Introduction
Chronic lower extremity wounds are an important
health concern and the prevalence of these is close to 1.3% in the
population (1,2). These wounds are most likely
associated with venous disease, diabetes and pressure. The most
common chronic wound is the venous leg ulcer which accounts for
40–70% of lower extremity wounds (3). A significant number of individuals
with venous leg ulcers suffer from impaired mobility, depression,
social anxiety and a poor self esteem. Chronic wounds are also
significantly associated with diabetes; close to 15% of the
diabetic population develop diabetic foot ulcers, which may result
in lower extremity amputations (4). These conditions have a profound
effect on the quality of life of patient and mortality rates, as
well as health care costs (2,3,5).
Understanding the mechanisms of wound healing is pertinent in
developing therapeutic strategies for the treatment of chronic
wounds.
Wound healing is comprised of an intricate balance
of distinct but overlapping phases of inflammation and resolution,
leading to tissue repair and tissue remodeling. Immediately after
wounding, the initial phase of wound healing, the inflammatory
phase begins; neutrophils infiltrate the wound area and initiate
the process of debridement of devitalized tissue. To invade
microbes they release reactive oxygen species (ROS), antimicrobial
peptides, proteases and form extracellular traps. These short-lived
neutrophils are then engulfed by resident macrophages and thus the
inflammatory stage subsides. The second phase is the tissue
formation, where epithelialization and newly formed granulation
tissue consisting of endothelial cells, macrophages and fibroblasts
tend to cover the wound area and restore tissue integrity. The
third phase is the tissue remodeling one in which extracellular
matrix molecules form and cell-cell interactions occur, and
eventually facilitate wound healing (6–8).
If, however, the inflammatory stage is prolonged, there is an
impairment in wound healing. Chronic non-healing or slow-healing
wounds are often caused by exaggerated inflammation due to the
persistence of neutrophils in tissue (9).
Cold-inducible RNA-binding protein (CIRP) belongs to
a family of cold-shock proteins and is constitutively expressed in
low levels in a variety of tissues (10–12). It is, however, highly expressed
during hypothermia, hypoxia and ultraviolet radiation (10,13–15). Recently, we demonstrated that CIRP
expression is increased in the blood of patients who were admitted
to intensive care units due to hemorrhagic shock (16). In animal models, deficiency in
CIRP led to decreased inflammation and mortality after hemorrhagic
shock (16). Wound healing is
compromised by a prolonged inflammatory phase, as observed in
chronic wounds, which results in exaggerated inflammation. Thus, we
hypothesized that a deficiency in CIRP would improve cutaneous
wound healing.
In order to examine this hypothesis, in this study,
we aimed to determine whether a deficiency in CIRP would accelerate
the inflammation phase and improve wound healing. We created wounds
on the dorsum of wild-type (WT) and CIRP-knockout
(CIRP−/−) mice and examined the wound healing
process.
Materials and methods
Experimental animals
Male adult (8–10
weeks old) C57BL/6 (WT) mice were purchased from Taconic (Albany,
NY, USA). In total, 4 C57BL/6 mice were included in the wound
closure experiments conducted to generate the data shown in
Fig. 1 and 4–6 mice were included
for the data generated from the day 3 and day 7 experiments. The
mice were allowed to acclimatize for 5 days in a
temperature-controlled environment with a 12-h light/dark cycle and
fed a standard chow diet and were allowed access to water ad
libitum. A male adult CIRP−/− mouse (8–10
weeks old) was a gift from Dr Jun Fujita (Kyoto University, Kyoto,
Japan) and was bred in our facility for experimental purposes. All
experiments were conducted in accordance with the Guide for the
Care and Use of Laboratory Animals from the National Institutes of
Health. The protocol was approved by the Institutional Animal Care
and Use Committee of the Feinstein Institute for Medical Research,
Manhasset, NY, USA.
Mouse model of dorsal cutaneous
wounds
On the day of the surgery, the mice were
anesthetized by isoflurane inhalation, dorsum-shaved and washed
with 10% povidone-iodine. A 2.0 cm full-thickness circular wound
was created on the dorsum using a trephine. The wounds were
extended to the muscle layer, but the panniculus carnosas was kept
intact. Prior to the skin incision, 1% lidocaine was injected
intradermally at the surgical site. Bleeding was stopped by
compression and the wounds were covered with a dressing (Tegaderm)
and changed every other day until the end of experiment.
Measurement of wound size
The wound area was traced on a transparency film
every other day until day 14 and wound closure was calculated using
NIH ImageJ software. The wound closure rate was calculated
according to the following formula as described previously
(17): wound closure rate (%) =
[(Areaday 0 − Areaday n)/Areaday
0] ×100; where Areaday 0 is the initial wound area
on day 0 and the Areaday n is the area on day 'n' after
wounding.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
On days 3 and 7 after wounding, the mice were
euthanized by CO2 asphyxiation. Skin tissues 1–2 mm in
length and in thickness beyond the wound edge and away from the
center were excised and harvested. One half was frozen in liquid
nitrogen and the other half was immersed in 10% formalin. Total RNA
was extracted from the frozen tissues and formalin-fixed tissues
was used for histological assessment. Total RNA was extracted from
the skin tissues using TRIzol reagent (Invitrogen, Carlsbad, CA,
USA) and reverse transcribed into cDNA and subjected to RT-qPCR
using the ∆∆Cq method with primers specific for mouse TNF-α. The
PCR reaction was carried out as described previously (17). The levels were normalized against
those of mouse β-actin. The primer sequences used were as follows:
mouse TNF-α forward, 5′-AGA CCC TCA CAC TCA GAT CAT CTT C-3′ and
reverse, 5′-TTG CTA CGA CGT GGG CTA CA-3′ and mouse β-actin
forward, 5′-CGT GAA AAG ATG ACC CAG ATC A-3′ and reverse, 5′-TGG
TAC GAC CAG AGG CAT ACA G-3′.
Histological and immunohistochemical
analyses
The skin tissues were fixed in 10% buffered
formalin, paraffin-embedded and sectioned into 5-µm-thick
sections. The sections were stained with hematoxylin and eosin
(H&E) and examined under a light microscope (Nikon Eclipse
Ti-S; Nikon Instruments, Inc., Melville, NY, USA). The
paraffin-embedded skin tissue was deparaffinized, immunostained
with either anti-Gr-1 antibody (Cat no. 108402; Biolegend, San
Diego, CA, USA) or anti-CD31 antibody (Cat no. sc-1506; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) and detected with NovaRED
substrate (Vector Laboratories, Burlingame, CA, USA) as described
previously (18).
Statistical analysis
All data are expressed as the mean values ± SEM
(n=4–6 animals/group) and analyzed by one-way analysis of variance
(ANOVA) and compared using the Student-Newman-Keuls (SNK) test for
multiple comparisons and the Student's t-test for comparisons
between 2 groups. The differences in values were considered
significant with a value of P<0.05.
Results
Wound closure rate is accelerated in
CIRP−/− mice
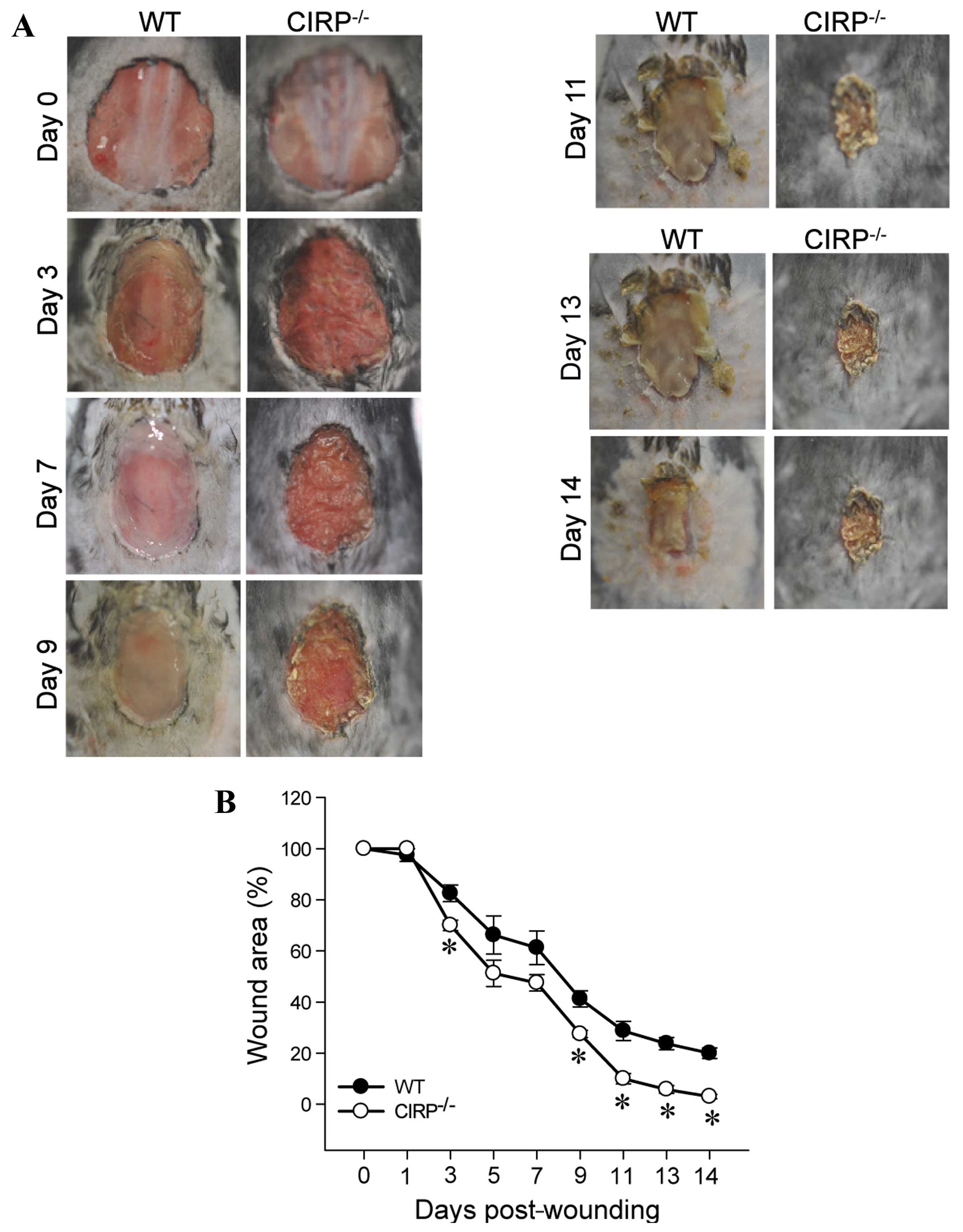
We first determined the rate of wound closure in the
CIRP−/− as compared to the WT mice. As early as day 3,
there were visual differences in the wounds between the 2 groups of
mice and the wound area also began to decrease in size in the
CIRP−/− mice (Fig. 1).
This difference in the size of the wound area between the 2 groups
of mice was more evident by day 9; the size of the wound area was
significantly decreased in the CIRP−/− mice (P=0.007).
The size of the wound area remained significantly smaller in the
CIRP−/− mice from days 9 to 14 and the greatest
significant difference between the 2 groups of mice was observed on
day 14 (P<0.001). These results demonstrated that a deficiency
in CIRP accelerated the cutaneous wound closure rate.
Wound-associated inflammation is resolved
early in CIRP−/− mice
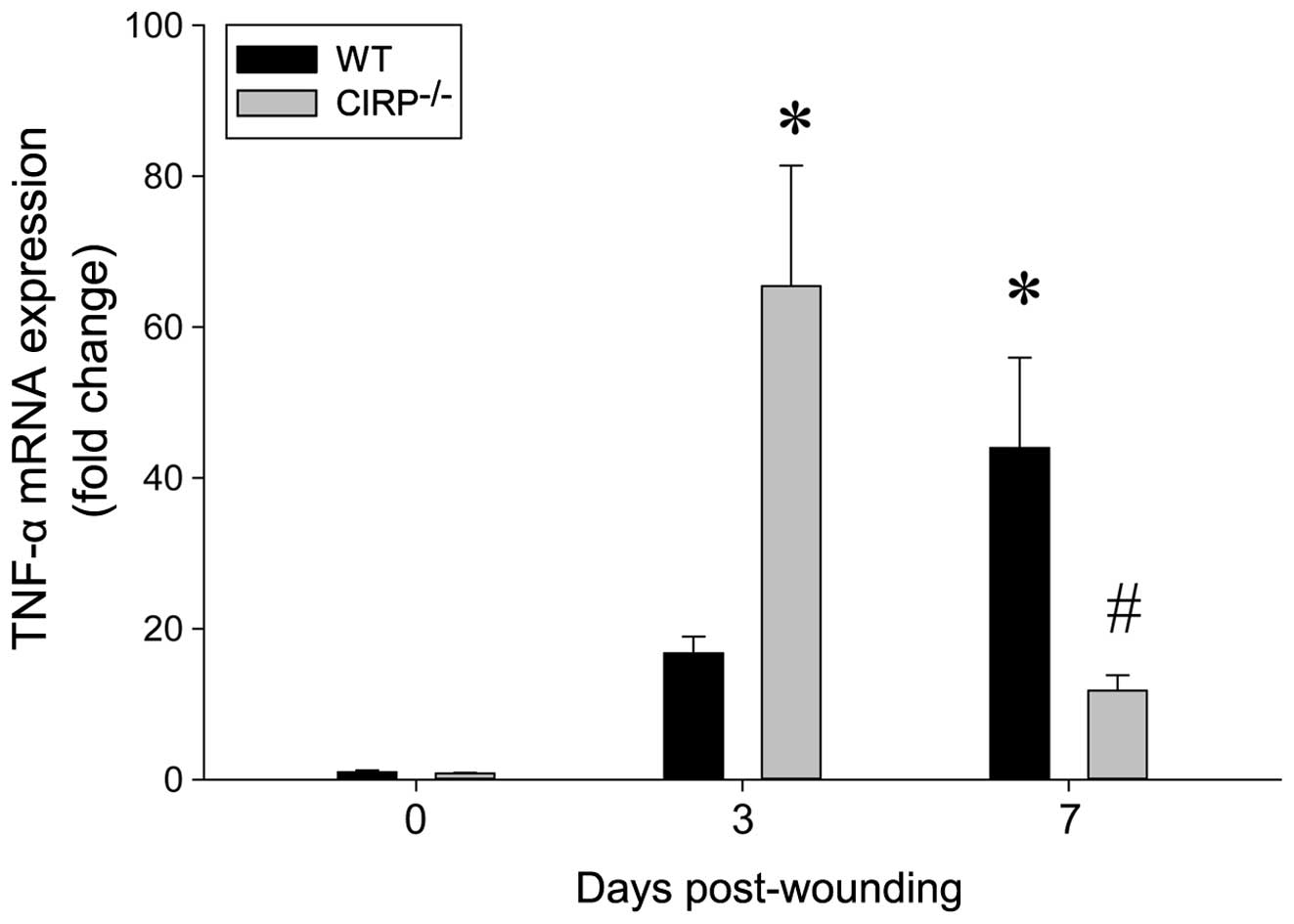
To determine the resolution of inflammation after
wounding, the mRNA expression of TNF-α in the wounded skin tissue
was assessed. In the WT mice, TNF-α expression was significantly
increased by 16-fold on day 3. In the CIRP−/− mice,
while TNF-α expression was also increased by day 3, the levels were
increased by 65-fold (Fig. 2). Of
note, while TNF-α expression was further increased by day 7 in the
WT mice, these levels were reduced in the CIRP−/− mice
(Fig. 2). The decrease in these
levels on day 7 presented a statistically significant difference
from the levels on day 3 in the WT and CIRP−/− mice.
Histological integrity after wounding is
improved in CIRP−/− mice
Histological integrity was assessed by H&E
staining of the skin tissue obtained from the wounds on days 3 and
7 in the WT and CIRP−/− mice. In the WT mice on day 3,
there was a major disorganization of the dermis layer and the
appearance of the infiltration of immune cells into the wounded
tissue, whereas in the CIRP−/− mice, the dermis layer
was more organized and the tissue appears more intact (Fig. 3). On day 7, granulation tissue
began to form and the infiltration of inflammatory cells was
observed in both the WT and CIRP−/− mice; however, the
appearance of infiltrated immune cells was more evident in the WT
than in the CIRP−/− mice.
Wound-associated neutrophil infiltration
is attenuated in CIRP−/− mice
To assess neutrophil infiltration into the wounded
skin, the tissue sections were stained with Gr-1 antibody, a
surface marker of neutrophils. On day 7, compared to the
CIRP−/− mice, more Gr-1 staining was observed in the WT
mice, indicating the persistence of neutrophil infiltration and
subsequent inflammation (Fig.
4).
Wound-associated angiogenesis is
accelerated in CIRP−/− mice
After the inflammatory phase, the process of wound
healing progresses to the angiogenesis phase where endothelial
cells from the bone marrow are migrated to the wounded tissue for
neovascularization (8). On day 7
after wounding, the tissues were stained with CD31, a marker of
angiogenesis. In the CIRP−/− mice, there were dense
staining of CD31 as compared to the WT mice, indicating that by day
7, the CIRP−/− mice had already progressed towards the
angiogenesis phase, while the WT mice remained at the inflammatory
phase of wound healing (Fig.
4).
Discussion
Chronic or non-healing wounds are very prevalent in
clinical practice and most of these wounds are associated with
venous insufficiency, diabetes mellitus, pressure necrosis and
vasculitis (19,20). Despite their differences in
origin, these wounds fail to progress through normal wound repair
and remain in the inflammatory state (21). Wound healing involves a complex
mechanism of an intricate balance of distinct phases namely, the
inflammatory phase, tissue formation and tissue remodeling. The
inflammatory response is the initial process in wound healing.
Chronic or non-healing wounds are caused by an exaggerated
inflammatory response due to the prolonged presence of neutrophils
in the wounded area (8). Thus,
identifying factors that may be responsible for such neutrophil
persistence will be helpful in developing therapeutic strategies
against impaired wound healing.
Our study demonstratred that a deficiency in CIRP
significantly decreased the size of the wound area as compared to
the WT counterpart, indicating the acceleration of wound healing in
mice. The wound closure rate correlated with the inflammatory phase
by day 3 in both the WT and CIRP−/− mice, but there was
a significant increase in the TNF-α mRNA expression levels in the
CIRP−/− mice compared to the WT mice. This increase in
TNF-α expression correlated with the increase in neutrophil
infiltration into the wounded tissue, as evidenced by Gr-1
staining. By contrast, on day 7, TNF-α expression was significantly
decreased in the CIRP−/− mice and there was a
concomitant decrease in neutrophil infiltration, whereas in the WT
mice, TNF-α expression remained high and a considerable level of
Gr-1 staining was also present. This acceleration of the resolution
of inflammation on day 7 in the CIRP−/− mice correlated
with the second phase, i.e., tissue formation, as observed by the
increased expression of CD31, as a measure of neovascularization.
Finally, the histological integrity was improved as early as day 3
in the CIRP−/− mice, as observed by an organized and
intact dermis layer, whereas in the WT mice, the dermis layer was
disordered and the appearance of immune cell infiltration was
observed even on day 7. These data suggest that a deficiency in
CIRP significantly accelerates the wound healing process in
mice.
In physiological wound repair, within a few hours of
injury, neutrophils which are entrapped and aggregated in the
blood, clot, and transmigrate across the endothelial cell wall of
blood capillaries which have been activated by pro-inflammatory
cytokines, i.e., TNF-α (8,22).
Activated endothelial cells express various adhesion molecules for
leukocyte adhesion at the wound site (22). The infiltrating neutrophils are
the initial components of the inflammatory phase. They not only
invade pathogens, but are also involved in tissue degradation and
tissue formation (8). Recruited
neutrophils begin the debridement of devitalized tissue and
phagocytosis of infectious agents. In this period. they release
ROS, cationic peptides and proteases and promote wound healing
(23). In general, neutrophil
infiltration ceases within a few days and the apoptotic neutrophils
are engulfed by macrophages at the wound site (24,25). While neutrophil infiltration is
essential for wound healing, the persistence of neutrophils at the
wound site leads to a prolonged inflammatory state and impaired
wound healing (7).
One main factor in causing chronic inflammation in
cutaneous wound healing is ROS. Leukocytes recruited to the wounded
site are a rich source of ROS (26). Endothelial cells and fibroblasts
present in the wound also contribute to the production of ROS
(27,28). In addition to direct damage to the
cell membrane and structural proteins of the extracellular matrix,
ROS activate various signaling pathways, leading to the
upregulation of pro-inflammatory cytokines, such as TNF-α (29). Therefore, the unbalance of the
oxidant/antioxidant microenvironment in chronic wounds is
responsible for the prolonged inflammatory state and impaired
healing. Diabetic hyperglycemia in diabetes or enhanced hydrostatic
pressure as in pressure ulcers can enhance the inflammatory
response. Tissue hypoxia, bacterial components, foreign agents and
necrotic tissue are all local stimuli which sustain the influx of
neutrophils and macrophages to the wound site (8,19,30).
The mechanisms through which a deficiency in CIRP
accelerates the inflammatory state and the resolution of
inflammation in wound healing have not yet been elucidated. CIRP is
known to be expressed in high levels during stress conditions such
as cold, ultraviolet exposure and hypoxia (13–15). Recently, we demonstrated that
hemorrhage associated with the release of TNF-α was significantly
reduced in CIRP−/− mice and that the blockade of CIRP
using antisera to CIRP in experimental models of hemorrhagic shock
and sepsis attenuated inflammatory response and mortality (16). In addition, we demonstrated
increased circulation levels of CIRP in patients admitted to the
surgical intensive care unit with hemorrhagic shock and have
identified CIRP as a mediator of inflammation (16). Shock and sepsis are tissue injury
indications that are associated with an exaggerated systemic
inflammatory response (31,32). It can thus be speculated that the
exaggerated inflammatory response in chronic wounds or non-healing
wounds is mediated by CIRP. Future studies are warranted to confirm
this hypothesis.
In conclusion, in the present study, we demonstrated
that a deficiency in CIRP accelerated the wound closure rate and
the inflammatory phase, which led to the resolution of inflammation
and neovascularization, and in the improvement of histological
integrity during the wound healing process. Therefore, CIRP may be
responsible for delayed wound healing in disease conditions, such
as diabetes, and venous and pressure ulcers. Therapeutic
interventions for blocking CIRP may thus prove to be beneficial for
the treatment of cutaneous wounds and a more rapid healing
process.
Acknowledgments
The authors would like to thank Dr Michael
Kuncewitch (Hofstra North Shore-LIJ School of Medicine, Manhasset,
NY, USA), Dr Mahendar Ochani and Dr Xiaoling Qiang (The Feinstein
Institute for Medical Research, Manhasset, NY, USA) for their
expert technical assistance.
References
|
1
|
Bickers DR, Lim HW, Margolis D, Weinstock
MA, Goodman C, Faulkner E, Gould C, Gemmen E and Dall T; American
Academy of Dermatology Association; Society for Investigative
Dermatology: The burden of skin diseases: 2004 a joint project of
the American Academy of Dermatology Association and the Society for
Investigative Dermatology. J Am Acad Dermatol. 55:490–500. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Phillips TJ: Chronic cutaneous ulcers:
Etiology and epidemiology. J Invest Dermatol. 102:38S–41S. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Margolis DJ, Bilker W, Santanna J and
Baumgarten M: Venous leg ulcer: Incidence and prevalence in the
elderly. J Am Acad Dermatol. 46:381–386. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pecoraro RE, Reiber GE and Burgess EM:
Pathways to diabetic limb amputation. Basis for prevention.
Diabetes Care. 13:513–521. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Abbade LP and Lastória S: Venous ulcer:
Epidemiology, physio-pathology, diagnosis and treatment. Int J
Dermatol. 44:449–456. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Diegelmann RF: Excessive neutrophils
characterize chronic pressure ulcers. Wound Repair Regen.
11:490–495. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dovi JV, Szpaderska AM and DiPietro LA:
Neutrophil function in the healing wound: Adding insult to injury?
Thromb Haemost. 92:275–280. 2004.PubMed/NCBI
|
|
8
|
Eming SA, Krieg T and Davidson JM:
Inflammation in wound repair: Molecular and cellular mechanisms. J
Invest Dermatol. 127:514–525. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jun JI, Kim KH and Lau LF: The
matricellular protein CCN1 mediates neutrophil efferocytosis in
cutaneous wound healing. Nat Commun. 6:73862015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nishiyama H, Higashitsuji H, Yokoi H, Itoh
K, Danno S, Matsuda T and Fujita J: Cloning and characterization of
human CIRP (cold-inducible RNA-binding protein) cDNA and
chromosomal assignment of the gene. Gene. 204:115–120. 1997.
View Article : Google Scholar
|
|
11
|
Nishiyama H, Danno S, Kaneko Y, Itoh K,
Yokoi H, Fukumoto M, Okuno H, Millán JL, Matsuda T, Yoshida O and
Fujita J: Decreased expression of cold-inducible RNA-binding
protein (CIRP) in male germ cells at elevated temperature. Am J
Pathol. 152:289–296. 1998.PubMed/NCBI
|
|
12
|
Nishiyama H, Xue JH, Sato T, Fukuyama H,
Mizuno N, Houtani T, Sugimoto T and Fujita J: Diurnal change of the
cold-inducible RNA-binding protein (Cirp) expression in mouse
brain. Biochem Biophys Res Commun. 245:534–538. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sheikh MS, Carrier F, Papathanasiou MA,
Hollander MC, Zhan Q, Yu K and Fornace AJ Jr: Identification of
several human homologs of hamster DNA damage-inducible transcripts.
Cloning and characterization of a novel UV-inducible cDNA that
codes for a putative RNA-binding protein. J Biol Chem.
272:26720–26726. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wellmann S, Bührer C, Moderegger E, Zelmer
A, Kirschner R, Koehne P, Fujita J and Seeger K: Oxygen-regulated
expression of the RNA-binding proteins RBM3 and CIRP by a
HIF-1-independent mechanism. J Cell Sci. 117:1785–1794. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xue JH, Nonoguchi K, Fukumoto M, Sato T,
Nishiyama H, Higashitsuji H, Itoh K and Fujita J: Effects of
ischemia and H2O2 on the cold stress protein
CIRP expression in rat neuronal cells. Free Radic Biol Med.
27:1238–1244. 1999. View Article : Google Scholar
|
|
16
|
Qiang X, Yang WL, Wu R, Zhou M, Jacob A,
Dong W, Kuncewitch M, Ji Y, Yang H, Wang H, et al: Cold-inducible
RNA-binding protein (CIRP) triggers inflammatory responses in
hemorrhagic shock and sepsis. Nat Med. 19:1489–1495. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Idrovo JP, Yang WL, Jacob A, Ajakaiye MA,
Cheyuo C, Wang Z, Prince JM, Nicastro J, Coppa GF and Wang P:
Combination of adrenomedullin with its binding protein accelerates
cutaneous wound healing. PLoS One. 10:e01202252015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Giangola MD, Yang WL, Rajayer SR, Nicastro
J, Coppa GF and Wang P: Growth arrest-specific protein 6 attenuates
neutrophil migration and acute lung injury in sepsis. Shock.
40:485–491. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Singer AJ and Clark RA: Cutaneous wound
healing. N Engl J Med. 341:738–746. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gosain A and DiPietro LA: Aging and wound
healing. World J Surg. 28:321–326. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Loots MA, Lamme EN, Zeegelaar J, Mekkes
JR, Bos JD and Middelkoop E: Differences in cellular infiltrate and
extracellular matrix of chronic diabetic and venous ulcers versus
acute wounds. J Invest Dermatol. 111:850–857. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Szpaderska AM, Egozi EI, Gamelli RL and
DiPietro LA: The effect of thrombocytopenia on dermal wound
healing. J Invest Dermatol. 120:1130–1137. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schäfer M and Werner S: Oxidative stress
in normal and impaired wound repair. Pharmacol Res. 58:165–171.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Martin P: Wound healing - aiming for
perfect skin regeneration. Science. 276:75–81. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Martin P and Leibovich SJ: Inflammatory
cells during wound repair: The good, the bad and the ugly. Trends
Cell Biol. 15:599–607. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Weiss SJ: Tissue destruction by
neutrophils. N Engl J Med. 320:365–376. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Campisi J: Replicative senescence: An old
lives' tale? Cell. 84:497–500. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mendez MV, Stanley A, Park HY, Shon K,
Phillips T and Menzoian JO: Fibroblasts cultured from venous ulcers
display cellular characteristics of senescence. J Vasc Surg.
28:876–883. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wenk J, Foitzik A, Achterberg V,
Sabiwalsky A, Dissemond J, Meewes C, Reitz A, Brenneisen P,
Wlaschek M, Meyer-Ingold W and Scharffetter-Kochanek K: Selective
pick-up of increased iron by deferoxamine-coupled cellulose
abrogates the iron-driven induction of matrix-degrading
metalloproteinase 1 and lipid peroxidation in human dermal
fibroblasts in vitro: A new dressing concept. J Invest Dermatol.
116:833–839. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Eming SA, Smola H and Krieg T: Treatment
of chronic wounds: State of the art and future concepts. Cells
Tissues Organs. 172:105–117. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Martin GS: Sepsis, severe sepsis and
septic shock: Changes in incidence, pathogens and outcomes. Expert
Rev Anti Infect Ther. 10:701–706. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Martin GS, Mannino DM, Eaton S and Moss M:
The epidemiology of sepsis in the United States from 1979 through
2000. N Engl J Med. 348:1546–1554. 2003. View Article : Google Scholar : PubMed/NCBI
|