Introduction
Vascular remodeling is a complex pathophysiological
process which is implicated in a number of cardiovascular diseases,
such as atherosclerosis, hypertension, and restenosis following
angioplasty. There is considerable evidence indicating that shear
stress, particularly low and oscillatory shear stress, plays a
critical role in vascular remodeling. For example, clinical and
pathological data have revealed that atherosclerotic lesions occur
preferentially at bifurcations and branch points, suggesting that
low and disturbed shear stress correlate with vascular remodeling
and atherogenesis (1). Even in
healthy subjects, carotid intima-media thickness (IMT), a surrogate
marker for early atherosclerosis, has been linked to low shear
stress in the common carotid artery (2). In addition, shear stress is also
responsible for the vascular remodeling associated with restenosis
following angioplasty (3,4).
Vascular remodeling induced by low shear stress
involves multiple cell types, processes, various genes and
signaling pathways. Of these, the excessive proliferation and
migration of vascular smooth muscle cells (VSMCs) are the main
pathological events leading to neointima formation and lumen
narrowing. It is well known that platelet-derived growth factor
(PDGF) is the major driving factor promoting abnormal VSMC
proliferation and migration involved in vascular remodeling
(5). Additionally, low shear
stress promotes the production of reactive oxygen species (ROS) in
a direct or indirect manner, and ROS can function as an
intracellular second messenger to regulate many downstream
signaling pathways, including those modulating the proliferation
and migration of VSMCs (6,7).
Therefore, identifying new genes and signaling pathways that can
indicate changes in wall shear stress or ROS levels and
subsequently control the development of vascular remodeling is of
great interest.
Nuclear receptors are a superfamily of transcription
factors that are involved in diverse pathological functions.
Several members of this superfamily play pivotal roles in
atherosclerosis and vascular remodeling and have emerged as
potential therapeutic targets for the treatment of cardiovascular
diseases (8–10). The orphan nuclear receptor, Nur77
[also known as TR3 or nuclear receptor subfamily 4, group A, member
1 (NR4A1)], is an immediate early gene that plays a crucial role in
the functional regulation of cell differentiation, proliferation,
apoptosis, and inflammation. Previously, the biological effects of
Nur77 in cardiovascular diseases, including atherosclerosis,
vascular remodeling, cardiac ischemia/reperfusion injury and
cardiac hypertrophy, have gained considerable attention (11–13). In 2000, Nur77 was first identified
as one of 40 smooth muscle activation-specific genes (smags) in
activated VSMCs, and it was noted that it is expressed in
atherosclerotic lesions (14).
Moreover, it has also been noted that Nur77 inhibits VSMC
proliferation and attenuates neointima formation (15). Consistent with this previous
study, more recent research from the same group indicated that
Nur77 inhibits outward remodeling in SMC-specific overexpression of
Nur77 transgenic mice (16).
However, the direct role of Nur77 in the PDGF-induced VSMC
proliferation and migration and vascular remodeling induced by low
shear stress has not been extensively investigated as of yet.
In this study, we used a mouse model of vascular
remodeling, which was induced by partial ligation of the left
common carotid artery (LCCA). To the best of our knowledge, this is
the first study that used Nur77-deficient mice to define the
precise role of Nur77 in vascular remodeling induced by low shear
stress. Our results suggest that oxidative stress is an important
trigger for the upregulation of Nur77 during vascular remodeling,
which in turn suppresses the proliferation and migration of VSMCs
and the development of vascular remodeling.
Materials and methods
Mice
All animal experiments were performed according to
the Shanghai Jiao Tong University School of Medicine (Shanghai,
China) guidelines for the ethical care of animals. The protocol was
approved by the Committee on the Ethics of Animal Experiments of
the Shanghai Jiao Tong University School of Medicine [Permit no.
(2013)-75]. Wild-type (WT) and Nur77 knockout (Nur77-KO) mice with
a C57BL/6 background were purchased from Jackson Laboratory, (Bar
Harbor, ME, USA) and were bred at Shanghai Biomodel Organism
Science & Technology Development Co., Ltd. Male WT and Nur77-KO
mice (6–8 weeks of age, weighing 20–25 g) were used in this study
and were randomly assigned to 2 groups (n=8 per group): partial
ligation of the LCCA and the sham-operated control. All surgical
procedures were performed under a dissecting microscope. All
animals were sacrificed 4 weeks after the procedure.
Animal surgery
Partial ligation of the LCCA induces low shear
stress in the LCCA and was performed as described previously
(17). In brief, the mice were
anesthetized with an intraperitoneal injection of pentobarbital
sodium (50 mg/kg), and body temperature was maintained at 37°C
using heating pads. After blunt dissection to expose the distal
branches of the LCCA, blood flow was reduced by ligating all
branches of the LCCA apart from the left thyroid artery. After
ensuring that blood flow was present, the incision was closed with
a suture. Sham ligation involved placing sutures, but no ligation
was undertaken.
Tissue collection and processing
The mice were perfused with ice-cold isotonic
saline, after which some left carotid arteries were isolated and
stored at −80°C until use. Others were fixed with 4%
paraformaldehyde overnight, and were then embedded in optimal
cutting temperature compound (OCT). OCT-embedded sections
(8-µm-thick) were cut for use every 200 µm over a 2
mm length of carotid artery, from the distal bifurcation of carotid
artery specimens.
Histological staining
The OCT-embedded sections were stained with
hematoxylin and eosin (H&E; Sigma, St. Louis, MO, USA) using a
standard protocol. Picrosirius red (Sigma) and Verhoeff-Van Gieson
(Genmed Scientifics, Arlington, VA, USA) stains were used for
staining collagen and elastin, respectively. For staining collagen,
the sections were fixed with 4% paraformaldehyde for 20 min and
then washed with double-distilled water 3 times for 5 min. The
sections were then immersed in 0.1% picric acid (Sigma) solution
and dehydrated using absolute ethyl alcohol (Zhenxing Chemical,
Shanghai, China) after being washed twice with 0.5% acetic acid.
Elastin was determined according to the manufacturer's instructions
provided with the Elastin Stain kit (Genmed Scientifics). All
images were captured using an Olympus digital camera (Olympus,
Tokyo, Japan) and analyzed using ImagePro Plus software.
Immunofluorescence staining
OCT-embedded sections were washed with
phosphate-buffered saline (PBS) and then fixed in 4%
paraformaldehyde for 20 min, after which time the sections were
permeabilized with 0.2% Triton X-100 (Sigma) for 8 min. The
sections were blocked with 5% fetal bovine serum (FBS; Gibco, Grand
Island, NY, USA) for 30 min and incubated with primary antibodies
against Nur77 (1:100; #ab13851; Abcam, Cambridge, UK), smooth
muscle (SM)-α-actin (1:300; #ab21027; Abcam), matrix
metalloproteinase-9 (MMP-9) (1:100; #ab38898; Abcam) and
proliferating cell nuclear antigen (PCNA) (1:100; #AJ1594a; Abgent,
San Diego, CA, USA) overnight, followed by further staining with
secondary antibodies labeled with red fluorescence (1:300; #A31570;
donkey-anti-mouse; 555 nm) and green fluorescence (1:300; #A21206;
donkey-anti-rabbit; 488 nm) (both from Invitrogen, Carlsbad, CA,
USA) for 60 min. After staining the nuclei with
4,6-diamidino-2-phenylindole (DAPI; blue fluorescence; Beyotime,
Shanghai, China), the fluorescence signal was acquired using a
confocal microscope (Zeiss LSM 710; Carl Zeiss, Oberkochen,
Germany).
Terminal
deoxynucleotidyltransferase-mediated dUTP nick-end labelling
(TUNEL) assay
TUNEL staining was performed using the In
Situ Cell Death Detection kit (Roche Diagnostics, Madison, WI,
USA). Briefly, the LCCA segments were fixed in 4% paraformaldehyde
for 20 min and permeabilized with 0.1% Triton X-100 and 0.1% sodium
citrate in PBS on ice for 2 min. Tissue samples were then incubated
with TUNEL reagent at 37°C for 50 min. Following incubation, the
nuclei were stained with DAPI for another 8 min. Images were
acquired using a confocal microscope (Zeiss LSM 710).
Detection of ROS
2′,7′-Dichlorodihydrofluorescein diacetate (DCFH-DA;
Sigma) was used to measure the ROS levels in the cultured VSMCs, as
previously described (18).
Dihydroethidium (DHE; Sigma) staining was used for the in
situ detection of ROS levels in frozen tissue sections, which
were mounted in OCT-embedding compound and frozen at −20°C.
Briefly, unfixed frozen cross-sections were incubated with DHE (5
µmol/l) at 37°C for 30 min in a humidified chamber protected
from light, followed by a 5-min wash in PBS. Images were obtained
by confocal microscopy (Zeiss LSM 710).
Cell culture
Primary VSMCs were isolated from the thoracic aortic
arteries of Sprague-Dawley rats (6–8 weeks of age) using mechanical
dissociation, as previously described (19). Briefly, the medial layer of the
rat thoracic aorta was isolated surgically and minced into
approximately 1-mm2 pieces, which were plated into 6-cm
dishes for culture in DMEM with 10% FBS, 100 U/ ml penicillin, 100
µg/ml streptomycin, and incubated at 37°C, 5%
CO2. VSMCs were characterized by immunofluorescence
staining for SM-α-actin. VSMCs at passages 3–8 were used in all
experiments. Twenty-four hours prior to drug treatment, the VSMCs
were transferred to serum-free medium for the duration of the
experiment. The VSMCs were treated with the vehicle (solvent
control) or various reagents, including PDGF (R&D Systems,
Minneapolis, MN, USA), H2O2 (Sigma) and the
antioxidant, N-acetyl cysteine (NAC; Sigma), as described in the
figure legends.
Western blot analysis
Whole cell lysates were prepared and quantitated by
Bradford assay. Equal amounts of protein per lane were subjected to
SDS-PAGE, transferred onto a nitrocellulose membranes, and western
blot analysis was performed using antibodies against Nur77
(1:1,000; #ab109180; Abcam), β-actin (1:5,000; #ab6276; Abcam), GFP
(1:1,000; #ab290; Abcam), PCNA (1:1,000; #AJ1594a; Abgent), cyclin
D1 (1:1,000; #2978; Cell Signaling Technology, Danvers, MA, USA)
and horseradish peroxidase-conjugated secondary antibodies
(1:5,000; #115-035-003/111-035-003, Jackson ImmunoResearch
Laboratories, West Grove, PA, USA). Protein bands were detected
using enhanced chemiluminescence (Millipore, Billerica, MA, USA),
and quantification was performed using Quantity One 4.4.0 software
(Bio-Rad, Hercules, CA, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the cells using TRIzol
reagent (Invitrogen) according to the manufacturer's instructions.
The purity of the total RNA was assessed by ultraviolet
spectrophotometry. Total RNA (2 µg) was reverse transcribed
into cDNA. cDNA was then used as the template in a 20-µl
qPCR reaction, which was completed using the ABI Prism 7300
real-time PCR system (Applied Biosystems, Foster City, CA, USA).
The primers used were as follows: β-actin forward, 5′-GGC ATC GTC
ACC AAC TGG GAC-3′ and reverse, 5′-CGA TTT CCC GCT CGG CCG TGG-3′;
Nur77 forward, 5′-GCT CAT CTT CTG CTC AGG CCT-3′ and reverse,
5′-CAG ACG TGA CAG GCA GCT GGC-3′.
Cell counts and cell counting kit-8
(CCK-8) assay
For cell counts, primary rat VSMCs were plated into
a 24-well plate and were infected with green fluorescent protein
(GFP)-Nur77 adenovirus (Ad-GFP-Nur77) and GFP control adenovirus
(Ad-GFP) for 48 h. The cells were subsequently stimulated with PDGF
(20 ng/ml) for another 24 h. The cells were then trypsinized and
counted using a hemocytometer and an Olympus inverted microscope.
For the CCK-8 assay, primary VSMCs were plated into a 96-well
plate. The cells were infected with adenovirus and stimulated with
PDGF as mentioned above. Cell proliferation was assessed by CCK-8
assay (Yeasen, Shanghai, China), according to the manufacturer's
instructions.
In vitro scratch-wound assay
The primary rat VSMCs were cultured on 6-well plates
and infected with Ad-GFP-Nur77 or Ad-GFP for 48 h. Confluent cells
were growth-arrested and scraped using sterilized 10-µl
pipette tips, washed with PBS, and stimulated with PDGF (20 ng/ml)
for 24 h. The cells were visualized on an Olympus inverted
microscope.
Statistical analysis
Values are expressed as the means ± standard error
of the mean (SEM). Differences between groups were compared by
two-tailed Student's t-tests. All tests were two-sided, and a value
of p<0.05 was considered to indicate a statistically significant
difference.
Results
Nur77 is highly expressed in neointimal
VSMCs in the ligated carotid arteries following vascular remodeling
induced by low shear stress
Partial ligation of the LCCA was performed to induce
low shear stress and to generate a model of carotid artery
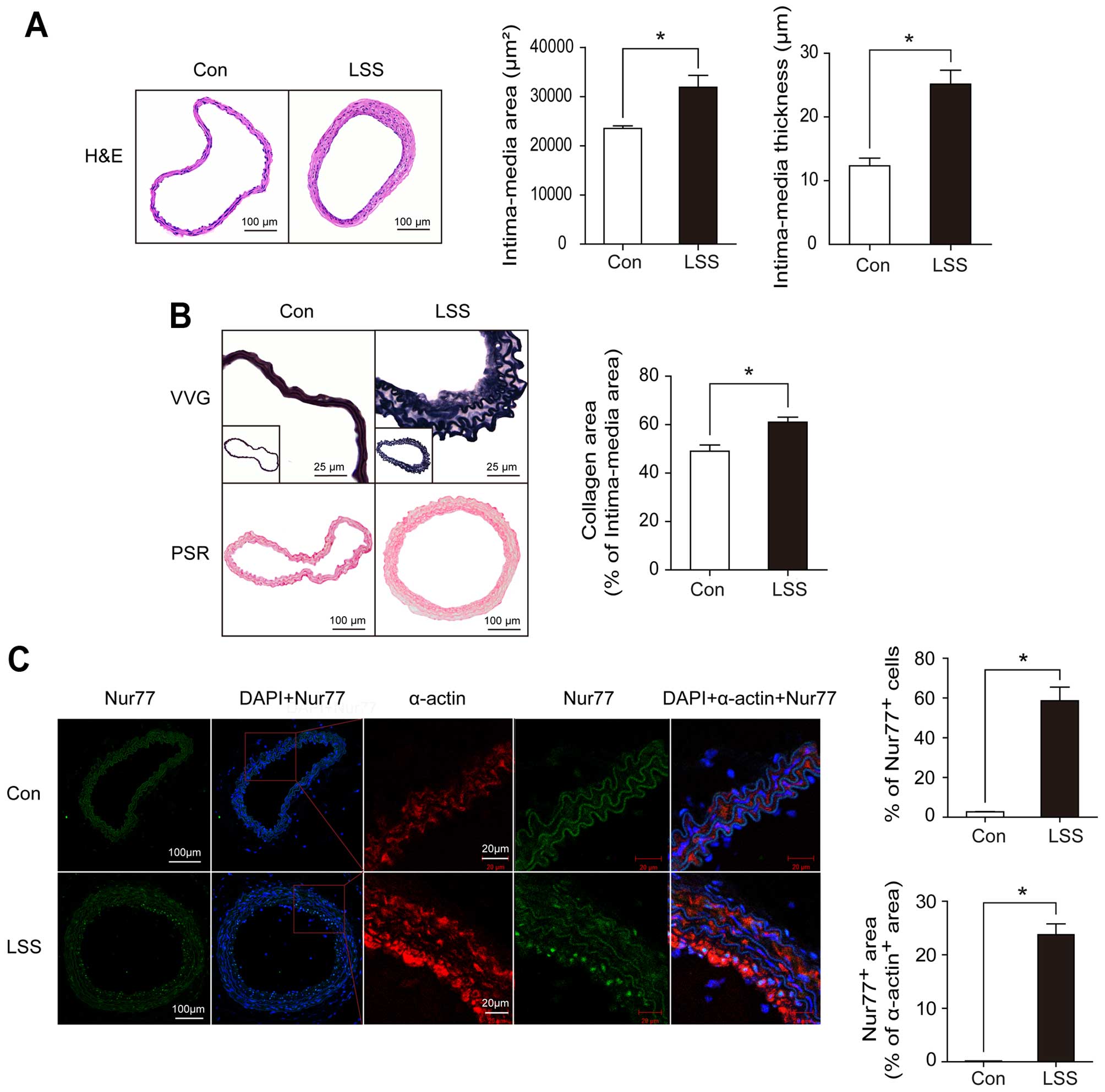
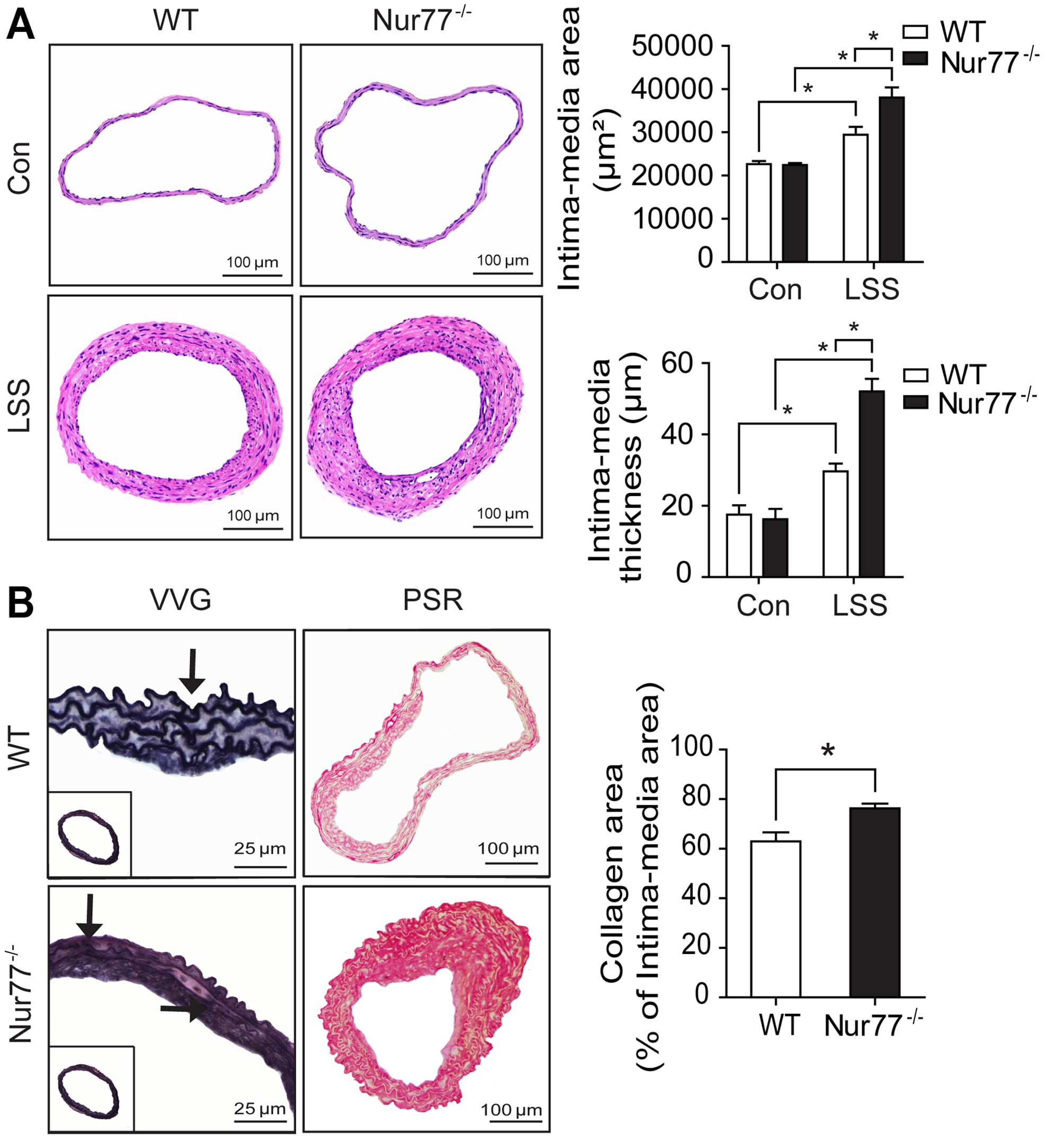
remodeling, as previously described (17). As shown in Fig. 1A, partial carotid ligation
resulted in significant vascular remodeling, as assessed by H&E
staining and quantification of the intimamedia area
(20,324.65±1,143.16 vs. 32,793.74±1,940.77 µm2,
p<0.05) and IMΤ (12.35±1.29 vs. 25.16±2.02 µm,
p<0.05). Accordingly, in the ligated carotid arteries, we noted
severe elastin disruption and more collagen deposition, as assessed
by Verhoeff-Van Gieson staining and picrosirius red staining,
respectively (Fig. 1B).
To determine whether Nur77 plays a role in the
vascular remodeling induced by low shear stress, we examined the
expression of Nur77 in neointimal VSMCs in the ligated carotid
arteries by dual immunofluorescence staining with specific
antibodies against Nur77 or SM-α-actin. As shown in Fig. 1C, Nur77 was highly expressed in
the neointimal VSMCs in the ligated carotid arteries following
vascular remodeling compared to the VSMCs in the arteries from the
sham-operated control. Moreover, Nur77 was localized predominantly
in the neointimal VSMCs in the ligated carotid arteries.
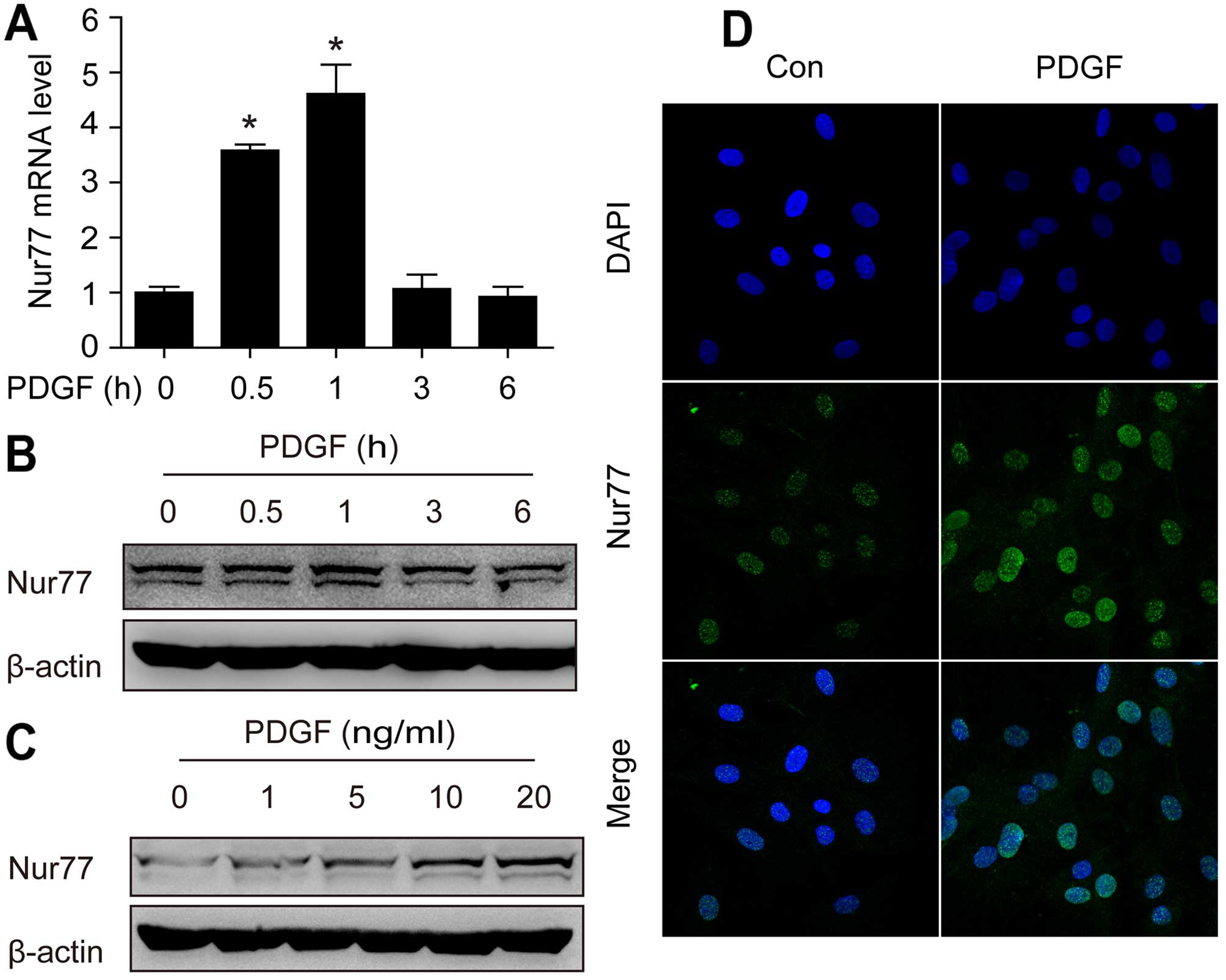
Nur77 expression is upregulated by PDGF
in a ROS-dependent manner in primary rat VSMCs
PDGF is known to be involved in vascular remodeling
induced by low shear stress (20). Therefore, we examined the
expression of Nur77 in rat VSMCs in response to PDGF stimulation.
As shown in Fig. 2A, stimulation
with PDGF (20 ng/ml) induced a rapid increase in Nur77 mRNA levels,
peaking at 1 h. Subsequently, the protein expression of Nur77 was
examined by western blot analysis and immunofluorescence staining.
As shown in Fig. 2B and C, PDGF
stimulation led to rapid increase in the protein expression of
Nur77 in a dose-dependent manner. Immunofluorescence staining
revealed similar results (Fig.
2D).
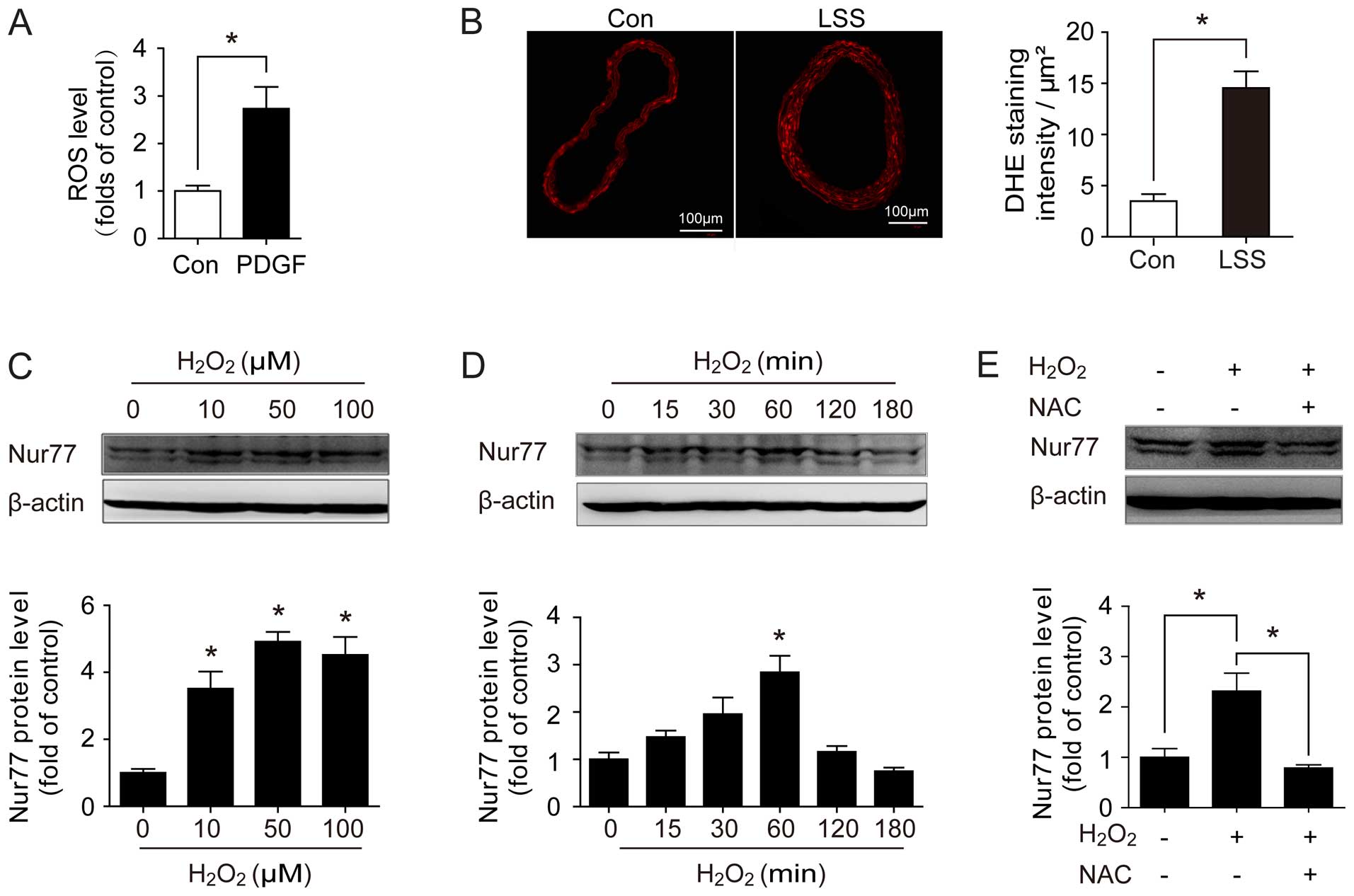
A growing body of evidence indicates that oxidative
stress plays a critical pathophysiological role in vascular
remodeling (21–24). Therefore, to determine whether
Nur77 expression is mediated by oxidative stress, we examined
whether Nur77 expression was induced following an induction of ROS
production. As shown in Fig. 3A,
stimulation with PDGF increased the intracellular ROS levels in
vitro in the primary VSMCs. Similarly, the ROS levels were
significantly increased in the ligated carotid arteries (Fig. 3B). Furthermore, we examined the
expression of Nur77 in response to H2O2
stimulation. The results revealed that H2O2
stimulation induced an upregulation in Nur77 expression in a
dose-dependent manner (Fig. 3C).
An obvious increase in Nur77 protein expression was noted following
15 min of exposure to H2O2, and reached a
plateau at 1 h (Fig. 3D).
Moreover, the upregulation of Nur77 expression was blocked by
treatment with the antioxidant, NAC (Fig. 3E), suggesting that the
upregulation of Nur77 is ROS-dependent. Taken together, these
results suggest that ROS may be the direct or indirect reason for
the upregulation of Nur77 during vascular remodeling.
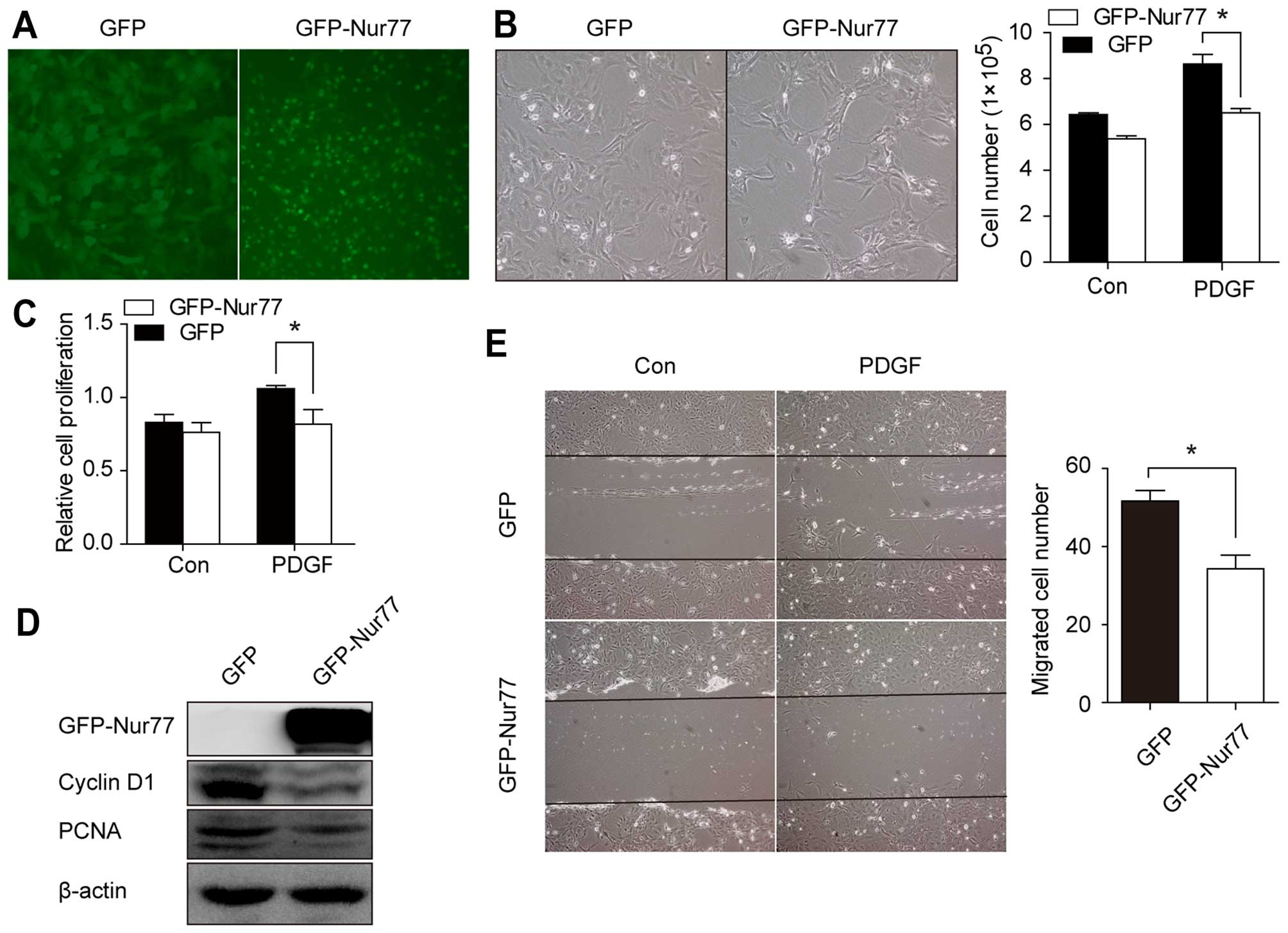
Nur77 inhibits the PDGF-induced
proliferation and migration of VSMCs
To establish the functional significance of Nur77,
we examined the effects of Nur77 overexpression on the PDGF-induced
proliferation and migration of VSMCs. We infected the VSMCs with
Ad-GFP-Nur77 or Ad-GFP (Fig. 4A).
As shown in Fig. 4B and C, the
Ad-GFP-Nur77-infected cells exhibited decreased proliferation
following stimulation with PDGF compared with the Ad-GFP-infected
cells. Consistent with this result, the levels of cyclin D1 and
PCNA, which reflect the ability of VSMCs to proliferate, were
markedly decreased in the Ad-GFP-Nur77-infected cells (Fig. 4D). Additionally, the in
vitro scratch-wound assay revealed decreased VSMC migration in
the Ad-GFP-Nur77-infected cells compared with the Ad-GFP-infected
cells (Fig. 4E). These results
suggest that Nur77 inhibits the proliferation and migration of
VSMCs.
Nur77 deletion enhances vascular
remodeling induced by low shear stress
In order to further define the in vivo role
of Nur77 in vascular remodeling induced by low shear stress,
partial carotid ligation was performed in age- and gender-matched
WT and Nur77-deficient mice. Four weeks following surgery, the
Nur77-deficient mice exhibited markedly enhanced vascular
remodeling compared with the WT mice, as assessed by H&E
staining (Fig. 5A). Quantitative
morphometric analysis revealed a marked increase in the
intima-media area (38,08 0±2,317 vs. 29,420±1,851
µm2, p<0.05) and IMΤ (52.01±3.27 vs.
29.54±2.76 µm, p<0.05) in the ligated carotid arteries
from the Nur77-deficient mice compared to those from the WT mice
(Fig. 5A). Verhoeff-Van Gieson
staining revealed more severe elastin disruption following partial
carotid ligation in the Nur77-deficient mice (Fig. 5B). Similarly, picrosirius red
staining, indicating collagen deposition, was more pronounced in
the ligated carotid arteries from the Nur77-deficient mice
(Fig. 5B).
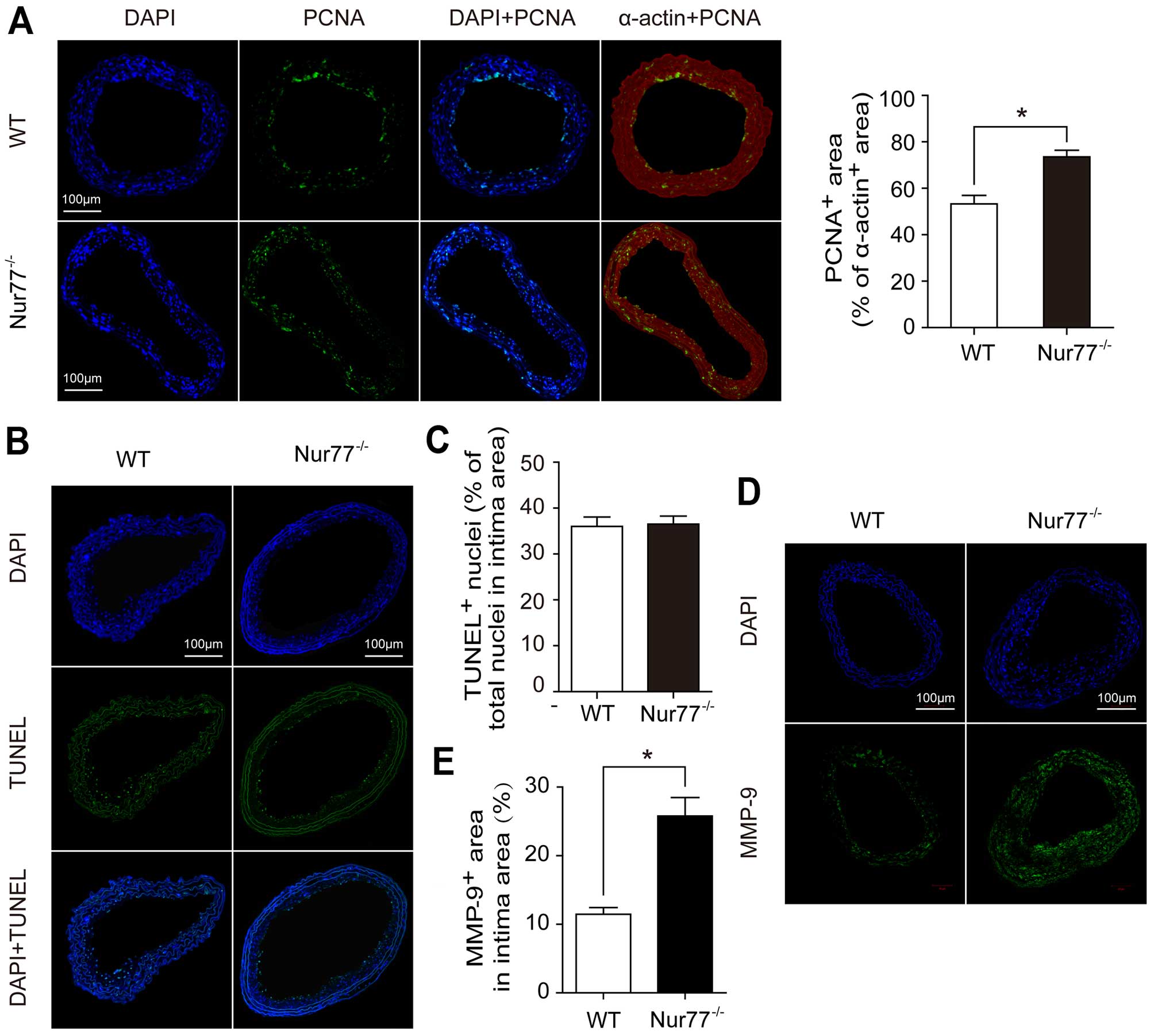
Dual immunofluorescence staining for PCNA and
SM-α-actin was performed in the ligated carotid arteries. The ratio
of the PCNA-positive area to the SM-α-actin-positive area was
significantly increased in the ligated carotid arteries from the
Nur77-deficient mice compared to those from the WT mice (73.17±1.89
vs. 51.50±3.18%, p<0.05) (Fig.
6A). On the contrary, there was no significant difference
observed in the number of apoptotic vascular cells between the
Nur77-deficient and WT mice (Fig. 6B
and C). Moreover, immunofluorescence staining for MMP-9
revealed that the size of the MMP-9-positive area in the intima
area was significantly increased in the ligated arteries from the
Nur77-deficient mice compared to those from the WT mice (25.79±2.69
vs. 11.46±0.96%, p<0.05) (Fig. 6D
and E). Taken together, these results suggest that Nur77
inhibits vascular remodeling induced by low shear stress in
vivo by suppressing VSMC proliferation and MMP-9
production.
Discussion
Vascular remodeling is an important common
pathophysiological process which is associated with a number of
cardiovascular diseases. Moreover, vascular remodeling is a complex
and heterogeneous process, which can be triggered and worsened by
various cardiovascular risk factors, such as local hemodynamic
dysfunction, hypertension and dyslipidemia. Also, shear stress,
particularly low and oscillary shear stress, is known to play a
crucial pathophysiological role in vascular remodeling-related
cardiovascular complications, such as carotid IMT, coronary
atherosclerosis, and even restenosis following angioplasty
(1–4).
The most important finding of the current study is
that, for the first time (to the best of our knowledge), we
demonstrate that Nur77 deletion enhances vascular remodeling
induced by low shear stress. The ligated carotid arteries from
Nur77-deficient mice exhibited enhanced VSMC proliferation and
MMP-9 production compared with those from WT mice. Furthermore,
when the VSMCs were infected with Ad-GFP-Nur77, they exhibited a
significantly decreased proliferation and migration following
stimulation with PDGF. These findings demonstrate that Nur77 is an
important negative regulator of the proliferation and migration of
VSMCs and the development of low shear stress-induced vascular
remodeling. Although some previous studies have examined the role
of Nur77 in the biological behaviors of VSMCs and vascular
remodeling (15,16,25,26), this study, to the best of our
knowledge, is the first to use Nur77-deficient mice to investigate
the role which Nur77 plays in vascular remodeling induced by low
shear stress, which may be the most common situation to mimic human
vascular remodeling. This observation is consistent with the
findings of previous studies (14–16) and strongly suggests that Nur77
inhibits the development of vascular remodeling.
Another important finding of this study was that the
upregulation of Nur77 was ROS-dependent. We were able to
demonstrate that Nur77 expression was increased in primary VSMCs
following stimulation with PDGF or following vascular remodeling
in vivo. In line with our data, it has been previously
demonstrated that Nur77 expression is induced by diverse stimuli,
including inflammatory stimuli, growth factors and cytokines, and
it is also upregulated during atherosclerosis and vascular
remodeling (15,27,28). However, the underlying mechanisms
through which Nur77 expression is regulated remain unclear.
Previous research has suggested that oxidative stress is critical
in vascular remodeling-related cardiovascular diseases (29). Accumulating data indicate that
various risk factors can trigger vascular remodeling through the
excessive generation of ROS (30). In this study, we observed
increased levels of ROS both following stimulation of primary VSMCs
in vitro with PDGF and following flow-induced vascular
remodeling in vivo. Based on this result, we suggest that
oxidative stress is the common reason for the upregulation of Nur77
during vascular remodeling. In the present study, we provided
evidence that Nur77 acts as a sensor of oxidative stress and
subsequently mediates the suppression of VSMC proliferation and
migration following vascular remodeling induced by low shear
stress.
From a therapeutic standpoint, our results have
clinical implications for the treatment of vascular
remodeling-related cardiovascular diseases. Modulating the
expression or transcriptional activity of Nur77 represents a novel
approach to inhibiting vascular remodeling. Pires et al
demonstrated that the enhanced transcriptional activity of Nur77 by
6-mercaptopurine exerted protective effects against neointima
formation (31). Moreover, it was
demonstrated that α-lipoic acid, which enhanced the cytoplasmic
localization of Nur77 in VSMCs, triggered VSMC apoptosis and
inhibited neointima formation in rat carotid arteries following
balloon injury (32). Coupled
with our new data, these findings suggest that Nur77 is a potential
therapeutic target in the treatment of vascular remodeling-related
cardiovascular diseases, such as atherosclerosis and restenosis
following angioplasty.
In conclusion, in the present study, we demonstrate
that Nur77 deletion enhances vascular remodeling induced by low
shear stress. Our findings indicated that oxidative stress is the
common trigger for the upregulation of Nur77 during vascular
remodeling. Moreover, it can be noted that the increased
ROS-dependent expression of Nur77 in turn inhibits the
proliferation and migration of VSMCs and the development of
vascular remodeling induced by low shear stress. Our study
identified Nur77 as a potential novel therapeutic target for
vascular remodeling. However, the identity of the downstream
targets of Nur77 and the detailed mechanisms through which VSMCs
function is regulated remain to be elucidated.
Acknowledgments
This study was supported by grant nos. 81070239,
30971185 and 81370399 from the National Natural Science
Foundation;, and grant no. 10JC1409400 from the Shanghai Municipal
Natural Science Foundation.
References
|
1
|
Chatzizisis YS, Jonas M, Coskun AU, Beigel
R, Stone BV, Maynard C, Gerrity RG, Daley W, Rogers C, Edelman ER,
et al: Prediction of the localization of high-risk coronary
atherosclerotic plaques on the basis of low endothelial shear
stress: an intravascular ultrasound and histopathology natural
history study. Circulation. 117:993–1002. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Carallo C, Irace C, Pujia A, De Franceschi
MS, Crescenzo A, Motti C, Cortese C, Mattioli PL and Gnasso A:
Evaluation of common carotid hemodynamic forces. Relations with
wall thickening. Hypertension. 34:217–221. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Post MJ, Borst C and Kuntz RE: The
relative importance of arterial remodeling compared with intimal
hyperplasia in lumen renarrowing after balloon angioplasty. A study
in the normal rabbit and the hypercholesterolemic Yucatan micropig.
Circulation. 89:2816–2821. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wentzel JJ, Gijsen FJ, Stergiopulos N,
Serruys PW, Slager CJ and Krams R: Shear stress, vascular
remodeling and neointimal formation. J Biomech. 36:681–688. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Qi YX, Jiang J, Jiang XH, Wang XD, Ji SY,
Han Y, Long DK, Shen BR, Yan ZQ, Chien S, et al: PDGF-BB and TGF-β1
on cross-talk between endothelial and smooth muscle cells in
vascular remodeling induced by low shear stress. Proc Natl Acad Sci
USA. 108:1908–1913. 2011. View Article : Google Scholar
|
|
6
|
Matlung HL, Bakker EN and VanBavel E:
Shear stress, reactive oxygen species, and arterial structure and
function. Antioxid Redox Signal. 11:1699–1709. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lyle AN and Griendling KK: Modulation of
vascular smooth muscle signaling by reactive oxygen species.
Physiology (Bethesda). 21:269–280. 2006. View Article : Google Scholar
|
|
8
|
Kratzer A, Buchebner M, Pfeifer T, Becker
TM, Uray G, Miyazaki M, Miyazaki-Anzai S, Ebner B, Chandak PG,
Kadam RS, et al: Synthetic LXR agonist attenuates plaque formation
in apoE−/− mice without inducing liver steatosis and
hypertriglyceridemia. J Lipid Res. 50:312–326. 2009. View Article : Google Scholar :
|
|
9
|
Pruthi D, McCurley A, Aronovitz M, Galayda
C, Karumanchi SA and Jaffe IZ: Aldosterone promotes vascular
remodeling by direct effects on smooth muscle cell
mineralocorticoid receptors. Arterioscler Thromb Vasc Biol.
34:355–364. 2014. View Article : Google Scholar :
|
|
10
|
Tobiasova Z, Zhang L, Yi T, Qin L, Manes
TD, Kulkarni S, Lorber MI, Rodriguez FC, Choi JM, Tellides G, et
al: Peroxisome proliferator-activated receptor-γ agonists prevent
in vivo remodeling of human artery induced by alloreactive T cells.
Circulation. 124:196–205. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hamers AA, Vos M, Rassam F, Marinković G,
Kurakula K, van Gorp PJ, de Winther MP, Gijbels MJ, de Waard V and
de Vries CJ: Bone marrow-specific deficiency of nuclear receptor
Nur77 enhances atherosclerosis. Circ Res. 110:428–438. 2012.
View Article : Google Scholar
|
|
12
|
Cheng Z, Völkers M, Din S, Avitabile D,
Khan M, Gude N, Mohsin S, Bo T, Truffa S, Alvarez R, et al:
Mitochondrial translocation of Nur77 mediates cardiomyocyte
apoptosis. Eur Heart J. 32:2179–2188. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang RH, He JP, Su ML, Luo J, Xu M, Du XD,
Chen HZ, Wang WJ, Wang Y, Zhang N, et al: The orphan receptor TR3
participates in angiotensin II-induced cardiac hypertrophy by
controlling mTOR signalling. EMBO Mol Med. 5:137–148. 2013.
View Article : Google Scholar :
|
|
14
|
de Vries CJ, van Achterberg TA, Horrevoets
AJ, ten Cate JW and Pannekoek H: Differential display
identification of 40 genes with altered expression in activated
human smooth muscle cells. Local expression in atherosclerotic
lesions of smags, smooth muscle activation-specific genes. J Biol
Chem. 275:23939–23947. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Arkenbout EK, de Waard V, van Bragt M, van
Achterberg TA, Grimbergen JM, Pichon B, Pannekoek H and de Vries
CJ: Protective function of transcription factor TR3 orphan receptor
in atherogenesis: decreased lesion formation in carotid artery
ligation model in TR3 transgenic mice. Circulation. 106:1530–1535.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bonta PI, Matlung HL, Vos M, Peters SL,
Pannekoek H, Bakker EN and de Vries CJ: Nuclear receptor Nur77
inhibits vascular outward remodelling and reduces macrophage
accumulation and matrix metalloproteinase levels. Cardiovasc Res.
87:561–568. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sullivan CJ and Hoying JB: Flow-dependent
remodeling in the carotid artery of fibroblast growth factor-2
knockout mice. Arterioscler Thromb Vasc Biol. 22:1100–1105. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang J, Li H, Chen YY, Wang XJ, Shi GY, Hu
QS, Kang XL, Lu Y, Tang XM, Guo QS and Yi J: Anthraquinones
sensitize tumor cells to arsenic cytotoxicity in vitro and in vivo
via reactive oxygen species-mediated dual regulation of apoptosis.
Free Radic Biol Med. 37:2027–2041. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jovinge S, Hultgårdh-Nilsson A, Regnström
J and Nilsson J: Tumor necrosis factor-alpha activates smooth
muscle cell migration in culture and is expressed in the
balloon-injured rat aorta. Arterioscler Thromb Vasc Biol.
17:490–497. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Raines EW: PDGF and cardiovascular
disease. Cytokine Growth Factor Rev. 15:237–254. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kunsch C and Medford RM: Oxidative stress
as a regulator of gene expression in the vasculature. Circ Res.
85:753–766. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Souza HP, Souza LC, Anastacio VM, Pereira
AC, Junqueira ML, Krieger JE, da Luz PL, Augusto O and Laurindo FR:
Vascular oxidant stress early after balloon injury: evidence for
increased NAD(P)H oxidoreductase activity. Free Radic Biol Med.
28:1232–1242. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Szocs K, Lassègue B, Sorescu D, Hilenski
LL, Valppu L, Couse TL, Wilcox JN, Quinn MT, Lambeth JD and
Griendling KK: Upregulation of Nox-based nad(p)h oxidases in
restenosis after carotid injury. Arterioscler Thromb Vasc Biol.
22:21–27. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
de Graaf R, Tintu A, Stassen F,
Kloppenburg G, Bruggeman C and Rouwet E: N-acetylcysteine prevents
neointima formation in experimental venous bypass grafts. Br J
Surg. 96:941–950. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
de Waard V, Arkenbout EK, Vos M, Mocking
AI, Niessen HW, Stooker W, de Mol BA, Quax PH, Bakker EN and
VanBavel E: TR3 nuclear orphan receptor prevents cyclic
stretch-induced proliferation of venous smooth muscle cells. Am J
Pathol. 168:2027–2035. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang L, Gong F, Dong X, Zhou W and Zeng Q:
Regulation of vascular smooth muscle cell proliferation by nuclear
orphan receptor nur77. Mol Cell Biochem. 341:159–166. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shao Q, Shen LH, Hu LH, Pu J, Qi MY, Li
WQ, Tian FJ, Jing Q and He B: Nuclear receptor Nur77 suppresses
inflammatory response dependent on COX-2 in macrophages induced by
oxLDL. J Mol Cell Cardiol. 49:304–311. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pei L, Castrillo A, Chen M, Hoffmann A and
Tontonoz P: Induction of NR4A orphan nuclear receptor expression in
macrophages in response to inflammatory stimuli. J Biol Chem.
280:29256–29262. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Higashi Y, Noma K, Yoshizumi M and Kihara
Y: Endothelial function and oxidative stress in cardiovascular
diseases. Circ J. 73:411–418. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yung LM, Leung FP, Yao X, Chen ZY and
Huang Y: Reactive oxygen species in vascular wall. Cardiovasc
Hematol Disord Drug Targets. 6:1–19. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pires NM, Pols TW, de Vries MR, van Tiel
CM, Bonta PI, Vos M, Arkenbout EK, Pannekoek H, Jukema JW, Quax PH
and de Vries CJ: Activation of nuclear receptor Nur77 by
6-mercap-topurine protects against neointima formation.
Circulation. 115:493–500. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim HJ, Kim JY, Lee SJ, Kim HJ, Oh CJ,
Choi YK, Lee HJ, Do JY, Kim SY, Kwon TK, et al: α-Lipoic acid
prevents neointimal hyperplasia via induction of p38
mitogen-activated protein kinase/Nur77-mediated apoptosis of
vascular smooth muscle cells and accelerates postinjury
reendothelialization. Arterioscler Thromb Vasc Biol. 30:2164–2172.
2010. View Article : Google Scholar : PubMed/NCBI
|