Introduction
Osteoarthritis (OA) is one of the most common
degenerative joint disorders, and it affects elderly individuals in
particular (1–3). It is characterized by the
progressive loss of the cartilage matrix, a reduction in bone mass,
the destruction of articular cartilage and osteophyte formation
(4,5). The prevalence of this disease has
been reported as continuing to increase, and this brings with it an
enormous financial burden and related impairment of quality of life
(6,7). However, to date, limited available
disease-modifying treatment options for patients with OA are
available due to the uncertain etiology of the disease (8). Currently, the only registered
systemic oral drug therapy for OA is analgesics or
anti-inflammatory agents to relieve symptoms (8,9).
The functional roles of microRNAs (miRNAs or miRs)
in OA have increasingly attracted attention (10–12). miRNAs are short, small non-coding
RNAs molecules that are involved in various human diseases
(13,14). miRNAs regulate gene expression by
repressing messenger RNA (mRNA) translation and promoting mRNA
degradation (15). Previous
studies have confirmed that a number of miRNAs, such as has-miR-21,
has-miR-23a, hsa-miR-24, hsa-miR-27, hsa-miR-93, hsa-miR-100,
hsa-miR-140, hsa-miR-145, hsa-miR-146a and hsa-miR-675 are
associated with the development of OA (16–21). In addition, it has been previously
demonstrated that A disintegrin-like and metalloprotease
(reprolysin type) with thrombospondin type 1 motif, 5 (ADAMTS5,
also known as aggrecanase-2) is involved in the development of OA
(22). Of the ADAMTS family,
ADAMTS4 and 5 are known to be the most efficient aggrecanases, and
have been regarded as the most likely candidates for OA
pathogenesis (23). Moreover, a
study using animals indicated that the deletion of ADAMTS5 in mice
with OA can prevent early aggrecan depletion and cartilage
destruction (24). In addition,
in another previous study, Matsukawa et al suggested that
miR-125b regulates the expression of ADAMTS4 in OA chondrocytes
(25).
However, to date, and to the best of our knowledge,
there are limited studies available investigating the effects of
hsa-miR-15a on OA, and the question of whether hsa-miR-15a
regulates the expression of ADAMTS5 remains unanswered. Therefore,
in the present study, we aimed to examine the effects of
hsa-miR-15a on OA, as well as the associated mechanisms.
Materials and methods
Specimen selection
Femoral condyles or tibial plateaus were selected as
specimens. Human OA cartilage was harvested from patients who
underwent total knee replacement surgery (10 males and 6 females;
mean age, 70±4 years) at the Department of Orthopaedic Surgery,
Peking Union Medical College Hospital (Beijing, China). OA was
diagnosed according to the clinical and radiological evaluation
criteria published by the American College of Rheumatology (ACR)
(26). In addition, none of the
patients from whom the samples were collected had received
intra-articular steroid therapy in the 3 months prior to the
surgery. Healthy (control) articular cartilage was obtained from
donors who had died (by car accident or due to cardiovascular and
cerebrovascular diseases such as severe heart failure, or malignant
tumors), within 12 h of death (8 males and 5 females; mean age,
67±5 years). The normal healthny individuals had no previous
history of joint disorder. This study was approved by the Medical
Ethics Committee of Peking Union Medical College Hospital (Beijing,
China) and informed consent was acquired from all participants.
Cell culture and transient
transfection
After collecting the cartilage specimens, the
tissues were diced and digested in 1.5 mg/ml collagenase type 2
(CLS-2; Worthington, Lakewood, NJ, USA) in Dulbecco's modified
Eagle's medium (DMEM; Invitrogen, Carlsbad, CA, USA) for 16 h at
37°C. The DMEM solution was supplemented with 10% fetal bovine
serum (FBS), 100 U/ml penicillin and 100 μg/ml streptomycin
(all from Sigma, St. Louis, MO, USA). The isolated cells were then
passed through a 100-μm cell strainer (Falcon;
Becton-Dickinson Labware GmbH, Heidelberg, Germany), pelleted and
washed with medium. The cultures were maintained in DMEM containing
10% FBS in a humidified incubator containing 5% CO2 at
37°C.
The cells were subsequently seeded in a 48-well
prior to transfection. After post-culturing for 18–24 h, when the
density of the chondrocytes had reached 70%, the cells were
transfected using Lipofectamine 2000 (Invitrogen) according to a
previously described method (27). In the present study, hsa-miR-15a
mimics, pcDNA3/enhanced green fluorescent protein
(EGFP)-ADAMTS5-3′-untranslated region (3′-UTR), antisense
oligonucleotides (ASO)-hsa-miR-15a, ASO-NC (negative control),
pcDNA3/EGFP-ADAMTS5-3′-UTR mutant and pDsRed2-N1, along with the
plasmids were constructed and synthesized by Sangon Biotech Co.,
Ltd. (Shanghai, China) based on standard protocols (28). Furthermore, ADAMTS5 expression was
suppressed using the small interfering RNA (siRNA) synthesized by
Sangon Biotech Co., Ltd. in both human OA and normal chondrocytes.
A negative control (siNC) was also performed concurrently on each
specimen. The sequence of the ADAMTS5 siRNA was 5′-AAGAUAAGCG
CUUAAUGUCUU-3′. Cells were collected 48 h after transfection for
subsequent assays.
Analysis of miRNA expression
After post-culturing for 24 h, total RNA was
extracted from the cells using an miRNA Isolation kit (Ambion,
Austin, TX, USA) according to the manufacturer's instructions. The
relative expression of levels miRNAs (specific for has-miR-15a)
were determined using TaqMan® MicroRNA assays (Applied
Biosystems, Foster City, CA, USA) with the comparative
2−ΔΔCT method. Total RNA was reverse transcribed into
complementary DNA (cDNA) using MuLV reverse transcriptase (Life
Technologies, Carlsbad, CA, USA), followed by quantification using
the QuantiTect SYBR-Green real-time-polymerase chain reaction kit
(Qiagen SA, Hilden, Germany). Primers for miR-15a were designed and
obtained from the TaqMan miRNA assays. U6 snRNA (Applied
Biosystems) was used as a loading control for miRNA expression. The
primer sequence for stem-loop RT-PCR hsa-miR-15a was
5′-CTCAACTGGTGTCGTGGAGTCGGC AATTCAGTTGAGCACAAACC-3′. The following
primers were used for PCR: hsa-miR-15a forward, 5′-ACACT
CCAGCTGGGTAGCAGCACATAATGG-3′ and reverse, 5′-TGGTGTCGTGGAGTCG-3′;
U6 forward, 5′-CTCGCTT CGGCAGCACA-3′ and reverse,
5′-AACGCTTCACGAATTTGCGT-3′.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
We extracted total RNA from the chondrocytes using
TRIzol reagent (Invitrogen) according to the manufacturer's
instructions. The purity of the total RNA was assessed with the
A260/A280 ratio, and values of 1.8 and 2.0 were considered good.
Total RNA was reverse transcribed into cDNA and amplified, and was
subsequently quantified using RT-qPCR with SYBR-Green Ex Taq on a
LightCycler 480 (Roche Applied Science, Indianapolis, IN, USA).
Primers for ADAMTS5 were designed and obtained from Invitrogen. The
glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene was used as a
reference. The PCR conditions included 1 prede-naturation cycle of
5 min at 95°C, 40–50 cycles of 95°C for 30 sec, 58–62°C for 30 sec,
and 72°C for 30 sec, and a final extension at 72°C for 5 min.
Relative quantification was calculated using the 2−ΔΔCT
method.
Bioinformatics analysis
Target genes of hsa-miR-15a were predicted by
bioinformatics analysis using TargetScan 6.2 (http://www.targetscan.org) and/or microRNA.org (http://www.microrna.org).
Luciferase reporter assay
The 3′-UTR of ADAMTS5 was PCR-amplified, cloned into
the psiCHECK-2 vector and co-transfected with the control or
hsa-miR-15a precursor into the chondrocytes. After 48 h of
transfection, luciferase activity was evaluated using the
Dual-luciferase activity assay system (DLR; Promega, Madison, WI,
USA).
Measurement of DNA content
Purified total DNA was quantified using Quant-iT™
PicoGreen® dsDNA reagent (Invitrogen) according to the
manufacturer's instructions. Fluorescence was measured using a
fluorescence microplate reader (Tecan Polarion, Stevenage, UK) at
an excitation/emission wavelength of 480/520 nm. Lambda DNA (Sigma)
was used as marker for determination of the DNA quantity.
Proteoglycan analysis
Total glycosaminoglycans (GAGs) were quantified
using a 1,9-dimethylmethylene blue (DMMB) spectrophotometric
analysis according to a previously described method (29). Briefly, following the addition of
DMMB, the mixture was assayed for GAG at an absorbance of 525 nm.
Chondroitin sulfate C (Sigma) was used as a reference.
Collagenase activity assay
After collecting the culture medium, collagenase
activity assay was performed to determine the collagenase activity
using an Enzchek Gelatinase/Collagenase assay kit (Invitrogen)
according to the manufacturer's instructions. The assays were
carried out in reaction buffer containing DQ Collagen Fluorescein
conjugate at room temperature. Fluorescence was determined using a
fluorescence micro-plate reader (SpectraMAX Gemini XS; Molecular
Devices, Sunnyvale, CA, USA) at 485 nm excitation and 538 nm
emission. Collagenase activity was determined using the formula
provided with the assay kit and normalized to the weight of the
explant. Collagenase type IV (Clostridium) was used as a
reference standard.
Western blot analysis
Following culture for 24 h, cell protein was
extracted using protein lysis buffer. Protein concentration was
assessed using the Bio-Rad DC protein assay kit (Bio-Rad,
Ivry-sur-Seine, France). The protein was separated by 10% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
was then transferred to polyvinylidene difluoride (PVDF) membranes
(Bio-Rad, Hercules, CA, USA). The membranes were blocked in a
sealed in 5% fresh non-fat dry milk for 2 h and were then incubated
with the primary anti-type II collagen antibody (1:1,000 dilution;
MAB1330; Chemicon, Millipore, Billerica, CA, USA) and primary
anti-ADAMTS5 antibody (1:100 dilution; ab45042; Abcam, Cambridge,
UK) for 2 h at room temperature or overnight at 4°C, followed with
horseradish peroxidase-conjugated anti-mouse secondary antibody
(accession numbers ab195239). An anti-GAPDH antibody (sc-365062;
Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) was used as a
loading control. Finally, the samples were tested using enhanced
chemiluminescence and densitometric analysis. For relative
expression analysis, NIH Image J software (NIH Image, Bethesda, MD,
USA) was performed to determine band intensity.
Statistical analysis
In the present study, the statistical package for
the social sciences (SPSS) (version 17.0; SPSS Inc., Chicago, IL,
USA) was used to perform statistical analysis. A student's t-test
was performed to study statistical comparisons. A p-value <0.05
was considered to indicate a statistically significant
difference.
Results
Expression of hsa-miR-15a is decreased in
human OA chondrocytes
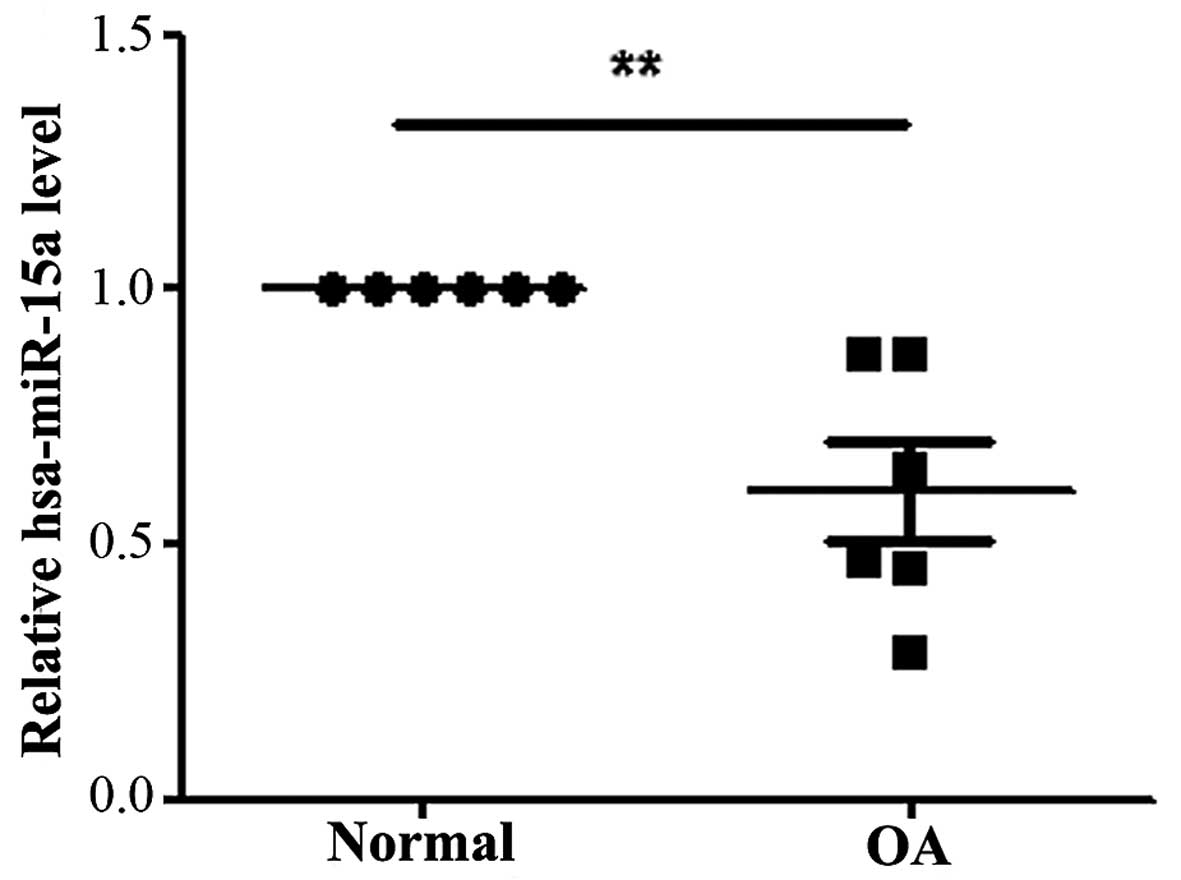
To dertermine the expression levels of hsa-miR-15a
in human OA chondrocytes, we determined the levels of hsa-miR-15a
in human OA cartilage specimens and normal cartilage tissues by
RT-qPCR. As shown in Fig. 1, the
expression level of hsa-miR-15a was significantly decreased in the
OA chondrocytes compared with the normal cartilage tissue
(P<0.05).
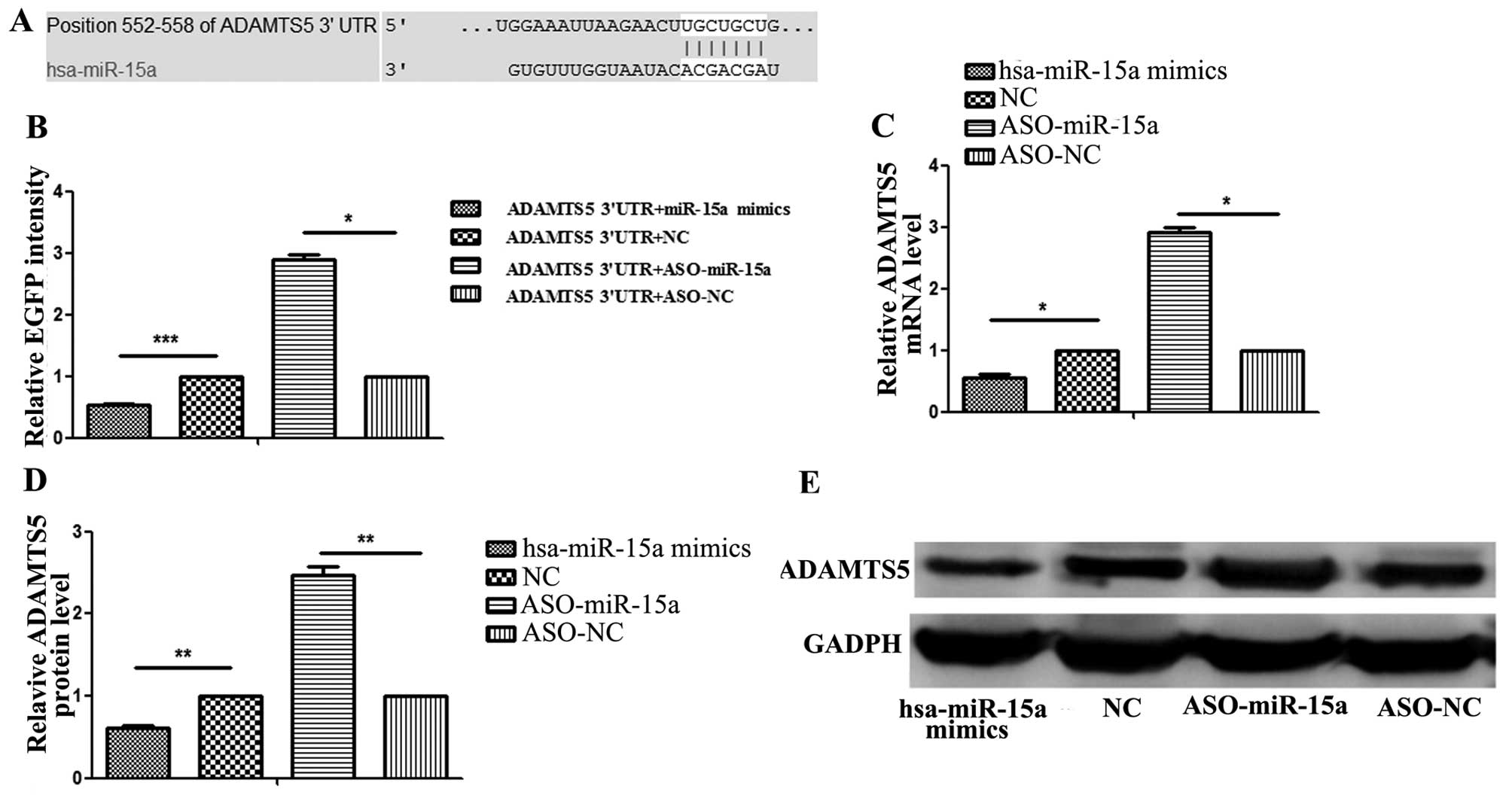
ADAMTS5 gene 3′-UTR carries a putative
hsa-miR-15a binding site and is negatively regulated by
hsa-miR-15a
To predict whether the ADAMTS5 gene mRNA 3′-UTR
contains a hsa-miR-15a binding site, TargetScan 6.2 and microRNA.org were used to confirm the prediction. The
results revealed that a binding site for hsa-miR-15a was in the
ADAMTS5 mRNA 3′-UTR (Fig.
2A).
In order to confirm that has-miR-15a binds to this
region and leads to translational repression, we cloned the
putative binding site into the pcDNA3/EGFP plasmid downstream of
the EGFP reporter construct and co-transfected this plasmid into
the cells with ASO-hsa-miR-15a or ASO-negative control (NC). We
found that the relative EGFP intensity was significantly higher in
the cells that were co-transfected with ASO-miR-15a compared with
the cells co-transfected with ASO-NC. In addition, we cloned the
putative binding site into pcDNA3/EGFP downstream of the EGFP
reporter construct and co-transfected this plasmid with hsa-miR-15a
mimics or NC. The results revealed that the relative EGFP intensity
was significantly lower in the cells co-transfected with
has-miR-15a mimics compared with those co-transfected with NC
(Fig. 2B). Additionally, we
performed RT-qPCR and western blot analysis to measure the mRNA and
protein expression levels of ADAMTS5, respectively. Both RT-qPCR
(Fig. 2C) and western blot
analysis (Fig. 2D and E) revealed
that the ADAMTS5 expression levels were significantly lower in the
has-miR-15a mimics-transfected group compared with the
NC-transfected group (P<0.05); however these levels were
significantly higher in the ASO-miR-15a-transfected group compared
with the ASO-NC-transfected group (P<0.05), respectively. These
results suggest that hsa-miR-15a binds directly to the 3′-UTR of
ADAMTS5 mRNA and inhibits gene expression. These results also
indicate that ADAMTS5 is a direct target of hsa-miR-15a.
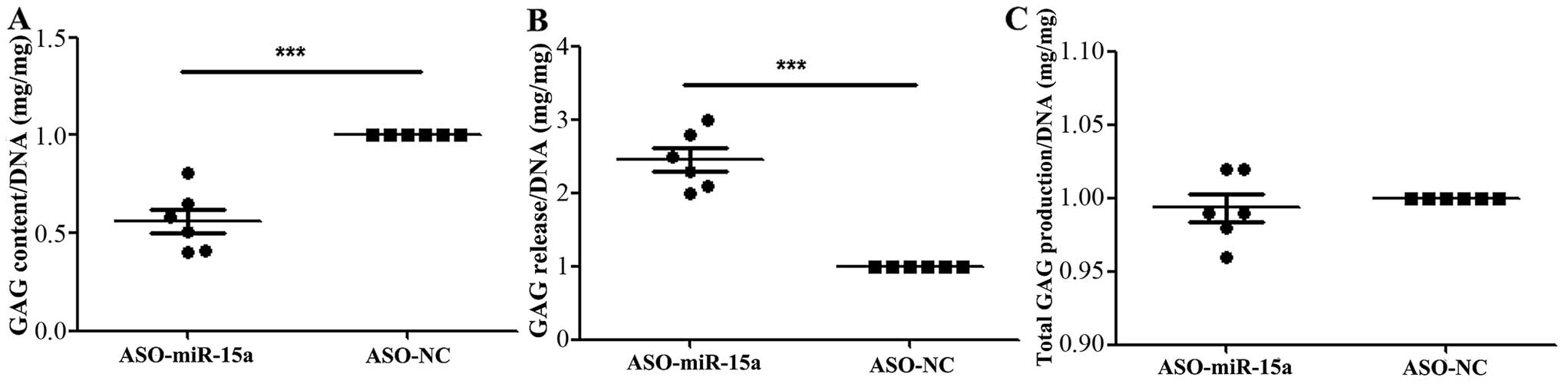
Downregulation of hsa-miR-15a expression
decreases the aggregation of proteoglycan and increases the release
of proteoglycan
The amount of proteoglycans was significantly
decreased in the cell substrate generated by the OA chondrocytes
transfected with ASO-miR-15a compared to the ASO-NC-transfected
group (Fig. 3A), while the amount
of proteoglycans was significantly increased in the culture medium
compared to the ASO-NC-transfected group (Fig. 3B). However, as regards the total
amount of proteoglycans, there were no significant differences
observed between the ASO-miR-15a-transfected group and the controls
(ASO-NC) (Fig. 3C). The total
amount of proteoglycans was measured by combining the amount found
in the cell substrate and that released into the medium at the end
of the culture period.
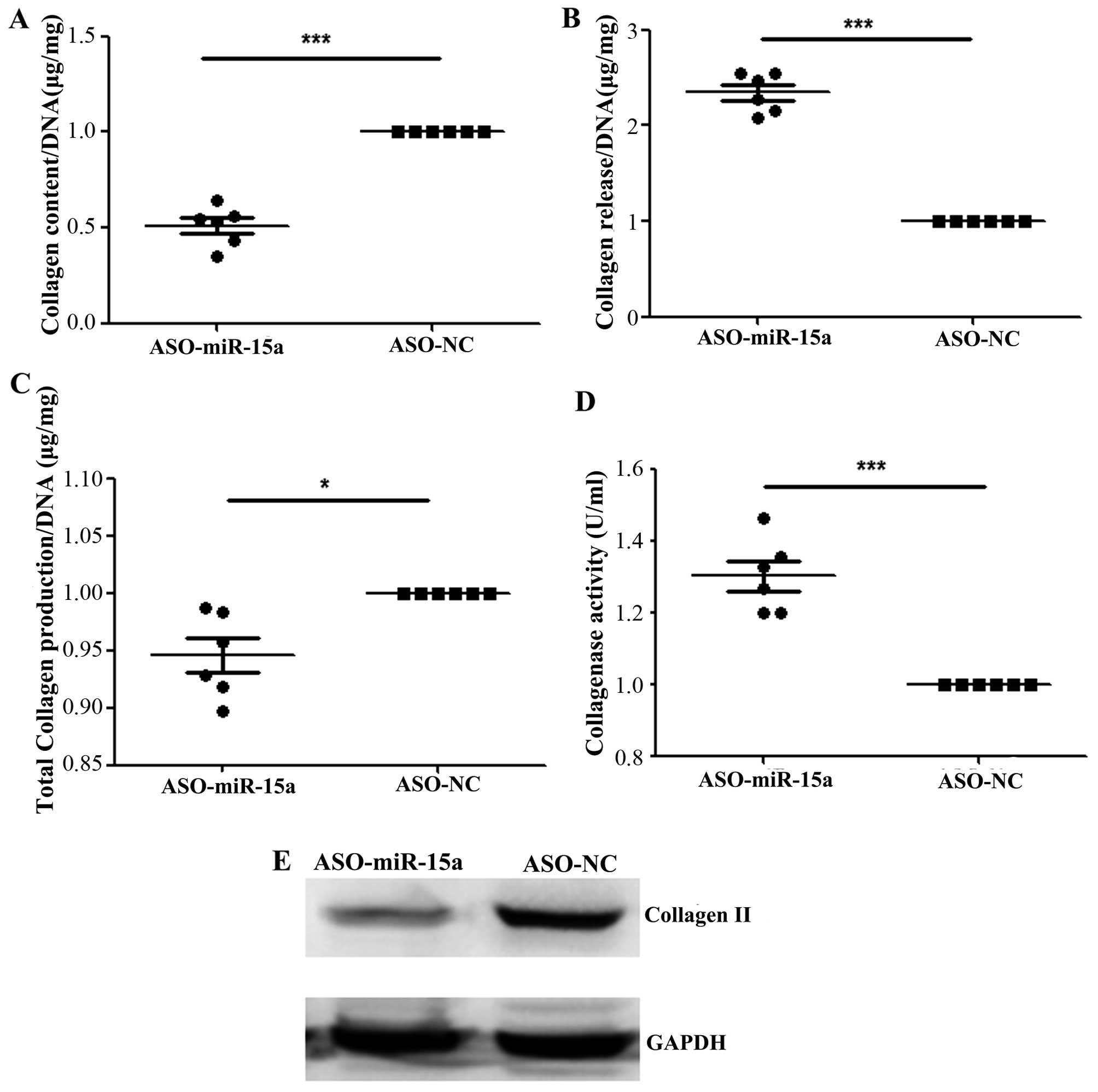
Downregulation of hsa-miR-15a expression
decreases the aggregation of collagen and increases the release of
collagen
The amount of collagen was significantly decreased
in the cell substrate generated by the OA chondrocytes transfected
with ASO-miR-15a compared to the ASO-NC-transfected group (Fig. 4A), while the amount of collagen
was significantly increased in the culture medium of the cells
transfected with ASO-miR-15a compared to the ASO-NC-transfected
group (Fig. 4B). In addition, the
total amount of collagen was significantly decreased in the
ASO-miR-15a-transfected group compared with the controls (ASO-NC)
(Fig. 4C). However, collagenase
activity was significantly increased in ASO-miR-15a-transfected
group compared with the controls (Fig. 4D). Moreover, the protein
expression of collagen II was significantly lower (0.75 ± 0.14 vs.
1.88 ± 0.16, with a 40% decrease) in the ASO-miR-15a-transfected
group compared with the ASO-NC-transfected group (Fig. 4E). These results indicate that the
decreased expression of hsa-miR-15a inhibits the aggregation of
collagen, while the overexpression of hsa-miR-15a promotes the
aggregation of collagen.
Downregulation of ADAMTS5 increases the
aggregation of proteoglycan and decreases the release of
proteoglycan
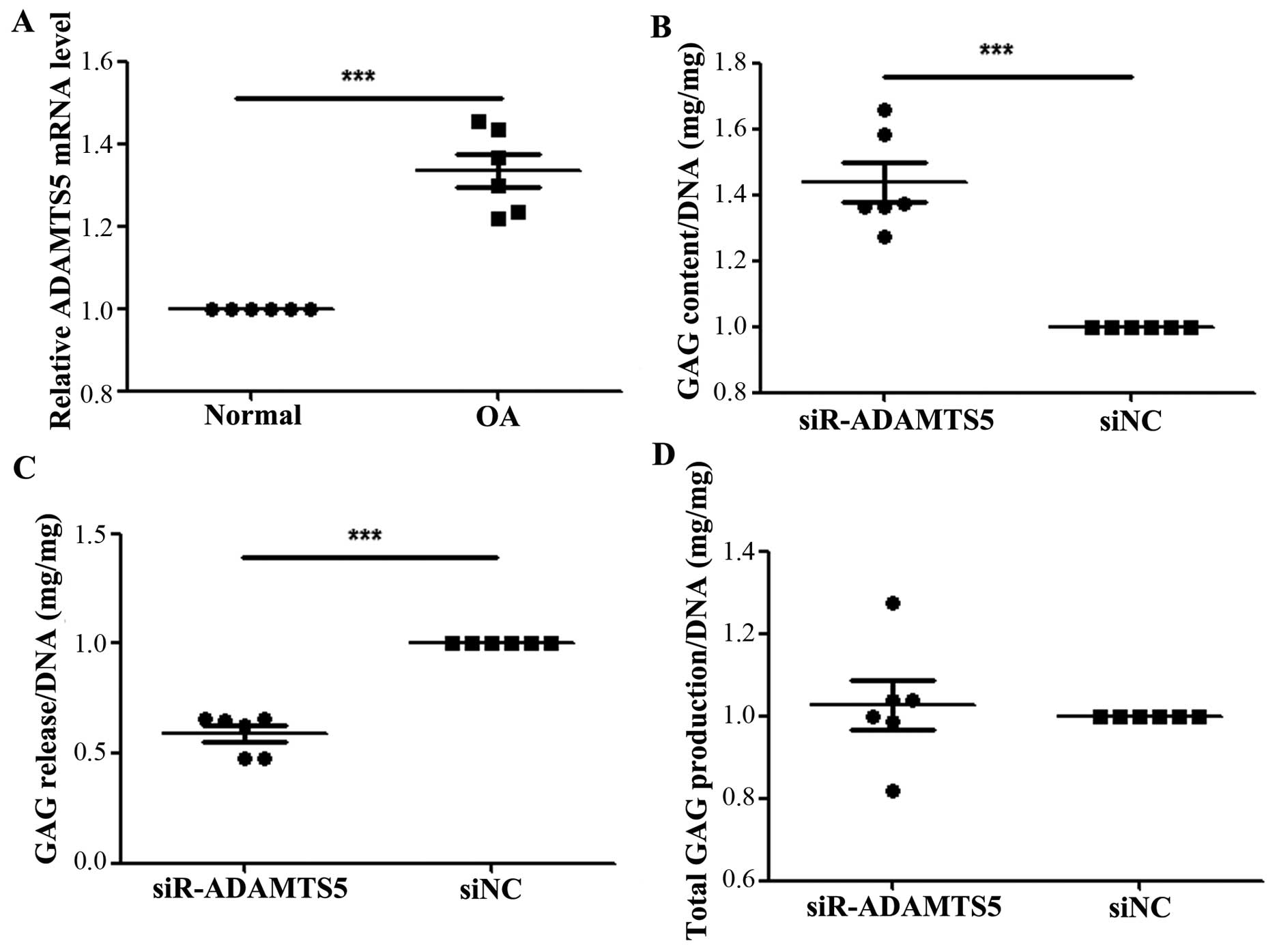
We performed RT-qPCR to measure the expression
levels of ADAMTS5 in human OA chondrocytes, as well as in normal
cartilage tissue. As shown in Fig.
5A, the expression level of ADAMTS5 was significantly increased
in the OA chondrocytes compared with the normal cartilage tissue
(P<0.05). To confirm a more definitive function of ADAMTS5 in
the development of OA, we used ADAMTS5 siRNA to transfect human OA
chondrocytes. The results revealed that the amount of proteoglycan
was significantly increased in the chondrocyte substrate of the
siR-ADAMTS5 group compared with that of the silenced negative
control (siNC) group (Fig. 5B),
whereas the amount released into the medium was significantly
decreased compared with siNC group (Fig. 5C). No significant differences were
observed in the total amount of proteoglycans between the 2 groups
(Fig. 5D).
Downregulation of ADAMTS5 increases the
aggregation of collagen and decreases the release of collagen
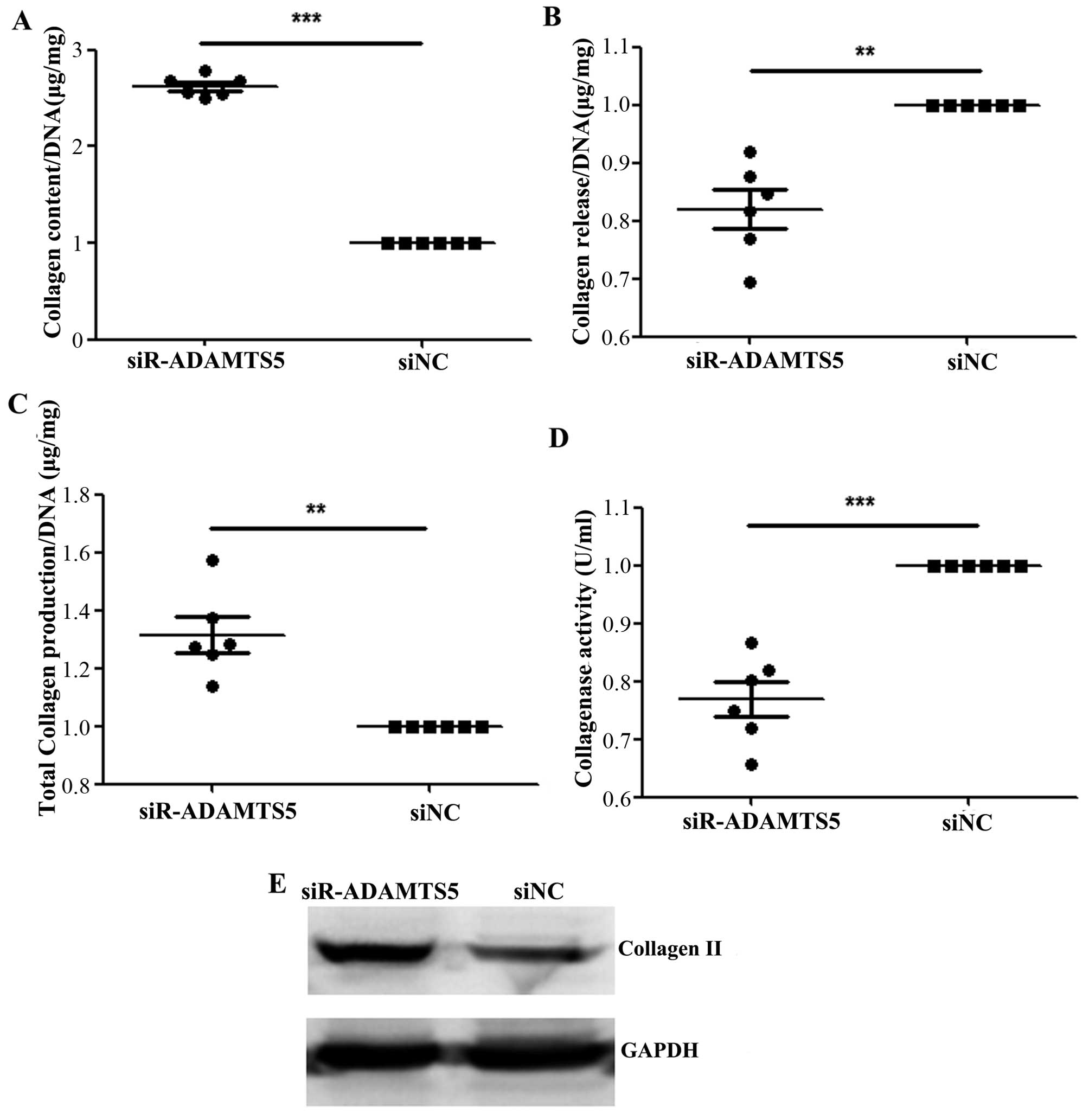
The amount of collagen was significantly increased
in the chondrocyte substrate of the siR-ADAMTS5 group compared with
that of the siNC group (Fig. 6A),
whereas the amount released into the medium was significantly
decreased compared with the siNC group (Fig. 6B). In addition, the total amount
of collagen was significantly increased in the siR-ADAMTS5 group
compared with the siNC group (Fig.
6C), while the collagenase activity was significantly lower in
the siR-ADAMTS5 group than that in the siNC group (Fig. 6D). Furthermore, the protein
expression of collagen II was significantly higher (2.22 ± 0.04 vs.
1.04 ± 0.02, with a 50% increase) in the siR-ADAMTS5 group compared
with the siNC group (Fig.
6E).
Discussion
miRNAs are involved in normal skeletal development
(30), and their levels are
associated with the pathogenesis of degenerative disorders, such as
OA (31,32). Hence, it is necessary to elucidate
the function of miRNA in the OA. In the present study, we examined
the expression and role of hsa-miR-15a in the pathogenesis of OA.
We found that hsa-miR-15a was expressed at lower levels in OA
chondrocytes compared with normal healthy control tissue,
suggesting that hsa-miR-15a plays an important role in the
pathogenesis of OA. Using TargetScan6.2 and microRNA.org to screen the targets of hsa-miR-15,
ADAMTS5 was predicted to be a target of hsa-miR-15a, and the
binding site was found in the ADAMTS5 gene 3′-UTR. Moreover, the
expression of ADAMTS5 was found to be negatively regulated by
hsa-miR-15a. In addition, ADAMTS5, as a key enzyme in OA, has been
reported to play a role in the degradation of articular cartilage
(24). Thus, we hypothesized that
hsa-miR-15a may exert protective effects against OA through the
negative regulation of the expression of ADAMTS5.
In order to prove this hypothesis, we first
determined the expression level of has-miR-15a in both OA and
normal chondrocytes. As previously mentioned, the expression level
of has-miR-15a was significantly decreased in the OA chondrocytes
compared with the normal chondrocytes. Subsequently, we further
assessed the prediction revealed by bioinformatics analysis that
ADAMTS5 is targeted by has-miR-15a. Luciferase reporter assay
confirmed the prediction, and demonstrated that ADAMTS5 is directly
targeted by hsa-miR-15a at its 3′-UTR. In addition, we also
determined the expression mRNA and protein levels of ADAMTS5 when
the level of hsa-miR-15a had been altered. By examining the
overexpression (by transfection with hsa-miR-15a mimics) or
downregulation (by transfection with ASO-hsa-miR-15a) of the level
of hsa-miR-15a, we found that the expression level of ADAMTS5
inversely correlated with hsa-miR-15a.
As a key enzyme in OA, in this study, ADAMTS5 was
found to be markedly increased in OA chondrocytes, which was in
line with the findings of previous studies (22,33,34). ADAMTS5 (namely aggrecanase-2) are
members of the ADAMTS family. Together with ADAMTS4 (namely
aggrecanase-1), they have the ability to cleave the aggrecan core
protein (35,36), resulting in the destruction of
cartilage function, which is subsequently followed by irreversible
collagen degradation (22,37,38).
Previous research has confirmed that ADAMTS4 and ADAMTS5 are the
most efficient aggrecanases in vitro, but ADAMTS5 presents
more actively than ADAMTS4, and rather than ADAMTS4, ADAMTS5
participates in the early stages of development of OA (33,39). Moreover, the deletion of ADAMTS5
protects against OA by decreasing aggrecan degradation (40). Additionally, the intervention of
aggrecanases has been considered as an effective therapeutic method
for treatment of OA (37). In the
present study, we demonstrated the important role of ADAMTS5 in the
pathogenesis of OA. To confirm the function of ADAMTS5, we further
silenced the expression of ADAMTS5. Our results indicated that the
aggregation of proteoglycan and the collagen content were markedly
increased, but the release of proteoglycan and collagen was
significantly decreased following the suppression of ADAMTS5.
Moreover, the total collagen production was significantly higher,
and collagenase activity was markedly lower in the
siR-ADAMTS5-transfected group compared with the siNC-transfected
group. However, the silencing of ADAMTS5 did not have a marked
effect on the total proteoglycan production, indicating that
diminished breakdown induced the increased retention of
proteoglycan content, rather than the synthetic activity of
proteoglycan being increased. These results are in accordance with
those of a previous study conducted by Vonk et al (41), which suggests that ADAMTS5 plays
an important role in the pathogenesis of OA through the inhibition
of the aggregation of proteoglycan and collagen content and the
increase in the collagenase activity.
The expression of hsa-miR-15a was downregulated in
this study to confirm the protective effects of has-miR-15a against
OA. Opposite effects were observed in the amount of proteoglycan
and collagen in the cellular matrix and medium, the expression
level of collagen II, and collagenase activity compared with the
effects revealed by the suppression of ADAMTS5 expression. Our
results demonstrated that the aggregation of proteoglycan and
collagen content were significantly decreased, but the release of
proteoglycan and collagen was markedly increased in the
ASO-miR-15a-transfected compared with the normal group (ASO-NC).
Moreover, the total collagen production was significantly lower,
and collagenase activity was markedly higher following the
downregulation of miR-15a. These data suggest that hsa-miR-15a
exerts protective effects against OA by suppressing the expression
of ADAMTS5.
In conclusion, to the best of our knowledge, this
study provides the first evidence that has-miR-15 plays a
protective role in OA by directly targeting ADAMTS5. Our results
provide significant clinical evidence for future studies
investigating the beneficial effects of targeting ADAMTS5 as a
biological method for the treatment of OA.
References
|
1
|
Blalock D, Miller A, Tilley M and Wang J:
Joint instability and osteoarthritis. Clin Med Insights Arthritis
Musculoskelet Disord. 8:15–23. 2015.PubMed/NCBI
|
|
2
|
Lee AS, Ellman MB, Yan D, Kroin JS, Cole
BJ, van Wijnen AJ and Im HJ: A current review of molecular
mechanisms regarding osteoarthritis and pain. Gene. 527:440–447.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Loeser RF, Goldring SR, Scanzello CR and
Goldring MB: Osteoarthritis: a disease of the joint as an organ.
Arthritis Rheum. 64:1697–1707. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pereira D, Peleteiro B, Araujo J, Branco
J, Santos RA and Ramos E: The effect of osteoarthritis definition
on prevalence and incidence estimates: a systematic review.
Osteoarthritis and cartilage/OARS. Osteoarthritis Res Soc.
19:1270–1285. 2011. View Article : Google Scholar
|
|
5
|
Zhang Y, Jia J, Yang S, Liu X, Ye S and
Tian H: MicroRNA-21 controls the development of osteoarthritis by
targeting GDF-5 in chondrocytes. Exp Mol Med. 46:e792014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Litwic A, Edwards MH, Dennison EM and
Cooper C: Epidemiology and burden of osteoarthritis. Br Med Bull.
105:185–199. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Buckwalter JA, Saltzman C and Brown T: The
impact of osteoarthritis: implications for research. Clin Orthop
Relat Res. 427(Suppl): S6–S15. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wieland HA, Michaelis M, Kirschbaum BJ and
Rudolphi KA: Osteoarthritis - an untreatable disease? Nat Rev Drug
Discov. 4:331–344. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Flower RJ: The development of COX2
inhibitors. Nat Rev Drug Discov. 2:179–191. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Goldring MB and Marcu KB: Epigenomic and
microRNA-mediated regulation in cartilage development, homeostasis,
and osteoarthritis. Trends Mol Med. 18:109–118. 2012. View Article : Google Scholar :
|
|
11
|
Wu C, Tian B, Qu X, Liu F, Tang T, Qin A,
Zhu Z and Dai K: MicroRNAs play a role in chondrogenesis and
osteoarthritis (Review). Int J Mol Med. 34:13–23. 2014.PubMed/NCBI
|
|
12
|
Le LT, Swingler TE and Clark IM: Review:
The role of microRNAs in osteoarthritis and chondrogenesis.
Arthritis Rheum. 65:1963–1974. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sethupathy P and Collins FS: MicroRNA
target site polymorphisms and human disease. Trends Genet.
24:489–497. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Djuranovic S, Nahvi A and Green R:
miRNA-mediated gene silencing by translational repression followed
by mRNA deadenylation and decay. Science. 336:237–240. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Seeliger C1, Karpinski K, Haug AT, Vester
H, Schmitt A, Bauer JS and van Griensven M: Five freely circulating
miRNAs and bone tissue miRNAs are associated with osteoporotic
fractures. J Bone Miner Res. 29:1718–1728. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Iliopoulos D, Malizos KN, Oikonomou P and
Tsezou A: Integrative microRNA and proteomic approaches identify
novel osteoarthritis genes and their collaborative metabolic and
inflammatory networks. PLoS One. 3:e37402008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Swingler TE, Wheeler G, Carmont V, Elliott
HR, Barter MJ, Abu-Elmagd M, Donell ST, Boot-Handford RP,
Hajihosseini MK, Münsterberg A, et al: The expression and function
of microRNAs in chondrogenesis and osteoarthritis. Arthritis Rheum.
64:1909–1919. 2012. View Article : Google Scholar
|
|
19
|
Yu C, Chen WP and Wang XH: MicroRNA in
osteoarthritis. J Int Med Res. 39:1–9. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dudek KA, Lafont JE, Martinez-Sanchez A
and Murphy CL: Type II collagen expression is regulated by
tissue-specific miR-675 in human articular chondrocytes. J Biol
Chem. 285:24381–24387. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tardif G, Hum D, Pelletier JP, Duval N and
Martel-Pelletier J: Regulation of the IGFBP-5 and MMP-13 genes by
the microRNAs miR-140 and miR-27a in human osteoarthritic
chondrocytes. BMC Musculoskelet Disord. 10:1482009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bondeson J, Wainwright S, Hughes C and
Caterson B: The regulation of the ADAMTS4 and ADAMTS5 aggrecanases
in osteoarthritis: a review. Clin Exp Rheumatol. 26:139–145.
2008.PubMed/NCBI
|
|
23
|
Little CB and Fosang AJ: Is cartilage
matrix breakdown an appropriate therapeutic target in
osteoarthritis - insights from studies of aggrecan and collagen
proteolysis? Curr Drug Targets. 11:561–575. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Glasson SS, Askew R, Sheppard B, Carito B,
Blanchet T, Ma HL, Flannery CR, Peluso D, Kanki K, Yang Z, et al:
Deletion of active ADAMTS5 prevents cartilage degradation in a
murine model of osteoarthritis. Nature. 434:644–648. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Matsukawa T, Sakai T, Yonezawa T, Hiraiwa
H, Hamada T, Nakashima M, Ono Y, Ishizuka S, Nakahara H, Lotz MK,
et al: MicroRNA-125b regulates the expression of aggrecanase-1
(ADAMTS-4) in human osteoarthritic chondrocytes. Arthritis Res
Ther. 15:R282013. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Altman R, Asch E, Bloch D, Bole G,
Borenstein D, Brandt K, Christy W, Cooke TD, Greenwald R, Hochberg
M, et al: Development of criteria for the classification and
reporting of osteoarthritis. Classification of osteoarthritis of
the knee Diagnostic and Therapeutic Criteria Committee of the
American Rheumatism Association. Arthritis Rheum. 29:1039–1049.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lafont JE, Talma S and Murphy CL:
Hypoxia-inducible factor 2alpha is essential for hypoxic induction
of the human articular chondrocyte phenotype. Arthritis Rheum.
56:3297–3306. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang XJ, Ye H, Zeng CW, He B, Zhang H and
Chen YQ: Dysregulation of miR-15a and miR-214 in human pancreatic
cancer. J Hematol Oncol. 3:462010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pieper JS, van der Kraan PM, Hafmans T,
Kamp J, Buma P, van Susante JL, van den Berg WB, Veerkamp JH and
van Kuppevelt TH: Crosslinked type II collagen matrices:
Preparation, characterization, and potential for cartilage
engineering. Biomaterials. 23:3183–3192. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Papaioannou G, Lisse T and Kobayashi T:
miRNAs in bone formation and homeostasis. microRNA in Regenerative
Medicine. Sen CK: Elsevier Inc; London: pp. 350–380. 2015
|
|
31
|
Miyaki S and Asahara H: Macro view of
microRNA function in osteoarthritis. Nat Rev Rheumatol. 8:543–552.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Beyer C, Zampetaki A, Lin NY, Kleyer A,
Perricone C, Iagnocco A, Distler A, Langley SR, Gelse K, Sesselmann
S, et al: Signature of circulating microRNAs in osteoarthritis. Ann
Rheum Dis. 74:e182015. View Article : Google Scholar
|
|
33
|
Gendron C, Kashiwagi M, Lim NH, Enghild
JJ, Thøgersen IB, Hughes C, Caterson B and Nagase H: Proteolytic
activities of human ADAMTS-5: comparative studies with ADAMTS-4. J
Biol Chem. 282:18294–18306. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Thirunavukkarasu K, Pei Y and Wei T:
Characterization of the human ADAMTS-5 (aggrecanase-2) gene
promoter. Mol Biol Rep. 34:225–231. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Abbaszade I, Liu RQ, Yang F, Rosenfeld SA,
Ross OH, Link JR, Ellis DM, Tortorella MD, Pratta MA, Hollis JM, et
al: Cloning and characterization of ADAMTS11, an aggrecanase from
the ADAMTS family. J Biol Chem. 274:23443–23450. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tortorella MD, Burn TC, Pratta MA,
Abbaszade I, Hollis JM, Liu R, Rosenfeld SA, Copeland RA, Decicco
CP, Wynn R, et al: Purification and cloning of aggrecanase-1: a
member of the ADAMTS family of proteins. Science. 284:1664–1666.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Verma P and Dalal K: ADAMTS-4 and
ADAMTS-5: Key enzymes in osteoarthritis. J Cell Biochem.
112:3507–3514. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Song RHD, Tortorella MD, Malfait AM,
Alston JT, Yang Z, Arner EC and Griggs DW: Aggrecan degradation in
human articular cartilage explants is mediated by both ADAMTS-4 and
ADAMTS-5. Arthritis Rheum. 56:575–585. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Stanton H, Rogerson FM, East CJ, Golub SB,
Lawlor KE, Meeker CT, Little CB, Last K, Farmer PJ, Campbell IK, et
al: ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and
in vitro. Nature. 434:648–652. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Majumdar MK, Askew R, Schelling S, Stedman
N, Blanchet T, Hopkins B, Morris EA and Glasson SS: Double-knockout
of ADAMTS-4 and ADAMTS-5 in mice results in physiologically normal
animals and prevents the progression of osteoarthritis. Arthritis
Rheum. 56:3670–3674. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Vonk LA, Kragten AH, Dhert WJ, Saris DB
and Creemers LB: Overexpression of hsa-miR-148a promotes cartilage
production and inhibits cartilage degradation by osteoarthritic
chondrocytes. Osteoarthritis and cartilage/OARS. Osteoarthritis Res
Soc. 22:145–153. 2014. View Article : Google Scholar
|