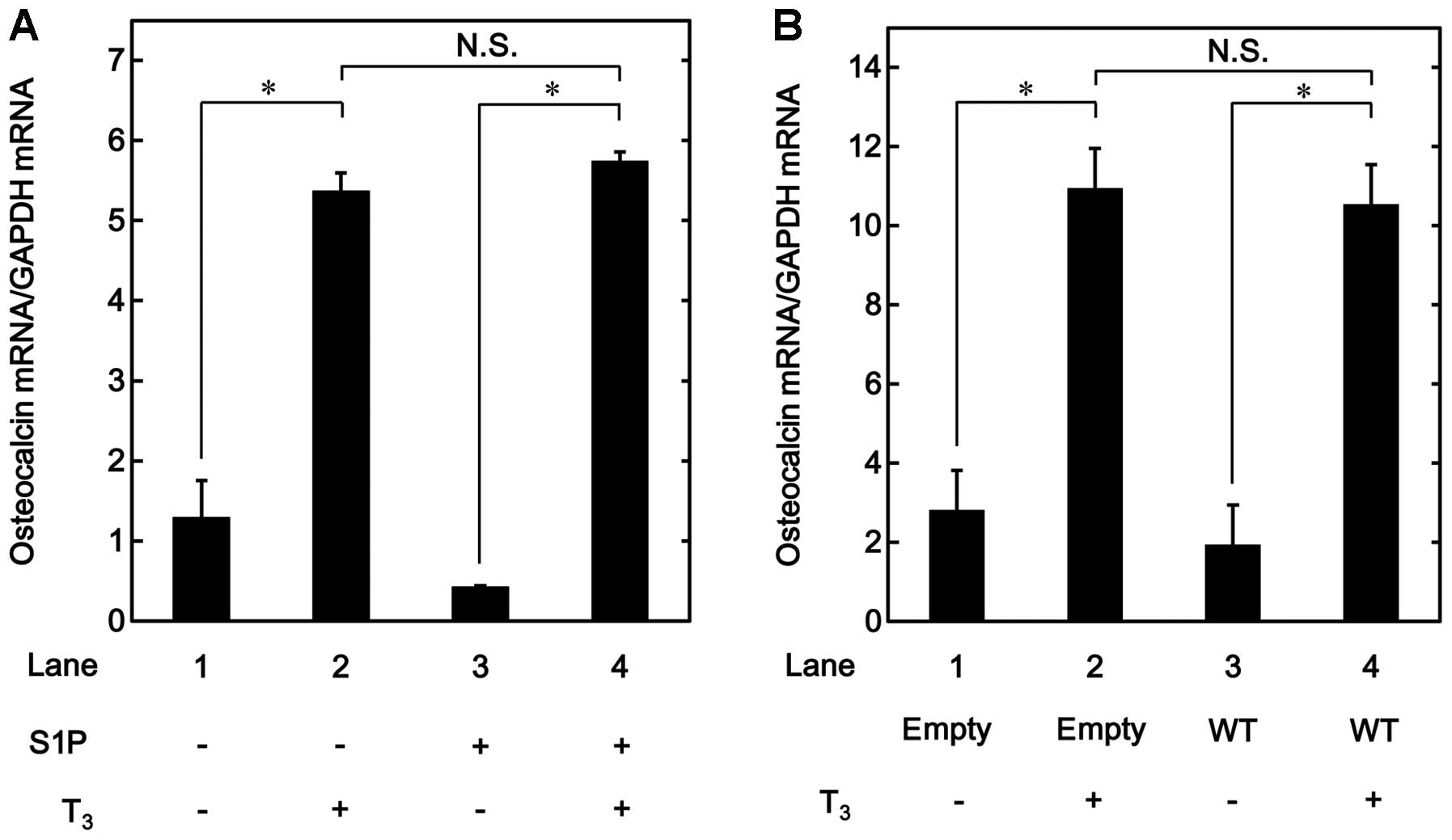
Effects of T3 on the OC mRNA
expression in the sphingosine-1-phosphate-treated MC3T3-E1 cells
and the HSP27-overexpressing cells
Our previous study reported that unphosphorylated
HSP27 has an inhibitory effect on the OC synthesis induced by
T3 in osteoblast-like MC3T3-E1 cells (17). In order to investigate whether the
suppressive effect of unphosphorylated HSP27 on
T3-stimulated OC release is due to a transcriptional
event, the effect of T3 on OC mRNA expression was
examined in the MC3T3-E1 cells treated with
sphingosine-1-phosphate, which is a physiological inducer of
unphosphorylated HSP27 protein expression in these cells, as
described in our previous studies (16,17). Sphingosine-1-phosphate, which
alone had a limited effect on the levels of OC mRNA, did not affect
the increase in the OC mRNA levels stimulated by T3
(Fig. 1A).
The effects of T3 on OC mRNA expression
were investigated in the HSP27-overexpressing MC3T3-E1 cells, which
were transiently transfected with a WT HSP27 plasmid. There were no
significant differences between the HSP27-overexpressing cells and
the control cells in terms of the mRNA expression levels of OC
stimulated by T3 (Fig.
1B). Our previous study demonstrated that HSP27 in the
HSP-overexpressing cells exists in an unphosphorylated form
(17).
HSP27 interacts with eIF4E, but not eIF4G
or 4E-BP1, in MC3T3-E1 cells with sphingosine-1-phosphate
treatment
It is generally established that 4E-BP1 competes
with eIF4G and regulates the association of eIF4E and eIF4G to form
the active component for the initiation of protein translation
(24). HSP27 reportedly interacts
with eIF4E, thus resulting in the prevention of eIF4E
ubiquitination and proteasomal degradation in advanced prostate
cancer cells (20). In addition,
it has been shown that HSP27 prevents translation by binding to
eIF4G (32). These findings led
us to hypothesize that HSP27 regulates the process of translation
in osteoblasts. In order to clarify the involvement of HSP27 in the
translation initiation process in osteoblast-like MC3T3-E1 cells,
whether HSP27 interacts with these molecules, including eIF4E,
eIF4G and 4E-BP1, was examined. Although the eIF4E protein was
co-immunoprecipitated with 4E-BP1 in the unstimulated MC3T3-E1
cells, in which HSP27 was hardly detected, it was not
co-immunoprecipitated with HSP27 or eIF4G. By contrast, eIF4E was
co-immunoprecipitated with HSP27 in addition to 4E-BP1, but not
eIF4G, in the sphingosine-1-phosphate-treated cells (Fig. 2A). eIF4G was not
co-immunoprecipitated with HSP27, eIF4E or 4E-BP1 in MC3T3-E1 cells
with or without sphingosine-1-phosphate treatment (Fig. 2B). The 4E-BP1 protein was
co-immunoprecipitated with eIF4E, but not with HSP27 or eIF4G in
these cells treated with or without sphingosine-1-phosphate
(Fig. 2C). Additionally, the
HSP27 protein was co-immunoprecipitated with eIF4E, but not with
eIF4G or 4E-BP1, in MC3T3-E1 cells treated with
sphingo-sine-1-phosphate, whereas it was not co-immunoprecipitated
with eIF4E, eIF4G or 4E-BP1 in the unstimulated MC3T3-E1 cells
(Fig. 2D). Furthermore, HSP27,
eIF4E, eIF4G and 4E-BP1 were not co-immunoprecipitated with normal
rabbit IgG (Fig. 2E).
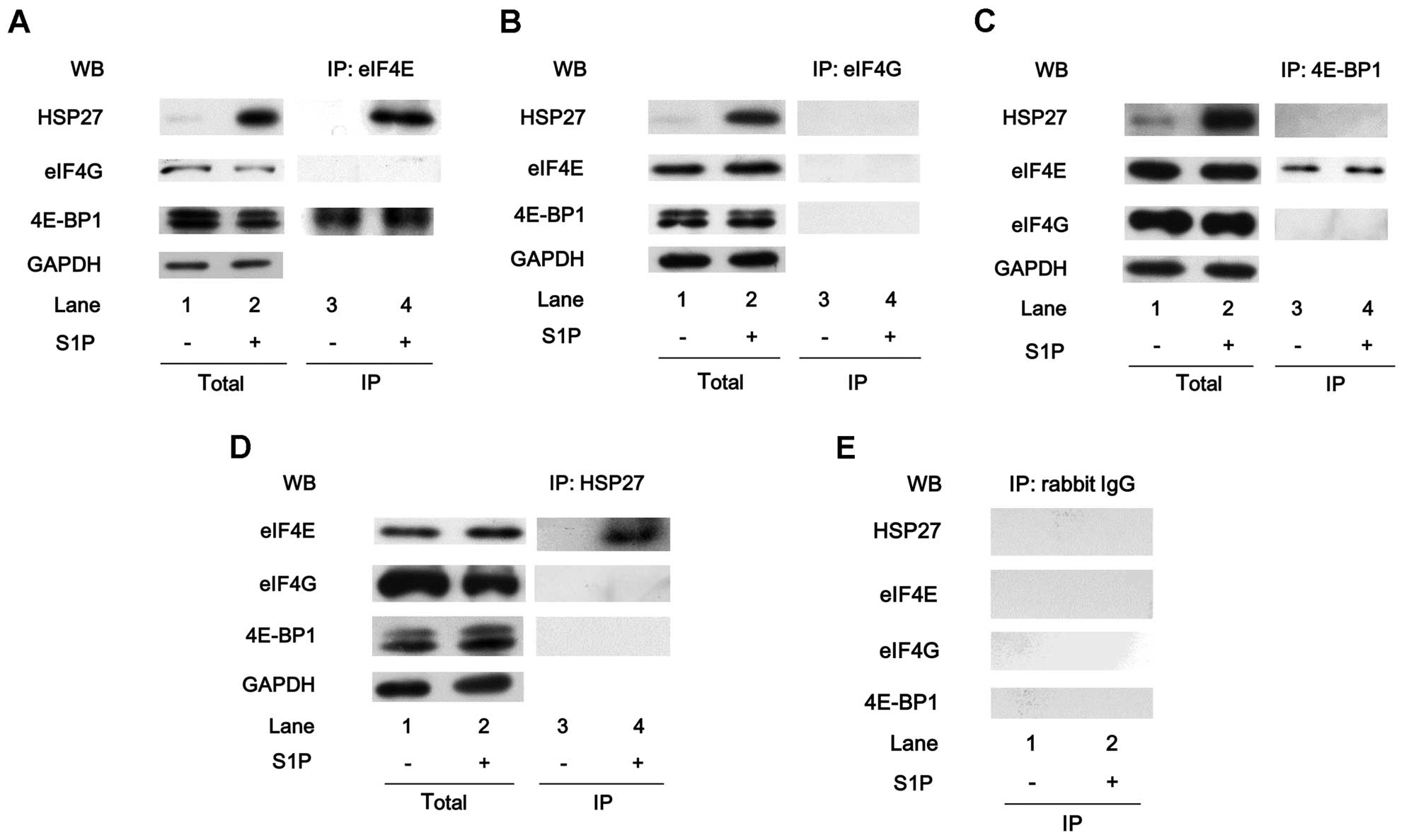 | Figure 2Heat-shock protein 27 (HSP27)
interacts with eukaryotic translation initiation factor 4E (eIF4E),
but not eIF4G or 4E-binding protein 1 (4E-BP1), in MC3T3-E1 cells
with sphingosine-1-phosphate (S1P) treatment. The cultured cells
were pre-treated with 30 µM of S1P or vehicle for 6 h. (A)
The expression levels of HSP27, eIF4G, 4E-BP1 and
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in the
pre-immunoprecipitated cell lysates of the cells pre-treated with
S1P or vehicle were determined by western blot analysis (total).
The cell lysates were immunoprecipitated with eIF4E antibodies,
followed by western blot analysis using antibodies for HSP27, eIF4G
and 4E-BP1, respectively. (B) The expression levels of HSP27,
eIF4E, 4E-BP1 and GAPDH in the pre-immunoprecipitated cell lysates
of the cells pre-treated with S1P or vehicle were determined by
western blot analysis (total). The cell lysates were
immunoprecipitated with eIF4G antibodies, followed by western blot
analysis using antibodies for HSP27, eIF4E and 4E-BP1,
respectively. (C) The expression levels of HSP27, eIF4E, eIF4G and
GAPDH in the pre-immunoprecipitated cell lysates of the cells
pre-treated with S1P or vehicle were determined by a western blot
analysis (total). The cell lysates were immunoprecipitated with
4E-BP1 antibodies, followed by western blot analysis using
antibodies for HSP27, eIF4E and eIF4G, respectively. (D) The
expression levels of eIF4E, eIF4G, 4E-BP1 and GAPDH in the
pre-immunoprecipitated cell lysates of the cells pre-treated with
S1P or vehicle were determined by a western blot analysis (total).
The cell lysates were immunoprecipitated with HSP27 antibodies,
followed by western blot analysis using antibodies for eIF4E, eIF4G
and 4E-BP1, respectively. (E) The cell lysates were
immunoprecipitated with normal rabbit IgG, followed by western blot
analysis using antibodies for HSP27, eIF4E, eIF4G and 4E-BP1,
respectively. IP, immunoprecipitated; WB, western blot
analysis. |
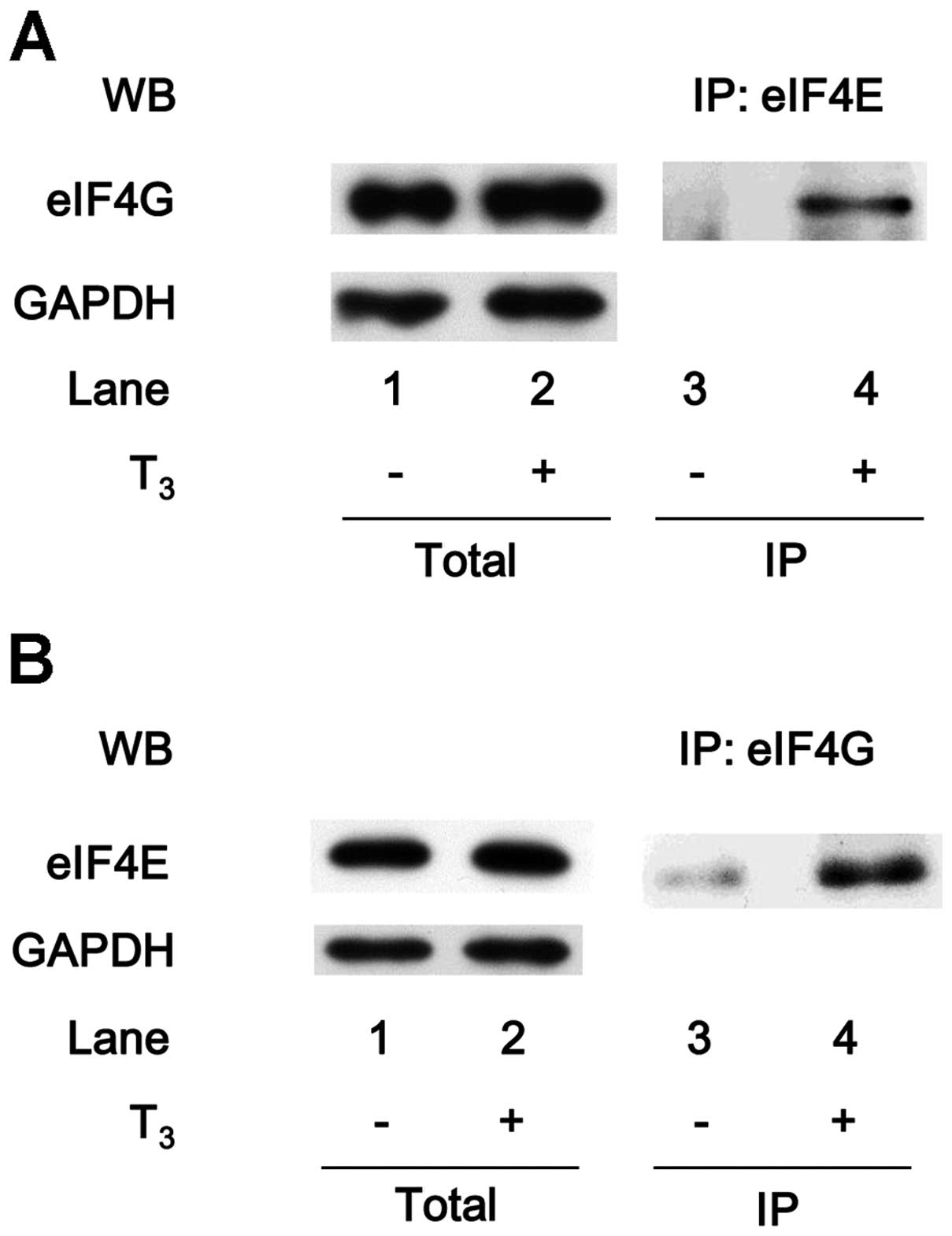
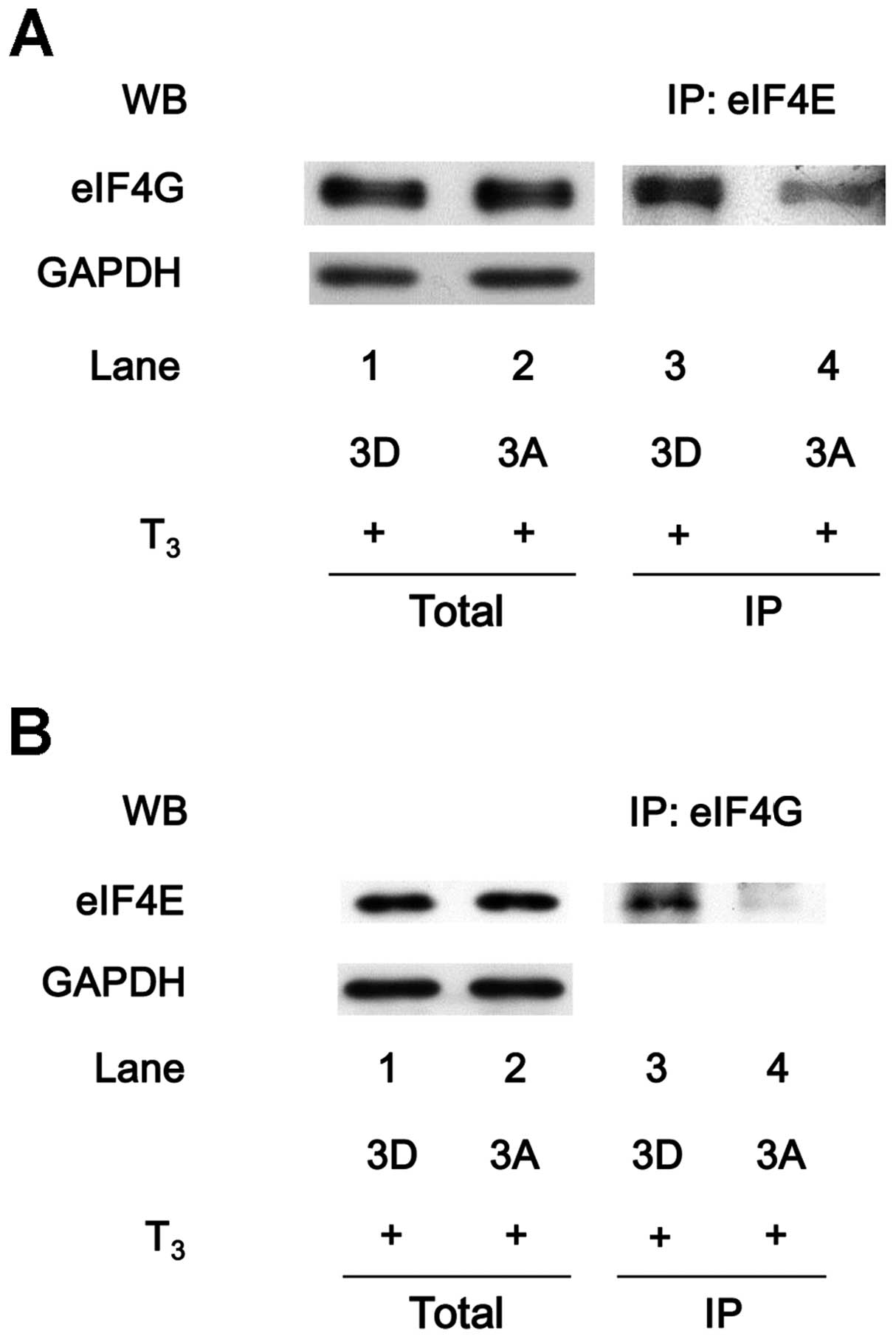
Association of eIF4E with eIF4G under
T3 stimulation in MC3T3-E1 cells
It is well recognized that eIF4E binds to 4E-BP1
under the unstimulated condition, and 4E-BP1 competes with eIF4G
for a single binding site on eIF4E (24). In response to stimulation, eIF4E
binds to the mRNA 5′ cap structure, mediating the initiation of
translation. eIF4E subsequently separates from 4E-BP1 and interacts
with eIF4G, which serves as a scaffold protein for the assembly of
eIF4E and eIF4A to form the eIF4F complex (23,24). eIF4E was co-immunoprecipitated
with eIF4G in the T3-stimulated MC3T3-E1 cells (Fig. 3A). In addition, eIF4G was
co-immunoprecipitated with eIF4E in the T3-stimulated
cells (Fig. 3B).
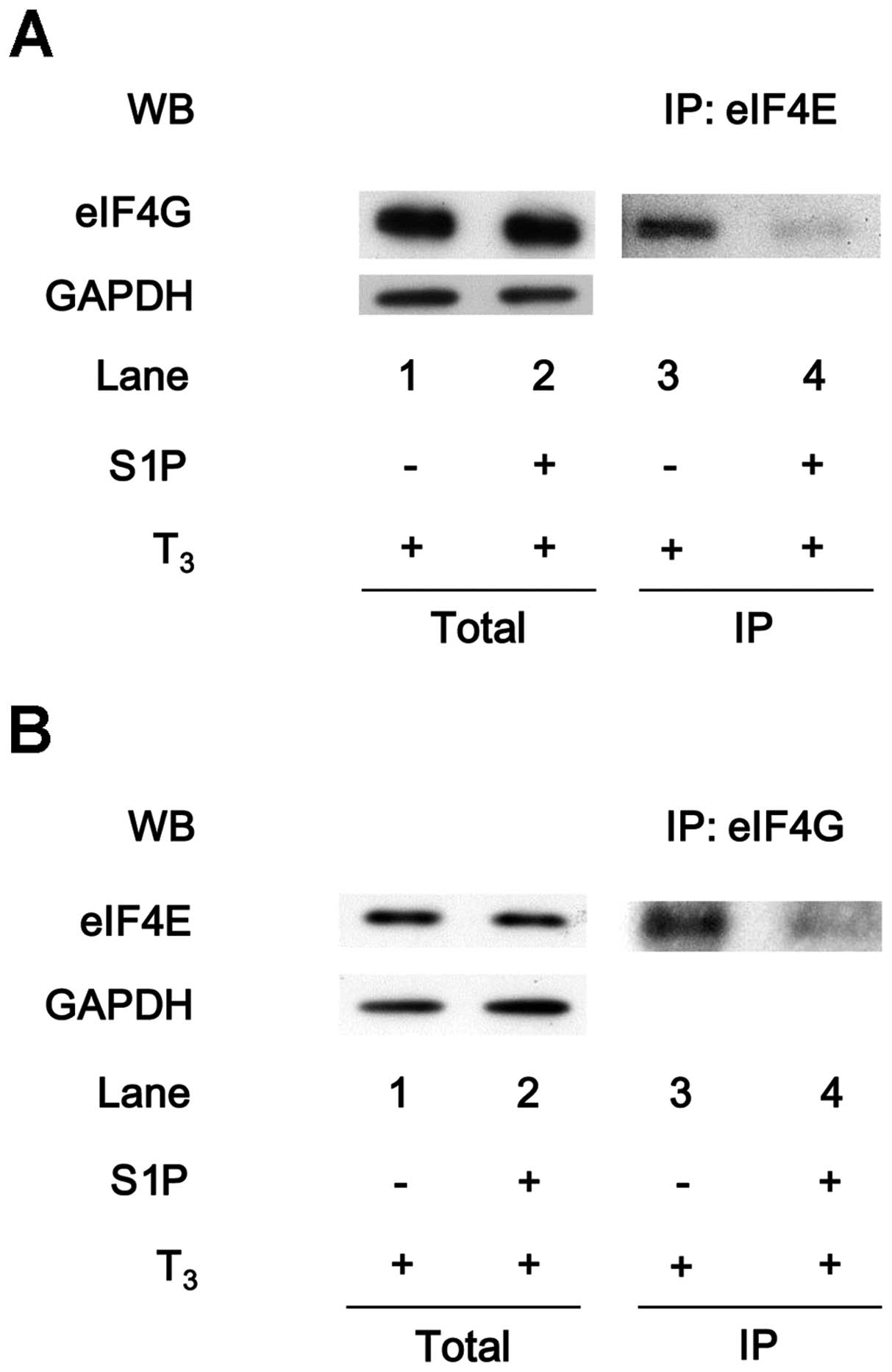
Suppression by sphingosine-1-phosphate of
the T3-induced association of eIF4E with eIF4G in
MC3T3-E1 cells
To clarify the effects of HSP27 on translation in
osteoblast-like MC3T3-E1 cells, the effect of
sphingosine-1-phosphate on the T3-induced binding of
eIF4E with eIF4G was examined. As presented in Fig. 3A, the eIF4E protein was
co-immunoprecipitated with eIF4G in the T3-stimulated
cells. The levels of eIF4E co-immunoprecipitated with eIF4G were
markedly reduced in the sphingosine-1-phosphate-treated cells
(Fig. 4A). Additionally, the
amount of eIF4G protein that co-immunoprecipitated with eIF4E under
T3 stimulation was decreased by sphingosine-1-phosphate
treatment (Fig. 4B).
HSP27 associates with eIF4E in the
HSP27-overexpressing MC3T3-E1 cells
The interaction between HSP27 and translational
molecules, such as eIF4E, eIF4G and 4E-BP1, were also examined in
the HSP27-overexpressing cells. The expression levels of eIF4E,
eIF4G and 4E-BP1 were not different between the
HSP27-overexpressing MC3T3-E1 cells and the control cells (Fig. 5A, lanes 1 and 2). In addition, the
HSP27 in the cells transiently transfected with WT HSP27 cDNA was
an unphosphorylated form (data not shown). The HSP27 protein was
co-immunoprecipitated with eIF4E, but not with eIF4G or 4E-BP1, in
the HSP27-overexpressing cells (Fig.
5A, lane 4), whereas eIF4E, eIF4G and 4E-BP1 were not
co-immunoprecipitated with HSP27 in the control cells (Fig. 5A, lane 3). By contrast, the eIF4E
protein in the HSP27-overexpressing cells was co-immunoprecipitated
with HSP27 and 4E-BP1 (Fig. 5B,
lane 4).
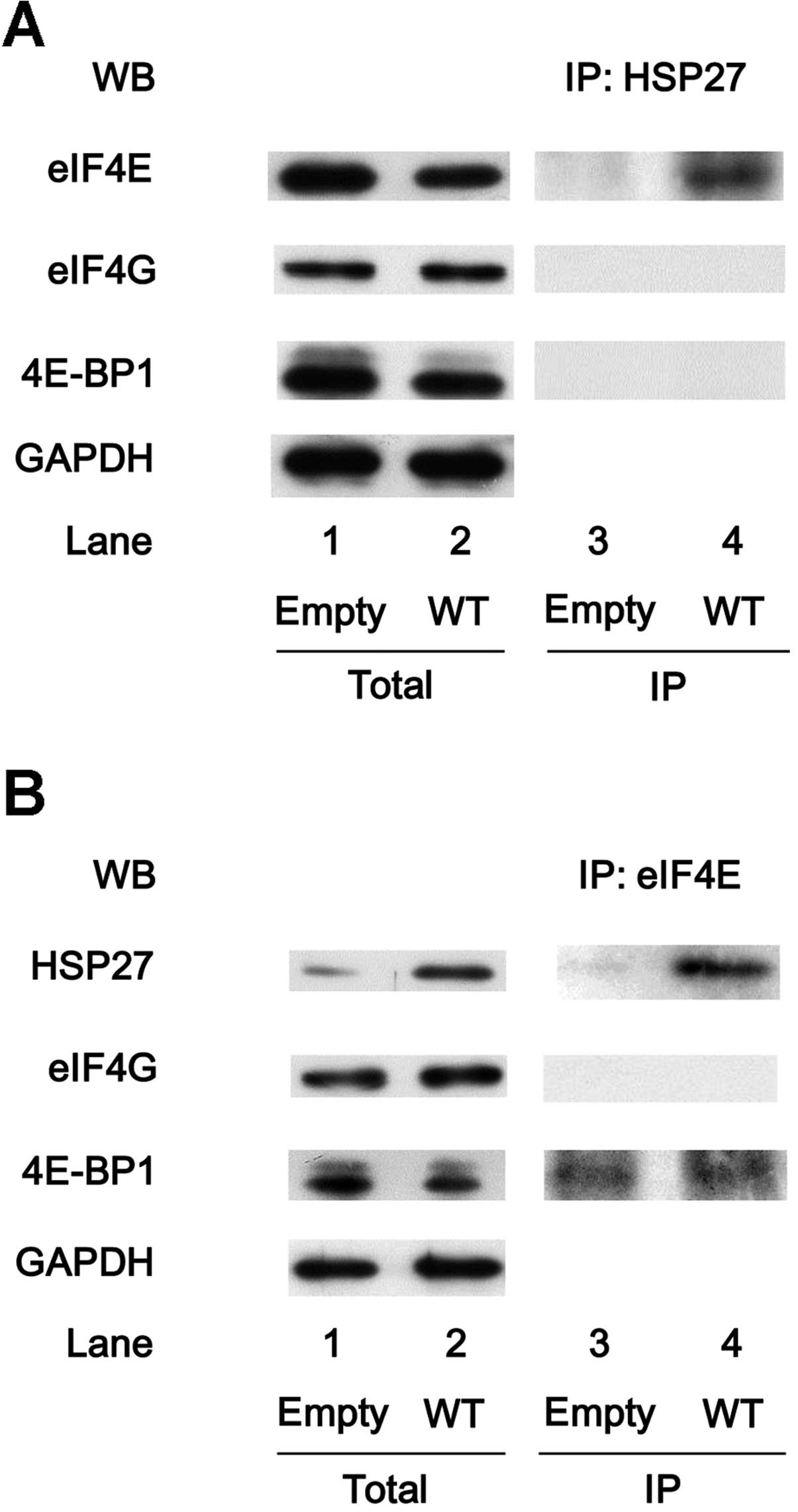 | Figure 5Heat-shock protein 27 (HSP27)
associates with eukaryotic translation initiation factor 4E (eIF4E)
in the HSP27-overexpressing MC3T3-E1 cells. The cultured cells were
transiently transfected with the control empty vector (empty) or
the wild-type (WT) HSP27 vector. (A) The expression levels of
eIF4E, eIF4G, 4E-binding protein 1 (4E-BP1) and
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in the
pre-immunoprecipitated cell lysates of the control cells or WT
HSP27-overexpressing cells were determined by western blot analysis
(total). The cell lysates were immunoprecipitated with HSP27
antibodies, followed by western blot analysis using antibodies for
eIF4E, eIF4G and 4E-BP1, respectively. (B) The expression levels of
HSP27, eIF4G, 4E-BP1 and GAPDH in the pre-immunoprecipitated cell
lysates of the control cells or the WT HSP27-overexpressing cells
were determined by western blot analysis (total). The cell lysates
were immunoprecipitated with eIF4E antibodies, followed by western
blot analysis using antibodies for HSP27, eIF4G and 4E-BP1,
respectively. IP, immunoprecipitated; WB, western blot
analysis. |
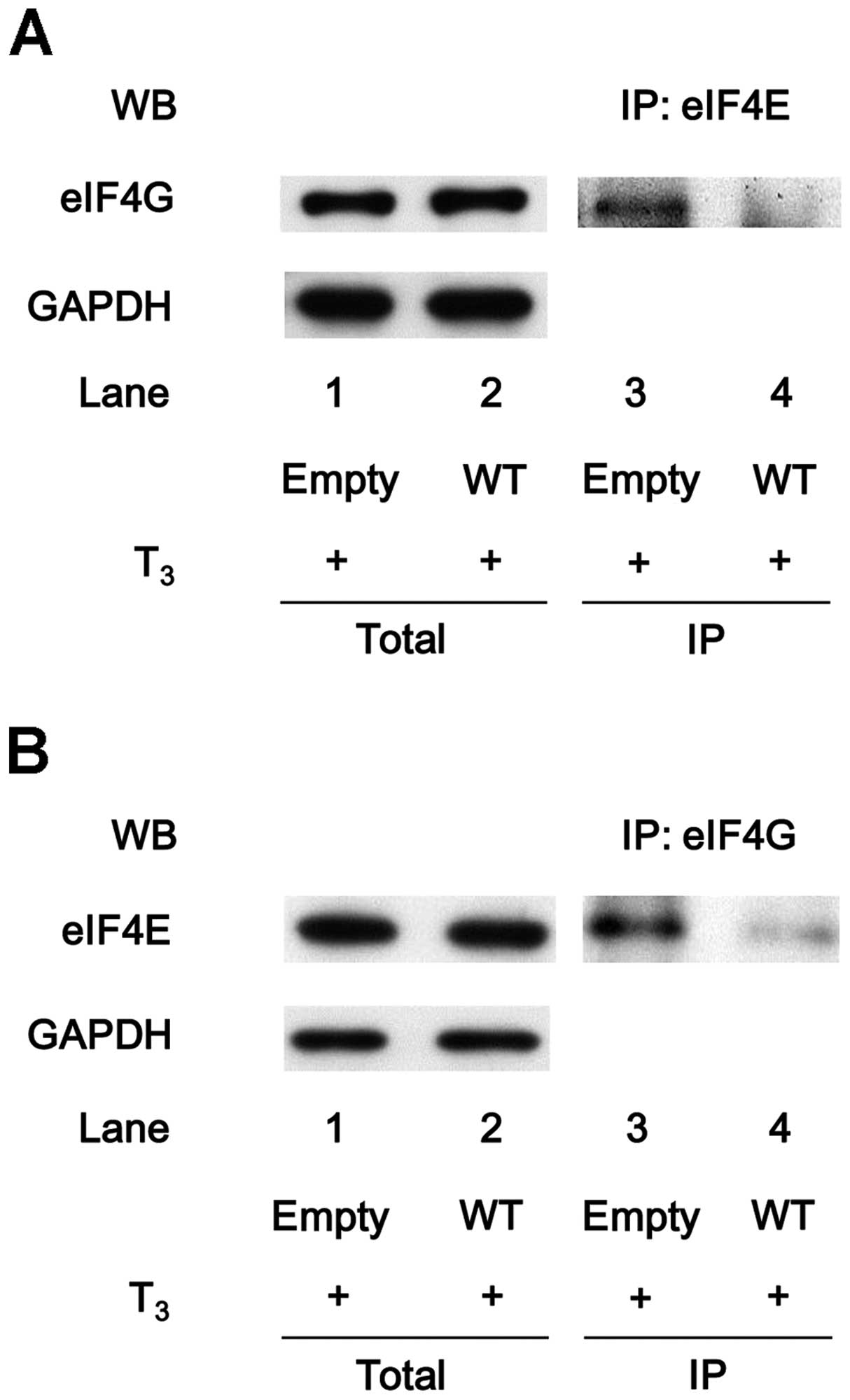
eIF4E does not associate with eIF4G in
the HSP27-over-expressing MC3T3-E1 cells with T3
stimulation
The association of eIF4E with eIF4G in the
HSP27-overexpressing MC3T3-E1 cells under T3 stimulation
was further investigated. However, the levels of eIF4E protein
co-immunoprecipitated with eIF4G were markedly attenuated in the
T3-stimulated WT HSP27-transfected cells compared with
the T3-stimulated empty vector-transfected cells
(Fig. 6A). In addition, the
levels of eIF4G co-immunoprecipitated with eIF4E were decreased in
the WT HSP27-transfected cells under the condition of T3
stimulation (Fig. 6B).
Suppression of the T3-induced
association of eIF4E with eIF4G in the unphosphorylatable
HSP27-overexpressing MC3T3-E1 cells
It is well known that HSP27 undergoes different
types of post-translational modifications, such as phosphorylation
(1). HSP27 is phosphorylated at
three sites (Ser-15, Ser-78 and Ser-82) (5). Previously, we reported that p38
mitogen-activated protein (MAP) kinase is involved in the
phosphorylation of HSP27 in the sphingosine-1-phosphate-treated
MC3T3-E1 cells (17). Using two
stable mutant HSP27-transfected MC3T3-E1 cell lines, the 3A and the
3D HSP27-overexpressing cells, which mimic the unphosphorylated and
phosphorylated status of HSP27, respectively, our previous study
demonstrated that the T3-stimulated OC release is
significantly decreased in the 3A HSP27-overexpressing cells
compared with that in the 3D HSP27-overexpressing cells (17). Therefore, to clarify whether the
inhibitory effect of unphosphorylated HSP27 on the
T3-stimulated OC synthesis is due to the suppression of
the association of eIF4E with eIF4G, the binding of eIF4E and eIF4G
under stimulation by T3 in these two types of mutant
HSP27-transfected MC3T3-E1 cells was examined. The eIF4E protein
was co-immunoprecipitated with eIF4G in the 3D HSP27-overexpressing
cells stimulated by T3 (Fig. 7A, lane 3). However, under
T3 stimulation, the levels of eIF4E
co-immunoprecipitated with eIF4G were markedly lower in the 3A
HSP27-overexpressing cells than those in the 3D
HSP27-overexpressing cells (Fig.
7A, lane 4). Furthermore, the levels of eIF4G
co-immunoprecipitated with eIF4E were decreased in the
T3-stimulated 3A HSP27-overexpressing cells in
comparison with those in the T3-stimulated 3D
HSP27-overexpressing cells (Fig.
7B).
Discussion
In the present study, the molecular targets of HSP27
were investigated using osteoblast-like MC3T3-E1 cells, on the
basis of our previous study, which showed that unphosphorylated,
but not phosphorylated, HSP27 acts as a negative regulator in
T3-induced OC synthesis in these cells (17). Therefore, whether the OC mRNA
expression levels stimulated by T3 are affected in
MC3T3-E1 cells in which HSP27 expression is induced was examined.
The T3-stimulated OC mRNA expression levels were hardly
affected in the MC3T3-E1 cells pre-treated with
sphingosine-1-phosphate, a physiological sphingomyelin metabolite,
which is capable of inducing the unphosphorylated form of HSP27 in
these cells, compared with the cells without
sphingosine-1-phosphate pre-treatment. In addition, the expression
levels of OC mRNA induced by T3 did not show any
significant differences between the WT HSP27-transfected cells and
the empty vector-transfected cells. The HSP27 in the
HSP27-overexpressing MC3T3-E1 cells exists in an unphosphorylated
form. Based on these findings, it appears unlikely that the
suppressive effects of unphosphorylated HSP27 on the
T3-induced OC synthesis are exerted at a point upstream
of transcription in osteoblast-like MC3T3-E1 cells.
The association between HSP27 and molecules involved
in translation, including eIF4E, eIF4G and 4E-BP1, was
investigated. During the mRNA translation process, eIF4E has a
crucial role as the mRNA cap-binding protein (21). In response to stimulation, eIF4E
binds to the mRNA 5′ cap structure and forms the eIF4F complex with
its cofactors, eIF4G and eIF4A. The eIF4F complex contributes to
the ribosomal recruitment of mRNA, the rate-limiting step in
translation initiation (21–23). The eIF4E protein was
co-immunoprecipitated with 4E-BP1, but not with eIF4G, in the
unstimulated MC3T3-E1 cells without HSP27 induction by
sphingosine-1-phosphate. In the unstimulated cells with
sphingosine-1-phosphate pre-treatment, eIF4E was
co-immunoprecipitated with not only 4E-BP1, but also HSP27.
Additionally, the HSP27 protein was markedly co-immunoprecipitated
with eIF4E, but not with eIF4G or 4E-BP1, and 4E-BP1 was
co-immunoprecipitated with eIF4E, but not HSP27. Furthermore, a
similar phenomenon was observed regarding the interaction of HSP27
with eIF4E, eIF4G and 4E-BP1 in the MC3T3-E1 cells that had been
transfected with the WT HSP27 vector. Thus, it is possible that
HSP27 may bind to eIF4E instead of 4E-BP1. Therefore, these
findings suggest that eIF4E exists as two independent forms, a
4E-BP1- and a HSP27-associated form, in unstimulated MC3T3-E1 cells
with HSP27 induction, whereas eIF4E exists as one form, a
4E-BP1-associated form, in the cells without HSP27 induction.
It is firmly established that 4E-BP1 separates from
eIF4E, and subsequently eIF4G binds to eIF4E at the same binding
site as 4E-BP1 under the stimulated condition (24). The association of eIF4E with eIF4G
in osteoblast-like MC3T3-E1 cells was found to increase in response
to T3 stimulation. In order to clarify the role of HSP27
in the initiation of translation in osteoblast-like MC3T3-E1 cells,
the binding of eIF4E and eIF4G in the T3-stimulated
cells with sphingosine-1-phosphate pre-treatment was examined. The
levels of eIF4E co-immunoprecipitated with eIF4G were markedly
attenuated in the MC3T3-E1 cells pre-treated with
sphingosine-1-phosphate. The association of eIF4E with eIF4G in the
T3-stimulated MC3T3-E1 cells transfected with the WT
HSP27 vector was further investigated, and the levels of eIF4E
co-immunoprecipitated with eIF4G in these cells were much weakened
compared with those in the empty vector-transfected cells. Our
previous study showed that the HSP27 in the MC3T3-E1 cells
transfected with WT HSP27 vector is an unphosphorylated form
(17). Taking all these findings
into account, it is most likely that unphosphorylated HSP27
attenuates eIF4E-eIF4G binding under T3 stimulation,
resulting in the downregulation of the translation initiation
process in osteoblasts.
HSP27 (HSPB1) is currently known to undergo several
types of post-translational modifications, including
phosphorylation, suggesting that the modifications alter the HSP27
functions (5). Therefore, whether
the phosphorylation of HSP27 affects its binding to eIF4E in
osteoblast-like MC3T3-E1 cells was investigated. Our previous study
reported that HSP27 is phosphorylated via p38 MAP kinase activation
in sphingosine-1-phosphate-induced MC3T3-E1 cells (17). Using two mutant HSP27-transfected
MC3T3-E1 cell lines, unphosphorylatable HSP27-overexpressing cells
(3A) and phospho-mimic HSP27-overexpressing cells (3D), eIF4E was
markedly co-immunoprecipitated with eIF4G in 3D cells stimulated by
T3, whereas the levels of eIF4E co-immunoprecipitated
with eIF4G were reduced in the T3-stimulated 3A cells.
Our previous study reported that the OC release induced by
T3 in 3A cells is suppressed in comparison with that in
the 3D cells (17). Taking these
results into account, it is most likely that unphosphorylated, but
not phosphorylated, HSP27 associates with eIF4E under the
unstimulated condition in osteoblast-like MC3T3-E1 cells, and that
the formation of the HSP27-eIF4E complex downregulates the
translation initiation process under stimulation via suppression of
the eIF4E-eIF4G interaction. It is possible that the induction of
HSP27 and its phosphorylation have a critical role in the
translation process of T3-stimulated OC synthesis in
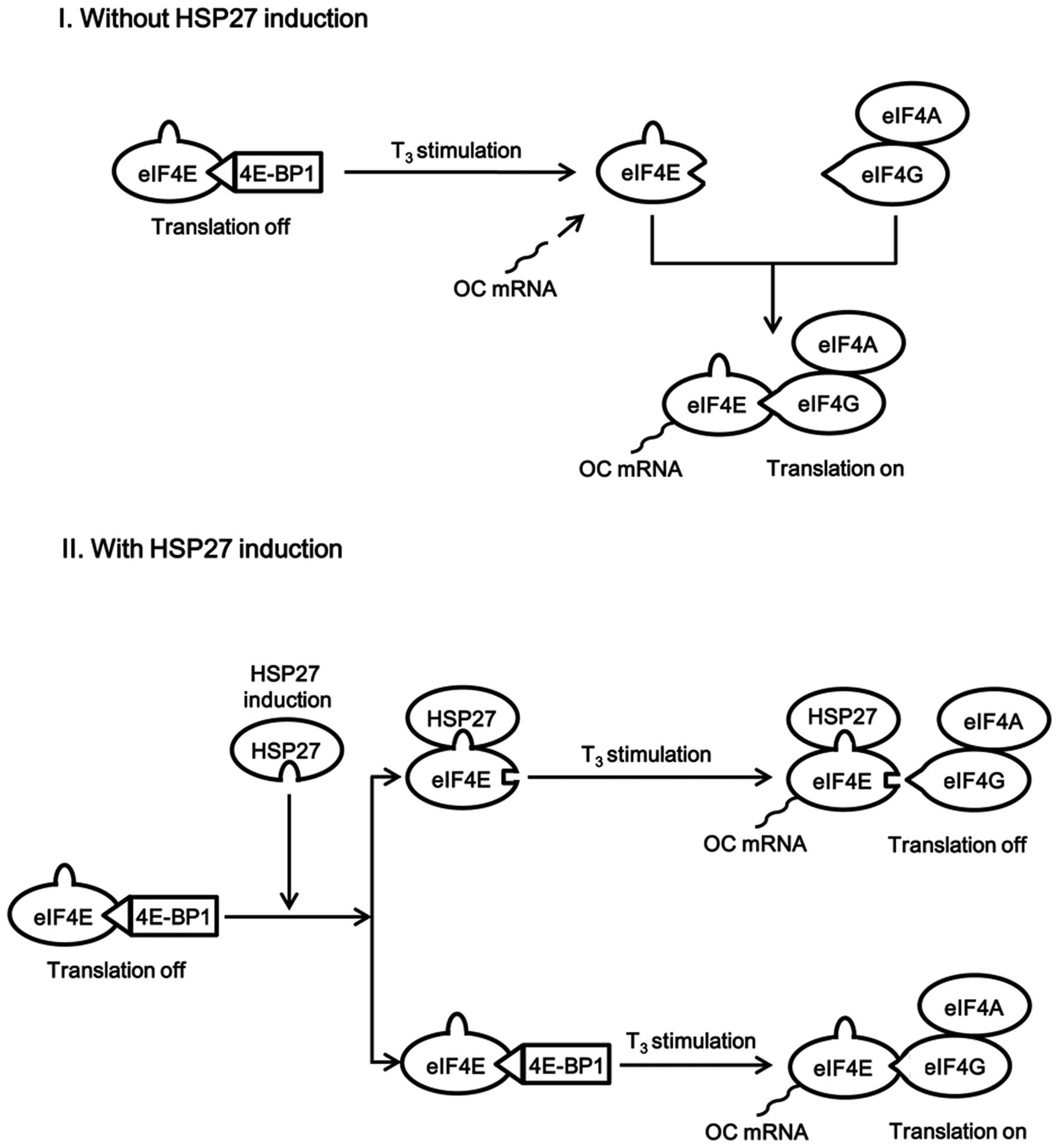
osteoblast-like MC3T3-E1 cells. A schematic illustration of the
potential mechanism underlying these effects of HSP27 is presented
in Fig. 8.
It is well known that HSP27 normally exists in an
aggregated form, but when it is phosphorylated, a conformational
change occurs that leads to the formation of dimers (5–7).
Our previous study demonstrated that HSP27 reduces the release of
vascular endothelial growth factor (VEGF) induced by TGF-β via a
post-transcriptional mechanism in osteoblast-like MC3T3-E1 cells in
which HSP27 expression is induced (33). In addition, phosphorylated HSP27
changes its localization from the cytosol to the perinuclear region
in these cells, and acts as a functional regulator of the
endoplasmic reticulum, contributing to the modulation of
T3-induced OC synthesis (17). Taking all of these findings into
account, it is possible that the change in the conformation or
localization of HSP27 by its phosphorylation contributes to the
regulation of the translation initiation process, thereby affecting
the synthesis of proteins, such as OC and VEGF in osteoblasts.
Phosphorylated HSP27 is reportedly involved in the pathogenesis of
conditions, such as diabetic kidney disease and viral infections
(34,35). Further investigation is necessary
to clarify the precise role of HSP27 and its phosphorylation status
in osteoblasts.
In conclusion, the present results strongly suggest
that the phosphorylation status of HSP27 has a role in switching
its binding to eIF4E, resulting in regulation of the translation
initiation process in osteoblasts.
Acknowledgments
The authors are grateful to Dr Yumiko Kurokawa for
her skillful technical assistance. The present study was supported
in part by a Grant-in-Aid for Scientific Research (no. 19591042)
from the Ministry of Education, Culture, Sports, Science and
Technology of Japan and the Research Funding for Longevity Sciences
(nos. 23-9 and 25-4) from the National Center for Geriatrics and
Gerontology (NCGG) of Japan.
References
|
1
|
Mymrikov EV, Seit-Nebi AS and Gusev NB:
Large potentials of small heat shock proteins. Physiol Rev.
91:1123–1159. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kampinga HH, Hageman J, Vos MJ, Kubota H,
Tanguay RM, Bruford EA, Cheetham ME, Chen B and Hightower LE:
Guidelines for the nomenclature of the human heat shock proteins.
Cell Stress Chaperones. 14:105–111. 2009. View Article : Google Scholar :
|
|
3
|
Kriehuber T, Rattei T, Weinmaier T,
Bepperling A, Haslbeck M and Buchner J: Independent evolution of
the core domain and its flanking sequences in small heat shock
proteins. FASEB J. 24:3633–3642. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dubińska-Magiera M, Jabłońska J, Saczko J,
Kulbacka J, Jagla T and Daczewska M: Contribution of small heat
shock proteins to muscle development and function. FEBS Lett.
588:517–530. 2014. View Article : Google Scholar
|
|
5
|
Kostenko S and Moens U: Heat shock protein
27 phosphorylation: Kinases, phosphatases, functions and pathology.
Cell Mol Life Sci. 66:3289–3307. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Landry J, Lambert H, Zhou M, Lavoie JN,
Hickey E, Weber LA and Anderson CW: Human HSP27 is phosphorylated
at serines 78 and 82 by heat shock and mitogen-activated kinases
that recognize the same amino acid motif as S6 kinase II. J Biol
Chem. 267:794–803. 1992.PubMed/NCBI
|
|
7
|
Hayes D, Napoli V, Mazurkie A, Stafford WF
and Graceffa P: Phosphorylation dependence of hsp27 multimeric size
and molecular chaperone function. J Biol Chem. 284:18801–18807.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Matsushima-Nishiwaki R, Takai S, Adachi S,
Minamitani C, Yasuda E, Noda T, Kato K, Toyoda H, Kaneoka Y,
Yamaguchi A, et al: Phosphorylated heat shock protein 27 represses
growth of hepatocellular carcinoma via inhibition of extracellular
signal-regulated kinase. J Biol Chem. 283:18852–18860. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kular J, Tickner J, Chim SM and Xu J: An
overview of the regulation of bone remodelling at the cellular
level. Clin Biochem. 45:863–873. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chim SM, Tickner J, Chow ST, Kuek V, Guo
B, Zhang G, Rosen V, Erber W and Xu J: Angiogenic factors in bone
local environment. Cytokine Growth Factor Rev. 24:297–310. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Uozaki H, Horiuchi H, Ishida T, Iijima T,
Imamura T and Machinami R: Overexpression of resistance-related
proteins (metallothioneins, glutathione-S-transferase pi, heat
shock protein 27, and lung resistance-related protein) in
osteosarcoma. Relationship with poor prognosis Cancer.
79:2336–2344. 1997.
|
|
12
|
Tiffee JC, Griffin JP and Cooper LF:
Immunolocalization of stress proteins and extracellular matrix
proteins in the rat tibia. Tissue Cell. 32:141–147. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Leonardi R, Barbato E, Paganelli C and Lo
Muzio L: Immunolocalization of heat shock protein 27 in developing
jaw bones and tooth germs of human fetuses. Calcif Tissue Int.
75:509–516. 2004. View Article : Google Scholar
|
|
14
|
Shakoori AR, Oberdorf AM, Owen TA, Weber
LA, Hickey E, Stein JL, Lian JB and Stein GS: Expression of heat
shock genes during differentiation of mammalian osteoblasts and
promyelocytic leukemia cells. J Cell Biochem. 48:277–287. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kozawa O, Niwa M, Matsuno H, Ishisaki A,
Kato K and Uematsu T: Stimulatory effect of basic fibroblast growth
factor on induction of heat shock protein 27 in osteoblasts: Role
of protein kinase C. Arch Biochem Biophys. 388:237–242. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kozawa O, Niwa M, Matsuno H, Tokuda H,
Miwa M, Ito H, Kato K and Uematsu T: Sphingosine 1-phosphate
induces heat shock protein 27 via p38 mitogen-activated protein
kinase activation in osteoblasts. J Bone Miner Res. 14:1761–1767.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kato K, Adachi S, Matsushima-Nishiwaki R,
Minamitani C, Natsume H, Katagiri Y, Hirose Y, Mizutani J, Tokuda
H, Kozawa O, et al: Regulation by heat shock protein 27 of
osteocalcin synthesis in osteoblasts. Endocrinology. 152:1872–1882.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Arrigo AP and Gibert B: HspB1, HspB5 and
HspB4 in human cancers: Potent oncogenic role of some of their
client proteins. Cancers (Basel). 6:333–365. 2014. View Article : Google Scholar
|
|
19
|
Gibert B, Eckel B, Fasquelle L, Moulin M,
Bouhallier F, Gonin V, Mellier G, Simon S, Kretz-Remy C, Arrigo AP,
et al: Knock down of heat shock protein 27 (HspB1) induces
degradation of several putative client proteins. PLoS One.
7:e297192012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Andrieu C, Taieb D, Baylot V, Ettinger S,
Soubeyran P, De-Thonel A, Nelson C, Garrido C, So A, Fazli L, et
al: Heat shock protein 27 confers resistance to androgen ablation
and chemotherapy in prostate cancer cells through eIF4E. Oncogene.
29:1883–1896. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kong J and Lasko P: Translational control
in cellular and developmental processes. Nat Rev Genet. 13:383–394.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gilbert RJ, Gordiyenko Y, von der Haar T,
Sonnen AF, Hofmann G, Nardelli M, Stuart DI and McCarthy JE:
Reconfiguration of yeast 40S ribosomal subunit domains by the
translation initiation multifactor complex. Proc Natl Acad Sci USA.
104:5788–5793. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sonenberg N and Gingras AC: The mRNA 5′
cap-binding protein eIF4E and control of cell growth. Curr Opin
Cell Biol. 10:268–275. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jia Y, Polunovsky V, Bitterman PB and
Wagner CR: Cap-dependent translation initiation factor eIF4E: An
emerging anticancer drug target. Med Res Rev. 32:786–814. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kubisch C, Dimagno MJ, Tietz AB, Welsh MJ,
Ernst SA, Brandt-Nedelev B, Diebold J, Wagner AC, Göke B, Williams
JA, et al: Overexpression of heat shock protein Hsp27 protects
against cerulein-induced pancreatitis. Gastroenterology.
127:275–286. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sudo H, Kodama HA, Amagai Y, Yamamoto S
and Kasai S: In vitro differentiation and calcification in a new
clonal osteogenic cell line derived from newborn mouse calvaria. J
Cell Biol. 96:191–198. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kozawa O, Tokuda H, Miwa M, Kotoyori J and
Oiso Y: Cross-talk regulation between cyclic AMP production and
phosphoinositide hydrolysis induced by prostaglandin E2
in osteoblast-like cells. Exp Cell Res. 198:130–134. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Matsushima-Nishiwaki R, Kumada T, Nagasawa
T, Suzuki M, Yasuda E, Okuda S, Maeda A, Kaneoka Y, Toyoda H and
Kozawa O: Direct association of heat shock protein 20 (HSPB6) with
phosphoinositide 3-kinase (PI3K) in human hepatocellular carcinoma:
Regulation of the PI3K activity. PLoS One. 8:e784402013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang W, Yang N and Shi XM: Regulation of
mesenchymal stem cell osteogenic differentiation by
glucocorticoid-induced leucine zipper (GILZ). J Biol Chem.
283:4723–4729. 2008. View Article : Google Scholar
|
|
30
|
Simpson DA, Feeney S, Boyle C and Stitt
AW: Retinal VEGF mRNA measured by SYBR green I fluorescence: A
versatile approach to quantitative PCR. Mol Vis. 6:178–183.
2000.PubMed/NCBI
|
|
31
|
Laemmli UK: Cleavage of structural
proteins during the assembly of the head of bacteriophage T4.
Nature. 227:680–685. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cuesta R, Laroia G and Schneider RJ:
Chaperone hsp27 inhibits translation during heat shock by binding
eIF4G and facilitating dissociation of cap-initiation complexes.
Genes Dev. 14:1460–1470. 2000.PubMed/NCBI
|
|
33
|
Kato K, Tokuda H, Adachi S,
Matsushima-Nishiwaki R, Yamauchi J, Natsume H, Minamitani C,
Mizutani J, Otsuka T and Kozawa O: Role of heat shock protein 27 in
transforming growth factor-β-stimulated vascular endothelial growth
factor release in osteoblasts. Int J Mol Med. 27:423–428.
2011.PubMed/NCBI
|
|
34
|
Barutta F, Pinach S, Giunti S, Vittone F,
Forbes JM, Chiarle R, Arnstein M, Perin PC, Camussi G, Cooper ME,
et al: Heat shock protein expression in diabetic nephropathy. Am J
Physiol Renal Physiol. 295:F1817–F1824. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Singh D, McCann KL and Imani F: MAPK and
heat shock protein 27 activation are associated with respiratory
syncytial virus induction of human bronchial epithelial monolayer
disruption. Am J Physiol Lung Cell Mol Physiol. 293:L436–L445.
2007. View Article : Google Scholar : PubMed/NCBI
|