Introduction
Acute lung injury (ALI) is a disorder of acute lung
inflammation and is clinically characterized by the enhanced
permeability of the alveolar-capillary barrier and disordered
air-exchange function. It is also known as acute respiratory
distress syndrome (ARDS) (1). Its
typical pathological characteristics include injury to pulmonary
capillary endothelial cells and alveolar epithelial cells,
extensive pulmonary edema, microatelectasis, microthrombosis and
microcirculation disturbance (2).
Serious infection, trauma, shock, intoxication and inhalation of
toxic gases are the most common causes of ALI (3). ALI/ARDS is a common critical disease
with high morbidity and fatality rates (4). With improvements in medical
technology and comprehensive treatment methods, the fatality rate
has decreased to a certain extent; however, it remains as high as
30–40% (5). Moreover, the
mechanisms responsible for the development of ALI/ARDS are complex
and are not yet fully understood (4,5).
Currently, clinical therapy is mainly comprehensive, and no
specific treatment method is available (6). Consequently, studies on the
prevention and treatment of ALI/ARDS are of great importance
(4).
ALI is a disease characterized by direct or indirect
damage to lung epithelial and endothelial cells (7). The mechanisms responsible for the
development of this disease, however, remain to be elucidated
(7). It was originally considered
that the root cause of ALI is cell activation by pathogenic factors
and bodily fluids, which cause inflammatory response syndrome, and
pathological processes such as alveolar collapse, an imbalance in
the ventilation/perfusion ratio and a decrease in lung compliance.
The imbalance between inflammatory reactions and anti-inflammatory
reactions is of great significance in the development of ALI
(7,8). The disease can be divided into the
early and late stages according to its pathological characteristics
(9). Acute inflammation of the
lung tissue and damage to lung cells are the pathological
characteristics of the early stages of the disease (10). Pulmonary fibrosis and lung cell
damage are the main characteristics of the later stages of the
disease. In recent years, a certain amount of progress has been
made as regards the understanding of the mechanisms responsible for
ALI (11). The
inflammation/anti-inflammatory mechanism, oxidation/antioxidant
mechanism, coagulation/fibrinolysis system and cell apoptosis
mechanism have been identifed to be involved in the development of
ALI (12). In addition, in this
study, we investigated whether sulforaphane exerts
anti-inflammatory effects against lipopolysaccharide (LPS)-induced
ALI in mice through the Nrf2/ARE pathway.
Sulforaphane (1-isothiocyanate-4-methyl sulfonyl
butane) is also known as 'raphanin'. In terms of its chemical
components, these are derivatives of isothiocyanate which is
soluble in water with a relative molecular mass of 177.3 and
molecular formula as C6H11S2NO (13). Sulforaphane as an agonist of
nuclear factor-E2-related factor 2 (Nrf2), is extracted from
cruciferous vegetables (such as broccoli, brussels sprouts and
cabbage) (14). It has previously
been demonstrated that sulforaphane exerts protective effects on
multiple organs, such as the liver, lungs, kidneys, heart and
nervous system by activating Nrf2 (14). Sulforaphane has also been shown to
exert protective effects on mouse peritoneal macrophages positive
for Nrf2; however, these effects were attenuated in macrophages
negative for Nrf2 and the authors concluded that sulforaphane
exerts its anti-inflammatory via the activation of Nrf2 (15). Thus, in the present study, we
aimed to investigate the anti-inflammatory effects of sulforaphane
and the mechanisms through which it protects against LPS-induced
ALI. We also aimed to investigate the signaling pathways
involved.
Nrf2 is an important transcription factor and a key
regulator of the activation of antioxidant genes to exogenous
stimulations. Reactive oxygen species (ROS) give rise to the
disruption of the Nrf2/Kelch-like ECH-associated protein-1 (Keap1)
complex and Nrf2 from the cytoplasm into the nucleus where Keap1
dimerizes with antioxidant response element (ARE) DNA sequence
ultimately activates the expression of ARE-dependent genes.
Materials and methods
Materials
The interleukin-6 (IL-6), tumor necrosis factor-α
(TNF-α), prostaglandin E2 (PGE2) and nitric oxide (NO) commercial
enzyme-linked immunosorbent assay (ELISA) kits were purchased from
the Nanjing Jiancheng Bioengineering Institute, Nanjing, China. The
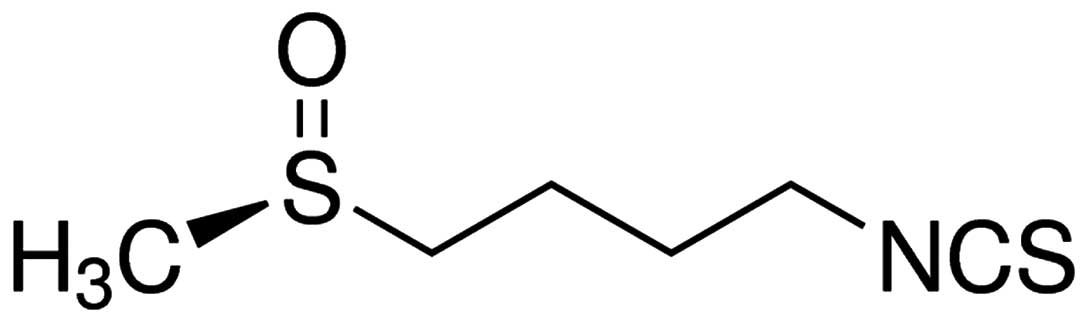
chemical structure of sulforaphane (≥95%; Sigma-Aldrich Co. LLC,
St. Louis, MO, USA) is illustrated in Fig. 1.
Animals
Male BALB/c mice (8–10 weeks old, weighing 18–20 g)
were purchased from the Second Hospital of Hebei Medical University
(Hebei, China). All experimental procedures were strictly in
accordance with the Guide for the Care and Use of Laboratory
Animals of The Second Hospital of Hebei Medical University. This
study was approved by the Ethics Committee of The Second Hospital
of Hebei Medical University. The male BALB/c mice were housed in
standard environmental conditions of light (12/12 h light/dark
cycle), temperature (22±2°C) and 50±10% humidity, with free access
to food and water.
Mouse model of ALI and experimental
groups
The BALB/c mice were randomly divided into 3 groups
as follows: the control, the model and sulforaphane-treated groups
(n=8 per group). Prior to the induction of ALI, sulforaphane (50
mg/kg) was administered by an intraperitoneal (i.p.) injection, as
previously described (16).
Seventy-two hours later, 25 µg LPS were instilled in 50
µl phosphate-buffered saline (PBS) to induce lung
injury.
Lactate dehydrogenase (LDH) assay
At 12 h after the LPS challenge, the mice were
sacrificed by decollation and lung tissue was acquired and mixed
with freshly prepared reaction mixture. The lung samples were
incubated with LPS in the dark for 30 min at room temperature. The
absorbance was measured using a microplate reader (BMG Labtech,
Ortenberg, Germany) at 490 nm.
Measurement of the wet-to-dry ratio of
the lungs
At 12 h after the LPS challenge, the mice were
sacrificed by decollation and lung tissue was acquired and the
right lungs were excised and weighed. Subsequently, the right lungs
were placed in an incubator at 80°C for 48 h and weighed. The
wet-to-dry ratio of the lungs was calculated as follows: dry
weight/wet weight ×100.
ELISA
At 12 h after the LPS challenge, lung samples were
acquired and lavaged with 500 µl sterile PBS. Lung samples
were centrifuged at 3,000 rpm for 10 min at 4°C. The IL-6, TNF-α
and PGE2 activities were analyzed using a microplate reader (BMG
Labtech), in accordance with the manufacturer's instructions
(Nanjing Jiancheng Bioengineering Institute).
Western blot analysis for nuclear
factor-κB (NF-κB), cyclooxy-genase-2 (COX-2) and matrix
metalloproteinase-9 (MMP-9) expression
At 12 h after the LPS challenge, lung samples were
acquired and lavaged with 500 µl sterile PBS. The lung
samples were homogenized with RIPA buffer (Beyotime Biotech,
Shanghai, China) and centrifuged at 3,000 rpm for 10 min at 4°C.
The protein concentration was determined with the BCA protein assay
(Beyotime Biotech). The homogenized samples were separated by 10%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) and electrophoretically transferred onto nitrocellulose
membranes (Millipore, Billerica, MA, USA), which were blocked with
5% non-fat milk with Tris-buffered saline for 2 h at room
temperature. The membranes were incubated overnight at 4°C with
primary antibodies to NF-κB (sc-109), COX-2 (sc-514489) and MMP-9
(sc-21733; all from Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA) overnight at 4°C followed by incubation with horseradish
peroxidase-conjugated secondary antibody (sc-69916; Santa Cruz
Biotechnology, Inc.) for 2 h at room temperature. All blots were
washed with enhanced chemiluminescence (ECL) reagents according to
the manufacturer's instructions and analyzed using Bio-Rad Quantity
One software (Bio-Rad, Hercules, CA, USA).
RNA isolation and quantitative PCR
(qPCR)
At 12 h after the LPS challenge, lung samples were
acquired and were used in the extraction of total RNA using TRIzol
reagent (Invitrogen, Carlsbad, CA, USA). Subsequently, the RNA was
purified using the RNeasy mini kit according to the manufacturer's
instructions (Qiagen, Valencia, CA, USA). cDNA was synthesized
using an iScript cDNA synthesis kit (Bio-Rad). The 7000 Sequence
Detection system (Applied Biosystems, Foster City, CA, USA) was
used to perform qPCR. The sequences of the primers used for gene
amplification were as follows: Nrf2 forward,
5′-CCCACAAGTTCGGCATCCAC-3′ and reverse, 5′-TGGCGATTCCTCTGGCGTCT-3′;
and β-actin forward, 5′-CGCGACATCAAGGAGAAGCTG-3′ and reverse,
5′-ATTGCCAATGGGTGATACCTG-3′.
Measurement of NO production
At 12 h after the LPS challenge, lung samples were
acquired and lavaged with 500 µl sterile PBS. The lung
samples were homogenized with RIPA buffer (Beyotime Biotech) and
centrifuged at 3,000 rpm for 10 min at 4°C. The supernatant was
incubated using a micro-plate reader (BMG Labtech), for 10 min at
room temperature and the optical density was measured at 540
nm.
Statistical analysis
Data are expressed as the means ± SD, and were
analyzed by one-way analysis of variance (ANOVA); variations
between the different groups were compared using the Tukey-Kramer
post hoc test. A P-value <0.05 was deemed asto indicate a
statistically significant difference.
Results
Anti-inflammatory effects of sulforaphane
on LDH activity in mice with LPS-induced ALI
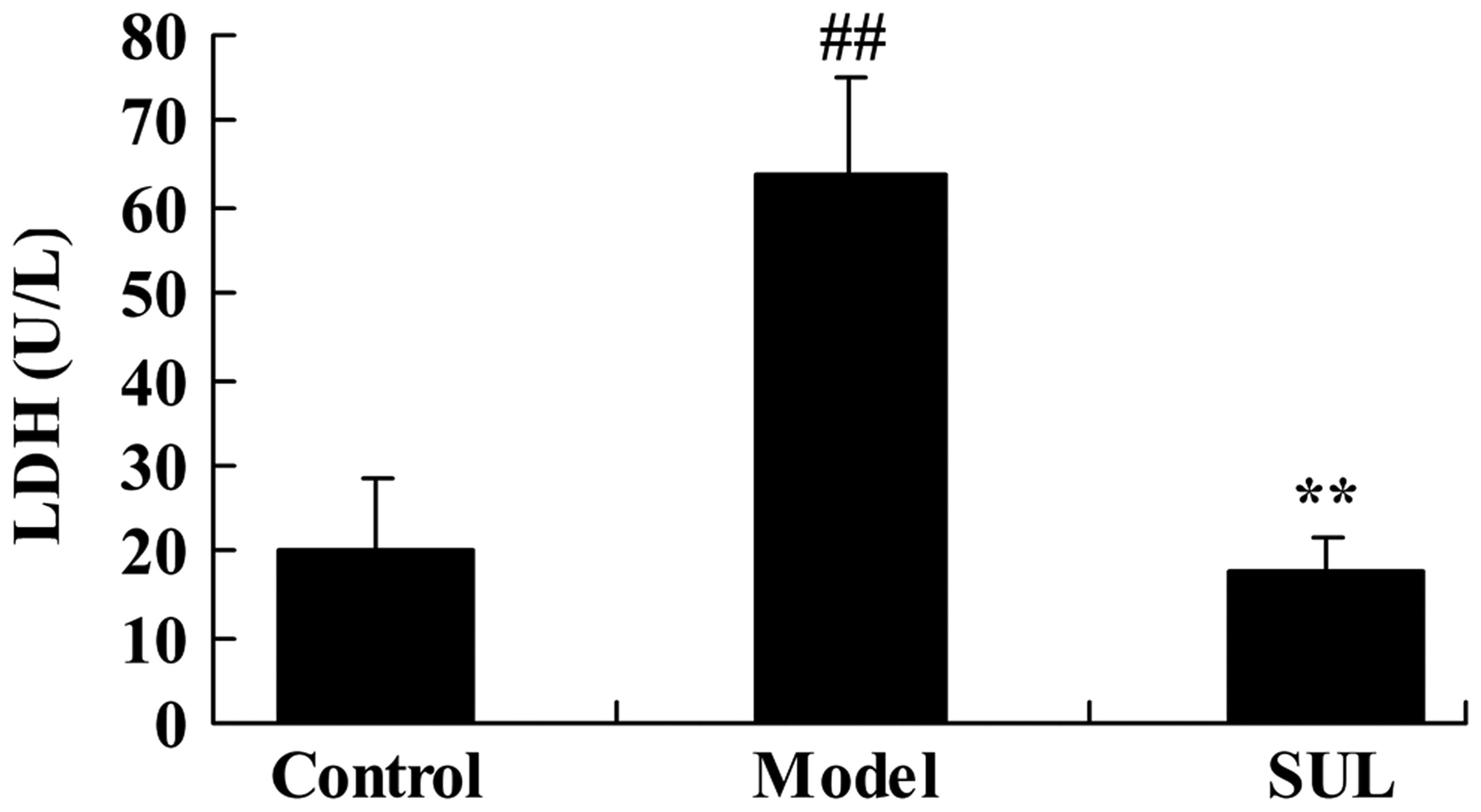
To evaluate the effects of sulforaphane on LDH
activity in mice with LPS-induced ALI, we established a model of
LPS-induced ALI and measured LDH activity. LPS effectively induced
LDH activity in the mice compared to the untreated control group
(Fig. 2). Treatment with
sulforaphane effectively inhibited LDH activity in the mice with
ALI (Fig. 2).
Anti-inflammatory effects of sulforaphane
on the wet-to-dry ratio of the lungs of mice with LPS-induced
ALI
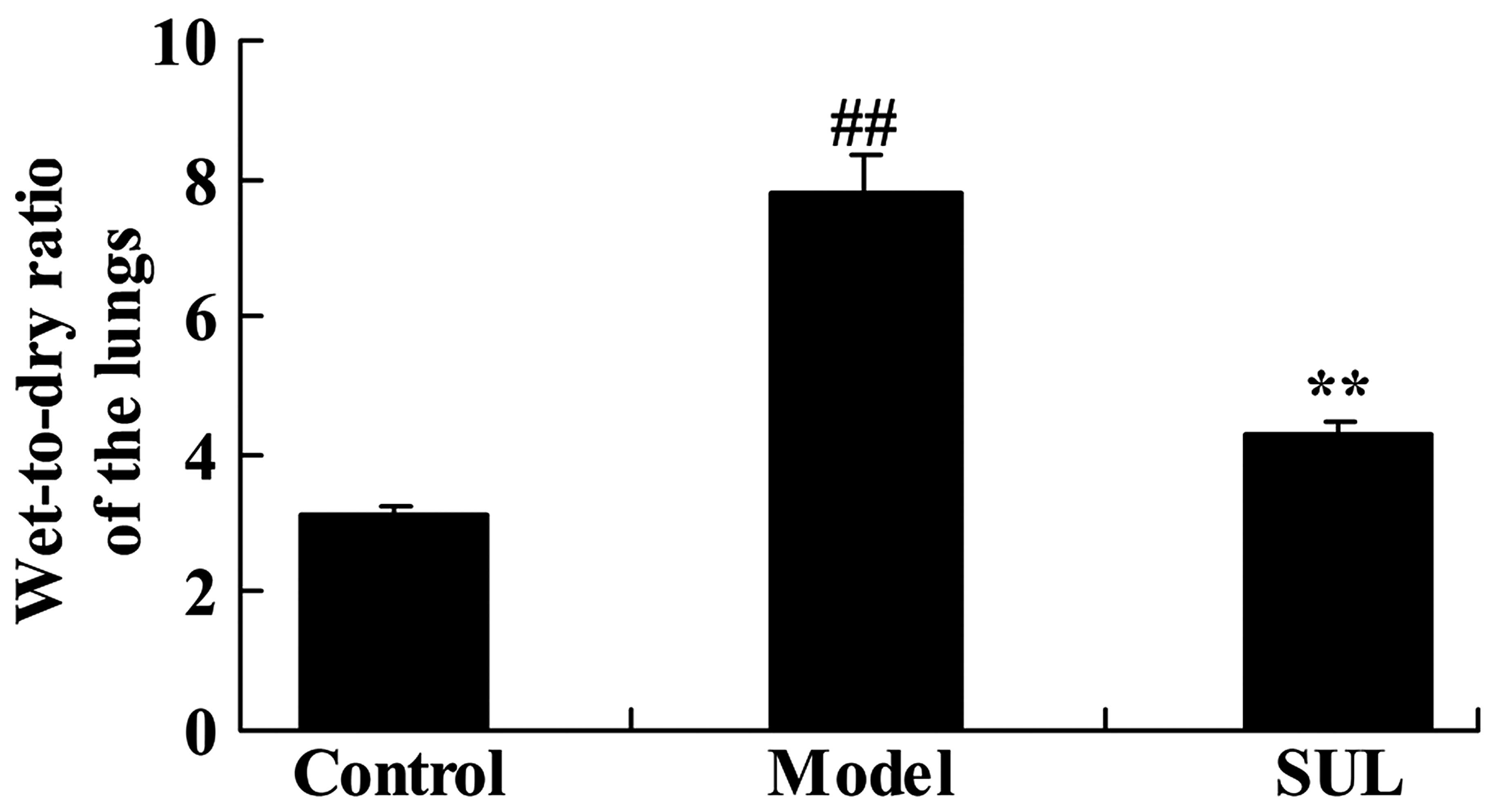
We subsequently determined the anti-inflammatory
effects of sulforaphane on the wet-to-dry ratio of the lungs of
mice with LPS-induced ALI. LPS-induced ALI notably increased the
wet-to-dry ratio of the lungs of mice with ALI, compared to lungs
from the mice in the control group (Fig. 3). We also noted that treatment
with sulforaphane decreased the wet-to-dry ratio, almost to the
same level as the control group. These data demonstrated that
pre-treatment with sulforaphane markedly reduced the wet-to-dry
ratio of lungs of mice with ALI.
Anti-inflammatory effects of sulforaphane
on the lungs of mice with LPS-induced ALI
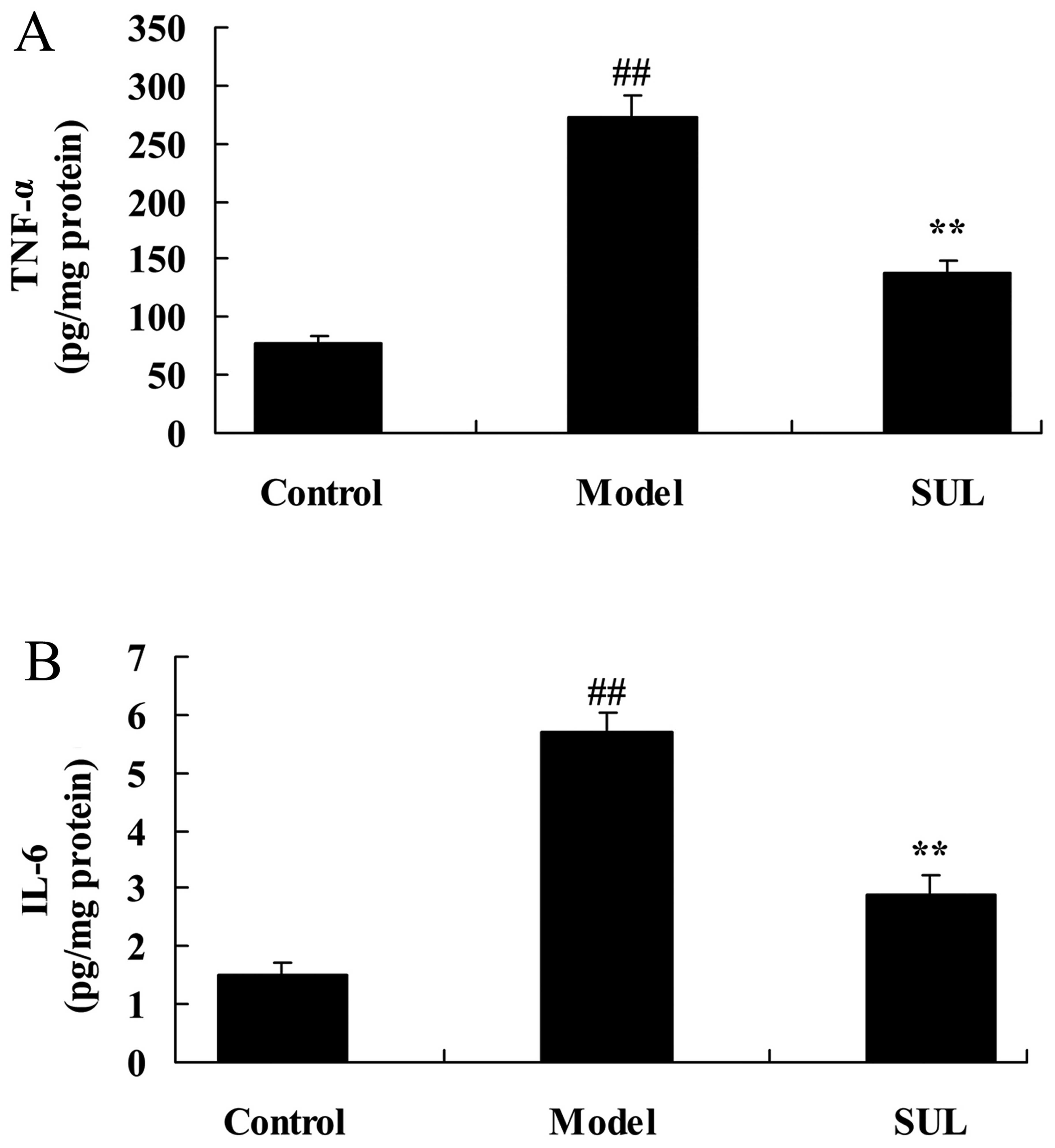
To examine the anti-inflammatory effects of
sulforaphane on the lungs of mice with LPS-induced ALI, the
activities of IL-6 and TNF-α were surveyed following the induction
of ALI by LPS. The IL-6 and TNF-α activities were markedly
increased in the mice with LPS-induced ALI, compared to the mice in
the untreated control group (Fig.
4). Treatment with sulforaphane led to a significant decrease
in IL-6 and TNF-α activities in the mice with LPS-induced ALI
(Fig. 4).
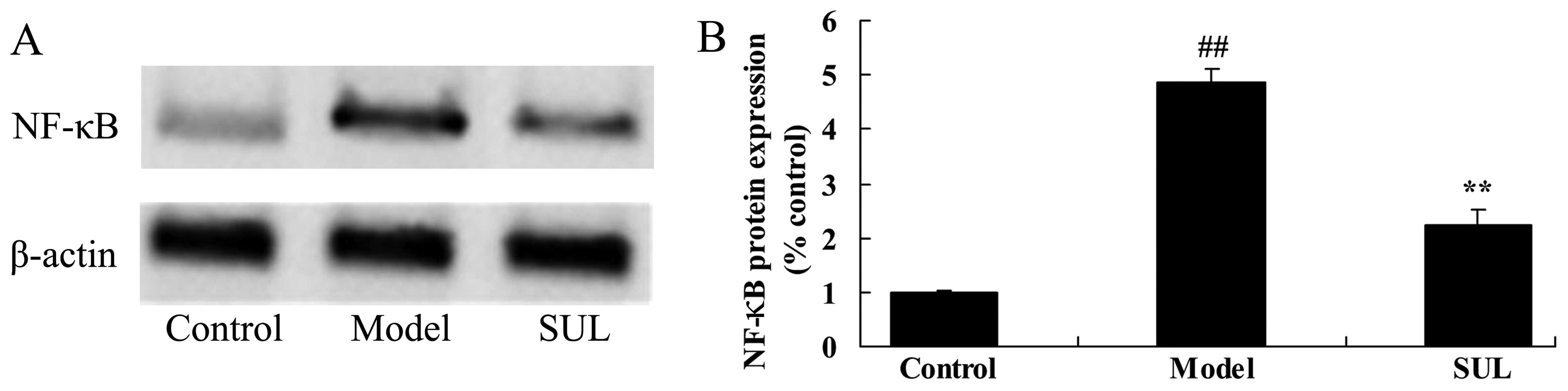
Anti-inflammatory effects of sulforaphane
on NF-κB protein expression in lungs of mice with LPS-induced
ALI
To examine the anti-inflammatory mechanisms of
action of sulforaphane on NF-κB protein expression in the lungs of
mice with LPS-induced ALI, western blot analysis was used to
measure the NF-κB protein expression levels. The results revealed
that NF-κB protein expression was higher in the lungs of mice with
LPS-induced ALI, compared to the lungs of mice in the control group
(Fig. 5). The
sulforaphane-treated mice exhibited a significant decrease in NF-κB
protein expression in the lungs compared to the mice with
LPS-induced ALI (Fig. 5).
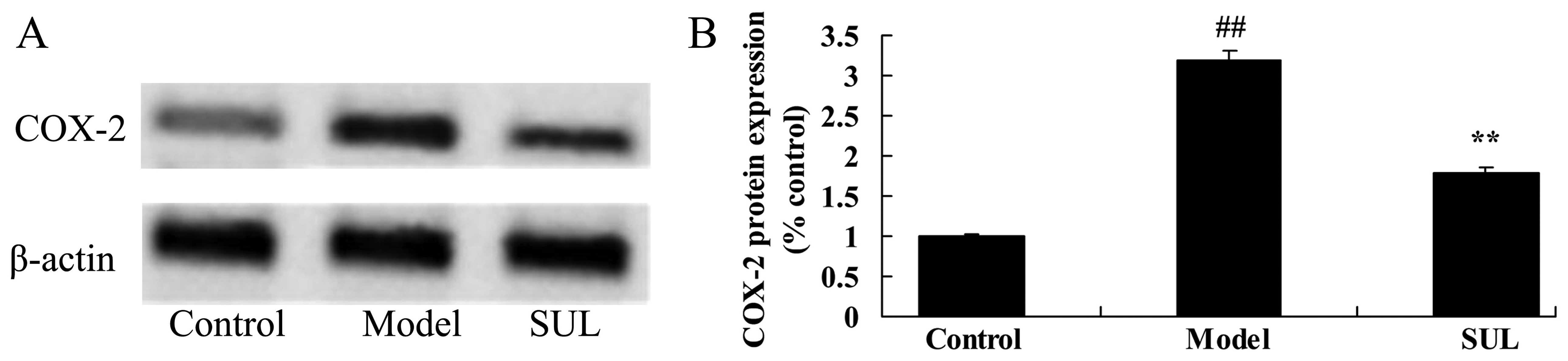
Anti-inflammatory effects of sulforaphane
on COX-2 protein expression in the lungs of mice with LPS-induced
ALI
As shown in Fig.
6, there was a significant increase in COX-2 protein expression
in the lungs of mice with LPS-induced ALI, compared to the mice in
the control group. However, treatment with sulforaphane
significantly suppressed COX-2 protein expression in the lungs of
mice with LPS-induced ALI (Fig.
6).
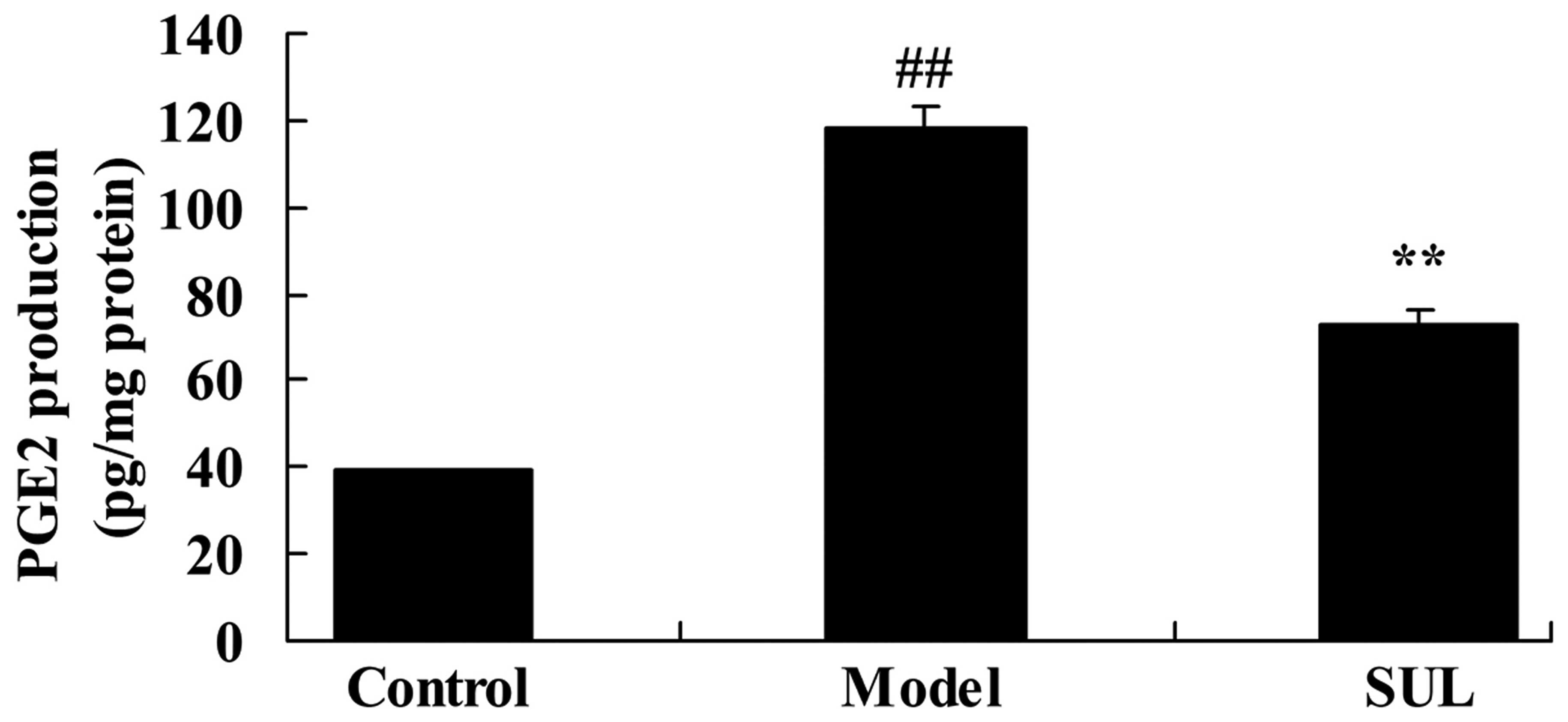
Anti-inflammatory effects of sulforaphane
on PGE2 production in the lungs of mice with LPS-induced ALI
PGE2 production in the lungs of mice with
LPS-induced ALI was significantly higher than that in the lungs of
mice in the control group (Fig.
7). We noted that PGE2 production was significantly reduced by
treatment with sulforaphane (Fig.
7).
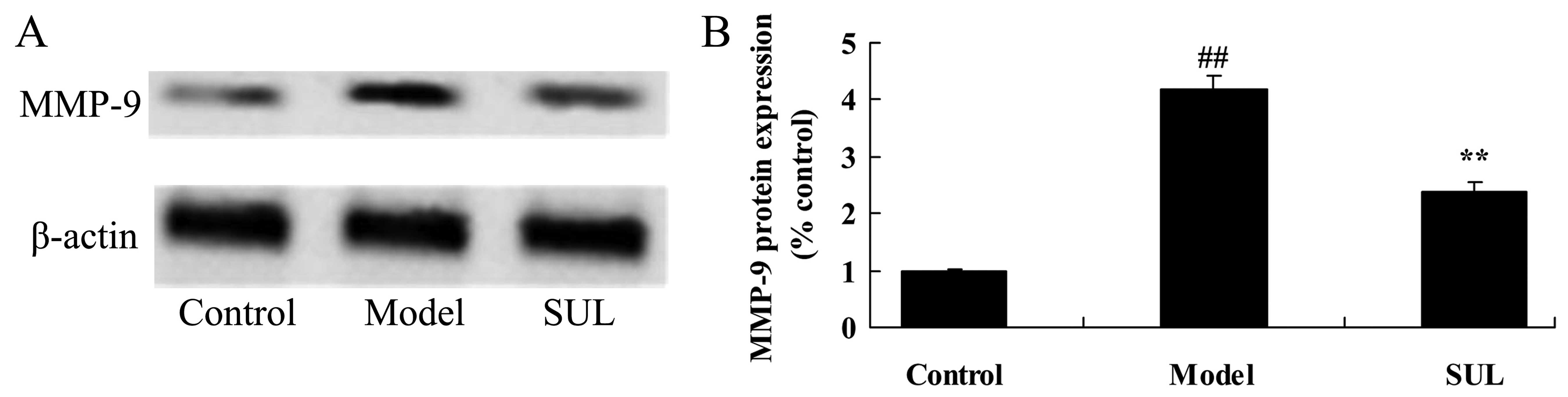
Anti-inflammatory effects of sulforaphane
on MMP-9 protein expression in the lungs of mice with LPS-induced
ALI
LPS markedly increased MMP-9 protein expression in
the lungs of mice with LPS-induced ALI. Western blot analysis was
used to measure the MMP-9 protein expression levels. MMP-9 protein
expression was markedly increased in the mice with LPS-induced ALI,
compared to the mice in the control group (Fig. 8). In the lungs of mice treated
with sulforaphane, we noted a statistically significant decrease in
MMP-9 protein expression (Fig.
8).
Anti-inflammatory effects of sulforaphane
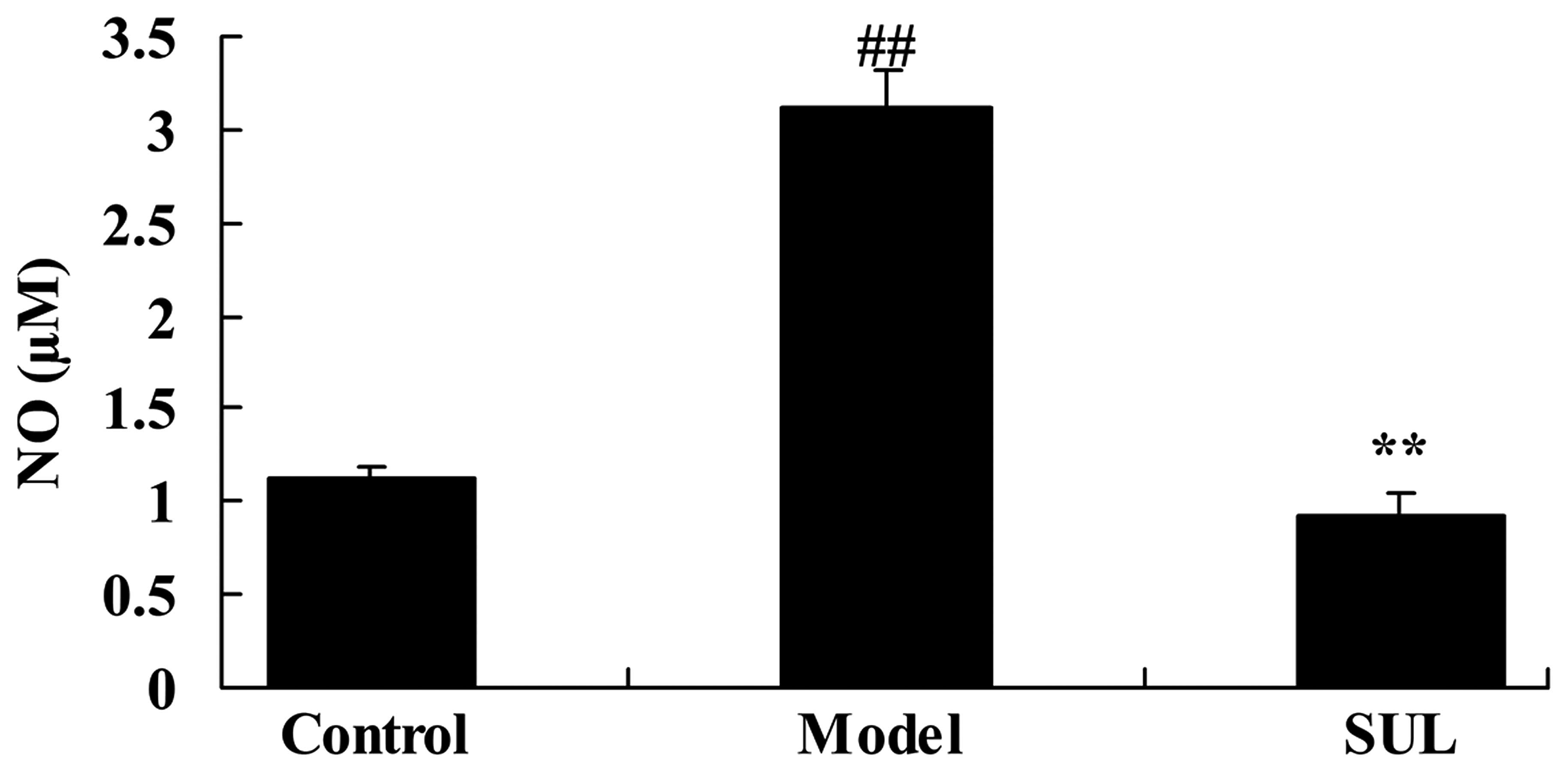
on NO production in the lungs of mice with LPS-induced ALI
There was a significant increase in the NO levels in
the lungs of mice with LPS-induced ALI, compared to the mice in the
control group (Fig. 9). However,
treatment with sulforaphane significantly decreased the NO levels
(Fig. 9).
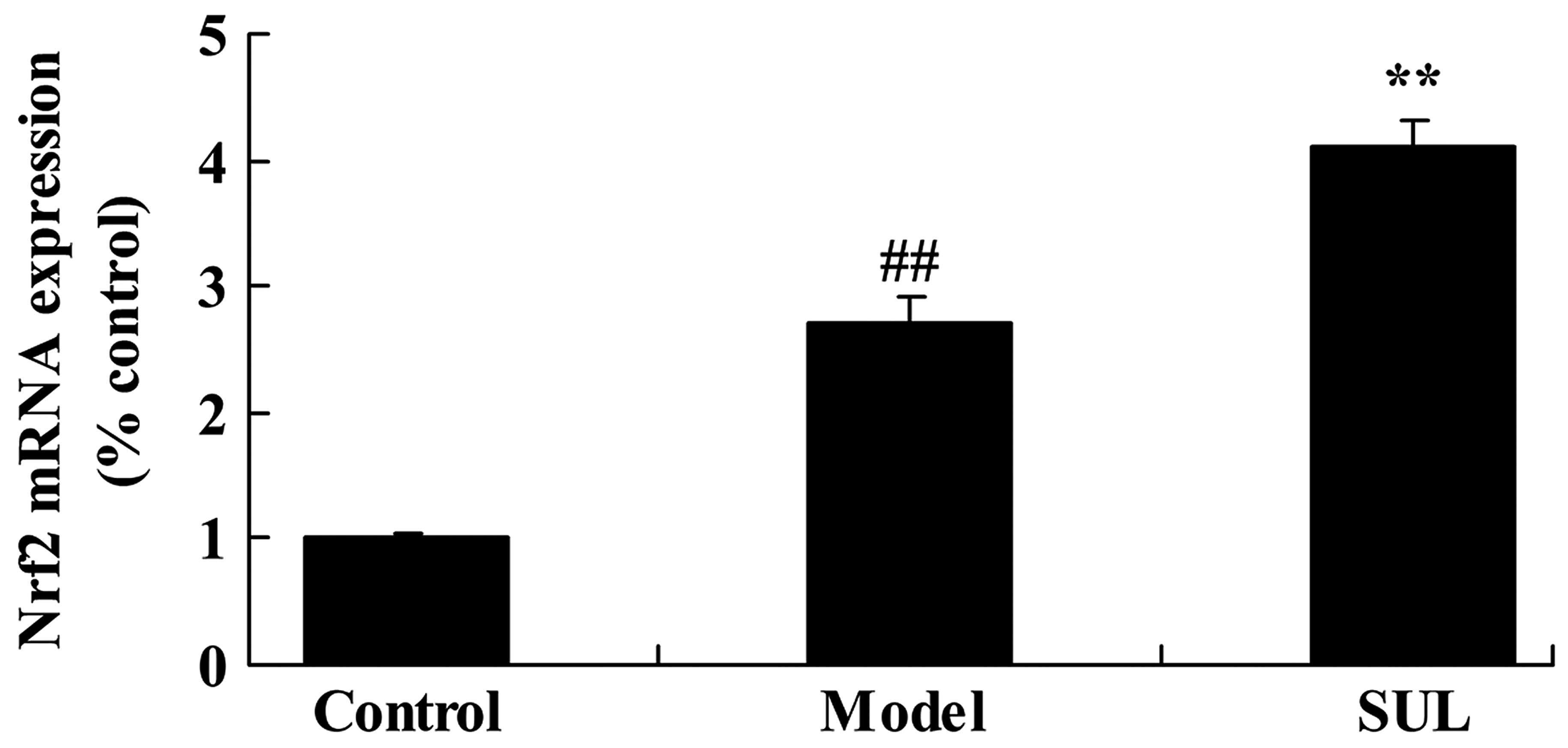
Anti-inflammatory effects of sulforaphane
on Nrf2 mRNA expression in the lungs of mice with LPS-induced
ALI
Compared to the untreated control group, Nrf2 mRNA
expression in the lungs of mice with LPS-induced ALI was
significantly increased (Fig.
10). Treatment with sulforaphane led to a further significant
increase in Nrf2 mRNA expression compared to the mice with
LPS-induced ALI not treated with sulforaphane (Fig. 10).
Discussion
ALI is a disorder of lung inflammation and is
clinically characterized by the enhanced permeability of the
alveolar-capillary barrier and disordered air-exchange function
(17). Its typical pathological
characteristics include injury to pulmonary capillary endothelial
cells and alveolar epithelial cells, extensive pulmonary edema,
microatelectasis, microthrombosis and microcirculation disturbance
(2). It commonly occurs following
serious infection, trauma, shock, intoxication and inhalation of
toxic gases. ALI (also known as ARDS) is a common critical disease
with a high mortality rate (4).
The mechanisms repsonsible for the development of ALI are quite
complex and have not yet been fully elucidated. Consequently, it is
still an important topic which requires further research in today's
medical industry. In the present study, we demonstrated that
sulforaphane significantly decreased LDH activity and the
wet-to-dry ratio of the lungs of mice with LPS-induced ALI.
Excessive lung inflammation response is a typical
pathological characteristic of ALI/ARDS (10). Its pathological characteristics
are widespread and are very difficult to control, leading to a
cascade reaction (12). According
to different pathological stages of inflammation, it can be divided
into the inflammatory exudation phase in the early stages and fiber
hyperplasia in the later stages of the disease (18). The administration of
anti-inflammatories constitutes a therapy specific to inflammatory
reactions, and such treatment can inhibit inflammatory reactions,
reduce damage and is an effective method of treating lung injury
during the early stages (19).
The findings of our study revealed that treatment with sulforaphane
significantly decreased the IL-6 and TNF-α activities in mice with
LPS-induced ALI. In a previous study, it was demonstrated that
sulforaphane inhibited the LPS-stimulated inflammatory response by
modulating NF-κB in human monocytes (20). Nallasamy et al indicated
that sulforaphane reduced vascular inflammation by interfering with
the NF-κB pathway in mice (21).
NF-κB is a nuclear factor present in the majority of
cells. It has a very important effect on the growth,
differentiation, adhesion, apoptosis and inflammation of cells
(22,23). While in a state of inactivity, it
combines with IKB in the cytoplasm in an inactive state. In cases
of external stimulation, IKB undergoes rapid phosphorylation
degradation so as to activate NF-κB and begin nuclear translocation
(11). After NF-κB is activated,
it inevitably regulates the gene expression of inflammatory
cytokines participating in the inflammatory reaction and mediates
the development of ALI (24,11). Epoxidase is an essential
rate-limiting enzyme which is involved in the process of
prostaglandin synthesis (25). It
has two isozymes, COX-1 and COX-2. COX-2 is an instant and early
gene. Its high expression is induced by a variety of genes
(26). The activation of NF-κB
induces the activation of COX-2, which may increase the levels of
the endogenous immune inhibitor, PGE2, which causes
immunosuppression following infection, thus increasing the
occurrence rate of lung injury and the fatality rate (27). In the present study, sulforaphane
significantly suppressed NF-κB and COX-2 protein expression in mice
with LPS-induced ALI. Nallasamy et al indicated that
sulforaphane reduced vascular inflammation by interfering with the
NF-κB pathway in mice (21). Shan
et al suggested that sulforaphane downregulates COX-2
expression by inhibiting NF-κB in human bladder T24 cells (28).
PGE2 is a prostaglandin which plays a complex
biological role in the ALI/ARDS inflammatory reaction process
(29). PGE2 synthase is the key
enzyme for the last step of PGE2 synthesis. Of the detected three
types of PGE2 synthase, microsomal prostaglandin E2
synthase-1 (mPGES-1), as the only induced enzyme, has been noted to
be upregulated in response to various types of inflammation
(29). In ventilator-associated
lung injury in mice, mPGES-1-derived PGE2 has been shown to play a
role in the onset of lung injury and pulmonary edema, and can
promote pathological reactions in a variety of effector cells and
can activate cytokines and inflammatory reactions (30,31). In the present study, we
demonstrated that treatment with sulforaphane significantly
decreased PGE2 production in mice with LPS-induced ALI. Choi et
al previously suggested that sulforaphane inhibits
IL-1β-induced proliferation by decreasing the production of MMP,
COX-2 and PGE2 (32).
It has previously been suggested that ALI may
trigger the activation of a variety of effector cells, inflammatory
reactions and cytokines (33).
MMPs are a family of genetically related endopeptidases whose
activity is zinc-dependent (33).
MMP-9 is an important member of the family (34). It has high hydrolysis specificity
for type IV and V collagen protein and gelatin (33). It plays a certain regulatory role
in matrix reconstruction. The endogenous inhibitor of MMP-9 is
tissue matrix metalloproteinase inhibitor-1 (TIMP-1) (34,35). Under physiological conditions, the
activity of MMP-9 is inhibited by TIMP-1 (35). Both are under an equilibrium state
(35). If an imbalance exists
between the two factors participating in airway remodeling, this
results in the development of ALI. In the present study, we found
that sulforaphane significantly decreased MMP-9 protein expression
in mice with LPS-induced ALI. Mao et al demonstrated that
sulforaphane attenuates MMP-9 expression following spinal cord
injury in mice (36).
NO has a wide range of biological functions, and
affects various pathological and physiological processes (37). Endogenous NO in pulmonary
circulation can attenuate hypoxic pulmonary vasoconstriction,
increase anoxic lung alveolar fluid filling and protect against
hypoxia, thus preventing ALI (38). NO inhibits neutrophil migration to
the endothelium, thus reducing the generation of inflammatory
cytokines (37). NO also inhibits
the activation of neutrophile granulocytes and decreases the
release of toxic oxidation products, which is conducive to
maintaining the integrity of the alveolar-capillary membrane and
repairing damage caused by ALI (39). In this study, treatment with
sulforaphane significantly decreased the NO levels in the lungs of
mice with LPS-induced ALI. Brandenburg et al demonstrated
that sulforaphane suppressed LPS-induced inflammation by decreasing
NO levels in rat primary microglia (40).
Nrf2 is an important regulator of cell oxidation. It
can regulate the expression of peroxiredoxins, generation II
metabolic enzymes, enhance the ability of cells to remove ROS,
maintain the oxidation-reduction equilibrium status in cells, and
reduce oxidative damage through interaction with ARE (41). Nrf2/ARE is an edogenous
antioxidant stress pathway (42).
It plays an important role in the treatment of a variety of
diseases (42). It has been
demonstrated that Nrf2 is a promising target for the treatment of
ALI (41). In the present study,
sulforaphane increased Nrf2 gene expression in the lungs of mice
with LPS-induced ALI. Chi et al demonstrated that
sulforaphane reduced apoptosis and protected against liver
injury-induced ischemic reperfusion by activating the Nrf2/ARE
pathway (14). Lin et al
revealed that sulforaphane suppressed LPS-induced inflammation
through the Nrf2 pathway in mouse peritoneal macrophages (15).
In conclusion, the findings of this study
demonstrate that sulforaphane protects against LPS-induced ALI in
mice by exerting anti-inflammatory effects, by mediating the
production of NF-κB, COX-2, PGE2 and NO and upregulating Nrf2
expression. Moreover, the present findings provide a scientific
foundation for the use of sulforaphane as a prophylactic treatment
for ALI in clinical settings.
References
|
1
|
Emr BM, Roy S, Kollisch-Singule M, Gatto
LA, Barravecchia M, Lin X, Young JL, Wang G, Liu J, Satalin J, et
al: Electroporation-mediated gene delivery of Na+,
K+-ATPase, and ENaC subunits to the lung attenuates
acute respiratory distress syndrome in a two-hit porcine model.
Shock. 43:16–23. 2015. View Article : Google Scholar
|
|
2
|
Wang L, Taneja R, Wang W, Yao LJ,
Veldhuizen RA, Gill SE, Fortin D, Inculet R, Malthaner R and Mehta
S: Human alveolar epithelial cells attenuate pulmonary
microvascular endothelial cell permeability under septic
conditions. PLoS One. 8:e553112013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ather JL, Alcorn JF, Brown AL, Guala AS,
Suratt BT, Janssen-Heininger YM and Poynter ME: Distinct functions
of airway epithelial nuclear factor-kappaB activity regulate
nitrogen dioxide-induced acute lung injury. Am J Respir Cell Mol
Biol. 43:443–451. 2010. View Article : Google Scholar :
|
|
4
|
Fard N, Saffari A, Emami G, Hofer S,
Kauczor HU and Mehrabi A: Acute respiratory distress syndrome
induction by pulmonary ischemia-reperfusion injury in large animal
models. J Surg Res. 189:274–284. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang B, Huang W, Han J and Liang Z: Study
of the role of epidermal growth factor on lung fluid transport in
rabbits with acute lung injury caused by endotoxin. Exp Ther Med.
4:611–614. 2012.PubMed/NCBI
|
|
6
|
Yan YM, Li YD, Song XL, Liu M, Diao F,
Wang Y, Sun Y, Wang ZH and Lu J: Therapeutic effects of inhaling
aerosolized surfactant alone or with dexamethasone generated by a
novel noninvasive apparatus on acute lung injury in rats. J Trauma
Acute Care Surg. 73:1114–1120. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bhandary YP, Velusamy T, Shetty P, Shetty
RS, Idell S, Cines DB, Jain D, Bdeir K, Abraham E, Tsuruta Y, et
al: Post-transcriptional regulation of urokinase-type plasminogen
activator receptor expression in lipopolysaccharide-induced acute
lung injury. Am J Respir Crit Care Med. 179:288–298. 2009.
View Article : Google Scholar :
|
|
8
|
Wang X, Zhang L, Duan W, Liu B, Gong P,
Ding Y and Wu X: Anti-inflammatory effects of triptolide by
inhibiting the NF-κB signalling pathway in LPS-induced acute lung
injury in a murine model. Mol Med Rep. 10:447–452. 2014.PubMed/NCBI
|
|
9
|
Schmidt AE and Adamski J; Education
Committee of the Academy of Clinical Laboratory Physicians and
Scientists: Pathology consultation on transfusion-related acute
lung injury (TRALI). Am J Clin Pathol. 138:498–503. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang ZY, Wu SN, Zhu ZZ, Yang BX and Zhu X:
Inhaled unfractionated heparin improves abnormalities of alveolar
coagulation, fibrinolysis and inflammation in endotoxemia-induced
lung injury rats. Chin Med J (Engl). 126:318–324. 2013.
|
|
11
|
Zhang JZ, Liu Z, Liu J, Ren JX and Sun TS:
Mitochondrial DNA induces inflammation and increases TLR9/NF-κB
expression in lung tissue. Int J Mol Med. 33:817–824.
2014.PubMed/NCBI
|
|
12
|
Irwin DC, Baek JH, Hassell K, Nuss R,
Eigenberger P, Lisk C, Loomis Z, Maltzahn J, Stenmark KR,
Nozik-Grayck E and Shetty S: Hemoglobin-induced lung vascular
oxidation, inflammation, and remodeling contribute to the
progression of hypoxic pulmonary hypertension and is attenuated in
rats with repeated-dose haptoglobin administration. Free Radic Biol
Med. 82:50–62. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dominguez-Perles R, Medina S, Moreno DA,
Garcia-Viguera C, Ferreres F and Gil-Izquierdo A: A new ultra-rapid
UHPLC/MS/MS method for assessing glucoraphanin and sulforaphane
bioavailability in human urine. Food Chem. 143:132–138. 2014.
View Article : Google Scholar
|
|
14
|
Chi X, Zhang R, Shen N, Jin Y, Alina A,
Yang S and Lin S: Sulforaphane reduces apoptosis and oncosis along
with protecting liver injury-induced ischemic reperfusion by
activating the Nrf2/ARE pathway. Hepatol Int. 9:321–329. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin W, Wu RT, Wu T, Khor TO, Wang H and
Kong AN: Sulforaphane suppressed LPS-induced inflammation in mouse
peritoneal macrophages through Nrf2 dependent pathway. Biochem
Pharmacol. 76:967–973. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Benedict AL, Mountney A, Hurtado A, Bryan
KE, Schnaar RL, Dinkova-Kostova AT and Talalay P: Neuroprotective
effects of sulforaphane after contusive spinal cord injury. J
Neurotrauma. 29:2576–2586. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang CT, Zhang L, Wu HW, Wei L, Xu B and
Li DM: Doxycycline attenuates acute lung injury following
cardiopulmonary bypass: Involvement of matrix metalloproteinases.
Int J Clin Exp Pathol. 7:7460–7468. 2014.
|
|
18
|
Chevalier S, Cury FL, Scarlata E, El-Zayat
E, Hamel L, Rocha J, Zouanat FZ, Moussa S, Scherz A, Elhilali M and
Anidjar M: Endoscopic vascular targeted photodynamic therapy with
the photosensitizer WST11 for benign prostatic hyperplasia in the
preclinical dog model. J Urol. 190:1946–1953. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Joo Choi R, Cheng MS and Shik Kim Y:
Desoxyrhapontigenin up-regulates Nrf2-mediated heme oxygenase-1
expression in macrophages and inflammatory lung injury. Redox Biol.
2:504–512. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Reddy SA, Shelar SB, Dang TM, Lee BN, Yang
H, Ong SM, Ng HL, Chui WK, Wong SC and Chew EH: Sulforaphane and
its methylcarbonyl analogs inhibit the LPS-stimulated inflammatory
response in human monocytes through modulating cytokine production,
suppressing chemotactic migration and phagocytosis in a NF-κB- and
MAPK-dependent manner. Int Immunopharmacol. 24:440–450. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nallasamy P, Si H, Babu PV, Pan D, Fu Y,
Brooke EA, Shah H, Zhen W, Zhu H, Liu D, et al: Sulforaphane
reduces vascular inflammation in mice and prevents TNF-α-induced
monocyte adhesion to primary endothelial cells through interfering
with the NF-κB pathway. J Nutr Biochem. 25:824–833. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Arslan S, Korkmaz Ö, Özbilüm N and Berkan
Ö: Association between NF-κBI and NF-κBIA polymorphisms and
coronary artery disease. Biomed Rep. 3:736–740. 2015.PubMed/NCBI
|
|
23
|
Mankan AK, Lawless MW, Gray SG, Kelleher D
and McManus R: NF-kappaB regulation: the nuclear response. J Cell
Mol Med. 13:631–643. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liang D, Sun Y, Shen Y, Li F, Song X, Zhou
E, Zhao F, Liu Z, Fu Y, Guo M, et al: Shikonin exerts
anti-inflammatory effects in a murine model of
lipopolysaccharide-induced acute lung injury by inhibiting the
nuclear factor-kappaB signaling pathway. Int Immunopharmacol.
16:475–480. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Trapani L, Segatto M, Ascenzi P and
Pallottini V: Potential role of nonstatin cholesterol lowering
agents. IUBMB Life. 63:964–971. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ethridge RT, Chung DH, Slogoff M, Ehlers
RA, Hellmich MR, Rajaraman S, Saito H, Uchida T and Evers BM:
Cyclooxygenase-2 gene disruption attenuates the severity of acute
pancreatitis and pancreatitis-associated lung injury.
Gastroenterology. 123:1311–1322. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Santos LA, Ribeiro EL, Barbosa KP, Fragoso
IT, Gomes FO, Donato MA, Silva BS, Silva AK, Rocha SW, França ME,
et al: Diethylcarbamazine inhibits NF-κB activation in acute lung
injury induced by carrageenan in mice. Int Immunopharmacol.
23:153–162. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shan Y, Wu K, Wang W, Wang S, Lin N, Zhao
R, Cassidy A and Bao Y: Sulforaphane down-regulates COX-2
expression by activating p38 and inhibiting NF-kappaB-DNA-binding
activity in human bladder T24 cells. Int J Oncol. 34:1129–1134.
2009.PubMed/NCBI
|
|
29
|
Grantham CJ, Izumi T, Lewis DH and Bakhle
YS: Effects of endotoxin-induced lung injury on the
pharmacokinetics of pros-taglandin E2 and adenosine in
rat isolated lung. Circ Shock. 26:157–167. 1988.PubMed/NCBI
|
|
30
|
Sun Y, Jia Z, Liu G, Zhou L, Liu M, Yang B
and Yang T: PPARγ agonist rosiglitazone suppresses renal
mPGES-1/PGE2 pathway in db/db Mice. PPAR Res. 2013:6129712013.
View Article : Google Scholar
|
|
31
|
Kono K, Toda S, Hora K and Kiyosawa K:
Direct hemoperfusion with a beta2-microglobulin-selective adsorbent
column eliminates inflammatory cytokines and improves pulmonary
oxygenation. Ther Apher Dial. 13:27–33. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Choi YJ, Lee WS, Lee EG, Sung MS and Yoo
WH: Sulforaphane inhibits IL-1β-induced proliferation of rheumatoid
arthritis synovial fibroblasts and the production of MMPs, COX-2,
and PGE2. Inflammation. 37:1496–1503. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Menezes LG, Uzuelli JA, Tefé-Silva C,
Ramos SG, Santos JE and Martinez JA: Acute lung injury induced by
the intravenous administration of cigarette smoke extract. J Bras
Pneumol. 39:39–47. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Qi B, Chen HL, Shang D, Dong Y, Zhang GX
and Yu L: Effects of hypoxia-inducible factor-1α and matrix
metalloproteinase-9 on alveolar-capillary barrier disruption and
lung edema in rat models of severe acute pancreatitis-associated
lung injury. Exp Ther Med. 8:899–906. 2014.PubMed/NCBI
|
|
35
|
Kim KH, Burkhart K, Chen P, Frevert CW,
Randolph-Habecker J, Hackman RC, Soloway PD and Madtes DK: Tissue
inhibitor of metalloproteinase-1 deficiency amplifies acute lung
injury in bleomycin-exposed mice. Am J Respir Cell Mol Biol.
33:271–279. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mao L, Wang HD, Wang XL, Qiao L and Yin
HX: Sulforaphane attenuates matrix metalloproteinase-9 expression
following spinal cord injury in mice. Ann Clin Lab Sci. 40:354–360.
2010.PubMed/NCBI
|
|
37
|
Liu H, Liang X, Wang D, Zhang H, Liu L,
Chen H, Li Y, Duan Q and Xie K: Combination therapy with nitric
oxide and molecular hydrogen in a murine model of acute lung
injury. Shock. 43:504–511. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xu D, Niu W, Luo Y, Zhang B, Liu M, Dong
H, Liu Y and Li Z: Endogenous estrogen attenuates hypoxia-induced
pulmonary hypertension by inhibiting pulmonary arterial
vasoconstriction and pulmonary arterial smooth muscle cells
proliferation. Int J Med Sci. 10:771–781. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lin HJ, Wang CT, Niu KC, Gao C, Li Z, Lin
MT and Chang CP: Hypobaric hypoxia preconditioning attenuates acute
lung injury during high-altitude exposure in rats via up-regulating
heat-shock protein 70. Clin Sci (Lond). 121:223–231. 2011.
View Article : Google Scholar
|
|
40
|
Brandenburg LO, Kipp M, Lucius R, Pufe T
and Wruck CJ: Sulforaphane suppresses LPS-induced inflammation in
primary rat microglia. Inflamm Res. 59:443–450. 2010. View Article : Google Scholar
|
|
41
|
Shan Y, Akram A, Amatullah H, Zhou DY,
Gali PL, Maron-Gutierrez T, González-López A, Zhou L, Rocco PR,
Hwang D, et al: ATF3 protects pulmonary resident cells from acute
and ventilator-induced lung injury by preventing Nrf2 degradation.
Antioxid Redox Signal. 22:651–668. 2015. View Article : Google Scholar
|
|
42
|
Yu JB, Shi J, Gong LR, Dong SA, Xu Y,
Zhang Y, Cao XS and Wu LL: Role of Nrf2/ARE pathway in protective
effect of electroacupuncture against endotoxic shock-induced acute
lung injury in rabbits. PLoS One. 9:e1049242014. View Article : Google Scholar : PubMed/NCBI
|