Introduction
Gastric cancer is one of the most common types of
cancer and the leading cause of cancer fatality worldwide (1). The overall prognosis is poor, with a
5-year survival rate of <40%, primarily due to recurrence and
distant metastasis (2). Recently,
gastric cancer stem cells (GCSCs) were shown to promote
tumorigenesis, aggressive growth, recurrence, metastasis and drug
resistance, generating opportunities for the development of novel
GCSC-targeted therapies in the treatment of gastric cancer
(3,4). Conventional anticancer treatments,
such as surgery, systemic chemotherapy and ablative therapy, which
can kill only differentiated cancer cells, result in tumor size
reduction; however, they are often linked with gastric cancer
relapses after some time. This relapse may be due to the presence
of the residual GCSCs, demonstrating the requirement to develop
drugs that specifically target cancer stem cells (CSCs).
GCSCs were first isolated and identified in 2009 and
exhibit the stem cell properties self-renewal, multiple
differentiation and strong tumorigenicity (5). Strong evidence has previously
demonstrated that GCSCs are the major cause of invasion (6). The source of GCSCs remains unknown,
although it has been postulated to originate from normal stem cells
(SCs) that have undergone malignant transformation or progenitor
cells that suffer oncogenic mutations and reactivation of
stemness-related properties (4).
Additionally, a previous study has indicated that
epithelial-to-mesenchymal transition (EMT)-related proteins were
associated with CSC-like properties in gastric cancer, which
suggested that the EMT can generate GCSCs, although the role of
this process remains a matter of debate (7). There are multiple signaling pathways
that are essential for the maintenance of CSCs, including the Notch
pathway, Hedgehog pathway, bone morphogenetic protein signaling,
epidermal growth factor pathway and Wnt pathway (8–10).
The Wnt pathways regulate cell growth, proliferation and survival,
and abnormal activity of this pathway has been shown to promote
self-renewal of CSCs, particularly in lung (11), breast (12) and colon cancer SCs (13). In addition, there is a strong
correlation between Wnt1 and CD44 expression and the grade of
gastric cancer. Stable overexpression of Wnt1 increased spheroid
formation of adenocarcinoma gastric (AGS) cells and enriched the
expression of Oct4 and CD44 (14). Also, Cai and Zhu (15) demonstrated that the Wnt/β-catenin
pathway is essential for the self-renewal of cancer stem-like cells
in human gastric cancer by detecting the expression levels of
β-catenin, c-myc, cyclin D1 and axin 2 in GCSCs. Therefore, given
the important role of the Wnt pathway in GCSCs, further
understanding of the regulation of Wnt pathways represents a viable
therapeutic approach to target GCSCs.
Evodiamine (Evo) is a natural chemical derived from
the plant Evodia rutaecarpa. Previously, Evo was shown to
have biological effects, including antitumor, antinociceptive and
vasorelaxant properties (16,17). The strong antitumor effects of Evo
occur via different mechanisms in a variety of tumors. In colon
cancer, Evo activated c-Jun N-terminal kinase, leading to
subsequent activation of apoptosis and G2/M arrest (18). Evo may inhibit transforming growth
factor-β1-induced EMT in NRK52E cells via the Smad and peroxisome
proliferator-activated receptor-γ pathway (19). Additionally, Evo inhibited growth
and induced apoptosis and autophagy in gastric cancer (20). Thus far, the effect of Evo on
GCSCs remains unclear.
In the present study, the effects of Evo on the
viability of GCSCs and signaling pathways regulating apoptosis and
self-renewal in GCSCs were investigated. These findings provide a
new option for the treatment of gastric cancer by specifically
targeting GCSCs.
Materials and methods
Cell lines and culture
Gastric cancer cell lines AGS and SGC7901 were
purchased from the Cell Bank of the Shanghai Branch of the Chinese
Academy of Sciences, Shanghai Institute of Cell Biology (Shanghai,
China). The gastric cancer cell lines were cultured in RPMI-1640
medium (Gibco, Thermo Fisher Scientific, Grand Island, NY, USA)
supplemented with 100 U/ml penicillin, 100 µg/ml
streptomycin and 10% fetal bovine serum, and maintained at 37°C in
a 5% CO2 humid atmosphere. Cancer stem cells were
enriched from these cells at 1×104 cells/well in
serum-free medium [20 µg/l epidermal growth factor
(Invitrogen, Carlsbad, CA, USA)], 1X B27 (Invitrogen), 20
µg/l basic fibroblast growth factor (Invitrogen), 0.4%
bovine serum albumin (Roche, Mannhein, Germany), 4 mg/l insulin
(Invitrogen) and 200 IU/ml penicillin/streptomycin (Sangon Biotech
Co., Ltd., Shanghai, China) on poly-HEMA-coated 6-well plates
(Corning, New York, NY, USA).
Reagents
Evo (Sigma-Aldrich, St. Louis, MO, USA) was
dissolved in dimethylsulfoxide (DMSO; Sigma-Aldrich) to generate a
30-mM stock solution and diluted in RPMI-1640 medium (Gibco) prior
to use. The final concentration of DMSO in all cell culture was
<0.5% and did not have any harmful effects on cell growth.
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
(MTS) was purchased from Promega (Madison, WI, USA). The Annexin
V-FITC reagent and propidium iodide (PI) were supplied by BD
Biosciences (San Jose, CA, USA), and the β-catenin/Tcf inhibitor
was purchased from Merck (Merck KGaA, Darmstadt, Germany).
MTS assay
CSCs enriched from AGS and SGC7901 cells were
dispensed (100 µl medium/well) in triplicate into a 96-well
plate at a density of 5×103 cells. Cells were treated
with (1, 2, 4 and 8 µM) or without Evo for 24, 48 and 72 h.
Subsequently, 20 µl MTS was added to each well and incubated
for 4 h. Absorbance was measured at 490 nm using a Thermo Varioskan
Flash Reader (Thermo Fisher Scientific, Cambridge, MA, USA). The
IC50 values for 24, 48 and 72 h were calculated using
the SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA).
Flow cytometry
Enriched CSCs treated with or without Evo (2
µM) for 48 h were digested and harvested in 1X binding
buffer (0.1 M Hepes, 1.4 M NaCl and 25 mM CaCl2). To the
solution (~1×105 cells), 5 µl of Annexin V and 5
µl of PI were added, and subsequently incubated at room
temperature in the dark for 15 min. Binding buffer (400 µl)
was added to each tube and samples were subjected to flow
cytometric analysis (BD Biosciences) within 1 h.
Western blot analysis
AGS and SGC7901 cells and CSCs enriched from them
were harvested and lysed in 350 ml radioimmunoprecipitation assay
buffer. Proteins were resolved using 12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and electrophoretically
transferred onto nitrocellulose membranes. Membranes were incubated
overnight at 4°C with the following primary monoclonal antibodies:
B-cell lymphoma 2 (Bcl-2; ab32124; rabbit monoclonal to human), Bax
(ab7977; rabbit polyclonal to human), caspase-3 (ab2171; mouse
monoclonal to human), β-catenin (ab6302; rabbit polyclonal to
human), c-Myc (ab32072; rabbit monoclonal to human), cyclin D1
(ab16663; rabbit monoclonal to human), E-cadherin (ab1416; mouse
monoclonal to human), vimentin (ab133260; rabbit monoclonal to
human), Slug (ab27568; rabbit polyclonal to human), Twist (ab50581;
rabbit polyclonal to human; 1:1,000–2,000; Epitomics, Burlingame,
CA, USA), Bmi1 (ab38295; rabbit polyclonal to human), Sox2
(ab97959; rabbit polyclonal to human), Oct4 (ab18976; rabbit
polyclonal to human), kruppel-like factor (KLF)4 (ab72543; rabbit
polyclonal to human; 1:500–1:1,000; Abcam, Cambridge, UK), and
glyceraldehyde-3-phosphate dehydrogenase (GAPDH; KC-5G4; mouse
monoclonal to human) (1:5,000; Kangcheng Biology Engineering Co.,
Ltd., Shanghai, China). After five washes, membranes were incubated
with a horseradish peroxidase-labeled goat anti-rabbit polyclonal
antibody (1:2;000; Jackson ImmunoResearch Laboratory, West Grove,
PA, USA) at room temperature for 1 h. The reactive bands were
detected using enhanced chemiluminescence (Cell Signaling
Technology, Beverley, MA, USA).
Sphere formation assay
AGS and SGC7901 cells were seeded in
poly-HEMA-coated 24-well plates (150 cells/well) with serum-free
medium, followed by incubation at 37°C in a 5% CO2
atmosphere for 5–7 days. Sphere formation efficiency was calculated
as the number of spheres/cell number × 100%.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Detection of mature ribonucleic acids (RNAs) was
performed using the TaqMan RT-qPCR method (Applied Biosystems,
Foster City, CA, USA) according to the manufacturer's protocol.
Total RNA was isolated from AGS and SGC7901 cells using the Rneasy
Mini kit (Qiagen, Hilden, Germany); reverse transcription was
performed following quantitation using PrimeScript RT reagent kit
with gDNA Eraser (Takara, Shiga, Japan). The reverse transcription
products were mixed with specific primers and probes, and RT-qPCR
was performed. The PCR program was as follows: 95°C for 30 sec,
followed by 40 cycles of 5 sec at 95°C and 34 sec at 60°C. The
primers and probe for Sox2 were as follows: Top,
5′-aatgccttcatggtgtgg-3′ and bottom, 5′-cttctccgtctccgacaaa-3′; and
probe, 5′Fam-agtttccactcggcgcccag-3′Tamra. The primers and probe
for KLF4 were as follows: Top, 5′-ggcactaccgtaaacacacg-3′ and
bottom, 5′-ctggcagtgtgggtcatatc-3′; and probe:
5′Fam-caggtcggaccacctcgcct-3′Tamra. The primers and probe for Oct4
were as follows: Top, 5′-gtggaggaagctgacaacaa-3′ and bottom,
5′-aacaaattctccaggttgcc-3′; and probe:
5′Fam-tctctttcgggcctgcacga-3′Tamra. Relative quantification of RNA
levels was normalized to GAPDH and presented using the ΔΔCt
method.
Drug resistance assay
AGS and SGC7901 cells were seeded in a 24-well plate
at a density of 1×104 cells/well in medium contained
oxaliplatin (1.5 µg/ml) (Jiangsu Hengrui Medicine Co., Ltd.,
Jiangsu, China) or oxaliplatin and Evo (2 µM/4 µM,
respectively). The total number of viable AGS and SGC7901 cells
were counted on days 3, 5 and 7.
Wound-healing assay
AGS and SGC7901 cells were grown to confluence in
6-cm dishes and wounds were generated using P-200 pipette tips.
Cells were treated with or without Evo (2 µM) for 48 h.
Phase contrast images were captured at 0 and 48 h.
Statistical analysis
All the data are presented as mean ± standard
deviation (SD) of triplicate experiments and were analyzed with
GraphPad Prism 5 (GraphPad Software, La Jolla, CA, USA) and SPSS
software, version 13.0. Data were checked for normality and equal
variances and transformed when necessary to meet the assumption of
normal distribution. Comparisons between the two groups were
performed using independent sample t-test. Differences between
multiple groups were determined using one-way analysis of variance
(ANOVA) with least significant difference test or Tamhane's T2 post
hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Evo inhibits proliferation of GCSCs
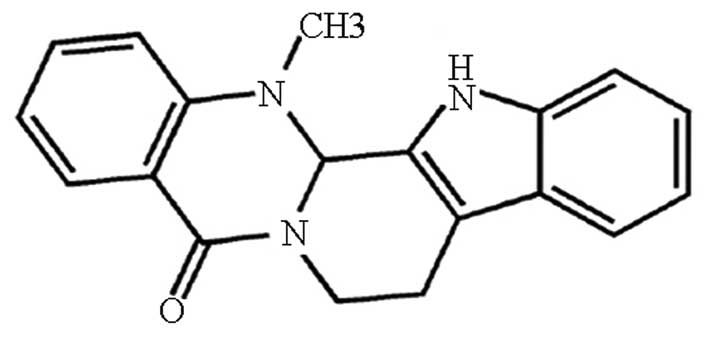
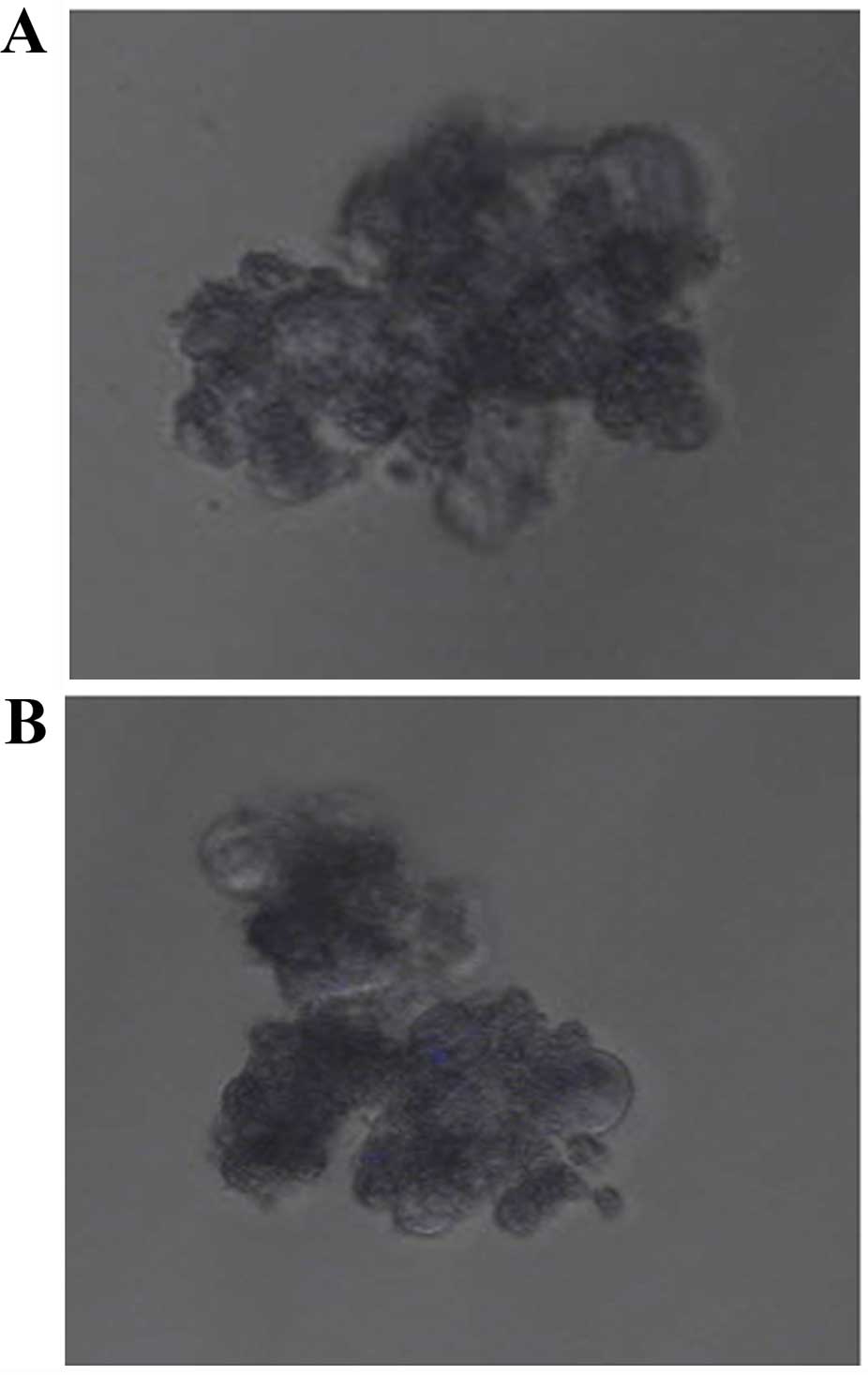
The structure of Evo is shown in Fig. 1. GCSCs were enriched from AGS
(Fig. 2A) and SGC7901 cells
(Fig. 2B), respectively, in
low-attachment plates with serum-free medium. In order to determine
the effect of Evo on cell proliferation, an MTS assay was
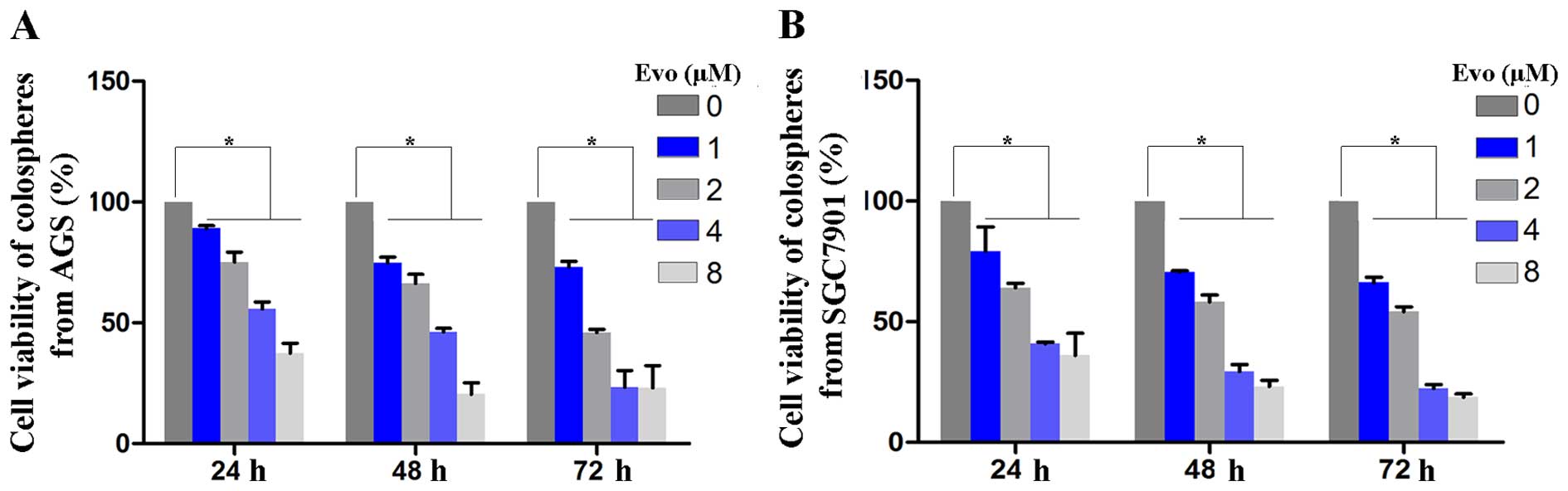
performed. Cell viability was significantly decreased in
Evo-treated cells in a dose- and time-dependent manner (Fig. 3). When the Evo concentration
reached 2 µmol/l, the cell survival rates were significantly
decreased by 24.78±2.1, 33.73±2.91 and 54±1.98% in AGS, and
35.91±2.68, 41.78±3.41 and 45.76±2.38% in SGC7901 cells (mean ± SD,
n=9, P<0.05) at 24, 48 and 72 h, respectively. The
IC50 values of Evo on GCSCs at 24, 48 and 72 h are shown
in Table I. The results showed
that evodiamine inhibited the proliferation of GCSCs in a dose- and
time-dependent manner.
 | Table IIC50 of evodiamine on
colonspheres enriched from AGS and SGC7901 cells. |
Table I
IC50 of evodiamine on
colonspheres enriched from AGS and SGC7901 cells.
| IC50 | 24 h | 48 h | 72 h |
|---|
| AGS, µM | 5.06 | 3.14 | 1.92 |
| SGC7901,
µM | 3.54 | 2.31 | 1.91 |
Evo induces apoptosis in GCSCs
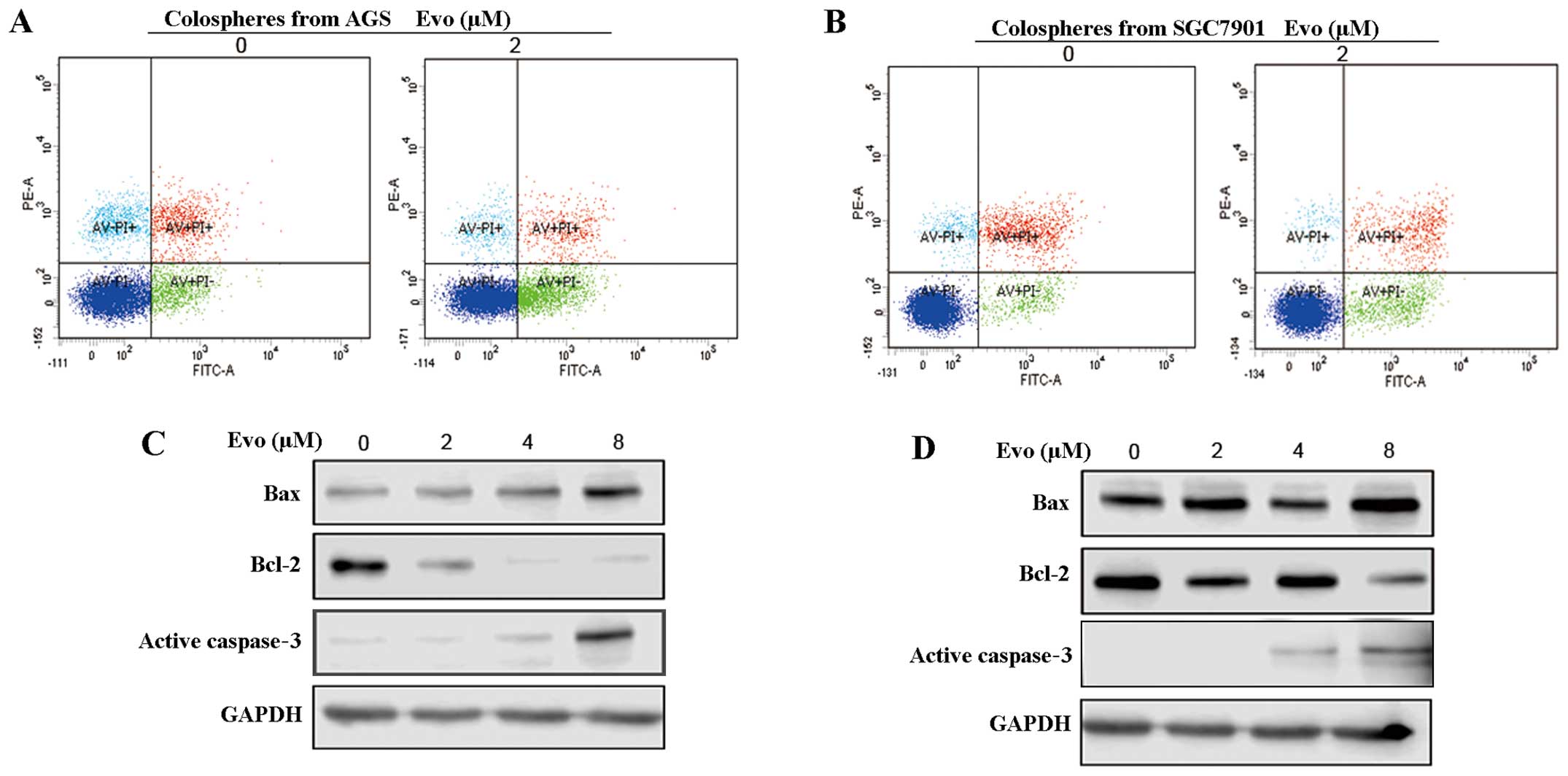
Flow cytometric analysis was performed to determine
the mechanism of Evo-mediated apotosis in GCSCs, and the percentage
of Annexin V-positive GCSCs was significantly increased by Evo. Evo
(2 µM, 48 h) increased rates of apoptosis from 11.6 to 23.9%
and 5.2 to 11.2% in GCSCs from AGS and SGC7901 cells, respectively
(Fig. 4A and B). These results
were confirmed with western blot analysis. Evo elevated the Bcl-2
family members, Bax and active caspase-3, and reduced Bcl-2 in
GCSCs (Fig. 4C and D). These
findings suggested that Evo activated caspase-3-dependent apoptosis
in GCSCs.
Evo inhibits the self-renewal of
GCSCs
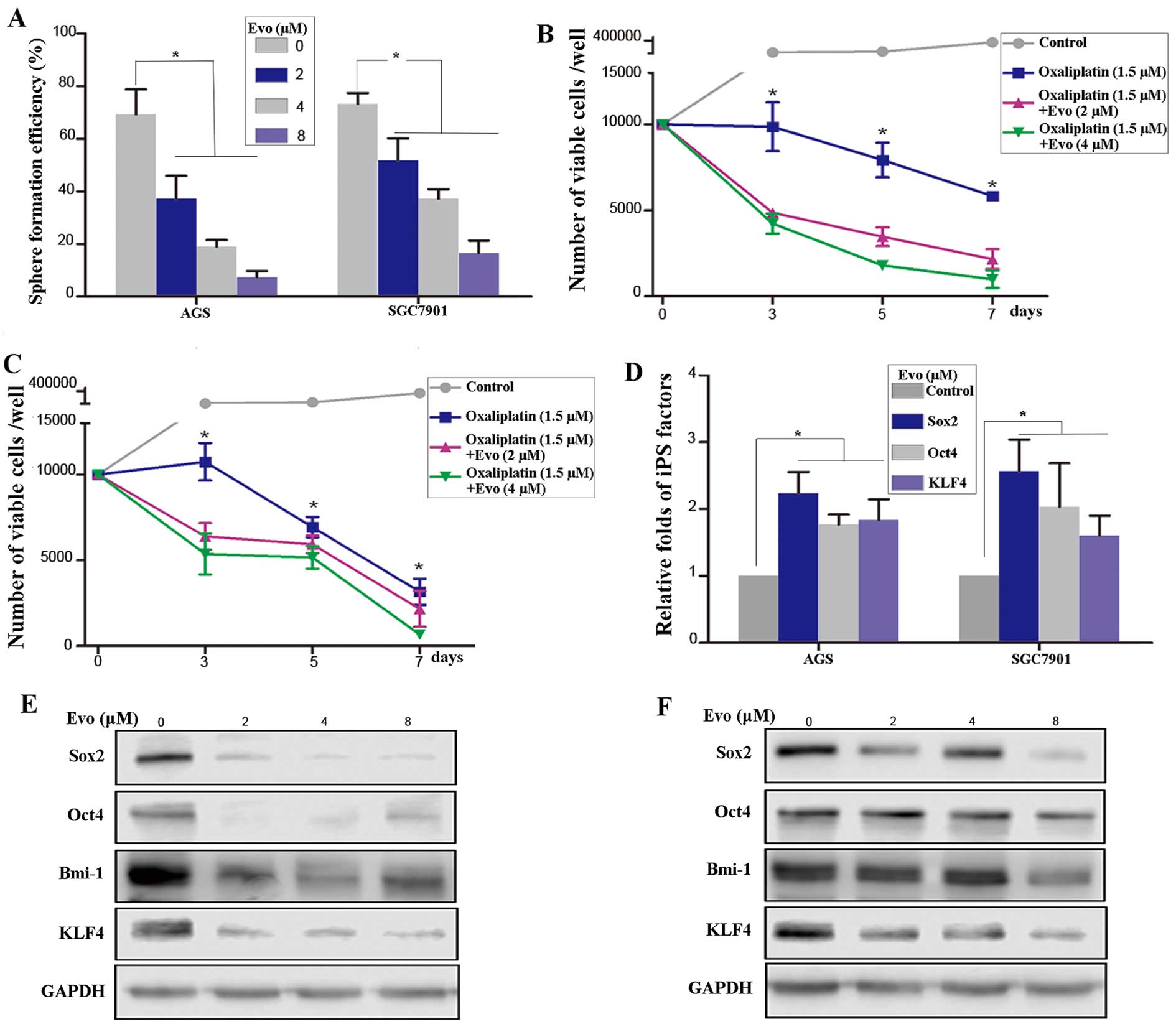
Sphere formation efficiency, a measure of
self-renewal, was significantly decreased in the AGS and SGC7901
cells treated with Evo (Fig. 5A).
Subsequently, the effect of Evo on drug resistance in gastric
cancer cells (GCCs) was examined. Combination treatment with Evo
and oxaliplatin significantly reduced the cell viability in a
dose-dependent manner relative to control and oxaliplatin alone in
AGS (Fig. 5B) and SGC7901
(Fig. 5C) cells. Finally, RT-qPCR
was performed to detect the expression levels of
induced-pluripotent stem (iPS) cell factors. The levels of Sox2,
KLF4 and Oct4 were all increased in GCSCs treated with Evo relative
to control (P<0.05, ANOVA) (Fig.
5D). Similarly, western blot analysis revealed that Evo
treatment decreased the expression of iPS factors in AGS (Fig. 5E) and SGC7901 (Fig. 5F) cells. Taken together, these
data indicated that Evo inhibited the self-renewal of GCSCs.
Evo confers resistance of EMT in
GCCs
As Evo inhibited anoikis and self-renewal of GCSCs,
and recent studies confirmed that the EMT is involved in resistance
to anoikis and generation of CSCs, it follows that Evo may induce
apoptosis and reduce stemness of GCCs by affecting the EMT process.
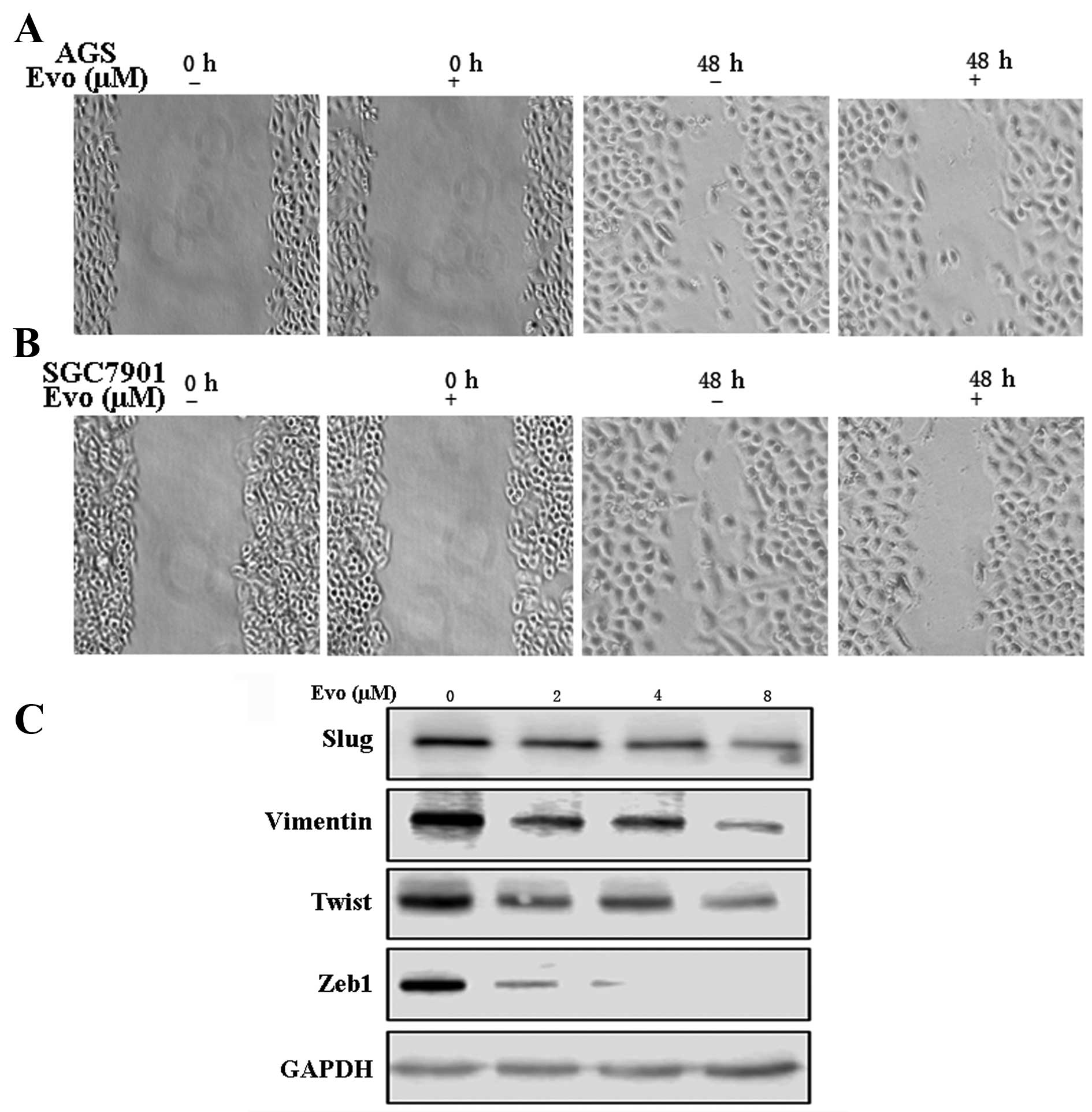
Therefore, the effect of Evo on the migratory properties of GCCs
was examined. The wound-healing migration assay showed that the
wound-healing ability of AGS and SGC7901 cells treated with Evo (2
µM for 48 h) was significantly decreased relative to
untreated controls (Fig. 6A and
B). Concordant with the aforementioned observations, Evo
reduced the expression of relevant EMT markers in GCCs. Western
blot analysis showed that Slug, Twist, Zeb1 and vimentin were
reduced with Evo treatment (Fig.
6C). In summary, Evo repressed EMT in GCCs.
Evo-mediated inhibition of GCSCs is
through the Wnt/β-catenin signaling pathway
The aforementioned experiments demonstrated a clear
effect of Evo on GCSCs; however, the molecular mechanism was
unclear. Abnormal activation of the Wnt pathway has been described
in CSCs, therefore Evo-mediated inhibition of GCSCs is possibly
through the Wnt pathway. Using western blot analysis, downstream
molecules of Wnt, including c-Myc and cyclin D1, were significantly
elevated in GCSCs (Fig. 7A) and a
β-catenin inhibitor and inhibition of the Wnt pathway decreased
sphere formation ability and stemness of GCCs (Fig. 7B). As the Wnt pathway is necessary
to maintain self-renewal of GSCS, whether Evo-mediated inhibition
of proliferation was via the Wnt pathway was investigated. The
expression levels of β-catenin, c-Myc and cyclin D1 in GCSCs were
all reduced by Evo in a dose-dependent manner (Fig. 7C and D). These findings implicated
a role for the Wnt/β-catenin signaling pathway in Evo-induced
regulation of GCSC self-renewal.
Discussion
Although CSCs represent only a minor subpopulation
of cells within a tumor, they are thought to have a major role in
tumor initiation and resistance to chemotherapy. CSCs have been
identified in solid tumors in lung, breast and colon cancer. These
cells represent a promising target for the development of novel
anticancer drugs that can inhibit all CSCs in a tumor and prevent
recurrence (21). Traditional
treatments, which receive significant attention in the field of
cancer research, often fail, demonstrating the necessity for novel
therapeutic options for the treatment of GC. For targeted
eradication of GCSCs, several novel strategies have been proposed,
including induction of apoptosis in the tumor, manipulation of GCSC
cell surface molecules, development of monoclonal antibodies,
modulation of the GCSC microenvironment and inhibition of GCSC
pathways (3). Treatment with Evo
induced apoptosis and inhibited the stemness of GCSCs by inhibiting
the Wnt pathway in the present study, thereby providing a
therapeutic approach to inhibit GCSC-targeted pathways.
Evo is widely used in Chinese herbal medicine, with
various effects. In vitro studies showed that Evo could
induce apoptosis and autophagy in GCCs (20,22). However, whether Evo inhibits
proliferation of GC through induction of apoptosis in GCSCs was
unclear. In the present study, Evo inhibited the proliferation of
GCSCs, as shown by the MTS assay and Evo induced apoptosis of
GCSCs, as apparent from flow cytometry. In addition, Evo (8
µM) reduced the expression of an antiapoptotic Bcl-2 family
member (such as Bcl-2) in a dose-dependent manner, whereas it
significantly elevated proapoptotic Bcl-2 family member proteins
(such as Bax), resulting in upregulation of the Bax/Bcl-2 ratio.
Thus, it is reasonable to suggest that Evo promoted apoptosis of
GCSCs. Additional studies are necessary to further delineate which
pathways are involved in Evo-induced apoptosis, the intrinsic or
extrinsic apoptotic pathways or both. In addition to the effects of
Evo on apoptosis, Evo reduced the spheres formation ability,
inhibited the drug resistance of oxaliplatin, and decreased the
expression of iPS factors of GCCs, revealing that Evo also
inhibited the self-renewal of GCSCs.
The Wnt pathway is a critical signaling axis that
regulates developmental processes in the embryo and maintains the
self-renewal and differentiation of stem cells (23). Inhibition of β-catenin was shown
to decrease the ability of gastric cancer cell sphere formation. In
addition, Evo downregulated the expression of β-catenin, cyclin D1
and c-Myc in GCSCs in a dose-dependent manner, suggesting that Evo
inhibited the self-renewal of GCSCs by regulating the Wnt
pathway.
The EMT is a fundamental process that is critical
for early embryo patterning during gastrulation, wound healing and
fibrotic disease (24). Aberrant
induction of the EMT has been shown to have a crucial role in the
origination, invasion and metastasis of various tumors, including
gastric cancer (25,26). Additionally, EMT endows cellular
plasticity and the properties of stemness in mammary epithelial
cells (27). The EMT has strong
link with iPS factors, such as Oct4 and Sox2 factors (28,29), suggesting that the EMT may give
rise to cancer stem cells. EMT is also correlated with activity in
the Wnt pathway. In particular, Slug, regulated by canonical Wnt
signaling, is critically important in EMT-induction of
transcription factors (30). In a
wound-healing assay, Evo significantly inhibited the migratory
properties of GCCs. This phenomenon was further corroborated by
detecting the expression of Slug, Twist, vimentin and Zeb1 in
SGC7901 cells treated with Evo. Notably, these were not detected in
AGS cells. The present findings suggest that Evo could inhibit the
expression of EMT factors in GCCs, perhaps leading to a decrease in
stemness of GCCs.
In conclusion, Evo significantly inhibited the
proliferation and self-renewal of GCSCs and decreased the
expression of EMT factors via downregulation of the Wnt pathway.
Given the role of the Wnt pathway in carcinogenesis in GCSCs, Evo
may be a potential novel antitumor agent for the treatment of
gastric cancer. However, bioavailability of Evo is low, due to its
poor water solubility, thereby limiting its anticancer efficacy
clinically. Future studies should aim to evaluate the effects of
Evo in vivo and to further develop its use as an anticancer
drug.
Acknowledgments
The authors would like to thank Ms. Theresa Fu for
English language editing. The present study was supported by the
Zhejiang Provincial Traditional Chinese Medicine Science Research
Foundation (no. 2015ZA010) and the Zhejiang Provincial Medical and
Health science Foundation (no. 2015KYB020).
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar
|
|
2
|
Fernández-Fernández FJ and Sesma P:
Gastric cancer. Lancet. 374:1594author reply 1594–1595. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Singh SR: Gastric cancer stem cells: A
novel therapeutic target. Cancer Lett. 338:110–119. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stojnev S, Krstic M, Ristic-Petrovic A,
Stefanovic V and Hattori T: Gastric cancer stem cells: Therapeutic
targets. Gastric Cancer. 17:13–25. 2014. View Article : Google Scholar
|
|
5
|
Takaishi S, Okumura T, Tu S, Wang SS,
Shibata W, Vigneshwaran R, Gordon SA, Shimada Y and Wang TC:
Identification of gastric cancer stem cells using the cell surface
marker CD44. Stem Cells. 27:1006–1020. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang L, Ping YF, Yu X, Qian F, Guo ZJ,
Qian C, Cui YH and Bian XW: Gastric cancer stem-like cells possess
higher capability of invasion and metastasis in association with a
mesenchymal transition phenotype. Cancer Lett. 310:46–52.
2011.PubMed/NCBI
|
|
7
|
Ryu HS, Park J, Kim HH, Kim WH and Lee HS:
Combination of epithelial-mesenchymal transition and cancer stem
cell-like phenotypes has independent prognostic value in gastric
cancer. Hum Pathol. 43:520–528. 2012. View Article : Google Scholar
|
|
8
|
Han ME and Oh SO: Gastric stem cells and
gastric cancer stem cells. Anat Cell Biol. 46:8–18. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mishra L, Shetty K, Tang Y, Stuart A and
Byers SW: The role of TGF-beta and Wnt signaling in
gastrointestinal stem cells and cancer. Oncogene. 24:5775–5789.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Clevers H, Loh KM and Nusse R: Stem cell
signaling. An integral program for tissue renewal and regeneration:
Wnt signaling and stem cell control. Science. 346:12480122014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mo XM, Li HH, Liu M and Li YT:
Downregulation of GSK3β by miR-544a to maintain self-renewal
ability of lung caner stem cells. Oncol Lett. 8:1731–1734.
2014.PubMed/NCBI
|
|
12
|
Zhao Z, Lu P, Zhang H, Xu H, Gao N, Li M
and Liu C: Nestin positively regulates the Wnt/β-catenin pathway
and the proliferation, survival and invasiveness of breast cancer
stem cells. Breast Cancer Res. 16:4082014. View Article : Google Scholar
|
|
13
|
Watanabe K, Biesinger J, Salmans ML,
Roberts BS, Arthur WT, Cleary M, Andersen B, Xie X and Dai X:
Integrative ChIP-seq/microarray analysis identifies a CTNNB1 target
signature enriched in intestinal stem cells and colon cancer. PLoS
One. 9:e923172014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mao J, Fan S, Ma W, Fan P, Wang B, Zhang
J, Wang H, Tang B, Zhang Q, Yu X, et al: Roles of Wnt/β-catenin
signaling in the gastric cancer stem cells proliferation and
salinomycin treatment. Cell Death Dis. 5:e10392014. View Article : Google Scholar
|
|
15
|
Cai C and Zhu X: The Wnt/β-catenin pathway
regulates self-renewal of cancer stem-like cells in human gastric
cancer. Mol Med Rep. 5:1191–1196. 2012.PubMed/NCBI
|
|
16
|
Kobayashi Y: The nociceptive and
anti-nociceptive effects of evodiamine from fruits of Evodia
rutaecarpa in mice. Planta Med. 69:425–428. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang S, Wang L, Shi Z, Zhong Z, Chen M and
Wang Y: Evodiamine synergizes with doxorubicin in the treatment of
chemoresistant human breast cancer without inhibiting
P-glycoprotein. PLoS One. 9:e975122014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chien CC, Wu MS, Shen SC, Ko CH, Chen CH,
Yang LL and Chen YC: Activation of JNK contributes to
evodiamine-induced apoptosis and G2/M arrest in human colorectal
carcinoma cells: A structure-activity study of evodiamine. PLoS
One. 9:e997292014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wei J, Li Z and Yuan F: Evodiamine might
inhibit TGF-beta1-induced epithelial-mesenchymal transition in
NRK52E cells via Smad and PPAR-gamma pathway. Cell Biol Int.
38:875–880. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang L, Liu X, Wu D, Zhang M, Ran G, Bi Y
and Huang H: Growth inhibition and induction of apoptosis in
SGC-7901 human gastric cancer cells by evodiamine. Mol Med Rep.
9:1147–1152. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Visvader JE and Lindeman GJ: Cancer stem
cells: Current status and evolving complexities. Cell Stem Cell.
10:717–728. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rasul A, Yu B, Zhong L, Khan M, Yang H and
Ma T: Cytotoxic effect of evodiamine in SGC-7901 human gastric
adenocarcinoma cells via simultaneous induction of apoptosis and
autophagy. Oncol Rep. 27:1481–1487. 2012.PubMed/NCBI
|
|
23
|
Van Camp JK, Beckers S, Zegers D and Van
Hul W: Wnt signaling and the control of human stem cell fate. Stem
Cell Rev. 10:207–229. 2014. View Article : Google Scholar
|
|
24
|
Lee JM, Dedhar S, Kalluri R and Thompson
EW: The epithelial-mesenchymal transition: New insights in
signaling, development, and disease. J Cell Biol. 172:973–981.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Montemayor-Garcia C, Hardin H, Guo Z,
Larrain C, Buehler D, Asioli S, Chen H and Lloyd RV: The role of
epithelial mesenchymal transition markers in thyroid carcinoma
progression. Endocr Pathol. 24:206–212. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao L, Li W, Zang W, Liu Z, Xu X, Yu H,
Yang Q and Jia J: JMJD2B promotes epithelial-mesenchymal transition
by cooperating with β-catenin and enhances gastric cancer
metastasis. Clin Cancer Res. 19:6419–6429. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Luo W, Li S, Peng B, Ye Y, Deng X and Yao
K: Embryonic stem cells markers SOX2, OCT4 and Nanog expression and
their correlations with epithelial-mesenchymal transition in
nasopharyngeal carcinoma. PLoS One. 8:e563242013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chiou SH, Wang ML, Chou YT, Chen CJ, Hong
CF, Hsieh WJ, Chang HT, Chen YS, Lin TW, Hsu HS, et al:
Coexpression of Oct4 and Nanog enhances malignancy in lung
adenocarcinoma by inducing cancer stem cell-like properties and
epithelial-mesenchymal transdifferentiation. Cancer Res.
70:10433–10444. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu ZQ, Li XY, Hu CY, Ford M, Kleer CG and
Weiss SJ: Canonical Wnt signaling regulates Slug activity and links
epithelial-mesenchymal transition with epigenetic Breast Cancer 1,
Early Onset (BRCA1) repression. Proc Natl Acad Sci USA.
109:16654–16659. 2012. View Article : Google Scholar : PubMed/NCBI
|