Introduction
Chronic hypoxia-induced pulmonary hypertension
(HPH), an incurable disease, is often a complication in patients
with chronic heart failure, chronic obstructive pulmonary disease
and sleep apnea (1). Pulmonary
arterial remodeling, a marker of severe and advanced HPH, is
primarily attributed to the abnormal proliferation of pulmonary
artery smooth muscle cells (PASMCs) (2,3).
Thus, the inhibition of the aberrant proliferation of PASMCs may
prove to be a novel therapeutic strategy for the treatment of
HPH.
Cyclin D1 and its associated cyclin-dependent
kinases (CDKs) are regulated proteins which are key in controlling
the re-entry of quiescent cells from the G0 to the G1 phase
(4). p27 blocks the G1-S
transition in the cell cycle, and is a negative regulator of the
protein kinases, cyclin/CDK (5).
The interaction of the two factors regulates cell proliferation and
apoptosis (6). The activity of
extracellular signal-regulated kinase 1/2 (ERK1/2), a major
mitogenic signaling pathway, has been shown to be significantly
enhanced in a variety of animal models of pulmonary arterial
hypertension (7). Previous
studies have revealed that 5-hydroxytryptamine (5-HT) and
platelet-derived growth factor (PDGF) induce the proliferation of
PASMCs through the activation of the phosphatidylinositol 3-kinase
(PI3K)-protein kinase B (AKT) signaling pathways (8,9).
Therefore, blocking these signaling pathways may play a key role in
the prevention and treatment of HPH.
Spermine (Sp), a polyamine, is a non-protein small
molecule with polyvalent positive charges and nitrogen. Polyamine
homeostasis is of great importance for cell survival (10). Ornithine decarboxylase (ODC) is
the key enzyme of polyamine biosynthesis, and spermidine/spermine
N1-acetyltransferase (SSAT) is the key enzyme in the terminal
degradation of polyamides. They are both associated with the
regulation of polyamine metabolism (11). However, to the best of our
knowledge, the role which Sp plays in PASMC proliferation has not
yet been elucidated. Cobalt chloride (CoCl2) is a
well-known hypoxia-mimetic agent that mimics the hypoxic response
in many aspects (12). Therefore,
in the present study, we established a hypoxia model by culturing
PASMCs with CoCl2, and then observed the changes in
polyamine metabolism following hypoxia. In addition, we examined
the effects of exogenous Sp on PASMC proliferation and the
underlying mechanisms.
Materials and methods
Materials
Sp was purchased from Sigma-Aldrich (St. Louis, MO,
USA). 5-Bromo-2′-deoxyuridine (BrdU) Labeling reagent and the
Detection Kit I were obtained from Roche Diagnostics GmbH
(Mannheim, Germany). Anti-cyclin D1 and anti-p27 antibodies were
from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
Anti-mitogen-activated protein kinase (MAPK), anti-PI3K p85 and
anti-AKT antibodies were all purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA). Alkaline phosphatase-labeled
goat anti-rabbit IgG antibody and goat anti-mouse IgG antibody were
both from Sigma-Aldrich.
Cell culture
Normal human PASMCs were acquired from the American
Type Culture Collection (PCS-100-023; ATCC, Mannassas, VA, USA. We
subcultured the cells, and cells at the 4th to 9th passages were
used in our experiments. The medium was composed of high glucose
Dulbecco's modified Eagle's medium (DMEM), 10% fetal bovine serum
(FBS) and 1% penicillin or streptomycin at 37°C, in a humidified
incubator with 5% CO2. After being conventionally
cultured for 48 h, the cells were serum-starved for 24 h in
serum-free medium. The control group consisted of cells cultured
with no special treatment for the next 24 h, whereas the hypoxia
group was cultured in with serum-free medium with 50 µM
CoCl2, and the treatment groups were treated with
various concentrations (0.1, 1, 10 and 100 µM) of Sp for 40
min prior to the induction of hypoxia.
Cell viability assay
Cell viability was measured using a Cell Counting
Kit-8 (CCK-8; Dojindo, Kumamoto, Japan). The PASMCs were seeded in
96-well plates at a density of 3×103 cells/well. After
being serum-starved for 24 h, various concentrations of
CoCl2 (10, 25, 50, 100, 300, 600 and 1,000 µM)
were added to the medium and the cells were cultured for 6, 12 and
24 h, respectively. We then used a microplate spectrophotometer
(BMG LABTECH, Offenburg, Germany) to detect the optical density
(OD) at 450 nm (A450).
Cell proliferation assay
PASMC proliferation was measured by
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay, CCK-8 assay and BrdU incorporation assay. For MTT assay, the
cells were seeded in 96-well plates at a density of
1×103 cells/well. The proliferation of the PASMCs in the
control, hypoxia and treatment groups was measured. The operating
protocols were according to the instructions provided by the
manufacturer of the MTT kit (Boster Institute of Biotechnology,
Wuhan, China). The absorbance [optical density (OD)] at 490 nm
(A490) was measured using a microplate spectrophotometer.
For CCK-8 assay, the cells were seeded in 96-well
plates at a density of 3×103 cells/well. At the end of
the treatment period, 10 µl CCK-8 was added to each well
followed by incubation for 1.5 h at 37°C. Subsequently, we detected
the OD value at 450 nm (A450) using a microplate
spectrophotometer.
For BrdU incorporation assay, the cells were seeded
in 12-well plates at a density of 3×104 cells/well. BrdU
was incorporated in proliferating cells according to the
manufacturer's instructions (Roche Diagnostics GmbH). For
evaluation by fluorescence microscopy (Nikon Corp., Tokyo, Japan),
we used an excitation wavelength in the range of 450–500 nm and
detection in the range of 515–565 nm.
Cell cycle analysis
In the present study, cell cycle progression was
determined by flow cytometry. The cells were harvested with 0.25
g/l trypsin in 6-well plates, re-suspended in 10 ml PBS, 1 ml 70%
ethanol was added, and the cells were then centrifuged (2,000 rpm,
5 min, 4°C) and washed with cold PBS. The cells were subsequently
dyed with 100 µl RNAse (1 mg/ml) and then 400 µl PI
(20 µg/ml). Following incubation for 30 min at room
temperature, the samples were run on a 7 Laser SORP BD LSR II
system (BD Biosciences, Franklin Lakes, NJ, USA) and the data was
analyzed using CellQuest software (BD Biosciences).
Western blot analysis
The cells were harvested and then lysed. Equal
amounts of proteins were boiled and separated with sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
electrophoretically transferred to nitrocellulose membranes. Equal
amounts of proteins were loaded into each lane of a 10% SDS-PAGE
gel, electrophoresed and transferred onto membranes. The membranes
were blocked with Tris-buffered saline (TBS) containing 5% non-fat
milk at room temperature for 1 h, and then incubated overnight at
4°C with the primary antibodies. The primary antibody dilutions
were as follows: 1:1,000 for ODC, SSAT, cyclin D1, p27,
phosphorylated (p-) or total ERK, PI3K, AKT and 1:10,000 for
β-actin. The membranes were incubated in TBST solution with
alkaline phosphatase-labeled secondary antibody (diluted 1:5,000)
for 1 h at room temperature on a shaker. Finally, antibody-antigen
complexes were detected using Western Blue Stabilized Substrate
(Promega, Madison, WI, USA) for alkaline phosphatase. The
intensities of the protein bands were quantified using a Bio-Rad
ChemiDoc™ EQ densitometer and Bio-Rad Quantity One software
(Bio-Rad Laboratories, Hercules, CA, USA).
Statistical analysis
All data are expressed as the means ± SE and
represent at least 3 independent experiments. Statistical
comparisons were made using a paired or unpaired t-test, and
one-way analysis of variance (ANOVA). A p-value <0.05 was
considered to indicate a statistically significant difference.
Results
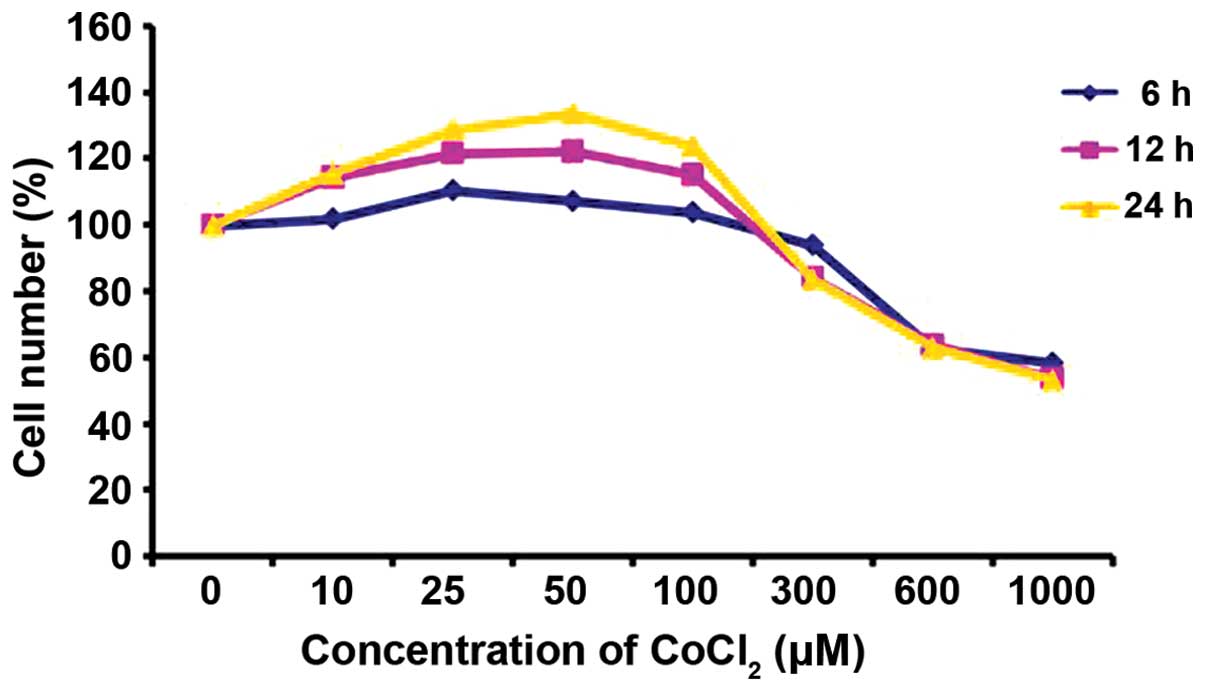
Effect of CoCl2 on PASMC
viability
The results of the CCK-8 assay indicated that the
number of PASMCs firstly increased and then decreased in a
concentration and time-dependent manner. The cells incubated in
control medium were considered 100% viable. CoCl2 at a
concentration of 10–100 µM increased the proliferation of
the PASMCs, and this trend was particularly evident when the cells
were incubated with 50 µM CoCl2 for 24 h;
however, when the cells were incubated with CoCl2 at a
concentration of 300–1,000 µM, the number of viable PASMCs
notably decreased (Fig. 1).
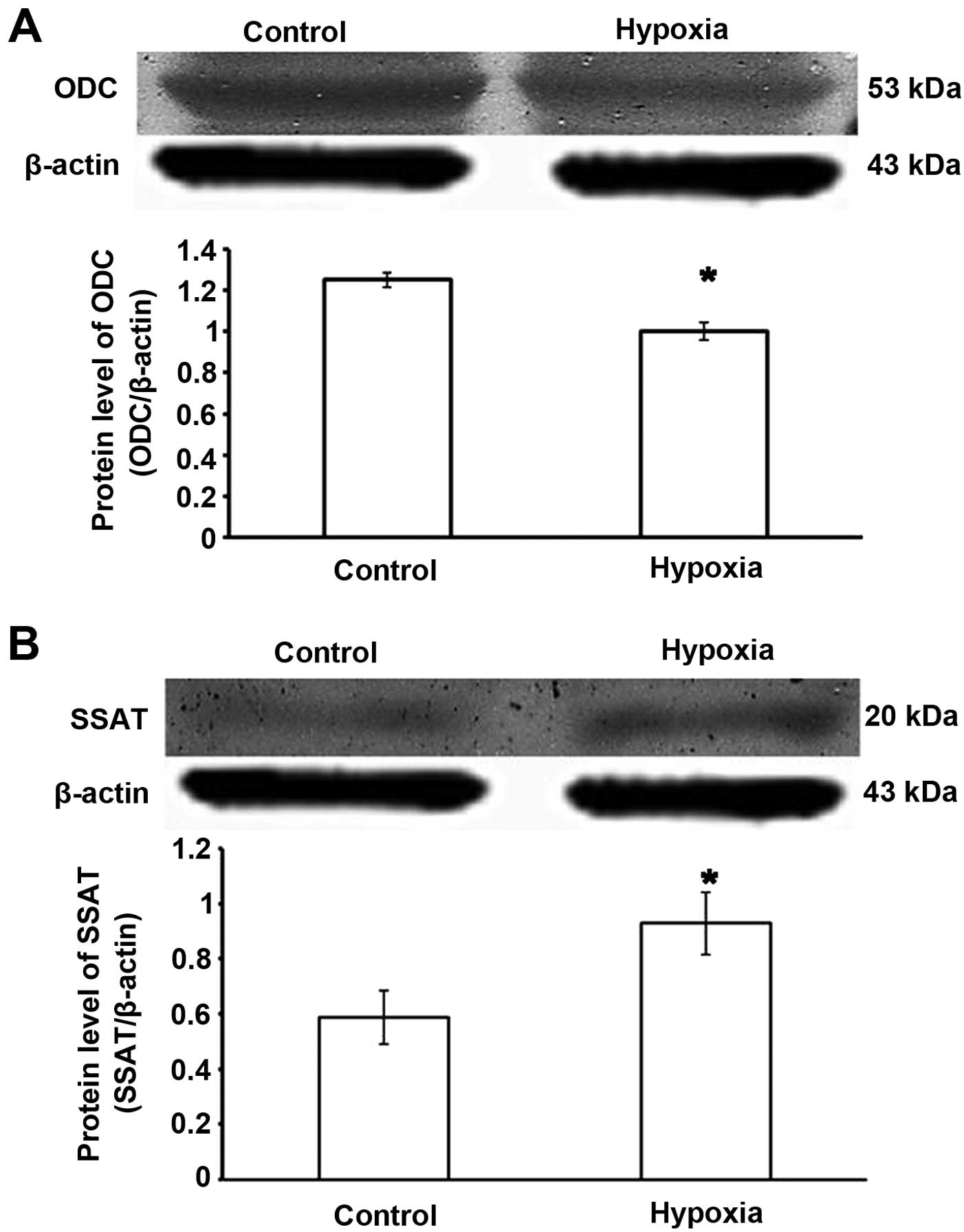
Changes to the key regulatory enzyme of
polyamine metabolism under hypoxic conditions
ODC is an enzyme that performs the first step in
polyamine biosynthesis; SSAT is the key regulatory enzyme for
polyamine catabolism (13,14).
Compared with the control group, the expression of ODC was
decreased and that of SSAT was significantly increased in the
hypoxia groups (p<0.05). These results indicated that hypoxia
induced a decrease in endogenous Sp (Fig. 2).
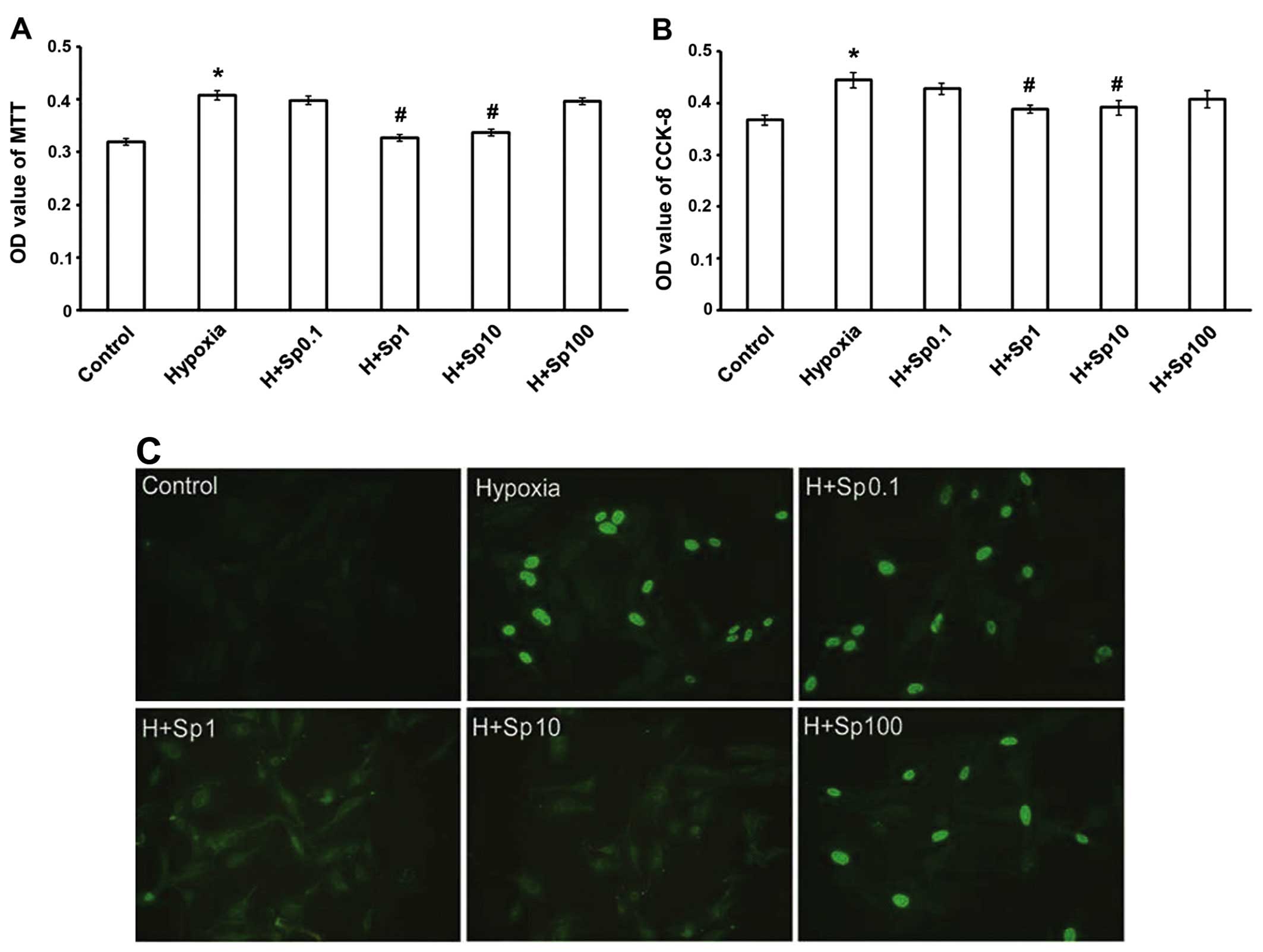
Exogenous Sp inhibits the increase in
PASMC proliferation caused by chemically-induced hypoxia
To demonstrate the effect of Sp on PASMC
proliferation, cell viability was examined by MTT assay (Fig. 3A). We found that exposure to
chemically induced hypoxia for 24 h significantly promoted PASMC
proliferation. At a concentration of 1 and 10 µM, Sp
inhibited PASMC proliferation compared with the hypoxia group
(p<0.05); however, Sp at 0.1 and 100 µM had no
significant effect on PASMC proliferation under hypoxic conditions
(p>0.05). The CCK-8 assay demonstrated similar results (Fig. 3B). To assess the population of
actively synthesizing DNA cells, a BrdU assay was utilized. The
fluorescence intensity of DNA reflects the ability of cells to
proliferate. Our results revealed that the induction of hypoxia
markedly increased cell proliferation compared with the control
group (p<0.05). Sp at 1 and 10 µM inhibited PASMC
proliferation compared with the hypoxia group (p<0.05); however,
the effect of Sp at 0.1 and 100 µM was not significant
(p>0.05; Fig. 3C).
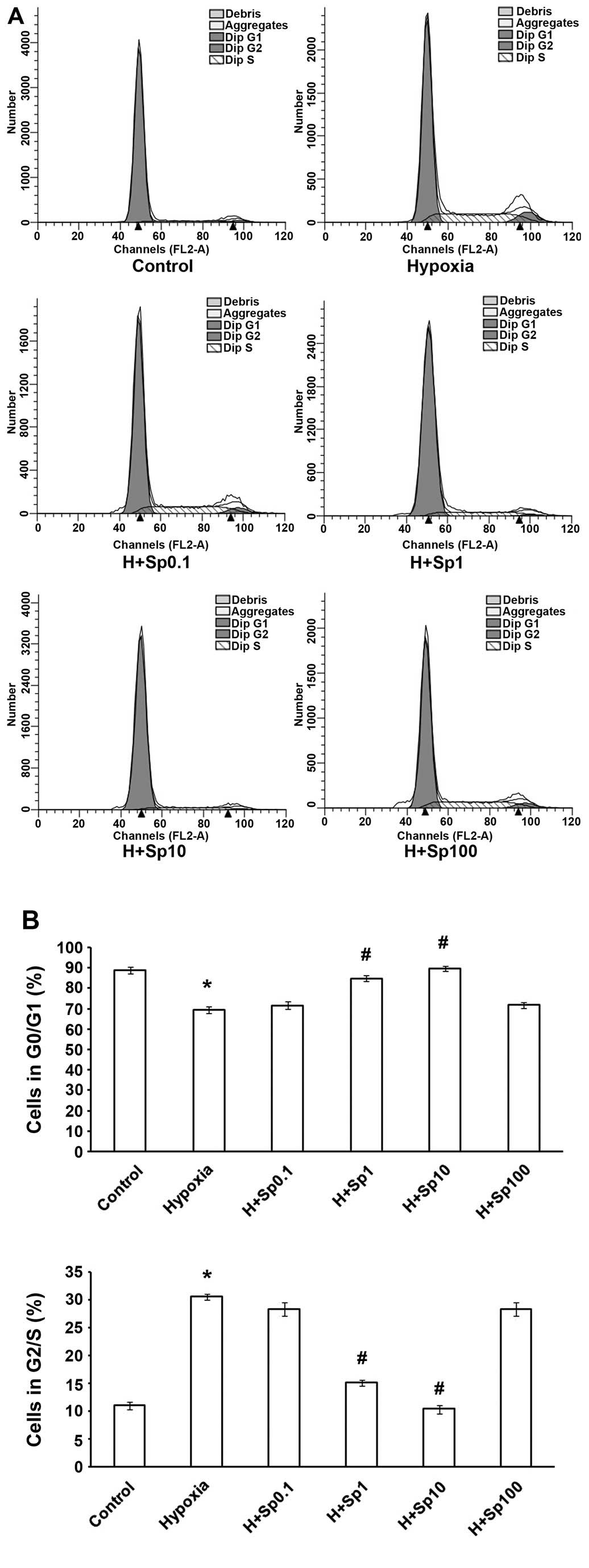
Exogenous Sp arrests PASMCs at the G1/G0
phase under hypoxic conditions
The cell cycle transition from the G1/G0 phase to
the G2/S phase reflects cell proliferation. Therefore, we wished to
determine whether Sp affects the cell cycle of PASMCs under hypoxic
conditions. As shown in Fig. 4,
the exposure of the PASMCs to hypoxia for 24 h caused the cells to
enter mitosis. Hypoxia also increased the number of PASMCs in the
G2/S phase and decreased the number of PASMCs in the G0/G1 phase
(p<0.05). However, treatment with Sp at 1 and 10 µM
weakened the effects of hypoxia on the PASMC cell cycle
(p<0.05), although Sp at 0.1 and 100 µM did not markedly
affect the cycle (p>0.05).
Exogenous Sp affects the protein
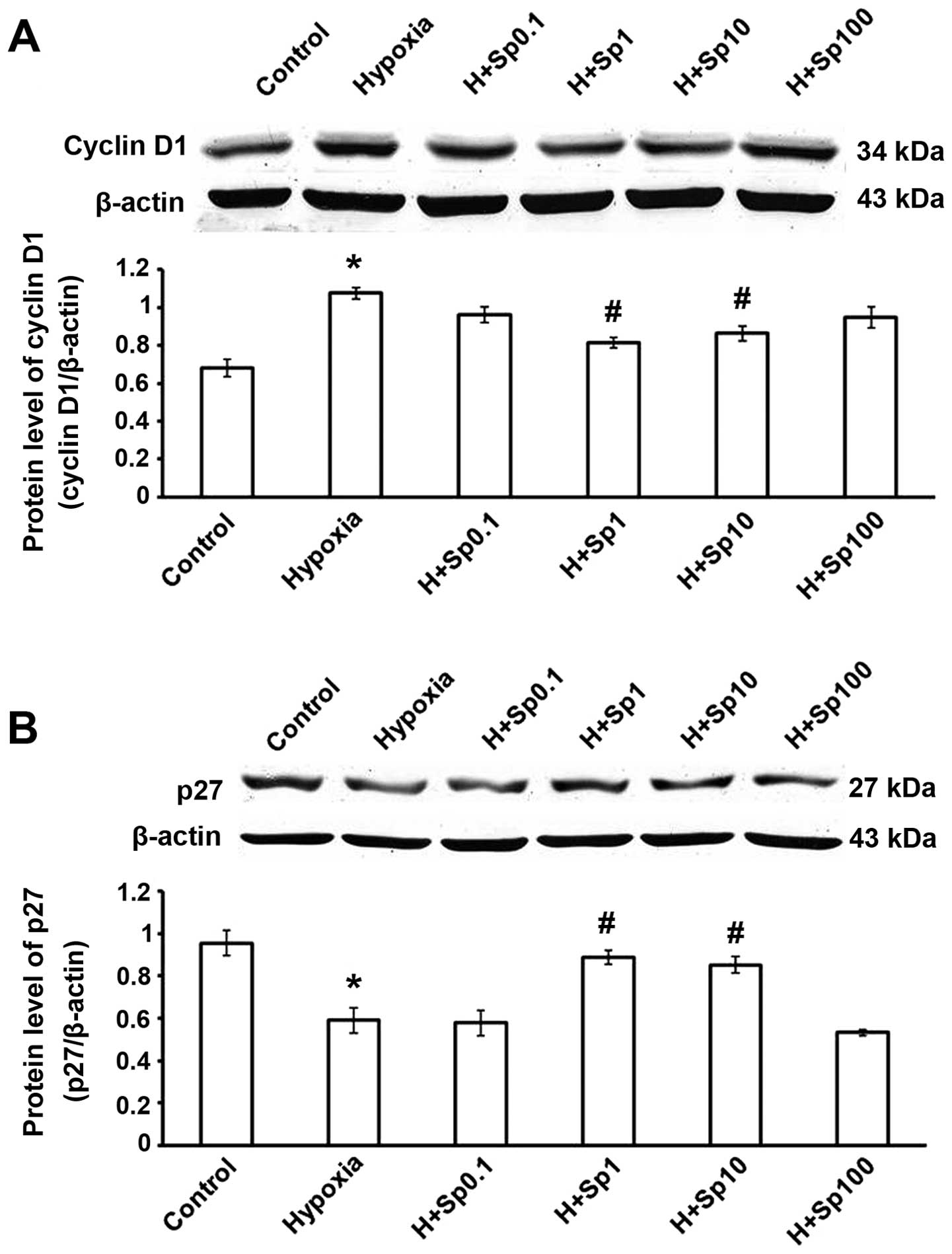
expression of cyclin D1 and p27 in cultured PASMCs
The expression of cyclin D1 was increased and that
of p27 was decreased in the hypoxia group (p<0.05 vs. control
group). Compared with the hypoxia group, treatment with Sp at 1 and
10 µM significantly decreased cyclin D1 expression, and
increased p27 expression (p<0.05); however, Sp at 0.1 and 100
µM did not have a significant effect (p>0.05; Fig. 5).
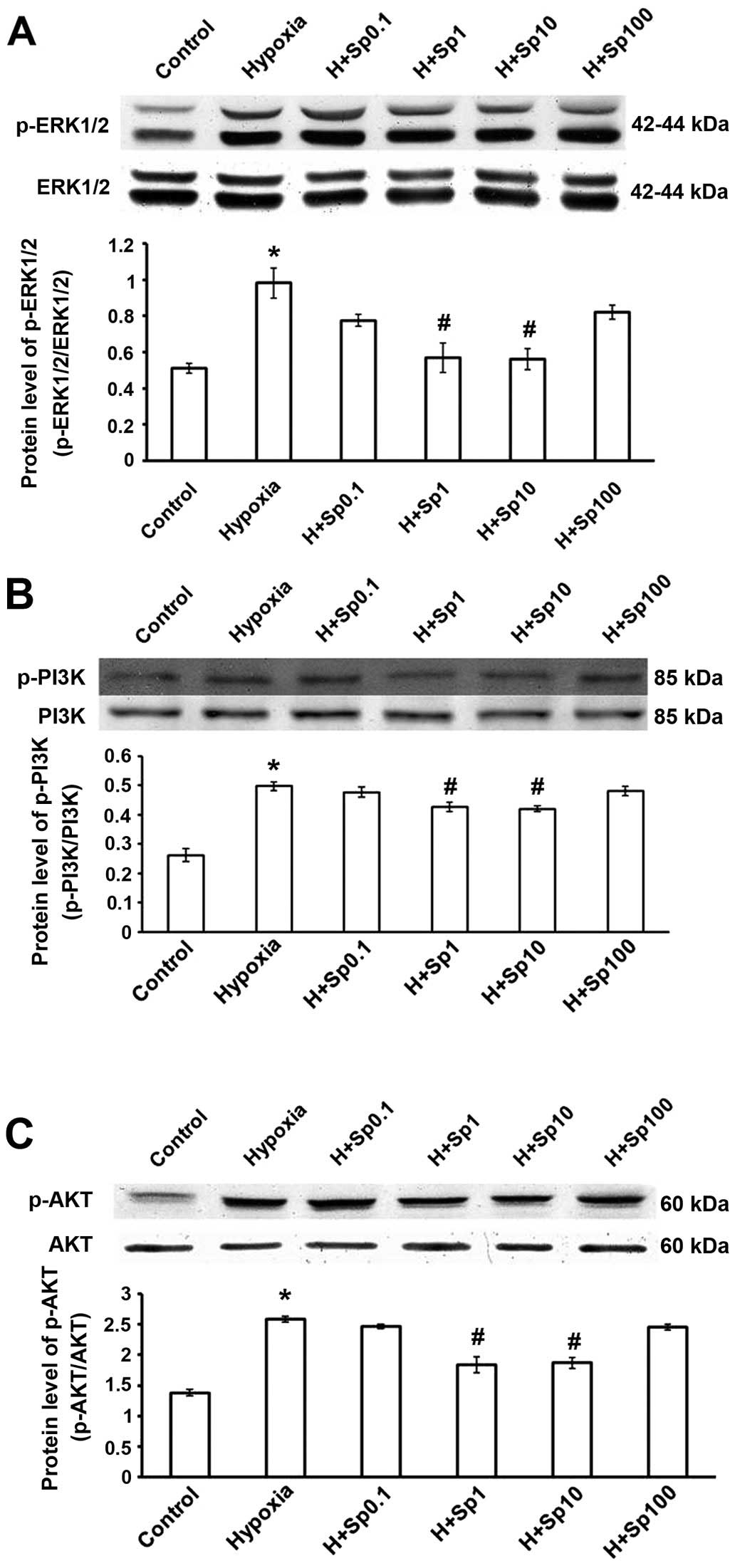
Protein expression of p-ERK, p-PI3K and
p-AKT in cultured PASMCs
Compared with the control group, the induction of
hypoxia promoted the phosphorylation of ERK1/2 (Fig. 6A) and increased PI3K and AKT
phosphorylation (Fig. 6B and C).
Treatment with Sp at 1 and 10 µM reversed the effects of
hypoxia on the MAPK and PI3K-AKT pathways (p<0.05); however, Sp
at 0.1 and 100 µM was not so effective at reducing the
effects of hypoxia (p>0.05). The total amount of ERK1/2, PI3K
and AKT proteins was not significantly altered in the different
groups.
Discussion
HPH, a chronic progressive disease with a poor
prognosis, is characterized by pulmonary vascular remodeling and a
persistent increase in arterial pressure (15). To explore the mechanisms
responsible for the development of HPH and to effectively treat
this condition, clinical observations and animal experiments are
commonly performed. In previous studies using cell models, injury
caused by chemically-induced hypoxia was used to construct HPH
models (16,17). CoCl2 is a well-known
hypoxia mimetic agent that mimics the hypoxic response in many
aspects (12). In the present
study, we examined the effects of CoCl2 at various
concentrations and treatment times on the number of viable PASMCs,
and we selected the concentration of 50 µM for 24 h to
establish the cell model of hypoxia, as was done in a previous
study (18).
Pulmonary arterial remodeling occurs primarily due
to the abnormal proliferation of PASMCs (2,3).
Therefore, the inhibition of hypoxia-induced PASMC proliferation
exerts anti-remodeling effects. The exact mechanisms responsible
for pulmonary arterial remodeling have, however, not been fully
elucidated thus far, although certain studies have demonstrated
that biologically active media, such as endothelin, vascular
endothelial growth factor, nitric oxide, prostacyclin and other
factors are involved in this pathological process (19,20). Moreover, the association between
pulmonary vascular remodeling and polyamine metabolism has not been
investigated to date, to the best of our knowledge.
Polyamines, including Sp, spermidine and putrescine,
are non-protein small molecules with polyvalent positive charges
and nitrogen. Sp, with four positive charges, has the most
prominent biological effect (10,21). Polyamines and their metabolic
enzymes play an essential role in a number of normal and
pathological processes. ODC and SSAT are involved in the regulation
of polyamine metabolism (11).
The transcription and translation of ODC promote cell growth and
differentiation (13). SSAT is
the central molecular regulator of polyamine metabolism (14,22). Polyamine catabolism plays an
important role in apoptosis and drug and stress responses, and is
involved in the etiology of certain pathological conditions
(including cancer) (23). It is
not yet clear, however, whether hypoxia, as a type of stress,
induces disrupts polyamine metabolism. The results of the present
study indicated that chemically-induced hypoxia caused a decrease
in ODC expression and an increase in SSAT expression in PASMCs. As
is known, ODC is one of the main enzymes involved in polyamine
biosynthesis, and SSAT is a key enzyme in the terminal degradation
of polyamine. Our results suggest that chemically-induced hypoxia
causes a decrease in endogenous Sp.
In this study, to examine the effects of Sp on PASMC
proliferation, cell viability was measured by MTT assay, CCK-8
assay and BrdU incorporation assay. Our results demonstrated that
chemically-induced hypoxia significantly promoted PASMC
proliferation and affected the key enzymes involved in polyamine
metabolism, ODC and SSAT. Our data also demonstrated that treatment
with exogenous Sp (1 and 10 µM) inhibited PASMC
proliferation.
Cell division consists of two consecutive processes,
which are divided into two stages: mitosis (M), the process of
nuclear division, and interphase, the interlude between two M
phases. In addition, the stages of interphase include the G1, S and
G2 phases. The G1, S, G2 and M phases are the traditional
subdivisions of the standard cell cycle. Cells in the G1 phase,
before commitment to DNA replication, enter into a resting state
termed G0. Cells in the G0 phase account for the major part of the
non-growing, non-proliferating cells in the human body (24). The cell cycle, a key therapeutic
target in vascular proliferation-associated diseases, is determined
by CDK and CDK inhibitors (CDKIs). Cyclin D1 has previously been
recognized as a proto-oncogene, whose main role is to promote cell
proliferation (25). Cell growth
begins at the G1 phase of the cell cycle, and cyclin D1 is a key
protein that regulates the cell cycle in the G1 phase. As one of
the main CDK inhibitors, p27 has been previously studied in
relation to its modulation of PASMC proliferation during mitogenic
stimulation, and its overexpression decreases PASMC proliferation
(26). Certain studies have
suggested that polyamines affect cell cycle progression by
regulating proteins, such as cyclins, CDKs and CDKIs (27,28). In the present study, we examined
the effects of chemically-induced hypoxia and exogenous Sp on the
cell cycle, and on the expression of cyclin D1 and p27. Our results
demonstrated that hypoxia promoted the entry of the PASMCs into
mitosis by increasing the number of PASMCs in the G2/S phase,
decreased the protein expression level of p27 and enhanced the
expression of cyclin D1. More importantly, we found that exogenous
Sp at 1 and 10 µM reversed the effects of hypoxia on the
cell cycle and the expression of related proteins. Therefore, the
effects of exogenous Sp are an important mechanism with which to
inhibit the proliferation of PASMCs.
ERK1/2 is the most important member of the MAPK
family, and can be activated by a variety of extracellular signals,
such as ischemia, hypoxia and hormones. ERK1/2 regulates the
expression of genes involved in cell growth, proliferation,
differentiation and apoptosis (29). Liu et al demonstrated that
ERK1/2 induced PASMC proliferation, possibly by increasing cyclin
D1 expression and promoting the DNA-binding transcription factor
early growth response 1 (Egr-1) and GATA binding protein 4 (GATA-4)
(30). Protein kinase B (PKB or
AKT) is a major factor of the PI3K signaling pathway. PI3K-AKT
signaling pathways regulate cell proliferation, differentiation,
survival, migration and other features (31). Previously, it has been reported
that PI3K-AKT is associated with PASMC proliferation (8,9,32).
In a rat model of pulmonary hypertension, the p-AKT level was shown
to be considerably increased, and this was accompanied by the
downregulation of p53 and p27 and the upregulation of cyclin D1
expression (33). In the present
study, we found that hypoxia promoted the expression of p-ERK1/2,
p-PI3K and p-AKT, and that Sp at 1 and 10 µM prevented the
effects of hypoxia on the MAPK and PI3K-AKT pathways.
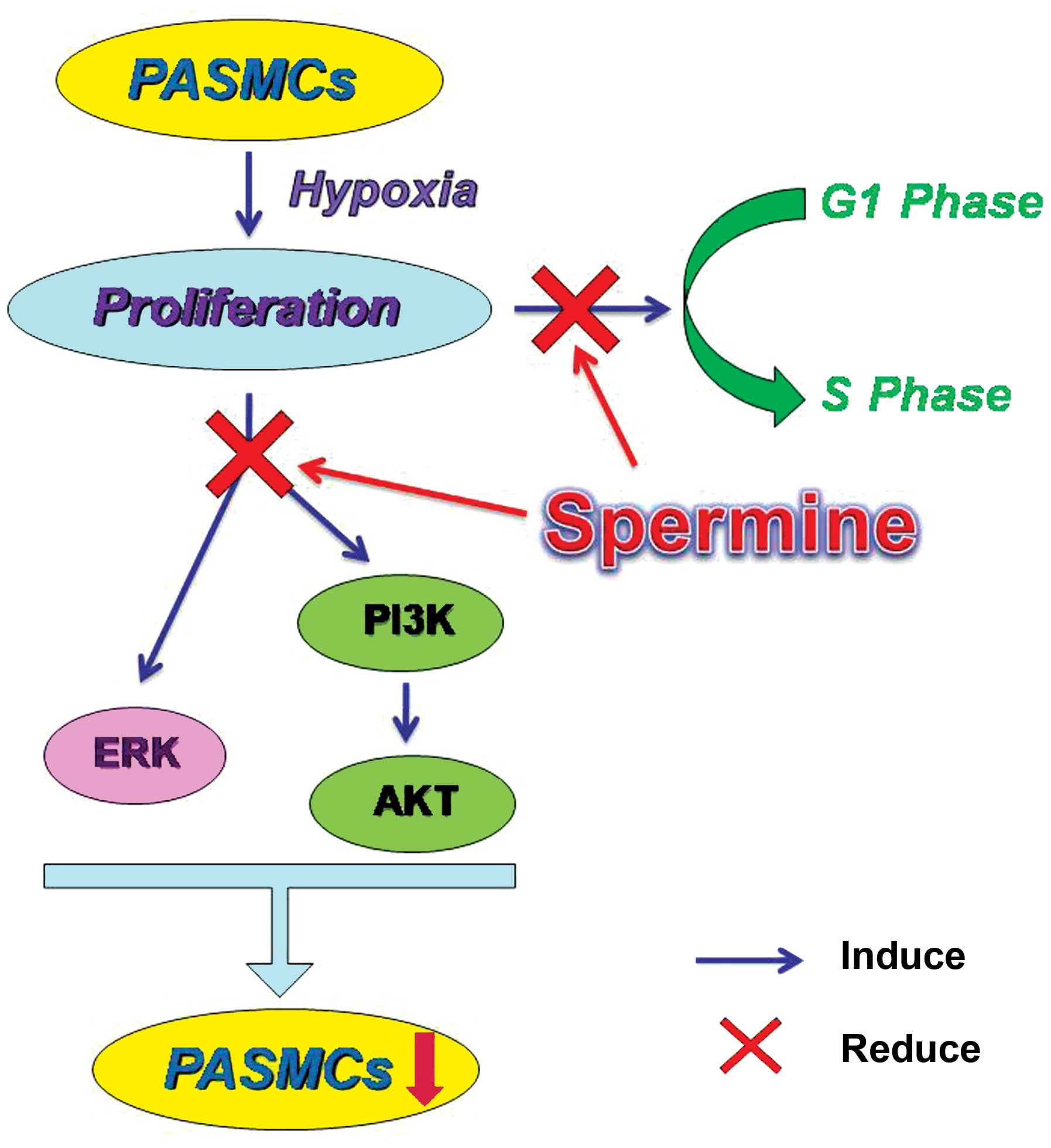
In conclusion, in the present study, we firstly
found that hypoxia caused polyamine metabolic disorder and human
PASMC proliferation, and exogenous Sp at 1 and 10 µM
inhibited the increase in PASMC proliferation caused by
chemically-induced hypoxia via the suppression of the ERK1/2- and
PI3K/AKT-associated pathways. As lower (0.1 µM) or higher
(100 µM) concentrations of Sp did not have a significant
effect, which may be related to the inbalance of polyamine
metabolism, further studies are warranted to investigate this
matter. It is thus suggested that Sp may serve as a novel, specific
and attractive therapeutic agent for the treatment of HPH (Fig. 7).
Abbreviations:
|
Sp
|
spermine
|
|
HPH
|
hypoxia-induced pulmonary
hypertension
|
|
PASMCs
|
pulmonary artery smooth muscle
cells
|
|
CoCl2
|
cobalt chloride
|
|
DMEM
|
Dulbecco's modified eagle's medium
|
|
FBS
|
fetal bovine serum
|
|
ODC
|
ornithine decarboxylase
|
|
SSAT
|
spermidine/spermine
N1-acetyltransferase
|
|
CCK-8
|
cell counting kit-8
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
|
BrdU
|
5-bromo-2′-deoxyuridine
|
|
AKT
|
protein kinase B
|
|
PI3K
|
phosphatidylinositol 3-kinase
|
|
ERK1/2
|
extracellular signal-regulated kinase
1/2
|
|
PDGF
|
platelet-derived growth factor
|
|
MAPK
|
mitogen-activated protein kinase
|
|
CDKIs
|
cyclin-dependent kinase inhibitors
|
|
CDK
|
cyclin-dependent kinases
|
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China (nos. 81070123,
81270311, 81270273 and 81200160), and the Natural Science
Foundation of Heilongjiang (no. LC201430).
References
|
1
|
Raguso CA, Guinot SL, Janssens JP, Kayser
B and Pichard C: Chronic hypoxia: common traits between chronic
obstructive pulmonary disease and altitude. Curr Opin Clin Nutr
Metab Care. 7:411–417. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stenmark KR, Fagan KA and Frid MG:
Hypoxia-induced pulmonary vascular remodeling: cellular and
molecular mechanisms. Circ Res. 99:675–691. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pak O, Aldashev A, Welsh D and Peacock A:
The effects of hypoxia on the cells of the pulmonary vasculature.
Eur Respir J. 30:364–372. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang G, Wu L, Bryan S, Khaper N, Mani S
and Wang R: Cystathionine gamma lyase deficiency and
overproliferation of smooth muscle cells. Cardiovasc Res.
86:487–495. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sherr CJ and Roberts JM: Inhibitors of
mammalian G1 cyclin-dependent kinases. Genes Dev. 9:1149–1163.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tsai YC, Lee YM, Hsu CH, Leu SY, Chiang
HY, Yen MH and Cheng PY: The effect of ferulic acid ethyl ester on
leptin-induced proliferation and migration of aortic smooth muscle
cells. Exp Mol Med. 47:e1802015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rabinovitch M: Molecular pathogenesis of
pulmonary arterial hypertension. J Clin Invest. 118:2372–2379.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu Y and Fanburg BL: Serotonin-induced
growth of pulmonary artery smooth muscle requires activation of
phosphatidylinositol 3-kinase/serine-threonine protein kinase
B/mammalian target of rapamycin/p70 ribosomal S6 kinase 1. Am J
Respir Cell Mol Biol. 34:182–191. 2006. View Article : Google Scholar
|
|
9
|
Goncharova EA: PI3K is required for
proliferation and migration of human pulmonary artery smooth muscle
cells. Am J Respir Cell Mol Biol. 283:354–363. 2002.
|
|
10
|
Heby O, Sarna GP, Marton LJ, Omine M,
Perry S and Russell DH: Polyamine content of AKR leukemic cells in
relation to the cell cycle. Cancer Res. 33:2959–2964.
1973.PubMed/NCBI
|
|
11
|
Hasegawa S, Nakano M, Hamana K, Taniguchi
Y, Iwasaki T, Kanda T, Suzuki T and Nagai R: Decrease in myocardial
polyamine concentration in rats with myocardial infarction. Life
Sci. 60:1643–1650. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Goldberg MA, Dunning SP and Bunn HF:
Regulation of the erythropoietin gene: evidence that the oxygen
sensor is a heme protein. Science. 242:1412–1415. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shantz LM and Pegg AE: Translational
regulation of ornithine decarboxylase and other enzymes of the
polyamine pathway. Int J Biochem Cell Biol. 31:107–122. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang Y, Devereux W, Stewart TM and Casero
RA Jr: Characterizatin of the interaction between the transcription
factors human polyamine modulated factor (PMF-1) and NF-E2-related
actor(Nrf-2) in the transcriptional regulation of the
spermidine/spermine N1-acetyltransferase (SSAT) gene. Biochem J.
355:45–49. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cogolludo A, Moreno L and Villamor E:
Mechanisms controlling vascular tone in pulmonary arterial
hypertension: implications for vasodilator therapy. Pharmacology.
79:65–75. 2007. View Article : Google Scholar
|
|
16
|
Hartwig K, Fackler V, Jaksch-Bogensperger
H, Winter S, Furtner T, Couillard-Despres S, Meier D, Moessler H
and Aigner L: Cerebrolysin protects PC12 cells from
CoCl2-induced hypoxia employing GSK3β signaling. Int J
Dev Neurosci. 38:52–58. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhong X, Lin R, Li Z, Mao J and Chen L:
Effects of Salidroside on cobalt chloride-induced hypoxia damage
and mTOR signaling repression in PC12 cells. Biol Pharm Bull.
37:1199–1206. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li Y, Liu G, Cai D, Pan B, Lin Y, Li X, Li
S, Zhu L, Liao X and Wang H: H2S inhibition of chemical
hypoxia-induced proliferation of HPASMCs is mediated by the
upregulation of COX-2/PGI2. Int J Mol Med. 33:359–366. 2014.
|
|
19
|
Humbert M, Morrell NW, Archer SL, Stenmark
KR, MacLean MR, Lang IM, Christman BW, Weir EK, Eickelberg O,
Voelkel NF and Rabinovitch M: Cellular and molecular pathobiology
of pulmonary arterial hypertension. J Am Coll Cardiol. 43(Suppl S):
pp. 13S–24S. 2004, View Article : Google Scholar
|
|
20
|
Jeffery TK and Morrell NW: Molecular and
cellular basis of pulmonary vascular remodeling in pulmonary
hypertension. Prog Cardiovasc Dis. 45:173–202. 2002. View Article : Google Scholar
|
|
21
|
Shah N, Thomas T, Shirahata A, Sigal LH
and Thomas TJ: Activation of nuclear factor kappaB by polyamines in
breast cancer cells. Biochemistry. 38:14763–14774. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Matsui-Yuasa I, Otani S, Yukioka K, Goto H
and Morisawa S: Two mechanisms of spermidine/spermine
N1-acetyltransferase-induction. Arch Biochem Biophys. 268:209–214.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Casero RA and Pegg AE: Polyamine
catabolism and disease. Biochem J. 421:323–338. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vermeulen K, Van Bockstaele DR and
Berneman ZN: The cell cycle: a review of regulation, deregulation
and therapeutic targets in cancer. Cell Prolif. 36:131–149. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Inaba T, Matsushime H, Valentine M,
Roussel MF, Sherr CJ and Look AT: Genomic organization, chromosomal
localization, and independent expression of human cyclin D genes.
Genomics. 13:565–574. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fouty BW, Grimison B, Fagan KA, Le Cras
TD, Harral JW, Hoedt-Miller M, Sclafani RA and Rodman DM: p27(Kip1)
is important in modulating pulmonary artery smooth muscle cell
proliferation. Am J Respir Cell Mol Biol. 25:652–658. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wilcken NR, Prall OW, Musgrove EA and
Sutherland RL: Inducible overexpression of cyclin D1 in breast
cancer cells reverses the growth-inhibitory effects of
antiestrogens. Clin Cancer Res. 3:849–854. 1997.PubMed/NCBI
|
|
28
|
Hong J, Shah NN, Thomas TJ, Gallo MA,
Yurkow EJ and Thomas T: Differential effects of estradiol and its
analogs on cyclin D1 and CDK4 expression in estrogen receptor
positive MCF-7 and estrogen receptor-transfected MCF-10AEwt5 cells.
Oncol Rep. 5:1025–1033. 1998.PubMed/NCBI
|
|
29
|
Roskoski R Jr: ERK1/2 MAP kinases:
structure, function, and regulation. Pharmacol Res. 66:105–143.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu Y, Suzuki YJ, Day RM and Fanburg BL:
Rho kinase-induced nuclear translocation of ERK1/ERK2 in smooth
muscle cell mitogenesis caused by serotonin. Circ Res. 95:579–586.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Faes S and Dormond O: PI3K and AKT:
Unfaithful partners in cancer. Int J Mol Sci. 16:21138–21152. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ogawa A, Firth AL, Smith KA, Maliakal MV
and Yuan JX: PDGF enhances store-operated Ca2+ entry by
upregulating STIM1/Orai1 via activation of Akt/mTOR in human
pulmonary arterial smooth muscle cells. Am J Physiol Cell Physiol.
302:C405–C411. 2012. View Article : Google Scholar
|
|
33
|
Ravi Y, Selvendiran K, Meduru S, Citro L,
Naidu S, Khan M, Rivera BK, Sai-Sudhakar CB and Kuppusamy P:
Dysregulation of PTEN in cardiopulmonary vascular remodeling
induced by pulmonary hypertension. Cell Biochem Biophys.
67:363–372. 2013. View Article : Google Scholar
|